Ventricular Arrhythmias in Heart Failure
Ventricular arrhythmias may present simultaneously with the diagnosis of systolic dysfunction or heart failure. Rarely, incessant or very frequent ventricular tachycardia (VT) can actually cause heart failure. More commonly, VT or ventricular fibrillation (VF) arises in patients with known systolic dysfunction as a result of aggravating factors such as recurrent ischemia, drug effects, or electrolyte abnormalities, or as a herald of progression of underlying heart failure. Therapeutic options and prognosis for ventricular arrhythmias in the setting of heart failure are constrained by the cause and the clinical severity of the heart failure. In patients with mild to modest symptoms of heart failure (New York Heart Association [NYHA] Classes I and II), the annual risk of sudden death ranges between 2% and 6%, accounting for most of the mortality. With more advanced heart failure (NYHA Classes III and IV), the risk rises to 5% to 12% per year.1 Although the frequency of ventricular arrhythmias increases as heart failure worsens, unexpected sudden death accounts for less than half of mortality in advanced heart failure. Those with the worst heart disease gain the least in terms of survival after an aborted arrhythmic event caused by death from pump failure and other related comorbidities.2,3
Evaluation of the Patient
Patients Presenting With Cardiac Arrest or Sustained Ventricular Tachycardia Without Prior Heart Failure Diagnosis
Ventricular arrhythmia or sudden cardiac death may be the initial manifestation of cardiac disease.4 With clinical stabilization, the focus is on diagnosis of the underlying cause and any precipitating factors, with emphasis on treatable conditions. Implantable cardioverter-defibrillators (ICDs) generally are indicated for secondary prevention, except in the setting of specific reversible causes or anticipated early death from refractory hemodynamic decompensation or other terminal conditions. Potentially reversible causes to be identified and treated include left ventricular (LV) dysfunction related to stunning or hibernation from ischemia, some of the inflammatory myocardiitides, thyroid dysfunction, and alcoholic cardiomyopathy (Figure 89-1).
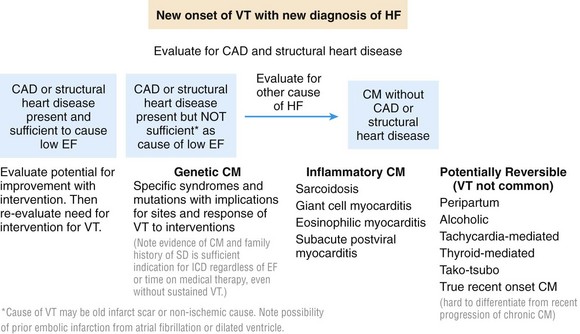
Figure 89-1 Examples of causes of cardiomyopathy with particular relevance for those associated with ventricular arrhythmias. CAD, Coronary artery disease; CM, cardiomyopathy; EF, ejection fraction; HF, heart failure; VT, ventricular tachycardia.
The electrocardiogram often provides the first clue to structural heart disease. Echocardiography is the first step taken to determine LV function, right ventricular (RV) function, and possible structural cardiac disease. Ventricular function is often worse after cardiac arrest or sustained VT, and reassessment after a period of recovery is typically necessary for a more accurate determination of the extent of underlying systolic impairment. In patients with low ejection fraction and extensive wall motion abnormalities, nuclear imaging may not be sensitive enough to exclude diffuse coronary artery disease. Hence, cardiac catheterization with coronary angiography should be performed to exclude or define coronary artery disease in patients for whom revascularization would be considered. Cardiac magnetic resonance (CMR) imaging is emerging as a valuable tool for detection of intramyocardial disease not readily evident on echocardiography, such as inflammation or focal scarring.5
Inflammatory Myocarditis
Cardiac sarcoidosis is a particularly important cause of cardiomyopathy that is often undetected until presentation with ventricular arrhythmias or a new conduction block. Less than half of patients with evidence of cardiac sarcoid at autopsy will have had clinically apparent cardiac involvement. Patchy noncaseating granulomas tend to involve the basal ventricular septum with disruption of cardiac conduction. When the right ventricle is predominantly involved, a normal left ventricular ejection fraction (LVEF) may be falsely reassuring. Sudden cardiac death accounts for two-thirds of terminal events in patients with overt cardiac sarcoidosis.6,7 Early presentation with heart block should prompt a search for cardiac sarcoidosis in patients with systolic dysfunction. In a review of unexplained heart block in patients younger than 55 years of age, cardiac sarcoid or giant cell myocarditis accounted for 25% of cases, and these patients had a high incidence of sudden death or ventricular tachycardia or the need for cardiac transplantation.8 Although endomyocardial biopsy provides a definitive diagnosis, sensitivity is low at 10% to 20% because of the patchy nature of the disease distribution. CMR with gadolinium enhancement and positron emission tomography (PET) scanning are becoming valuable aids in diagnosis.6,7
Giant cell myocarditis is generally characterized by a more fulminant course with worse outcomes than sarcoidosis. The granulomas are accompanied by a diffuse inflammatory infiltrate. Although generally considered as distinct entities on the basis of biopsy and presentation, sarcoidosis and giant cell myocarditis may represent different levels along a spectrum of inflammation with giant cells. Hearts showing sarcoidosis on endomyocardial biopsy have occasionally been found to have areas consistent with giant cell myocarditis after removal at the time of transplantation. Cases of patients in whom giant cell myocarditis has been stabilized for a year or longer by high-dose immunosuppressive therapy have been reported.9,10
Postviral myocarditis is probably much more common than has been recognized. More than half of patients presenting within 3 months of subacute heart failure symptoms and low ejection fraction will show improvement in the LV ejection fraction to about 40% within the next 6 to 12 months.10 Endomyocardial biopsy performed in these patients often shows mild hypertrophy without evidence of cellular inflammation or significant fibrosis. Although premature ventricular contractions (PVCs) may occur, sustained VT or VF is uncommon in early stages of presumed postviral myocarditis. It should be noted that Lyme disease has been reported rarely to cause clinical myocarditis with or without clinical conduction system disease.
