Ventricular Arrhythmias in Congenital Heart Disease
The reported prevalence of congenital heart disease (CHD) is 5.8 per 1000, with 11.9 per 1000 children and 4.1 per 1000 adults being affected. The prevalence of severe defects is 1.45 per 1000 children and 0.38 per 1000 adults. Of importance, between 1985 and 2000 the prevalence of severe CHD increased by 85% in adults but only by 22% in children.1 A clear change in mortality in CHD has been observed, with better survival in infancy and a trend towards death at older age.2 Improvements in survival are driven by a decreased mortality in moderate and severe forms of CHD in childhood, including tetralogy of Fallot (TOF), truncus arteriosus, atrioventricular septal defect, transposition of the great arteries (TGA), and univentricular hearts. This is likely the result of earlier surgical interventions and improved surgical techniques and outcomes. The increasing number of patients with repaired congenital heart disease joining the adult population requires the training of electrophysiologists with special interest in adult CHD as potentially life-threatening ventricular arrhythmias and sudden cardiac deaths (SCD) can still occur late after surgery.
The incidence of SCD in repaired CHD is 0.9 to 1.4 per 1000 patient-years, which is 25- to 100-fold higher than for the general population.3,4 In one population-based series, 90% of all sudden deaths occurred in four main categories of CHD, being TGA, TOF, aortic stenosis and aortic coarctation with an apparent time-dependent incremental risk in patients with left heart obstructions and TOF. Six to nine percent of patients after repair of TOF died suddenly after 21 to 35 years of follow-up (2% to 3% per decade), accounting for up to 50% of all deaths in this group.3,5,6 The highest incidence of SCD was found in patients with aortic stenosis with SCD rates between 10% and 13% after 15 to 20 years of follow-up.3,7 The majority of CHD-related SCD is presumably due to ventricular arrhythmias, either hemodynamically not tolerated sustained monomorphic reentrant ventricular tachycardia (SMVT), with a higher prevalence in patients who have undergone ventricular incision and patch closure of ventral septal defect (VSD), or monomorphic and polymorphic VT and ventricular fibrillation (VF) in the absence of surgical scars. Although data are lacking, the latter arrhythmia mechanisms may be similar to those observed in other cardiac diseases with pathologic hypertrophy, fibrosis, impairment of cardiac function, and ultimately heart failure. Impairment of right and left ventricular function is likely to result in altered ion channel and transporter function. Downregulation of K+ currents and APD prolongation, which is a consistent finding in ventricular myocytes from subjects with cardiac dysfunction, promotes early afterdepolarization. In addition, changes in Ca2+ handling proteins, which are also observed in heart failure, can cause diastolic Ca2+ leak from the sarcoplasmic reticulum, resulting in delayed afterdepolarization and triggered activity. Advanced hypertrophy can be due to chronic pressure overload in left heart obstructions and unrepaired TOF. Ventricular dysfunction occurs if the right ventricle serves as the systemic ventricle after an atrial switch operation for TGA or in congenitally corrected transposition of the great arteries (ccTGA). Left ventricular dysfunction may be due to longstanding cyanosis if TOF repair is performed at an older age or if chronic volume overload after palliative shunting has occurred. Furthermore, long-lasting volume overload owing to chronic pulmonary regurgitation after initial correction contributes to ventricular dysfunction. Understanding of the different mechanism of ventricular arrhythmogenesis in CHD is crucial for both risk stratification and treatment. Current data on late morbidity and mortality are based on patients who underwent repair as adolescents. Early surgical intervention and changes in the surgical strategy in particular for TOF and TGA might not only influence early mortality but can also affect the incidence and the potential mechanism of arrhythmias and perhaps late morbidity and mortality in adult CHD patients in the future. Detailed descriptions of the most common forms of CHD related to ventricular arrhythmias are provided in the following sections.
Ventricular Arrhythmias as Related to Specific Types of Congenital Heart Disease
Tetralogy of Fallot
Developmental and Anatomic Aspects
During normal development, the outlet portion of the heart needs to evolve from a single myocardial tube to a situation where the separated aorta and pulmonary trunk achieve their definitive positional relationship. This process requires proper septation of the outlet portion of the heart. Formation of the aortopulmonary septum (future outlet septum), orchestrated by neural crest cells, will result in separation of the common trunk into an aorta and pulmonary trunk. Asymmetrical, mainly subpulmonary myocardial contributions from the so-called second heart field will result in marked lengthening of the subpulmonary myocardium and will “push” the pulmonary trunk to its definitive position left anterior to the aorta.8 After proper development, the right ventricular outflow tract (RVOT) is characterized by the presence of a muscular subpulmonary infundibulum forming a circular muscular tube below the pulmonary valve. The posterior wall of the infundibulum, also known as the crista supraventricularis, is situated between the tricuspid valve and the pulmonary valve. The crista supraventricularis forms the summit of the ventriculo-infundibular fold (VIF), a fold of myocardium at the posterolateral wall of the RVOT, and extends toward the ventricular septum into the trabecula septomarginalis (Figure 102-1). The latter continues over the interventricular septum and contains the right bundle branch. The crista supraventricularis also encompasses the outlet septum (the muscular septum separating the aortic en pulmonary outlets), situated in between the ventriculo-infundibular fold and trabecula septomarginalis, which is small and not recognizable as a separate structure in the normal heart. In TOF, however, there is an anterior deviation of the outlet septum that, in contrast to normal, can be recognized as a separate structure, which is regarded as a pathognomonic feature of TOF (see Figure 102-1). The deviation of the outlet septum causes malalignment with the remainder of the ventricular septum, resulting in a subaortic, and in most cases perimembranous, ventricular septal defect. The deviation of the outlet septum also contributes to the subpulmonary stenosis. The amount of displacement and hypertrophy of the outlet septum determines the severity of the stenosis. The morphology of TOF encompasses a broad spectrum, with on the one end very slight malformations and cyanosis and on the other end severe forms of the disease with pulmonary atresia.
