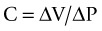15 Ventilation and Oxygenation Management
After reading this chapter, you should be able to:
• describe oxygen therapy, including low-flow and high flow devices, complications associated with oxygen therapy, and management priorities
• state nursing priorities for airway management strategies including laryngeal masks, endotracheal tubes and tracheostomy tubes
• summarise current knowledge on the physiological benefits, indications for use, associated monitoring priorities, complications, modes, settings and interfaces for non-invasive ventilation
• state the indications for use, associated monitoring priorities, complications, classification framework, modes and settings for invasive mechanical ventilation
• outline the weaning continuum and current evidence for optimising safe and efficient weaning from mechanical ventilation
• discuss ventilation management strategies for refractory hypoxaemia
• discuss ventilation management strategies for severe airflow limitation
Introduction
Supporting oxygenation and ventilation are two of the most common interventions in intensive care; in 2007–2008, approximately 41% of patients in Australian and New Zealand ICUs received invasive mechanical ventilation and 8% received non-invasive ventilation (NIV).1 The technology available for supporting oxygenation and ventilation is complex, ranging from simple interventions, such as nasal cannulae through to invasive mechanical ventilation and extracorporeal support. Additionally, the meaning of ventilator terminology is often unclear and terms may be used interchangeably. Critical care nurses must have a strong knowledge of the underlying principles of oxygenation and ventilation that will facilitate an understanding of respiratory support devices, associated monitoring priorities and risks.
Oxygen Therapy
Oxygen is required for aerobic cellular metabolism and ultimately for human survival, with some cells, such as those in the brain, being more sensitive to hypoxia than others. Refer to Chapter 13 for discussion of oxygen delivery and consumption, the oxyhaemoglobin dissociation curve, hypoxaemia and tissue hypoxia; this material provides rationales for clinical decisions regarding the administration of oxygen therapy or ventilation strategies. Oxygen therapy should be considered for patients with a significant reduction in arterial oxygen levels, irrespective of diagnosis and especially if the patient is drowsy or unconscious.
Complications
Hypoventilation and CO2 Narcosis
High-dose oxygen therapy may lead to hypoventilation, worsening hypercapnia and CO2 narcosis due to inhibition of the hypoxic drive in a small proportion of patients with chronic obstructive pulmonary disease (COPD). These patients require close monitoring of PaCO2 levels when oxygen therapy is instituted or increased. Although COPD patients frequently may have a lower baseline SpO2 (88–94% compared to 96–100% in patients with no lung pathology), treatment of hypoxia is still essential, and oxygen should not be withheld or withdrawn while hypoxia remains, even if hypercapnia worsens.2,3
Oxygen Toxicity
Administration of high concentrations of oxygen may lead to oxygen toxicity; symptoms include non-productive cough, substernal pain, reduced lung compliance, interstitial oedema, and pulmonary capillary haemorrhage. These symptoms may be mistakenly attributed to the underlying illness, especially in a sedated and ventilated patient. Many of the symptoms abate once the percentage or fraction of inspired oxygen (FiO2) is reduced, although irreversible pulmonary fibrosis may occur (see Box 15.1). The concentration and duration of oxygen exposure that induces oxygen toxicity varies between patients;4 the lowest possible FiO2 should therefore be used to achieve the target PaO2 or SpO2.
Oxygen Administration Devices
• patient factors: inspiratory flow rate, respiratory rate, tidal volume, respiratory pause
• oxygen device factors: oxygen flow rate, volume of mask/reservoir, air vent size, tightness of fit
Normal inspiratory flow in a healthy adult ranges between 25 and 35 L/min. Patients with respiratory failure tend to increase their flow demand from 50 up to 300 L/min. Patients in respiratory distress are characterised by high respiratory rates and low tidal volumes4,5 that can significantly decrease the FiO2 available via an oxygen delivery device, depending on the type in use.