Genetic Cardiomyopathy
Familial cardiomyopathies (CMs) account for 20% to 30% of cases of disease originally diagnosed as idiopathic dilated CM. Most cases are transmitted in an autosomal dominant pattern, but autosomal recessive, X-linked, and mitochondrial inheritance patterns are also well recognized. More than 30 genes have been identified as harboring mutations that cause familial dilated CM (DCM); however, mutations in these genes account for only half of the reported cases of familial DCM.11,12 Sarcomeric gene mutations account for one-third. Mutations in the gene coding for structural proteins of the nuclear lamina (lamin A and C) and in the SCN5A gene are particularly associated with conduction system disease and ventricular arrhythmias in association with DCM.12,13 Research findings suggest that abnormalities in the giant molecule titin may be among the most common causes of inherited CM frequently associated with ventricular arrhythmias.14
Arrhythmogenic RV cardiomyopathy (ARVC) due to defects in genes encoding for cardiac desmosomal proteins is predominantly an RV disease, but LV involvement is common and can precede RV manifestations.15 Approximately 50% of cases have a familial transmission with an autosomal dominant inheritance. Sudden death and ventricular arrhythmias are often the initial presentation frequently provoked by exercise. RV failure due to progressive myocardial loss is seen in later stages of the disease and may progress to a phase of biventricular failure that can mimic DCM. Autosomal recessive forms of ARVC due to defects in plakoglobin or desmoplakin result in cardiocutaneous syndromes that include Naxos disease and Carvajal syndrome.16
Tachycardia-Related Cardiomyopathy
Prolonged periods of all forms of tachycardia, including frequent PVCs, are well recognized as a cause of reversible CM. Ventricular arrhythmia is uncommon in tachycardia-related CM unless it is the causative tachycardia. The diagnosis of tachycardia CM is often a retrospective one based on recovery of ventricular function after treatment of the arrhythmia. In one retrospective analysis of 174 patients with idiopathic PVCs referred for catheter ablation, LV dysfunction was present in 33%.17 The smallest PVC burden resulting in LV dysfunction was 10% over a 24-hour period, but a 24% PVC burden best differentiated the group that demonstrated CM from the group with preserved LV function. Data also suggest that the probability of LV dysfunction is related to QRS duration of the PVCs; frequent PVCs with a wider QRS (>150 milliseconds) are associated with greater risk of CM.18 Frequent runs of nonsustained VT, PVCs, or supraventricular arrhythmias should also be considered a potential cause of deteriorating LV function in cases of previously stable cardiomyopathy of another origin.
Evaluation of Ventricular Arrhythmias in Patients With Known Heart Failure
The onset of ventricular arrhythmias in a previously stable heart failure patient may result from electrolyte abnormalities, ischemia, or intercurrent illness but often heralds the progression of underlying cardiac disease. Most clinically functional patients with LVEF <35% and long-standing heart failure symptoms will have an ICD in place. Analysis of the SCD-Heft trial data revealed that necessary ICD therapies were provided to 16% of patients over a mean follow-up of 45 months.19 The event rate is higher for patients who receive an ICD for secondary prevention.20 Several studies have shown an association of ICD shocks with reduced survival, with heart failure being an important cause of mortality.19,21
Ventricular arrhythmia in a patient with an ICD should prompt careful patient reassessment, even though the ICD may have promptly restored sinus rhythm. The original diagnosis may need to be reevaluated. In heart failure with known coronary artery disease, ischemia and infarction that contribute to hemodynamic deterioration and arrhythmias may occur without recognized angina. Baseline abnormalities of cardiomyopathy may mask typical diagnostic features; therefore perfusion stress imaging or coronary angiography is often required. In a significant proportion of patients after ICD shock, progressive ventricular remodeling is often evidenced by ventricular enlargement and declining LV ejection fraction.22 Development of bundle branch block by dys-synchronous contraction can further contribute to adverse ventricular remodeling.
Identifying the type of ventricular arrhythmia is helpful in determining the cause. Monomorphic VT usually represents reentry involving regions of scar from prior infarction, inflammation, or surgery. A mix of fibrosis and surviving strands of myocardium allows for reentrant VTs that have a high recurrence rate—20% per year.23 Polymorphic VT is often associated with changing arrhythmia substrates. Migratory circuits around scars can lead to polymorphic VT, but acute myocardial ischemia and electrolyte disturbances should be major considerations because they are often easier to correct. Adverse effects of drugs that prolong the QT interval are more common in heart failure. Torsade de pointes VT typically induced by drugs such as sotalol and dofetilide often requires specific interventions such as discontinuation of the offending drug, intravenous administration of magnesium, correction of electrolyte derangements, and, occasionally, pacing at faster rates.
Less frequently, implanted devices themselves may exert proarrhythmic effects. Pacing algorithms designed to minimize ventricular pacing are recognized to induce VT/VF through inadvertent induction of long-short intervals.24 Sinus tachycardia or supraventricular tachycardia detected in the VT zone of an ICD can trigger unnecessary antitachycardia pacing to induce VT or VF. The appearance of ventricular arrhythmia in close temporal proximity to the start of LV epicardial pacing should raise the possibility of related proarrhythmia.25 Inactivation of LV pacing may be necessary. If loss of cardiac resynchronization therapy (CRT) results in hemodynamic deterioration, cautious reinstitution of LV pacing after control of arrhythmia with drugs or ablation is attained is a possibility. Rarely, RV leads can trigger PVCs or sustained ventricular VT or VF. Repositioning of the lead has occasionally resulted in resolution of VT in such patients.26
Triage and Management of Heart Failure
Assessment of Compensation
Evaluation of heart failure disease severity is a major component of initial and serial management. Bedside assessment of baseline hemodynamic status allows broad triage into four hemodynamic profiles based on whether clinical evidence supports the presence of elevated filling pressures (wet or dry) and the adequacy or critical compromise of systemic perfusion (warm or cold) (Figure 89-2).27 In the setting of chronic heart failure, rales and peripheral edema are frequently absent despite severely elevated filling pressures, which should be suspected in patients with orthopnea or dyspnea on minimal exertion such as that associated with getting dressed. High pressures in chronic heart failure are best detected through experienced evaluation of jugular venous pressure and may also be identified from echocardiographic findings of dilated inferior vena cava and elevated pulmonary artery pressures. Hypoperfusion should be suspected in patients with a narrow pulse pressure, even if the systolic blood pressure is preserved, and in patients with vague mental status and cool calves and forearms. Many patients with hypoperfusion will have been intolerant of attempts to uptitrate ACEIs or β-blockers given to treat hypotension. Because functional capacity is not well correlated with the ejection fraction, careful questioning about daily activities and limiting symptoms is crucial for assessment of disease trajectory.