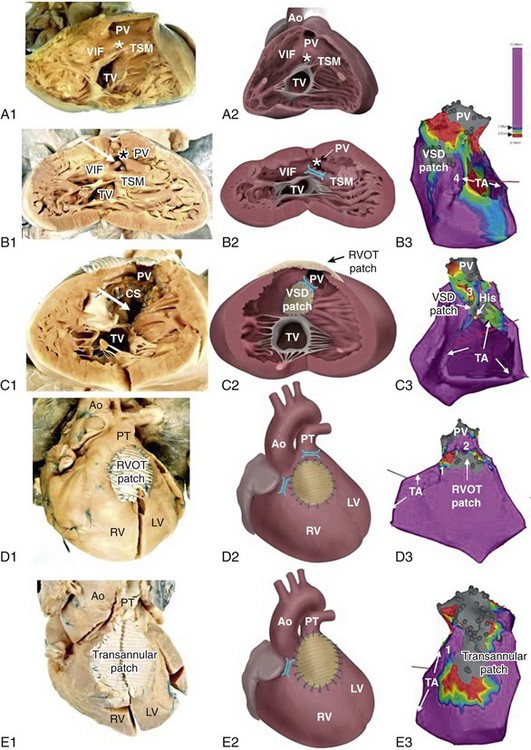
Figure 102-1 Tetralogy of Fallot (TOF) isthmus. A, Anatomic specimen (A1) and drawing (A2) of the normal heart, right ventricular view. The heart is sectioned parallel to the interventricular septum and opened to allow a view into the right ventricle (RV). The fibrous tissues of the tricuspid valve (TV) and pulmonary valve (PV) are separated by the muscular tissues of the crista supraventricularis (CS), that consists of the continuum of the ventriculoinfundibular fold (VIF) and trabecula septomarginalis (TSM). The outlet septum (location indicated by the asterisk) is also part of the continuum, but cannot be identified as a separate structure in the normal heart. B, Anatomic specimen (B1) and drawing (B2) of a heart with unoperated TOF, same view as in A. The outlet septum (indicated by the asterisk), that in this case is small and fibrous, has deviated from the other components of the crista supraventricularis in an anterior direction, thus narrowing the ostium of the PV. The dextroposed aorta can be seen through the ventricular septal defect (arrow), which has a muscular rim (substrate for anatomic isthmus 4). B3, Electroanatomic voltage map (3-D mapping system, CARTOXP, Biosense Webster, USA) for a patient with operated TOF in a modified left posterior view. Voltages are color coded according to the color bar; grey tags indicate unexcitable tissue. Anatomical isthmus 4 is indicated. C, Anatomic specimen (C1) and drawing (C2) of operated TOF, same view as in A and B. The VSD patch drawn in place in C2 has been folded to the right in C1 to expose the VSD (arrow in C1). Note the hypertrophic crista supraventricularis (CS), at which site an infundibulectomy has been performed (C1). C3, Electroanatomic voltage map of a patient with operated TOF in a modified anterior view (same color coding). Anatomic isthmus 3 is indicated. D, Anatomic specimen (D1) and drawing (D2) of operated TOF, with a right ventricular outflow tract (RVOT) patch, frontal view. D3, Electroanatomic voltage map of a patient with operated TOF in a modified anterior view (same color coding). Anatomic isthmuses 1 and 2 are indicated. E, Anatomic specimen and (E1) and drawing (E2) of operated TOF, with a transannular patch, frontal view. E3, Electroanatomic voltage map of a patient with operated TOF in a modified anterior view (same color coding). Anatomic isthmus 1 is indicated. Ao, Aorta; PT, pulmonary trunk; PV, pulmonary valve; TA, tricuspid annulus.
Type and Timing of Surgical Repair and Its Potential Relation to Risk Factors for Ventricular Arrhythmias
Total repair included (patch) closure of the perimembranous or muscular VSD and relief of the infundibular or valvular RVOT obstruction. This repair was initially performed through a vertical or transverse right ventriculotomy often combined with the use of an RVOT or a transannular patch to augment the restrictive RVOT or to relieve the stenosis of the pulmonary orifice. The malformation and type of repair are important determinants of potential reentrant tachycardia circuits, which is a common underlying mechanism of VT in repaired TOF.8 Areas of dense fibrosis owing to surgical incisions, but also patch material and the valve annuli, can form regions of conduction block that define reentry circuit borders and create intervening isthmuses of myocardial bundles that might contain the critical reentry circuit isthmus of a macroreentrant VT. Four anatomic isthmuses related to VT in repaired TOF have been identified9: isthmus 1 bordered by the tricuspid annulus and the scar or patch in the anterior RVOT, isthmus 2 between the pulmonary annulus and the RV free wall incision or RVOT patch sparing the pulmonary valve annulus, isthmus 3 between the pulmonary annulus and the VSD patch or septal scar, and isthmus 4 between the VSD patch or septal scar and the tricuspid annulus in patients with muscular VSDs (see Figure 102-1).
The right ventriculotomy and the frequent use of a transannular patch with consecutive pulmonary regurgitation and chronic volume overload often resulted in RV dilatation and dysfunction, which is associated with VT and SCD in the long term (Table 102-1). Consequently, a combined transatrial-transpulmonary approach has been introduced. Currently, patch augmentation is avoided or usually limited to the pulmonary annulus whenever possible. This approach does not only positively affect RV function but can also prevent the anatomic isthmuses 1 and 2.