Variable Flow Devices
High-flow Nasal Cannulae
High-flow nasal cannulae (HFNC) have slightly larger prongs that facilitate oxygen flow of up to 60 L/min leading to less air entrainment effect than with other oxygen delivery systems.5,6 HFNC generate low levels of end-expiratory pressure and can therefore reduce tachypnoea and work of breathing.7,8 The high gas flow may flush CO2 from the anatomical dead space preventing CO2 rebreathing and thereby decreasing PaCO2, although this is not well supported by the literature.9,10 These systems are also generally well-tolerated by the patient, but must be used with heated humidification to avoid drying the respiratory mucosa.8 HFNC are now used more frequently in clinical practice to avoid more invasive therapies but there is limited high-quality evidence on their use in adults.
Oxygen Masks
Loose-fitting oxygen masks include simple (Hudson) face masks, aerosol masks used in combination with heated humidification and nebuliser treatments, tracheostomy masks and face tents. All are considered low-flow or variable-flow devices, with the delivered FiO2 varying with patient demand. Flow rates ≥5 L/min minimise CO2 rebreathing. The addition of ‘tusks’ to a Hudson mask may increase the oxygen reservoir,11 but does not guarantee a consistent FiO2 and has probably been superseded by high-flow systems.12
Partial rebreather and non-rebreather masks have an attached reservoir bag that enables delivery of higher levels of FiO2. Both mask types have a one-way valve precluding expired gas entering the reservoir bag. A non-rebreather mask has two one-way valves on the mask preventing air entrainment.13 The maximum FiO2 delivery with non-rebreather masks is 0.85 with low flow demand, with a steep decline in FiO2 concentration at the alveoli level as the patient’s minute volume increases. Non-rebreather masks may perform worse than a Hudson mask without a reservoir bag.5
Venturi Systems
Venturi systems use the Bernoulli Effect to entrain gas via a side port; gas flow through a narrowing increases speed and gains kinetic energy, resulting in an area of low pressure that entrains room air through the side port. An FiO2 concentration can be selected by widening or narrowing the aperture in the Venturi device to a maximum FiO2 of 0.6. The FiO2 concentration using a Venturi system is less affected by changes in respiratory pattern and demand compared to other low-flow oxygen devices.5
Bag–Mask Ventilation
Bag–mask ventilation (BMV) with a self-inflating bag (and reservoir), non-return valve and mask delivers assisted ventilation at an FiO2 of 1. Addition of a positive end-expiratory pressure (PEEP) valve will improve oxygenation. Manual ventilation requires a good seal between the patient’s face and the mask; this may be difficult to achieve as a single operator. One person may be required to hold the mask and lift the patient’s chin, while another squeezes the bag. Effective bag–mask ventilation is confirmed when the chest visibly rises as the bag is squeezed as well as improved oxygen saturations.14 BMV may cause gastric insufflation, increasing the risk of vomiting and subsequent aspiration.
Airway Support
The most common cause of partial airway obstruction in an unconscious patient is loss of oropharyngeal muscle tone, particularly of the tongue. This may be alleviated by tilting their head slightly back and lifting the chin, or thrusting the jaw forward. The head-tilt/chin-lift manoeuvre is not used if cervical spine injury is suspected.15 The jaw-thrust manoeuvre may require two hands to maintain.16 If more prolonged support is required, an oro- or nasopharyngeal airway can be used that may also facilitate bag–mask ventilation.
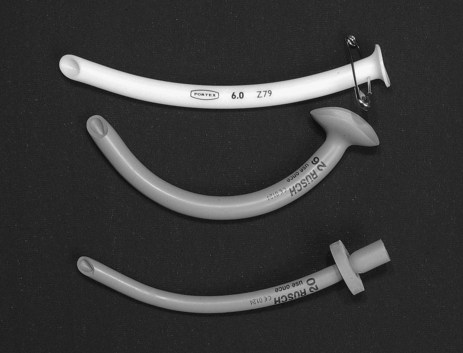
Oro- and Nasopharyngeal Airways
The Guedel oropharyngeal airway is available in various sizes (a medium-sized adult requires a size 4). The airway is inserted into the patient’s mouth past the teeth, with the end facing up into the hard palate, then rotated 180 degrees, taking care to bring the tongue forward and not push it back. Oropharyngeal airways are poorly tolerated in conscious patients and may cause gagging and vomiting.14
A nasopharyngeal airway (see Figure 15.1) is inserted through the nares into the oropharynx; it can be difficult to insert and require generous lubrication to minimise trauma. This type of airway should not be used for patients with a suspected head injury. As well as opening the airway, suction catheters can be passed to facilitate secretion clearance. Once inserted these airways are better tolerated than an oropharyngeal airway.