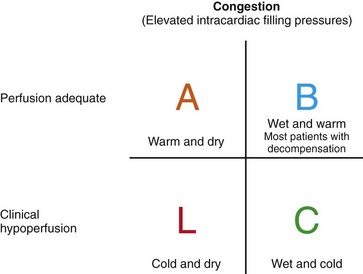
Figure 89-2 Simple 2 × 2 table to indicate the general hemodynamic profiles of patients with low ejection fraction. Most are able to maintain good perfusion without evidence of elevated intracardiac filling pressures—“warm and dry.” Profile B is the most common profile of decompensation—“warm and wet”—which requires thorough diuresis. The patient presenting with profile C—“cold and wet”—usually requires major intervention before procedures can be performed safely and may in some cases require triage for assessment of potential candidacy for transplantation or mechanical circulatory support. Very few patients present with profile L—“cold and dry”—unless they have recently been treated aggressively. Such patients generally do not have resting symptoms but may have very limited cardiac reserve. (Adapted from Nohria A, Tsang SW, Fang JC, et al: Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 41:1797-1804, 2003.)
Titration of Neurohormonal Antagonists
Baseline hemodynamic compromise requires aggressive correction, which, in most cases, should precede procedural interventions or initiation of neurohormonal antagonist therapies. β-Blocking agents and inhibitors of the renin-angiotensin system are remarkably beneficial over the long term but when given acutely can exacerbate hemodynamic compromise. When hemodynamic stabilization cannot be achieved, or when the hemodynamic profile remains uncertain, right-heart catheterization can be useful for clarifying right- and left-sided filling pressures, perfusion, and systemic and pulmonary vascular resistance to guide further therapy. Intravenous diuretic therapy is the mainstay of acute therapy for congestion. If inotropic therapy has been initiated to treat hypoperfusion and to improve diuretic response, neurohormonal antagonists should be initiated in small doses and escalated gradually. Careful uptitration over weeks to months is required to individualize “optimal” dosing, which frequently is lower than in trial populations, particularly for elderly patients.28
Heart Failure Disease Progression
The Pivotal Right Ventricle
The role of the right ventricle in the progression of heart failure has been underrecognized and still remains difficult to track because of the difficulty associated with routine imaging of this chamber. Some causes of heart failure such as sarcoidosis and ARVC can affect the right ventricle early. The causes of heart failure in younger patients often affect both ventricles similarly from the onset. However, the main challenge to the right ventricle in chronic heart failure is usually presented by progressive left-sided congestion, as worsening mitral regurgitation and higher LV filling pressures contribute to secondary pulmonary hypertension and subsequent right-heart failure. RV dilatation and tricuspid regurgitation then contribute to renal dysfunction and further fluid retention. Chronic congestion of the liver and of the digestive system leads to malnutrition and increases inflammatory mediators in late-stage disease. In addition to indicating worse outcomes with heart failure, serious RV dysfunction identified on echocardiography increases risk and decreases “bail-out” options for procedures because it is a contraindication to long-term mechanical circulatory support.29
Cardio-Renal Interactions
The interaction between the heart and the kidneys is complex and is integral to compensation and decompensation.30,31 For most patients, fluid retention usually begins before any clinical evidence of hypoperfusion is noted. Once attributed solely to decreased cardiac output, renal dysfunction with progressive heart failure is now recognized to be influenced more by elevated right-sided filling pressures. The rate of heart failure progression is accelerated by even modest degrees of renal impairment. The dynamic nature of renal function in advanced heart failure is crucial to recognize in relation to clearance of antiarrhythmic agents and risk of life-threatening hyperkalemia in the setting of aldosterone antagonists.
Risk Stratification
Within a given clinical status, risk factors include higher creatinine and blood urea nitrogen (BUN), lower systolic blood pressure, increased mitral and tricuspid regurgitation, higher ventricular filling pressures and pulmonary pressures, worse RV function, higher natriuretic peptide levels, and lower serum sodium. Risk scores can facilitate selection of risk groups for clinical trials, triage for referral for advanced therapies such as transplantation, and timing of discussions regarding patient preferences.32,33 However, most scores, such as the Seattle Heart Failure Score derived from heart failure trials or the Heart Failure Survival Score derived from transplant candidates, do not apply to general community populations, in which most patients are over the age of 70, many over 80, and have multiple comorbidities. In these populations, functional status, systolic blood pressure, renal function, and natriuretic peptide levels are the universal predictors.33 Of greatest relevance to clinical decisions about treating tachyarrhythmias are the identification of patients at high risk for hemodynamic decompensation with procedures and the competing risks of primary arrhythmias, heart failure events, and events resulting from comorbidities (Table 89-1).