Table 102-1
Risk Factors for Ventricular Tachycardia, Sudden Cardiac Death, and Adverse Events in Tetralogy of Fallot
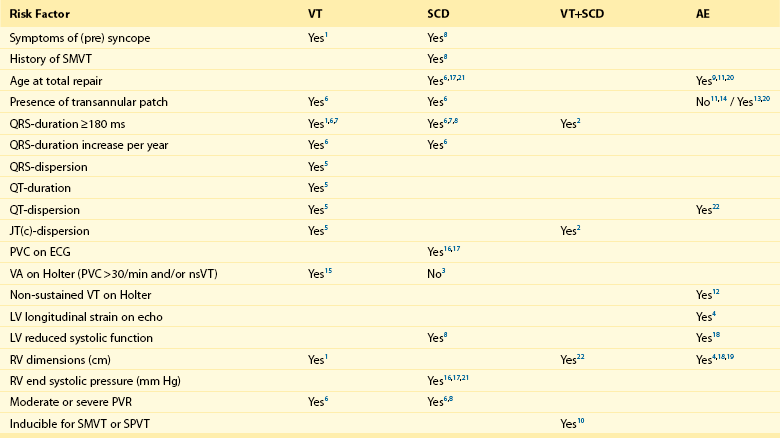
1: Balaji et al.; Am J Cardiol; 1997 Jul 15;80(2):160-3. (n=135)
2: Berul et al.; J Cardiovasc Electrophysiol; 1997 Dec;8(12):1349-56. (n=101)
3: Cullen et al.; J Am Coll Cardiol; 1994 Apr;23(5):1151-5. (n=86)
4: Diller et al.; Circulation; 2012 May 22;125(20):2440-6. (n=413)
5: Gatzoulis et al.; Circulation; 1997 Jan 21;95(2):401-4. (n=99)
6: Gatzoulis et al.; Lancet; 2000 Sep 16;356(9234):975-81. (n=793)
7: Gatzoulis et al.; Circulation; 1995 Jul 15;92(2):231-7. (n=178)
8: Ghai et al.; J Am Coll Cardiol; 2002 Nov 6;40(9):1675-80. (n=125)
9: Karamlou et al.; Ann Thorac Surg; 2006 May;81(5):1786-93; discussion 1793. (n=249)
10: Khairy et al.; Circulation; 2004 Apr 27;109(16):1994-2000. (n=252)
11: Murphy et al.; N Engl J Med, 1993 Aug 26;329(9):593-9. (n=163)
12: Khairy et al.; Circulation; 2008 Jan 22;117(3):363-70. (n=68)
13: Nollert et al.; J Am Coll Cardiol; 1997 Nov 1;30(5):1374-83. (n=490)
14: Nollert et al.; Thorac Cardiovasc Surg; 1997 Aug;45(4):178-81. (n=71)
15: Harrison et al.; J Am Coll Cardiol, 1997 Nov 1;30(5):1368-73. (n=210)
16: Garson et al.; Circulation 1979 Jun;59(6):1232-40. (n=207)
17: Garson et al.; J Am Coll Cardiol; 1985 Jul;6(1):221-7. (n=488)
18: Knauth et al.; Heart; 2008 Feb;94(2):211-6. Epub 2006 Nov 29. (n=88)
19: Ortega et al.; Am J Cardiol; 2011 May 15;107(10):1535-40. (n=39)
20: Jonsson et al.; Scand J Thorac Cardiovasc Surg; 1995;29(2):43-51. (n=165)
21: Jonsson et al.; Scand J Thorac Cardiovasc Surg; 1995;29(3):131-9. (n=141)
Myocardial histopathologic changes, in particular interstitial fibrosis of the muscular tissue of the crista supraventricularis, are more pronounced in patients who were operated at older age, specifically beyond the age of 4 years, and are thus subjected to long standing cyanosis (SaO2 < 80%) and higher end-diastolic RV pressure (>12 mm Hg).10 The clinical gold standard to detect ventricular fibrosis is late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR). Myocardial LGE was present in both the RV and LV of adults after repair of TOF and was related to increased age, and adverse clinical effects such as ventricular dysfunction and specifically RV LGE were associated with atrial and ventricular arrhythmias.11
Histopathologic changes can also contribute to the occurrence of complex ventricular ectopy, which has been considered a risk factor for fatal ventricular arrhythmias (VA). In particular, older age at repair has been associated with a higher grade of ventricular ectopy. Only 11% of patients in whom repair was performed between the ages of 4 and 15 years showed complex ectopy on Holter monitoring 6 to 12 months after operation, compared with 39.4% of patients who underwent surgery beyond the age of 15 years.10 The age dependency of the occurrence of complex ventricular ectopy could also be demonstrated in uncorrected patients. No significant ventricular ectopy (defined as Lown ≥ 2, <30 uniform PVCs/h) was observed in patients 0 to 7 years old. In contrast, 58% of patients 16 years or older had complex ectopy, with 21% having runs of nonsustained VT. Of interest in corrected patients, the relation of complex ventricular ectopy and time of repair persisted and was independent of the duration of follow-up or of the postoperative hemodynamic status.12 Although a higher grade of ectopy and nonsustained VT have been associated with VT inducibility, inconsistent data exist regarding their association with SCD. Accordingly, treatment of asymptomatic complex ventricular ectopy is not recommended.13 Primary repair in infancy before the age of 18 months is common practice since the early 90s and can be performed with low perioperative mortality. Avoiding long-standing prolonged hypoxemia and pressure overload can reduce the histopathologic changes and the substrate for slow conduction, complex ventricular ectopy, and fatal VA.
Despite early operation, progressive pulmonary regurgitation occurs in almost all patients after transannular patch repair and is an important reason for reintervention. Although often tolerated, pulmonary regurgitation ultimately leads to RV dilatation and dysfunction, which can be further aggravated by residual RVOT obstruction. Moderate to severe PR and abnormal RV hemodynamics in particular, and increased RV end-systolic pressure have been associated with VT and SCD. In addition, a wide QRS (>180 ms) and an increase in QRS duration have consistently been reported as risk factor for SMVT and SCD.5 In particular, RV dilatation but not restrictive RV physiology has been associated with QRS prolongation, referred to as mechanoelectrical interaction.14
Impairment of RV function and LV hemodynamics have important roles. A moderate to severe left ventricular systolic dysfunction—defined as ejection fraction (EF) of less than 39% and 20%, respectively—was also more common in patients with TOF and (aborted) SCD.15 In addition, an left ventricular end diastolic pressure (LVEDP) ≥ 12 mm Hg was a strong and independent predictor of appropriate ICD shocks in patients with TOF who received an ICD for primary prevention.16
Effect of Pulmonary Valve Replacement and Intraoperative Cryoablation on Ventricular Arrhythmias
In selected patients, a reduction in QRS duration after pulmonary valve replacement (PVR) was related to a reduction in RV EDV assessed by CMR, which further supports the occurrence of mechanoelectrical interaction.17 A QRS duration greater than 180 ms 6 months after PVR, or no reduction of the QRS duration postoperatively, were strong predictors of adverse events defined as all-cause mortality, reoperation for pulmonary regurgitation, heart failure or VT.18 In particular, patients with severely prolonged QRS duration that did not change postoperatively had the highest incidence of adverse events. Prolonged QRS duration can serve as one surrogate marker for the changes in mechanical forces that trigger changes on the cellular and subcellular level also known as mechanoelectrical coupling associated with arrhythmogenesis.