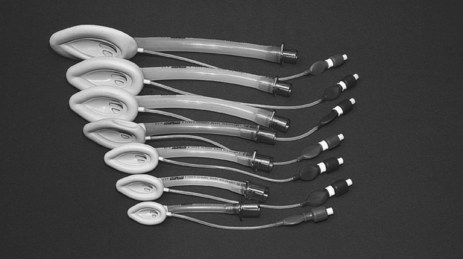
Laryngeal Mask Airway and Its Intubation
The classic laryngeal mask airway (cLMA) (see Figure 15.2) is positioned blindly into the pharynx to form a low-pressure seal against the laryngeal inlet. It is easier and quicker to insert than an endotracheal tube, and is particularly useful for operators with limited airway skills; the cLMA does not carry the same potentially fatal complications such as oesophageal intubation although the risk of aspiration remains.17
Mechanical ventilation can be delivered with low-airway pressures (less than 20 cmH2O) via a cLMA. This device is widely used in elective general anaesthesia,15 and can be used in critical care as an alternative to bag–mask ventilation17 or endotracheal intubation when initial attempts at intubation have failed.18 The ‘intubating’ LMA is most commonly used when a difficult intubation is anticipated or encountered. This device has a handle and is more rigid, wider and curved than the cLMA, enabling passage of a purpose-made endotracheal tube.17
Combitube
The combitube is more widely used in North America for emergency situations than in Australia and the UK.15 It is a dual-lumen, dual-cuff oesophageal-tracheal airway that enables ventilation if inserted into either the oesophagus or trachea. Inexperienced operators may find a combitube more difficult to insert correctly than a cLMA.19 Complications may occur in up to 40% of patients and include aspiration pneumonitis, pneumothorax, airway injuries and bleeding, oesophageal laceration and perforation and mediastinitis.20
Intubation
Endotracheal intubation is the ‘gold standard’ for airway support, providing airway protection in the presence of an airway oedema, absent gag, cough or swallow reflex. Intubation facilitates delivery of mechanical ventilation and pulmonary secretion clearance.16
Endotracheal Tubes
Endotracheal tubes (ETT) are available with internal diameters ranging from 2–10 mm (common adult sizes are 7–9 mm), and are up to 30 cm long. A longitudinal radio-opaque line allows visualisation of tube placement on a chest X-Ray. Markings at 1 cm intervals indicate the length from the distal end. Tubes are available with and without a distal cuff. Adults typically require a cuffed ETT to seal their trachea, facilitating positive pressure ventilation and preventing aspiration of oropharyngeal contents. Cuffs come in a range of profiles and volumes, but are commonly high-volume, low-pressure (see Figure 15.3).