Table 89-1
Indicators of Advanced Heart Failure
• Repeated (≥2) hospitalizations or ER visits for heart failure in the last year
• Persistent dyspnea with dressing or bathing, requiring rest
• Inability to walk one block on level ground because of dyspnea or fatigue
• Frequent systolic blood pressure <90 mm Hg
• Progressive deterioration in renal function (rise in creatinine and/or BUN)
• Progressive decline in serum sodium, usually to <133 mEq/L
• Intolerance of ACEIs caused by hypotension and/or worsening renal function
• Intolerance of β-blockers as the result of worsening heart failure symptoms or hypotension
• Recent need to escalate diuretics to maintain volume status, often reaching a daily furosemide equivalent dose of over 160 mg a day and/or use of supplemental metolazone therapy
Options for Advanced Heart Failure
Patients with persistent symptoms that limit daily life (Classes III and IV) despite standard medical therapy are generally considered to have advanced heart failure, usually with LVEF <25% and more than two hospitalizations within a year, with common descriptors as provided in Table 89-1. Before designating patients as refractory, it is important to ensure that diuretics have been aggressively titrated to relieve all evidence of congestion, that salt and fluid restrictions have been emphasized, and that neurohormonal antagonists have been reevaluated and sometimes downtitrated to allow adequate blood pressure and renal function. Treatable rhythm disorders such as rapid ventricular response in AF and left bundle branch block (LBBB) with QRS ≥150 should have been addressed. A key point of triage is to consider the question, “Would you be surprised if this patient died within the next year?”
Cardiac Transplantation and Mechanical Circulatory Support
These advanced interventions that exchange one chronic disease for another are considered in many more patients than are ultimately found to be eligible. Cardiac transplantation is limited by the fixed donor supply to about 2200 patients per year in the United States, with 10-year survival now exceeding 50%, limited primarily by allograft vascular disease and malignancy. Approximately twice as many durable mechanical circulatory support devices are now implanted annually—about one-third for anticipated lifetime “destination” therapy and the others with hopeful anticipation of transplantation at some time in the future. Two-year survival with the currently implanted continuous flow devices now exceeds 60%, with a cerebrovascular event rate of about one in ten and higher rates of drive-line infection and gastrointestinal bleeding.34,35 The cost of the device, the hospitalizations, and the infrastructure required for perioperative and long-term support currently sum to more than $100,000 per life-year gained. Although the supply of devices is not constrained as the supply of donor hearts is, the high cost of this care mandates that device candidates be carefully selected from the estimated U.S. population of more than 150,000 patients with advanced heart failure who might benefit.34
Advanced Heart Failure in the Community
For patients with advanced heart failure in the general community, frailty and other comorbidities usually preclude the option of cardiac transplantation or lifetime mechanical circulatory support. Care teams making and accepting referrals for complex procedures should consider the overall picture of competing risks when contemplating further intervention. For patients with advanced heart failure, prognosis, patient preferences, and “what if” discussions should be reviewed at least annually, and again after milestones are reached, as indicated in Table 89-1. The end of life is too often preceded by an abrupt reversal in strategy within hours to days of death. In many cases, however, the priority of care ideally would shift more gradually from length of life to quality of life. Before actual decisions are made about the scene and supporting cast for the final days, it is often appropriate to increase focus on relieving the burden of symptoms and worries.
Management of Ventricular Arrhythmias in Heart Failure
Indications and Timing for ICD Implantation
ICDs provide the best means of primary and secondary prevention of sudden death resulting from VT or VF.36 The impact of ICDs in prolonging meaningful life depends primarily on underlying heart failure disease progression and the severity of other comorbidities. Successful termination of a potentially lethal ventricular arrhythmia extends meaningful life when the patient has well-compensated heart failure and returns to a good prearrhythmia functional state. The benefits of ICD therapy follow a U-shaped curve (Figure 89-3).37 Patients with mild stable heart failure are at low arrhythmic risk and consequently benefit little from an ICD during early follow-up. With increasing severity of heart failure, the incidence of VT/VF increases and the ICD becomes more valuable. However, as heart failure progresses farther to the point where pump failure outstrips arrhythmic death as the predominant risk, termination of VT/VF has minimal impact in prolonging meaningful life and occasionally can prolong death. In the absence of eligibility for CRT or transplantation, patients with refractory heart failure symptoms (Class IV) should not receive an ICD (Figure 89-4).
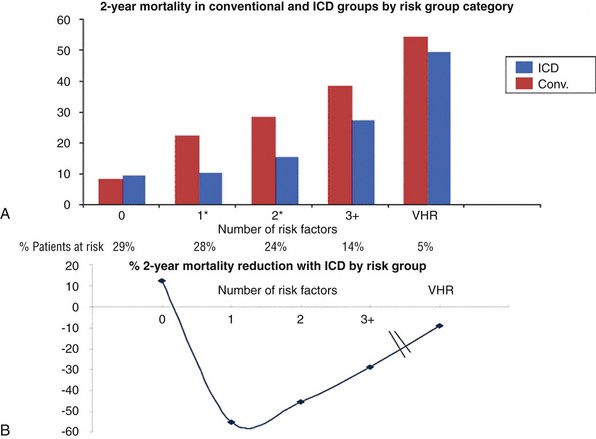
Figure 89-3 U-Shaped Curve for ICD Efficacy in Patients With Low LVEF Compared With Patients in the Conventional and ICD Arms of the MADIT Study of ICDs for Primary Prevention of Sudden Death
A, Shows 2-year Kaplan-Meier mortality rates for the two groups based on additional comorbidities defined as risk factors. B, Depicts the corresponding mortality reduction achieved with an ICD. The benefit derived from use of an ICD recedes as the number of risk factors increases. VHR, Very high risk. (From Goldenberg I, Vyas AK, Hall J, et al: Risk stratification for primary implantation of a cardioverter defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 51:288-296, 2008.)