Despite the remarkable reduction of RV volumes and hemodynamic improvement of RV function after PVR,19 simply replacing the valve might not eliminate the substrate for reentrant ventricular tachycardias; reentry was the underlying mechanism of all VTs that were also associated with a QRS greater than 180 ms in one series.14 After a late PVR performed in 98 patients 20 ± 9 years SD after TOF repair, VT occurred in 11, and death occurred in 6 patients after a mean follow-up of 2.8 ± 4.3 years. The 5- and 10-year measures of freedom of the composite outcome of death and VT was 80% and 41%, which was not different from a control group (n = 77) matched for age, pulmonary regurgitation, RV dilatation, and QRS duration and who did not undergo PVR.20 These data suggest that either PVR was performed too late, not leading to a favorable reverse remodeling despite a reduction in RV size, or VTs are the result of a fixed substrate not influenced by hemodynamic improvements. Of interest, five of seven patients who underwent PVR and concomitant (not further specified) intraoperative cryoablation experienced VT during follow-up. In contrast, map-guided intraoperative ablation was more effective to control VT recurrence. Of 44 patients with documented monomorphic sustained ventricular tachycardia (MSVT), 31 underwent cryoablation applied to the macroreentry site as assessed by combined endocardial and epicardial mapping during cardioplegic arrest. VT recurred in only three (10%) patients after ablation with a 96% freedom of VT after 7.5 years.21
Role of Programmed Electrical Stimulation
A positive programmed electrical stimulation (PES), usually defined as inducibility of a sustained monomorphic VT, provides an important tool to prove the presence of a substrate for reentrant VT, although VT based on triggered activity may also be inducible in the electrophysiology laboratory. Accordingly, only 16% of patients who had documented sustained VT were not inducible by PES.22
Consequently, PES has also been used to identify patients with TOF who are at risk of potential fatal ventricular arrhythmias. Inducibility of sustained VT is high in patients with presyncope and complex PVCs on Holter monitoring and is more likely in patients repaired at older age and when PES is performed late after surgery.23,24 Importantly, inducibility strongly depends on the applied induction protocol; MSVT induction required three extrastimuli in 70% and isoproterenol in 11% of the patients in two series.22,23 Using a complete PES protocol (two RV sites, 3 cycle lengths (CL), triples and isoproterenol) the diagnostic value (sensitivity, 77.4%; specificity, 79.5%) and the prognostic significance (relative risk, 5.0 for subsequent clinical VT or SCD) of PES in TOF patients is comparable with the postinfarct population.22 Of interest, the highest event rate (SCD, VT, or both) was observed in patients inducible for sustained polymorphic VT (SPVT). Although derived from a small number of patients, induction of SPVT may not be an unspecific finding and may reflect a different but potential fatal arrhythmogenic substrate.
Type of Ventricular Arrhythmias and the Underlying Substrate: Impact on Treatment
The majority of VA documented in TOF patients is monomorphic VT. The prevalence of SMVT was 14.2% in a recently conducted multicenter study including 556 TOF patients and was markedly increased after the age of 45. In contrast, in only 0.5% of the population VF could be documented.2 The incidence of VF in TOF patients may be underestimated as patients in the cohort are survivors. However, in 121 TOF patients who have received an ICD for primary or secondary prevention, 81.5% of all appropriately delivered therapy was for monomorphic VT. Of importance, VTs were fast with a median heart rate of 213 bpm (182-264).16
The feasibility of catheter ablation of VT in CHD with the majority performed in TOF patients has been reported (Table 102-2). In most of the earlier series and case reports, the targeted VTs were slow, hemodynamically tolerated, and therefore approachable by conventional mapping techniques, such as activation and entrainment mapping during ongoing VT. An intention to treat analysis was published by the group from the Boston Children’s Hospital.25 In this study, acute ablation failure was 50% because of noninducibility, hemodynamic instability, or anatomic reasons. Two recently published studies used substrate mapping techniques to target all inducible and, in particular, fast and poorly tolerated VTs, which are common.
Table 102-2
Published Data on Ventricular Tachycardia Ablation in Congenital Heart Disease
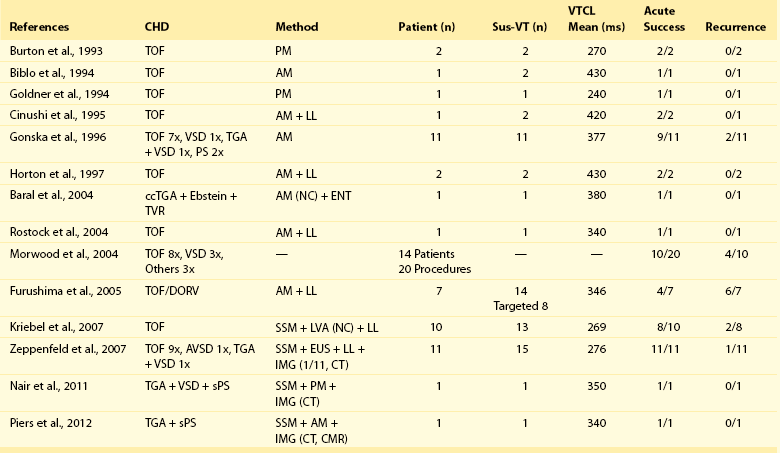
From Burton et al: Pacing Clin Electrophysiol 16:2319–2325, 1993; Biblo et al: Pacing Clin Electrophysiol 17:1556–1560, 1994; Goldner et al: Pacing Clin Electrophysiol 17:1441–1446, 1994; Chinushi et al: Pacing Clin Electrophysiol, 18(9 Pt 1):1713–1716, 1995; Gonska et al: Circulation 94:1902–1208, 1996; Horton et al: J Cardiovasc Electrophysiol 8:432–435, 1997; Baral et al: J Interv Card Electrophysiol 11:211–215, 2004; Rostock et al: Pacing Clin Electrophysiol 27(6 Pt 1):801–804, 2004; Morwood et al: Heart Rhythm 1:301–308, 2004; Furushima et al: J Electrocardiol 39:219–224, 2006; Kriebel et al: J Am Coll Cardiol 50:2162–2168, 2007; Zeppenfeld et al: Circulation 116:2241–2252, 2007; Nair et al: Indian Pacing Electrophysiol J 11:120–125, 2011; Piers et al: Circ Arrhythm Electrophysiol 5:e38–e40, 2012.