Endotracheal tubes may be reinforced with a wire coil embedded within the plastic for the entire length of the tube to prevent kinking and occlusion. These tubes are more commonly used in the operating room.21 The wire coils can however be irreversibly compressed by a strong bite that occludes the airway. Reinforced tubes also increase the risk of tracheal damage and therefore should be replaced with a standard endotracheal tube on arrival in the ICU. Most endotracheal tubes have a ‘Murphy eye’, an oval-shaped hole in the side of the tube between the cuff and the end of the tube that provides a patent aperture if the distal opening is occluded.22
Preparation for Intubation
Adequate preparation of the patient, equipment and environment, as well as strong knowledge of emergency procedures is important to ensure safe and efficient intubation. Up to 50% of patients undergoing endotracheal intubation in ICU will experience a complication; 28% will have a serious complication, including hypoxaemia, circulatory collapse, cardiac arrhythmia, cardiac arrest, oesophageal intubation, aspiration and death.23
Patient Preparation
Equipment and Drugs
All equipment should be checked immediately prior to intubation, including
• suction supply, with a range of Yankaeur and y-suction catheters
• laryngoscope blades and holder are compatible, with a functioning light
• appropriately-sized face mask
• manual ventilation (ambubag™) available and attached to oxygen supply
• ETT cuff inflated in sterile water to ensure no leaks and even inflation
• water-based lubricant of tube and cuff (while maintaining sterility)
• capnography (chemical CO2 detectors are often used in emergency situations)
Procedure
The patient is preoxygenated to minimise desaturation during apnoea and laryngoscopy, commonly via bag and mask, although other methods such as non-invasive ventilation have been suggested.24 Intubation in ICU is usually performed via laryngoscopy with insertion of an oral ETT. Intubation may be performed using a fibreoptic bronchoscope when difficulty is encountered, or for nasal intubation.
Oral vs Nasal Intubation
Oral intubation is preferred unless there are specific indications for nasal intubation. Oral intubation is easier to perform and allows use of a larger diameter tube. While nasal intubation provides better splinting for the ETT and facilitates oral hygiene, it can damage nasal structures, is contraindicated in skull fractures and increases the risk of maxillary sinusitis and ventilator-associated pneumonia.25
Cricoid Pressure
Cricoid pressure (Sellick manoeuvre) was introduced in the 1960s to prevent aspiration of gastric contents during intubation. The oesophagus lies behind and in line with the trachea. The cricoid cartilage, situated below the thyroid prominence, is a closed tracheal ring which, when compressed, closes the oesophagus while the trachea remains open. Cricoid pressure is performed by placing your thumb on one side of the patient’s trachea, middle finger on the other side and index finger directly on the cricoid.26 Although widely used over the last 50 years, its efficacy is being questioned as technique is frequently poor,27 and there is wide anatomical variation in the exact orientation of the oesophagus in relation to the trachea.28
Backwards, Upwards, Rightward Pressure Manoeuvre
The backwards, upwards, rightward pressure (BURP) manoeuvre on the thyroid cartilage was introduced in the mid-1990s to improve visualisation during difficult laryngoscopy. The patient’s jaw is thrust forward, so their head is in the ‘sniffing’ position. Place your thumb and third finger on either side of the thyroid cartilage and index finger on top. Pressure is applied in the sequence backwards (towards the spine), upwards (towards the head), rightward (towards the patient’s right side). This is easier to perform following administration of muscle relaxants.29,30
Cuff Management
Endotracheal and tracheostomy tube cuffs prevent airway contamination by pharyngeal secretions and gastric contents and loss of tidal volume during mechanical ventilation. The cuff does not secure the tube in the trachea. Cuff inflation pressures should be maintained at 20–30 cmH2O.31,32 Cuff inflation pressures ≤20 cmH2O (15 mmHg) are associated with an increased risk of aspiration and a 2.5-fold increase in ventilator-associated pneumonia (VAP).33 Conversely, tracheal wall damage may occur if cuff pressure exceeds the capillary perfusion pressure in the trachea (27–40 cmH2O/20–30 mmHg).
There are four methods described for assessing cuff inflation:
In Australia and New Zealand, CPM is the most common form of cuff pressure assessment,34 in contrast to the UK35 and North America36 where CPM is used infrequently. Cuff pressure varies with head and body position, tube position and airway pressures.37 The optimum frequency of cuff pressure monitoring is unclear; at a minimum it should be done post-intubation, on arrival in ICU and once per nursing shift. A persistent cuff leak or pressures of ≥30 cmH2O (22 mmHg) to generate a seal should be reviewed and referred to medical staff.