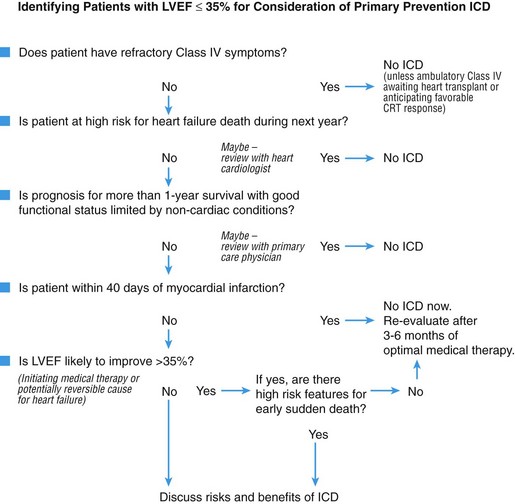
Figure 89-4 Algorithm for selection of patients with heart failure for primary prevention ICD implantation. (Adapted from Stevenson LW, Desai AS: Selecting patients for discussion of the ICD in primary prevention for sudden death in heart failure. J Cardiac Fail 12:407-412, 2006.)
The timing of ICD implantation for primary prevention of sudden death in heart failure should generally be deferred when ventricular function may improve spontaneously or with therapy. Current guidelines for patients with ischemic cardiomyopathy require that ventricular function must be reassessed 40 days after an acute myocardial infarction and 90 days after revascularization. These guidelines are supported by clinical trials showing limited benefit of ICDs in the early phase after an acute myocardial infarction, when recurrent ischemia, cardiac rupture, or pump failure can negate any benefit of arrhythmia termination by an ICD.38,39 For patients with nonischemic cardiomyopathy, the recommended waiting period for ICD implantation after initial diagnosis is more contentious. Studies that evaluated the time dependence of risk for sudden death relative to the time of diagnosis failed to demonstrate late versus early risk.36 Nevertheless, a significant number of patients with an initial diagnosis of nonischemic cardiomyopathy will improve spontaneously or with medical treatment. In the absence of syncope, high-risk events, or a family history of sudden death, it is appropriate to wait for reassessment of ejection fraction after a period of adequate medical therapy.
Imaging modalities such as CMR can be helpful in assessing risk of arrhythmia.40,41 In the study by Wu et al, myocardial fibrosis as detected by late gadolinium enhancement on CMR imaging was associated with an eightfold increased risk of adverse events that included cardiac death, ICD firing, and hospitalizations.5 The presence of replacement fibrosis as seen on magnetic resonance studies is used by many as an indication of the need for earlier ICD implant, although no large prospective trials have been conducted to support this strategy.
Introducing the ICD to the Patient
Patients have difficulty grasping percentages of mortality and survival, which may be 0 or 100% at any given time. Some patients with heart failure with and without ICDs have overestimated their projected life span and the likelihood that the ICD will save their lives. Many who have never undergone rhythm therapy feel that the device has already saved their life. Some assume that the ICD will treat the disease of heart failure and improve their symptoms, and few are aware that the ICD can prevent only one mode of death. It has been suggested that patients relate best to information given in the following form: “For 100 patients with disease like yours…” An information format outlining the benefits of the ICD for primary prevention of sudden death in heart failure has been developed based on findings from the SCD-HeFT and MADIT trials (Figure 89-5).42
Combining ICD Implantation With Cardiac Resynchronization Therapy
In selected patients with LVEF <30% to 35% and appropriate QRS prolongation, CRT can reduce symptoms, reverse ventricular remodeling, and reduce mortality with or without an ICD.36 CRT has been well studied in randomized clinical trials involving more than 6000 patients and has demonstrated favorable structural remodeling with improved LV function and reduced mitral regurgitation in approximately 70% of patients. Recent trials of CRT in less symptomatic patients (NYHA Classes I and II) show a reduction in composite end points of heart failure hospitalization and death, but mortality reduction is limited to Class II patients.43,44 CRT device implantation is more difficult than placement of a non-CRT pacemaker or ICD, and complication rates are greater, usually as a result of the additional manipulations required for the lead and its delivery systems. Lead dislodgment requiring revision is particularly more common.45 Patient selection for this therapy is therefore critical. In post hoc subgroup analyses of clinical trials, factors associated with the greatest benefit from CRT include nonischemic dilated cardiomyopathy, the presence of LBBB, and QRS duration >150 milliseconds.46
ICD Generator Replacement
With the 5- to 10-year longevity of modern ICDs, generator replacement for battery depletion accounts for 25% of overall ICD implants. Complications of ICD generator change are significant and can be as high as 5%. Hence, the default practice of generator replacement in all patients has recently been questioned.47 A number of patients will develop significant comorbidities and will progress in their heart failure status to Class IV, prompting a review of the need for continued ICD therapy. Anticipated survival with a reasonable quality of life remains the major indication for generator replacement and is the currently accepted indication for an initial ICD implant. Some patients will recover their LVEF over time. Decisions regarding generator change are more difficult in this population. If a diagnosis of reversible cardiomyopathy becomes clear, as with control of tachycardia or resolution of myocarditis, the impetus to consider discontinuation of ICD therapy is greater. However, it should be borne in mind that certain familial cardiomyopathies might demonstrate fluctuating levels of ventricular dysfunction while sudden death risk may remain unchanged (see earlier).
ICD Deactivation
When symptoms of heart failure or other terminal disease become refractory, it is the responsibility of the medical team to initiate discussions about ICD deactivation. Patients and families, who often have an unrealistic perception of the actual function and benefit of the ICD and the ways in which death can occur, will rarely broach this subject. This discussion seems to be easier for patients for whom initial ICD teaching included the possibility of deactivation (see Figure 89-5). Although it is strongly recommended, deactivation of the ICD is not mandatory for a peaceful death with dignity. It has been estimated that up to 30% of deaths in ICD recipients may be accompanied by shocks; however, the patients most likely to suffer repeated shocks while dying are those who have experienced repeated shocks before. VT storm is a rare mode of death in refractory Class IV heart failure, for which the most common terminal rhythm is progressive bradycardia with pulseless electrical activity that does not trigger defibrillation.