Using a noncontact mapping system consisting of a multielectrode balloon array that allows simultaneous acquisition of virtual unipolar electrograms, the activation sequence of 13 fast or nonsustained VTs in 10 patients could be obtained.26 Eleven of the 13 induced VTs were due to macroreentrant circuits, whereas two were due to microreentrant circuits. The anatomic location of the isthmus could be identified in all patients and was successfully targeted by a linear radiofrequency (RF) lesion in eight patients. In two patients, RF delivery was withheld because of the proximity of the His bundle. An alternative approach applies point-by-point electroanatomic voltage and activation mapping during stable sinus rhythm to obtain a three-dimensional reconstruction of all potential isthmuses by identifying the anatomic boundaries (see Figure 102-1).9 Peak-to-peak bipolar electrogram amplitudes can be displayed color-coded and projected on a three-dimensional shell of the RV. Electrograms greater than 1.5 mV are considered normal voltage. At sites with amplitudes less than 0.5 mV, high output pacing (10 mA, 2 ms) can be performed to identify unexcitable tissue, which is consistent with patch material or surgical scars. Connecting the anatomic isthmuses by linear RF lesions has been highly effective to treat all VTs, and in particular poorly tolerated ones, with promising long-term results. In the largest series to date, reporting on VT ablation targeting anatomic isthmuses in 31 adults with CHD (81% TOF; mean VTCL of 298 ± 74 ms) total procedural success defined as noninducibility of any VT was achieved in 22 adults (71%). None of the patients in whom complete procedural success was achieved had recurrence of a monomorphic VT during 43 ± 28 months of follow-up, whereas one patient with poor RV function experienced appropriate ICD discharge for VF. These data suggest that macroreentrant VTs based on an anatomic substrate can be treated effectively with catheter ablation; however, other VA can occur in patients with impaired RV function.9
Implantable Cardioverter Defibrillator
As discussed earlier, the risk of SCD in patients with surgically corrected TOF is estimated to be 1.2% to 1.8% 10 years after surgery and 2.5% 20 years after surgery. The SCD risk increases to 4% and 6%, respectively, 25 and 30 years after surgery. Several factors associated with SCD in patients with TOF have been identified (see Table 102-1); however, the prognostic value of each factor is limited. According to the AHA/ACC and ESC guidelines, an implantable cardioverter defibrillator (ICD) should be implanted in SCD survivors.13,27 Furthermore, an ICD should be implanted in patients with ventricular arrhythmias after failed catheter ablation. ICD implantation can be considered in patients after unexplained syncope without a defined and reversible cause. However, if symptoms of presyncope of syncope are reported, an induction protocol for both VT and supraventricular tachycardias may be helpful. Prophylactic implantation of ICDs is still under debate, but could be considered in the presence of LV/RV dysfunction, extensive fibrosis, and a QRS greater than 180 ms and if VT is inducible during electrophysiological study.13,27 In high-risk patients with TOF, ICD implantation has been shown to be effective for both primary and secondary prevention, with appropriate and effective shocks in more than 30% of patients.16 Of importance, more than 80% of the treated ventricular arrhythmias were monomorphic and fast VT (median heart rate, 212 beats/min), but 70% of the patients required ICD shocks to terminate the first arrhythmia. These fast VT are currently approachable by substrate-based ablation techniques, which should be considered in particular in patients with preserved RV and LV function. However, long-term efficacy data of catheter ablation of fast VT are lacking. Important device-related complications are inappropriate shocks, which were observed in 25% to 30% of all patients16,28 (actuarial rate 5.8% of patients per year) and are often due to supraventricular arrhythmias and late lead-related complications occurring in 20%.16
Transposition of the Great Arteries
Developmental and Anatomic Aspects
Transposition of the great arteries is the most common cyanotic heart disease in newborns, comprising 5% of all congenital heart diseases. It can occur isolated (simple TGA) or in association with other congenital malformations (complex TGA). In TGA, there is a concordant atrioventricular (AV) connection in combination with ventriculoarterial discordance, with the aorta connecting to the morphologic right ventricle, and the pulmonary trunk to the morphologic left ventricle. There is often a parallel course of the great arteries, with a right anterior position of the aorta with respect to the pulmonary orifice.29 From a developmental point of view, the abnormal position of the great arteries in TGA might be related to a deficient lengthening of the outflow tract during development, for which contributions from the so-called second heart field are necessary.8
Complex TGA is most commonly associated with ventricular septal defects (in approximately 40%) and left ventricular outflow tract (LVOT) or RVOT obstruction. In cases with infundibular VSDs, the outlet septum can often be recognized as a separate structure. Obstruction of the RVOT and LVOT are usually caused by rightward deviation and leftward deviation of the outlet septum, respectively, and can become more pronounced by hypertrophy of the outlet septum. In addition, muscle bundles, subvalvular fibrous tissues, or abundant AV valve tissues can contribute to narrowing of the RVOT and LVOT.29
Type and Timing of Surgical Repair and Its Potential Relation to Risk Factors for Ventricular Arrhythmias
Currently, most of the patients seen at the adult outpatient clinic have been treated with the atrial switch operation. SCD is the leading cause of late mortality and seems to be constant over time, with an estimated incidence of 4% ± 7% at 10 years and 9% ± 4% at 20 years of follow-up.3 In a retrospective study of 47 patients with sudden death (SCD; 13 aborted), the event occurred at a median age of 9.7 years (range, 0.3 to 32 years) and 7.9 years (range, 0.3 to 28.8 years) after surgery.30
Of importance, more than 80% of these SCDs occurred during exercise, likely because of an inability to increase cardiac output as a response to the increased demand, leading to low output cardiac failure. The latter can be worsened by coexisting conditions such as baffle obstruction, pulmonary vein stenosis, or ventricular dysfunction.30 Atrial arrhythmias that preceded SCD in 7% of the patients further impair AV transport and decrease RV filling. Right ventricular dysfunction may also lead to ventricular arrhythmia as a consequence of RV dilatation and tricuspid regurgitation, resulting in hemodynamic compromise and mechanoelectrical interaction (i.e., a relation of mechanical and electrical properties of the RV, due to which right ventricular dilation and life-threatening arrhythmias may occur.14 Ischemia of the systemic RV may further contribute to ventricular arrhythmogenesis.31 Late enhancement CMR studies have indicated the occurrence of myocardial fibrosis in the systemic right ventricle in patients after physiological correction, which may be relevant in the occurrence of ventricular arrhythmias.32
Although risk stratification is generally limited, symptoms of arrhythmias and heart failure and documented atrial flutter or fibrillation have been identified as predictors of SCD in patients after a Mustard or Senning procedure.30
The implication of supraventricular arrhythmias for the occurrence of ventricular arrhythmias is further supported by the observation that 50% of all appropriate ICD shocks in primary or secondary prevention ICD recipients were preceded by or coexisted with supraventricular tachycardia.33 Risk factors as identified in patients with TOF are of limited value. Only 1 of 23 patients who received an ICD for primary prevention experienced appropriate ICD therapy. Implantation was prompted by presyncope in 48% of patients, nonsustained (ns) VT in 48%, QRS duration ≥ 180 ms in 30%, right ventricular ejection fraction (RVEF) ≤ 35% in 35%, and inducible VT in 30.4%.
Type of Ventricular Arrhythmias and the Underlying Substrate: Effect on Treatment
The Senning and Mustard procedures result in physiological repair, after which the morphologic right ventricle supports the systemic circulation with the risk of right heart failure and related ventricular arrhythmias over time. Accordingly, more than 50% of all VAs prompting ICD therapy in TGA in patients receiving implants for primary or secondary prevention were polymorphic VT or VF.33 Sustained monomorphic VT is relatively uncommon, with an estimated incidence rate of 0.5% per year.3
Simple TGA does not require surgical incisions of the ventricles or patch material, which has been associated with monomorphic VT in the TOF population. Surgical incisions or patch material are however necessary in complex TGA, which is usually defined as the additional presence of a VSD requiring patch closure or other lesions like outflow tract obstruction requiring surgical intervention.30,34 Surgical scars and patch material can serve as anatomic boundaries facilitating macroreentrant VT as in TOF (Figure 102-2). In an adult cohort of 149 patients in which 107 (72%) had simple transposition, sustained VT was documented in seven patients. Four of these seven had complex TGA. VT occurred in 9.5% of patients with complex TGA, but in only 2.8% of patients with simple TGA.34 Sustained VT is rarely observed during childhood. The deterioration of RV function over time with electrical and structural remodeling, which is more often observed in complex TGA (42% vs. 2% at 20 years),35 is likely to contribute to the late occurrence of VT and SD.
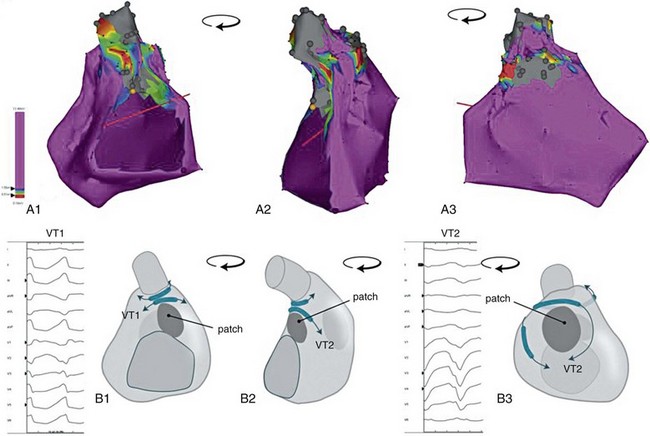
Figure 102-2 Potential propagation waves. A, Electroanatomic voltage map of a patient with operated TOF (closure of a perimembranous ventral septal defect, right ventricular outflow tract patch sparing the pulmonary vein, resection of infundibular muscle). Bipolar voltages are color coded according to the color bar; gray indicates inexcitable tissue; yellow tag indicates the position of the His bundle. The map is turned counterclockwise from a posterior (A1) to a right lateral (A2) to an anterior view (A3). B, Schematic of panel A. Potential anatomic isthmuses are indicated, as are potential directions of wavefront propagation during ventricular tachycardia. Schematic drawings in panels B1 to B3 correspond with the electroanatomic voltage maps in A1 to A3. Note that activation of the same isthmus in a counterclockwise or clockwise direction results in a different 12-lead electrocardiogram. Example provided for isthmus 3: counterclockwise activation results in VT 1, clockwise activation in VT2.
There are no specific data on medical treatment of VA in TGA, and only four case reports on catheter mapping and ablation of monomorphic VT, all of which were performed in patients with complex TGA (see Table 102-2). The underlying mechanism was reentry, and the critical reentrant circuit isthmus was located in an anatomic isthmus bordered by the VSD patch and the aortic valve in three patients, and in an area of septal transmural scar and at the aortic valve in one patient. In the latter patient, preprocedural imaging of the three-dimensional scar architecture by LGE CMR and its spatial relationship to the aortic valve identified the anatomic isthmus (see Figure 102-3). Integration of the CMR-derived anatomic information with the electroanatomic mapping data was applied to guide and facilitate ablation.36 Substrate imaging and image integration should be considered in all patients with CHD with complex anatomy and surgical scars.36
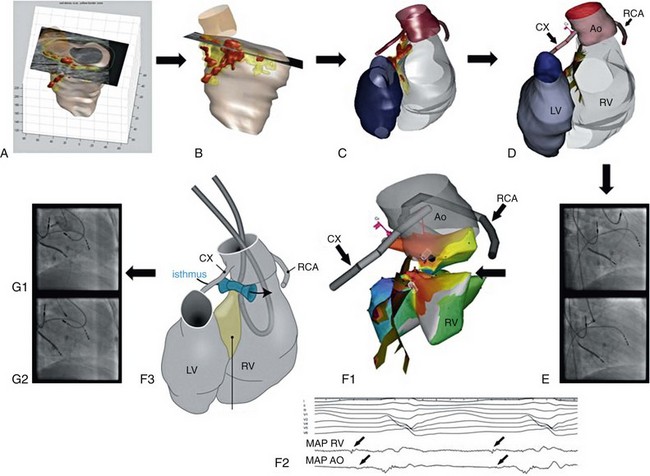
Figure 102-3 Transposition of the great arteries (TGA; including image integration). Ventricular tachycardia ablation in a patient with Mustard baffle and resection of a subpulmonary stenosis for complex TGA. Preprocedure contrast-enhanced cardiac magnetic resonance imaging (MRI) (A) was followed by image processing to reconstruct the anatomy and scar distribution based on signal intensity. Red areas indicate dense scar, and yellow areas indicate scar borderzone (B and C). D, Integration of MRI-derived scar projected on the endocardial surface of the right ventricle (RV) was performed using the position of the ostium of the right coronary artery (RCA) and circumflex branch of left coronary artery (CX) determined by contrast injection through the mapping catheter (E). C, D, and F demonstrate the same posterior view. After two-point image integration, limited activation mapping from the aorta (AO) and RV restricted to the area of interest showed a macroreentrant tachycardia (red indicates early activation, purple late activation ([F1] with middiastolic activity recorded from RV and AO [F2]). The schematic illustrates the identified anatomic isthmus based on contrast enhanced (CE)-magnetic resonance imaging (MRI), which was bordered by septal scar tissue and the aortic root. Transection of the isthmus required bipolar ablation delivered between two ablation catheters, one placed in the aortic root and one opposite in the RV as indicated on the map (F1), the schematic (F3), and the fluoroscopic image (G2). Radiofrequency (RF) delivery terminated ventricular tachycardia after 3 seconds (not shown). Before RF delivery, the distance between the catheter and the ostium of the RCX was estimated based on a contrast injection in the aortic root and was considered safe (G1).