Antiarrhythmic Drugs in Heart Failure
Amiodarone
Amiodarone has been most extensively studied in patients with heart failure. Apart from one study that showed increased noncardiac mortality in Class III heart failure, most studies have described neutral effects on mortality or benefit in reducing arrhythmia-related deaths.48 Amiodarone in combination with a β-blocker is the most effective pharmacologic intervention for reducing ICD shocks.49 Bradycardia remains a limitation and occurs in 1% to 7% of patients in large trials; it is more common in clinical use. In patients with sinus node or atrioventricular nodal disease, the drug has the potential for marked bradycardia and worsening heart failure symptoms. In addition, amiodarone may lead to ventricular pacing in patients with implanted defibrillators, thereby provoking ventricular dys-synchrony.
In stable patients, loading doses of amiodarone 600 to 800 mg daily are usually well tolerated. However, in patients with decompensated heart failure, high doses can potentially worsen clinical status by slowing heart rate without allowing for increased stroke volume. Slow titration can often avoid this initial negative inotropic effect. The noncardiac toxicity of amiodarone can impact the course of heart failure adversely. Hyperthyroidism can lead to acute hemodynamic instability, often with onset of AF with rapid ventricular rates and intractable ventricular arrhythmias. In one study, amiodarone-induced thyrotoxicosis was associated with a 2.7-fold increase in major adverse cardiac events, driven primarily by higher rates of ventricular arrhythmia requiring hospitalization.50 Amiodarone lung toxicity occurs at a rate of 1% per year, and risk increases with daily doses that exceed 300 mg. Differentiation from pulmonary congestion may require right-heart catheterization and/or bronchoalveolar lavage.
Other Class 3 Drugs
Dronedarone is a newer antiarrhythmic drug approved for the management of AF. Despite its structural similarity to amiodarone (but without the iodine moiety), the drug is less effective in control of AF. Of concern in heart failure patients is that two large studies demonstrated increased mortality.51,52 The mechanism for increased mortality is unclear. Dronedarone is currently avoided in heart failure patients.
Ablation for Ventricular Arrhythmias in Heart Failure
For patients with monomorphic VT related to previous myocardial infarction, experience with catheter ablation is considerable. In experienced centers, the procedure can reduce VT episodes and ICD shocks in 70% of patients with drug-refractory VT.23,53,54 The efficacy of catheter ablation for other types of heart disease such as nonischemic cardiomyopathy and arrhythmogenic RV cardiomyopathy is less well defined and is limited to single-center experiences. Reentrant circuits in these diseases tend to be located deeper within the myocardium and more often require epicardial ablation. Catheter ablation for VT generally requires significant expertise, and epicardial ablations carry higher risks.55 Nevertheless, catheter ablation can be life-saving in patients with incessant VT and in those presenting with electrical storm.56 In experienced centers, catheter ablation is now an early consideration in the course of management of recurrent monomorphic VT to avoid exposing patients to toxic drugs and risking multiple ICD shocks with associated posttraumatic stress disorder.23,57 Procedure-related mortality for VT ablation is in the range of 3%, but most deaths are due to continued VT when ablation attempts have failed to control VT.
In patients with advanced heart failure, prolonged procedure times, fluid load, and arrhythmias associated with ablation for VT can precipitate acute decompensation. Circulatory support is being used increasingly during VT ablation procedures.58 Strategies for management of uncommon but known complications of VT ablation such as ventricular perforation and damage to coronary arteries should be in place and should be tempered by considerations of overall prognosis in end-stage heart failure. In patients with heart failure and severe hemodynamic compromise, expedited review of potential eligibility for transplantation or long-term mechanical circulatory support before the procedure is performed may simplify difficult decisions if the ablation procedure does not go well. Even for eligible patients, use of an implantable circulatory support device is not indicated for urgent rescue from cardiogenic shock; for this purpose, a temporary external device would be used, with the potential to bridge to transplantation or more durable circulatory support if the patient stabilizes adequately. It should be recalled that the patient with severe RV dysfunction who is not a transplant candidate would not be eligible to go on to lifetime mechanical circulatory support, which with current devices provides support only for the left heart.
Ventricular Arrhythmias After Cardiac Transplantation
Allograft vasculopathy and ventricular dysfunction can lead to sudden death in a significant number of heart transplant patients. The documented incidence of VF as the first arrhythmia in sudden death is, however, lower (10%) in transplanted patients than in the general population, possibly secondary to sympathetic denervation.59 Both sustained and nonsustained cases of VT portend a poor prognosis when they occur late after transplantation. Mechanisms include ischemic myocardial damage from allograft vasculopathy, cumulative injury from repeated inflammatory reactions, and scarring due to rejection episodes.60 The exact role of ICDs in primary prevention is not well defined, and most centers use conventional criteria of risk that include severe LV dysfunction (EF <35%), syncope with inducible VT, and severe allograft vasculopathy. In a retrospective study of 36 transplant recipients undergoing ICD implant for primary and secondary prevention, appropriate shocks for VT or VF occurred in 28% of patients. It should be noted that defibrillator implants in heart transplant recipients are associated with a higher rate of complications, including infection and lead complications.61
Management of Ventricular Arrhythmias in Patients With Ventricular Assist Devices
The use of ventricular assist devices (VADs) as a bridge to transplantation and as destination “lifetime” therapy has become more common and successful.34 As a result, ventricular arrhythmias have gained greater importance in this patient population. Most patients who are candidates for LV assist devices (LVADs) will have ICDs already in place. The overall prevalence of ventricular arrhythmia in LVAD patients is similar to that in a population with comparable LV dysfunction and heart failure,62 but ventricular arrhythmias can resolve after LVAD, can persist, or can appear for the first time after LVAD. The LVAD procedure involves the addition of an inflow cannula to the LV apex, which increases the risk of ventricular arrhythmia through formation of a nidus for reentry as well as by mechanical stimulation of the endocardium by the cannula. The latter is more common when the LV is collapsed from intravascular volume depletion or as the result of suction events with high LVAD flow rates. Lowering the LVAD speed can often be helpful in reducing nonsustained VT that precipitates sustained arrhythmias.