Implantable Cardioverter Defibrillator
The risk of SCD in TGA is reported to be 4% after 10 years and 9% after 20 years (5 to 8 per 1000 patient-years). In the majority of patients who have undergone an atrial switch operation, ventricular arrhythmias are the underlying cause of SCD. It is important to note that ventricular fibrillation can be preceded by atrial arrhythmias in a significant number of these patients.33 Other reported risk factors for SCD are RV dysfunction, venous obstruction and a QRS duration of 140 ms or greater34; however, specific criteria that prompt ICD implantation have not been defined. An ICD should be implanted after an SCD episode and can be considered in patients with unexplained syncope without defined and reversible cause.13,27
In TGA patients after atrial switch, ICD implantation has been shown to be effective for secondary prevention. However, effectiveness of ICD implantation for primary prevention could not be demonstrated, and inducible VT on electrophysiological testing did not predict future events, suggesting that risk stratification is limited.33 It is important to recognize that implantation of an ICD can be technically challenging because of the presence of intraatrial baffles and implantation of the lead in a morphological left ventricle. In addition, after ICD implantation the number of inappropriate shocks (6.6% per year) and late lead-related complications are high.33,37
Other
Left Ventricular Outflow Tract Obstruction
Despite surgical repair, aortic stenosis has the highest incidence of SCD (5.4 per 1000 patient- years).3 LVOT obstruction comprises a variable group of patients with obstruction at the subvalvular, valvular, or supravalvular level. Valvular stenosis, which constitutes the largest group in congenital heart disease patients, is usually attributable to a bicuspid aortic valve. Congenital cases of subvalvular aortic stenosis are usually the result of a subaortic valvular membrane. Supravalvular aortic stenosis is less common and is often associated with other congenital malformations or syndromes, such as Williams syndrome, or related to previous surgery (e.g., the suture site in the ascending aortic wall after arterial switch for TGA).
The risk of SD in patients with aortic stenosis seems time dependent. Etiologies of SD were variable and included arrhythmic SD (often in the presence of significant residual valve dysfunction), cerebral and coronary complications (often related to aortic valve endocarditis), and low cardiac output failure secondary to acute ventricular failure.3 Other suggested mechanisms for the high occurrence of sudden cardiac death in this group include ventricular dysfunction and ventricular hypertrophy. Deficient coronary perfusion during exercise can play a role in patients with unoperated severe aortic stenosis. In asymptomatic patients with aortic stenosis, QRS duration and morphology were independently associated with the risk of SCD.38
Coarctation of the Aorta
The reported incidence of SCD in patients’ after repair of coarctation of the aorta is 1.3 per 1000 patient-years.3 Coarctation of the aorta can occur at different levels, but it is classically found at the so-called isthmus, just below the branching of the left subclavian artery. One third of patients have a bicuspid aortic valve. Even after surgical resection of the coarctation, patients can be remain prone to hypertension and concomitant left ventricular hypertrophy that can contribute to the increased incidence of SCD in this group.
Atrioventricular Septal Defect
In patients with atrioventricular septal defect (AVSD), the reported incidence of SCD is 0.9 per 1000 patient-years.3 AVSD is part of a broad morphologic spectrum that includes complete AVSD and partial AVSD. Extreme disbalance of the ventricles can result in hypoplastic right or left heart syndrome. AVSD can occur isolated and in association with other CHD, such as left ventricular outflow tract obstruction. AV conduction disorders can occur because of an abnormal position and morphology of the AV conduction system. Ventricular arrhythmias and SCD are less common and can be related to LVOT obstruction, the amount and duration of left AV valve regurgitation, the amount of ventricular disproportion, complete AV block, and the association with complex cardiac anomalies, although data are limited.
Congenitally Corrected Transposition of the Great Arteries
In ccTGA, there is discordance at both the atrioventricular and ventriculoarterial level. In the absence of atrial or ventricular septal defects or an open ductus arteriosus, patients will not exhibit shunting or cyanosis, and late complications of the disease are largely associated with the fact that the RV is the systemic ventricle, predisposing for right heart failure, and regurgitation of the often abnormal (Ebstein-like) tricuspid valve. Data on the occurrence of ventricular arrhythmias in this group are limited. Ventricular tachycardia and SCD can be related to ventricular dysfunction, AV valve regurgitation, or AV block. In a cross-sectional study examining the mode of death in patients with CHD, mortality in patients with ccTGA was 40% related to SCD.39
Univentricular Hearts
There is also an increased risk of ventricular arrhythmias that might be attributed to the morphology, function, and myocardial quality of the systemic ventricle (e.g., dilatation and presence of advanced ventricular hypertrophy of the systemic ventricle)and associated anomalies (e.g., non-compaction cardiomyopathy of the systemic ventricle). Myocardial fibrosis, as can be detected by CMR using LGE, was associated with dilatation, hypertrophy, and dysfunction of the systemic ventricle, with a higher prevalence of nsVT compared with patients without LGE on CMR.40
Eisenmenger Syndrome
Patients with Eisenmenger syndrome are at increased risk of SCD. In one series of 77 patients, SCD death occurred in 63% of patients, but was not related to arrhythmias.41 Nonsustained VTs were frequently present in this group, but did not result in SCD. Cardiac deaths are probably related to progressive heart failure and hypoxemia. Administration of drugs is problematic because many drugs are not tolerated and cause adverse events.
Implanted Cardioverter Defibrillator Therapy
Only small studies are available evaluating the cause of SCD in patients with ICDs; however, patients with LVOT obstruction, aortic coarctation, or univentricular heart are at higher risk of SD. The incidence of SCD remains high in postoperative patients with congenital aortic stenosis (3% after 10 years, 13% after 20 years, and 20% after 30 years). Although VA as the underlying cause of SCD is likely, only limited data are available. An ICD should be implanted after an aborted SCD and can be considered in patients with unexplained syncope without defined and reversible cause, similar to other adult structural heart disease guidelines.13,27 Complex anatomy and altered venous access (e.g., implantation of lead in an anatomic left ventricle, cavopulmonary connections) can hamper ICD implantation. Although potentially lifesaving, significant adverse effects on the quality of life have been reported in these often young patients, especially after ICD shocks.42 In addition, lead failure is relatively high, possibly because of a more active lifestyle in young patients.43
References
1. Marelli, AJ, Mackie, AS, Ionescu-Ittu, R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007; 115(2):163–172.
2. Khairy, P, Ionescu-Ittu, R, Mackie, AS, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010; 56(14):1149–1157.
3. Silka, MJ, Hardy, BG, Menashe, VD, et al. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998; 32(1):245–251.
4. Verheugt, CL, Uiterwaal, CS, van der Velde, ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010; 31(10):1220–1229.
5. Gatzoulis, MA, Balaji, S, Webber, SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000; 356(9234):975–981.
6. Murphy, JG, Gersh, BJ, Mair, DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993; 329(9):593–599.
7. Keane, JF, Driscoll, DJ, Gersony, WM, et al. Second natural history study of congenital heart defects. Results of treatment of patients with aortic valvar stenosis. Circulation. 1993; 87(2 Suppl):I16–I27.