Currently available durable devices support only the left ventricle (LVAD), leaving RV output vulnerable to ventricular arrhythmias, which thus can compromise LV inflow. Although rarely lethal, ventricular arrhythmias can cause syncope and injury in patients, particularly in the upright position. Management of ventricular arrhythmias in LVAD patients includes the use of antiarrhythmic drugs such as amiodarone, mexiletine, and quinidine. Catheter ablation is attempted in refractory cases but is technically more difficult because of the collapsed ventricle.63 Traversing the aortic valve may prove difficult, necessitating a transseptal approach.
Summary
The complex interaction between ventricular arrhythmia and systolic heart failure warrants careful incorporation of heart failure status and prognosis in decisions regarding management of VT or VF. Key points to consider are summarized in Table 89-2. Optimizing hemodynamic status before providing elective interventions for ventricular arrhythmias offers the best chance for successful outcomes.
Table 89-2
Ventricular Arrhythmias in Heart Failure: Key Points
• New-onset heart failure and ventricular tachyarrhythmias should raise concern for inflammatory heart disease.
• Evidence of hemodynamic compromise (high filling pressures with or without hypoperfusion) should be sought and the situation addressed before elective arrhythmia procedures are undertaken.
• Treatment with β-blockers and amiodarone can worsen heart failure acutely, although, if tolerated, it is often beneficial for the long term.
• Consider competing risks of primary arrhythmic events and death from pump failure or comorbidities when planning therapy to treat or prevent life-threatening arrhythmic events.
• Timely deactivation of ICD requires proactive consideration by the care team.
• Most anticipated heart failure deaths occur with bradycardia and/or pulseless electrical activity.
• Mechanical circulatory support for acute decompensation should be initiated with a short-term device rather than an implantable device as a bridge to medical stabilization or more durable mechanical support.
• Evidence of severe right ventricular failure will preclude mechanical support with current implantable devices for destination (lifetime) therapy.
References
1. Jessup, M, Abraham, WT, Casey, DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009; 119:1977–2016.
2. Koller, MT, Schaer, B, Wolbers, M, et al. Death without prior appropriate implantable cardioverter-defibrillator therapy: A competing risk study. Circulation. 2008; 117:1918–1926.
3. Levy, WC, Lee, KL, Hellkamp, AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009; 120:835–842.
4. Deo, R, Albert, CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012; 125:620–637.
5. Wu, K, Weiss, RG, Kitagawa, K, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in non-ischemic cardiomyopathy. J Am Coll Cardiol. 2008; 51:2414–2421.
6. Sekhri, V, DeLorenzo, LJ, Aronow, WS, et al. Cardiac sarcoidosis: A comprehensive review. Arch Med Sci. 2011; 4:546–554.
7. Iannuzzi, MC, Rybicki, BA, Teristein, AS. Sarcoidosis. N Engl J Med. 2007; 357:2153–2165.
8. Kandolin, R, Lehtonen, J, Kupari, M. Cardiac sarcoidosis and giant cell myocarditis as cause of atrio-ventricular block in young and middle aged adults. Circ Arrhythm Electrophysiol. 2011; 4:303–309.
9. Cooper, LT, Hare, JM, Tazelaar, HD, et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol. 2008; 102:1535.
10. Kindermann, I, Barth, C, Mahfoud, F, et al. Update on myocarditis. J Am Coll Cardiol. 2012; 59:779–792.
11. Hershberger, RE, Lindenfield, J, Mestroni, L, et al. Genetic evaluation of cardiomyopathy—Heart Failure Society of America practice guidelines. J Card Fail. 2009; 15:83.
12. Ackerman, MJ, Prior, SG, Willems, S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. Europace. 2011; 13:1077.
13. Van Tintelen, JP, Tio, RA, Kerstjens-Frederickse, WS, et al. Severe myocardial fibrosis caused by deletion of 5′ end of the lamin A/C gene. J Am Coll Cardiol. 2007; 49:2430.
14. Herman, DS, Lam, L, Taylor, MR, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012; 16:619–628.
15. Sen-Chowdhry, S, Syrris, P, Ward, D, et al. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of genetic expression. Circulation. 2007; 115:1710–1720.
16. Basso, C, Corrado, D, Marcus, FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2007; 373:1289–1300.
17. Baman, TS, Lange, DC, Ilg, KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010; 7:865.
18. Yokokawa, M, Kim, HM, Good, E, et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012; 9:1460–1464.
19. Poole, JE, Johnson, GW, Hellkamp, AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008; 359:1009–1017.
20. Saxon, LA, Hayes, DL, Gilliam, R, et al. Long term outcome after ICD and CRT implantation and influence of remote device follow-up: The ALTITUDE Survival Study. Circulation. 2010; 122:2359–2367.
21. Daubert, JP, Zareba, W, Cannon, DS, et al. for the MADIT II Investigators: Inappropriate implantable cardioverter-defibrillator shocks in the MADIT II study: Frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008; 51:1357–1365.
22. Sutton, MS, Lee, D, Rouleau, JL, et al. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation. 2003; 107:2577–2582.
23. Kuck, KH, Schaumann, A, Eckhardt, L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): A multicenter randomized controlled trial. Lancet. 2010; 375:31–40.
24. Sweeney, MO, Ruetz, LL, Belk, P, et al. Bradycardia pacing induced short long short sequences at the onset of ventricular tachyarrhythmias: A possible mechanism of proarrhythmia? J Am Coll Cardiol. 2007; 50:614–622.
25. Turitto, G, El-Sheriff, N. Cardiac resynchronization therapy: A review of proarrhythmic and antiarrhythmic mechanisms. Pacing Clin Electrophysiol. 2007; 30:115–122.
26. Lee, JC, Epstein, LM, Huffer, LL, et al. ICD lead proarrhythmia cured by lead extraction. Heart Rhythm. 2009; 6:613–618.