8. Scherptong, RW, Jongbloed, MR, Wisse, LJ, et al. Morphogenesis of outflow tract rotation during cardiac development: The pulmonary push concept. Dev Dyn. 2012.
9. Zeppenfeld, K, Schalij, MJ, Bartelings, MM, et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007; 116(20):2241–2252.
10. Chowdhury, UK, Sathia, S, Ray, R, et al. Histopathology of the right ventricular outflow tract and its relationship to clinical outcomes and arrhythmias in patients with tetralogy of Fallot. J Thorac Cardiovasc Surg. 2006; 132(2):270–277.
11. Babu-Narayan, SV, Kilner, PJ, Li, W, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006; 113(3):405–413.
12. Deanfield, JE, McKenna, WJ, Presbitero, P, et al. Ventricular arrhythmia in unrepaired and repaired tetralogy of Fallot. Relation to age, timing of repair, and haemodynamic status. Br Heart J. 1984; 52(1):77–81.
13. Baumgartner, H, Bonhoeffer, P, De Groot, NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010; 31(23):2915–2957.
14. Gatzoulis, MA, Till, JA, Somerville, J, et al. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995; 92(2):231–237.
15. Ghai, A, Silversides, C, Harris, L, et al. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol. 2002; 40(9):1675–1680.
16. Khairy, P, Harris, L, Landzberg, MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008; 117(3):363–370.
17. van Huysduynen, BH, van, SA, Swenne, CA, et al. Reduction of QRS duration after pulmonary valve replacement in adult Fallot patients is related to reduction of right ventricular volume. Eur Heart J. 2005; 26(9):928–932.
18. Scherptong, RW, Hazekamp, MG, Mulder, BJ, et al. Follow-up after pulmonary valve replacement in adults with tetralogy of Fallot: association between QRS duration and outcome. J Am Coll Cardiol. 2010; 56(18):1486–1492.
19. Vliegen, HW, van Straten, A, de Roos, A, et al. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of Fallot. Circulation. 2002; 106(13):1703–1707.
20. Harrild, DM, Berul, CI, Cecchin, F, et al. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009; 119(3):445–451.
21. Karamlou, T, Silber, I, Lao, R, et al. Outcomes after late reoperation in patients with repaired tetralogy of Fallot: the impact of arrhythmia and arrhythmia surgery. Ann Thorac Surg. 2006; 81(5):1786–1793.
22. Khairy, P, Landzberg, MJ, Gatzoulis, MA, et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair: a multicenter study. Circulation. 2004; 109(16):1994–2000.
23. Lucron, H, Marcon, F, Bosser, G, et al. Induction of sustained ventricular tachycardia after surgical repair of tetralogy of Fallot. Am J Cardiol. 1999; 83(9):1369–1373.
24. Chandar, JS, Wolff, GS, Garson, A, Jr., et al. Ventricular arrhythmias in postoperative tetralogy of Fallot. Am J Cardiol. 1990; 65(9):655–661.
25. Morwood, JG, Triedman, JK, Berul, CI, et al. Radiofrequency catheter ablation of ventricular tachycardia in children and young adults with congenital heart disease. Heart Rhythm. 2004; 1(3):301–308.
26. Kriebel, T, Saul, JP, Schneider, H, et al. Noncontact mapping and radiofrequency catheter ablation of fast and hemodynamically unstable ventricular tachycardia after surgical repair of tetralogy of Fallot. J Am Coll Cardiol. 2007; 50(22):2162–2168.
27. Epstein, AE, Dimarco, JP, Ellenbogen, KA, et al. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm. 2008; 5(6):934–955.
28. Koyak, Z, de Groot, JR, Van Gelder, IC, et al. Implantable cardioverter defibrillator therapy in adults with congenital heart disease: who is at risk of shocks? Circ Arrhythm Electrophysiol. 2012; 5(1):101–110.
29. Bartelings, MM, Gittenberger-De Groot, AC. Morphogenetic considerations on congenital malformations of the outflow tract. Part 2: Complete transposition of the great arteries and double outlet right ventricle. Int J Cardiol. 1991; 33(1):5–26.
30. Kammeraad, JA, van Deurzen, CH, Sreeram, N, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004; 44(5):1095–1102.
31. Millane, T, Bernard, EJ, Jaeggi, E, et al. Role of ischemia and infarction in late right ventricular dysfunction after atrial repair of transposition of the great arteries. J Am Coll Cardiol. 2000; 35(6):1661–1668.
32. Babu-Narayan, SV, Goktekin, O, Moon, JC, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005; 111(16):2091–2098.
33. Khairy, P, Harris, L, Landzberg, MJ, et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol. 2008; 1(4):250–257.
34. Schwerzmann, M, Salehian, O, Harris, L, et al. Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J. 2009; 30(15):1873–1879.
35. Roubertie, F, Thambo, JB, Bretonneau, A, et al. Late outcome of 132 Senning procedures after 20 years of follow-up. Ann Thorac Surg. 2011; 92(6):2206–2213.
36. Piers, SR, Dyrda, K, Tao, Q, et al. Bipolar ablation of ventricular tachycardia in a patient after atrial switch operation for dextro-transposition of the great arteries. Circ Arrhythm Electrophysiol. 2012; 5(2):e38–e40.
37. Khanna, AD, Warnes, CA, Phillips, SD, et al. Single-center experience with implantable cardioverter-defibrillators in adults with complex congenital heart disease. Am J Cardiol. 2011; 108(5):729–734.
38. Greve, AM, Gerdts, E, Boman, K, et al. Impact of QRS duration and morphology on the risk of sudden cardiac death in asymptomatic patients with aortic stenosis: the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) Study. J Am Coll Cardiol. 2012; 59(13):1142–1149.
39. Oechslin, EN, Harrison, DA, Connelly, MS, et al. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000; 86(10):1111–1116.
40. Rathod, RH, Prakash, A, Powell, AJ, et al. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol. 2010; 55(16):1721–1728.
41. Niwa, K, Perloff, JK, Kaplan, S, et al. Eisenmenger syndrome in adults: ventricular septal defect, truncus arteriosus, univentricular heart. J Am Coll Cardiol. 1999; 34(1):223–232.
42. Sears, SF, Jr., Conti, JB. Quality of life and psychological functioning of icd patients. Heart. 2002; 87(5):488–493.
43. Alexander, ME, Cecchin, F, Walsh, EP, et al. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004; 15(1):72–76.