27. Nohria, A, Tsang, SW, Fang, JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003; 41:1797–1804.
28. Nohria, A, Lewis, E, Stevenson, LW. Medical management of advanced heart failure. JAMA. 2002; 287:628–640.
29. Haddad, F, Doyle, R, Murphy, D, et al. Right ventricular function in cardiovascular disease, Part II. Pathophysiology, clinical importance and management of right ventricular failure. Circulation. 2008; 117:1717–1731.
30. Haywood, JT, Fonarow, GC, Costanzo, MR, et al. ADHERE Scientific Advisory Committee and Investigators: High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. J Card Fail. 2007; 13:422–430.
31. Butler, J, Chirovsky, D, Phatak, H, et al. Renal function, health outcomes and resource utilization in acute heart failure. Circ Heart Failure. 2010; 3:726–745.
32. Peterson, PA, Rumsfeld, JS, Ling, L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association Get With the Guidelines program. Circ Cardiovasc Qual Outcomes. 2010; 3:25–32.
33. Abraham, WT, Fonarow, GC, Albert, NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol. 2008; 52:347–356.
34. Kirklin, JK, Naftel, DC, Kormos, RL, et al. The fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Transplant. 2012; 31:117–126.
35. Stewart, GC, Givertz, MM. Mechanical circulatory support for advanced heart failure: Patients and technology in evolution. Circulation. 2012; 125:1304–1315.
36. Epstein, AE, DiMarco, JP, Ellenbogen, KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2008; 51:1–62.
37. Goldenberg, I, Vyas, AK, Hall, J, et al. Risk stratification for primary implantation of a cardioverter defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008; 51:288–296.
38. Steinbeck, G, Andresen, D, Seidl, K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009; 361:1427–1436.
39. Pouleur, AC, Barkoudah, E, Uno, H, et al. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010; 122:597–602.
40. Green, JJ, Berger, JS, Kramer, CM, et al. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2012; 5:370–377.
41. Iles, L, Pfluger, H, Lefkovits, L, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011; 57:821–828.
42. Stevenson, LW, Desai, AS. Selecting patients for discussion of the ICD in primary prevention for sudden death in heart failure. J Cardiac Fail. 2006; 12:407–412.
43. Tang, ASL, Wells, GA, Talajic, M, et al. Cardiac-resynchronization therapy for mild to moderate heart failure. N Engl J Med. 2010; 363:2385–2395.
44. Moss, AJ, Hall, WJ, Cannon, DS, et al. for the MADIT-CRT Trial Investigators: Cardiac resynchronization therapy for the prevention of heart failure events. N Engl J Med. 2009; 361:1329–1338.
45. van Rees, JB, de Bie, MK, Thijssen, J, et al. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: A systematic review of randomized clinical trials. J Am Coll Cardiol. 2011; 58:995–1000.
46. Zareba, W, Klein, H, Cygankiewicz, I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator trial—cardiac resynchronization therapy (MADIT-CRT). Circulation. 2011; 123:1061–1072.
47. Kramer, DB, Buxton, AE, Zimetbaum, PJ. Time for a change—a new approach to ICD replacements. N Engl J Med. 2012; 366:291–293.
48. Packer, DL, Prutkin, JM, Hellkamp, AS, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: Analysis from the sudden cardiac death in heart failure trial. Circulation. 2009; 120:2170–2176.
49. Connolly, SJ, Dorian, P, Roberts, RS, et al. Comparison of beta blockers, amiodarone plus beta blockers or sotalol for prevention of shocks from implantable cardioverter defibrillators. The OPTIC study: A randomized trial. JAMA. 2006; 295:165–171.
50. Yiu, KH, Jim, MH, Siu, CW, et al. Amiodarone induced thyrotoxicosis is a predictor of adverse cardiovascular outcome. J Clin Endocrinol Metab. 2009; 94:109–114.
51. Keber, L, Topr-Pedersen, C, McMurray, JJ. Dronedarone Study Group: Increases mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008; 358:2678–2687.
52. Connoly, SJ, Camm, AJ, Halperin, JL, et al. for PALLAS Investigators: Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011; 365:2268–2276.
53. John, RM, Stevenson, WG. Catheter-based ablation for ventricular arrhythmias. Curr Cardiol Rep. 2011; 13:399–406.
54. Stevenson, WG, Wilber, DJ, Natale, A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: The multicenter thermacool ventricular tachycardia ablation trial. Circulation. 2008; 118:2773–2782.
55. Bohnen, M, Stevenson, WG, Tedrow, UB, et al. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011; 8:1661–1666.
56. Carbucicchio, C, Santamaria, M, Trevisi, N, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: Short and long term outcomes in a prospective single center study. Circulation. 2008; 117:462–469.
57. Aliot, EM, Stevenson, WG, Almendral-Garrote, JM, et al. EHRA/HRS expert consensus on catheter ablation for ventricular arrhythmias. Heart Rhythm. 2009; 6:886–933.
58. Miller, MA, Dukkipati, SR, Mittmacht, AJ, et al. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J Am Coll Cardiol. 2011; 58:1363–1371.
59. Vaseghi, M, Lellouche, N, Ritter, H, et al. Mode and mechanism of death after orthotopic heart transplantation. Heart Rhythm. 2009; 6:503–509.
60. Thajudeen, A, Stecker, EC, Shehata, M, et al. Arrhythmias after heart transplantation: Mechanism and management. J Am Heart Assoc. 2012; 1:e001461.
61. Tsai, VW, Cooper, J, Garan, H, et al. The efficacy of implantable cardioverter-defibrillator in heart transplant recipients: Results from a multicenter registry. Circ Heart Fail. 2009; 2:197–201.
62. Anderson, M, Videbaek, R, Boesgaard, S, et al. Incidence of ventricular arrhythmias in patients on long term support with a continuous-flow assist device (HeartMate II). J Heart Lung Transplant. 2009; 28:733–735.
63. Dandemudi, G, Ghumman, W, Das, M, et al. Endocardial catheter ablation of ventricular tachycardia in patients with ventricular assist devices. Heart Rhythm. 2007; 4:1165–1169.