Endotracheal Tube Fixation
The purpose of ETT fixation is to maintain the tube in the correct position, prevent unintended extubation and facilitate mechanical ventilation while maintaining skin integrity and oral hygiene.38 ETT fixation methods include:
• tying cotton tape around the tube, then around the patient’s neck
• taping the tube to the patient’s face using medical adhesive tape
There is no evidence supporting a preferred method39 with each having specific strengths and weaknesses. Two nurses are required to prevent ETT dislodgement during fixation. Although there is also no evidence to recommend a preferred frequency, ETT fixation is generally changed at least daily, to allow assessment of the underlying skin with particular attention to the tops of the ears and corners of the mouth and to facilitate oral hygiene.38 The ETT position in the mouth is alternated at this time.
Confirmation of Tube Position
The correct position of the ETT distal end is 3–5 cm above the carina. A lip level of 20 cm for women and 22 cm for men should prevent endobronchial intubation, with the proximal end fixed at either the centre or the side of the mouth.40 Confirmation of the ETT position is required immediately following intubation and at regular intervals thereafter as movement of the tube can occur.
Chest auscultation is the traditional method to confirm ETT position. Observation of chest expansion is, however, unreliable, as the chest may appear to rise with oesophageal intubation. Conversely the chest may not rise with a correctly positioned tube if the patient is obese or has a rigid chest wall. Patients with left main bronchus intubation may exhibit bilateral breath sounds.41 End-tidal CO2 monitoring is the ‘gold standard’ method for confirming ETT placement. Disposable devices that change colour in the presence of CO2 are inexpensive and easy to use, but may be inaccurate during cardiopulmonary resuscitation, or if contaminated. Capnography is the most reliable technique to identify ETT placement in both arrest and non-arrest situations.18 Continuous end-tidal CO2 monitoring during intubation is recommended as a minimum standard by the College of Intensive Care Medicine of Australia and New Zealand.42
Tracheostomy
Tracheostomy may be required for upper airway obstruction, although it is most commonly performed for ICU patients who require prolonged mechanical ventilation. The advantages of tracheostomy over endotracheal intubation include decreased risk of laryngeal damage and subglottic stenosis, reduced airway resistance and deadspace which decreases the work of breathing and therefore supports weaning,43 and improved patient tolerance enabling reduction of sedation. The optimum time to perform tracheostomy remains contentious, and is often influenced by a patient’s diagnosis.44
Procedure
Tracheostomy can be performed using a surgical technique (ST) or percutaneous dilatational technique (PDT). PDT is contraindicated in patients with anatomical anomalies of the neck and serious bleeding disorders, and should be undertaken with caution in patients who are obese, have a cervical spine injury, coagulopathy, difficult airway or require high levels of ventilatory support.45 PDT is more commonly performed than ST in Australian and New Zealand ICUs.45
Tracheostomy Care
The aim of tracheostomy care is to keep the site free of infection, and prevent tube blockage or dislodgement. The site is cleaned with normal saline and fixation devices changed at least 12-hourly with two nurses to safely perform tape changes.46 Velcro tapes are easier to change and more comfortable than cotton tape.47 Lint-free or superabsorbent foam dressings may be placed under the flange to absorb secretions. Adequate humidification and suctioning will usually prevent tube obstruction (see later in this chapter). The use of inner cannulae has obviated the need for frequent tracheostomy tube changes. Single lumen (no inner cannula) tracheostomy tubes should be changed every 7–10 days.46
Complications of Endotracheal Intubation and Tracheostomy
Tube blockage, tube dislodgement and aspiration are major complications. Partial ETT or tracheostomy tube dislodgement can cause greater harm than complete removal because of delays in diagnosis and resultant aspiration or worsening gas exchange. Tube dislodgement is most likely to occur when turning the patient, if the patient is agitated or when nursing staff are distracted or on breaks.48 While physical restraint may be considered to prevent tube dislodgement, multiple studies noted patients were restrained at the time of self-extubation or device removal.49–54 Effective levels of analgesia and sedation is therefore most appropriate in minimising the risk of self-extubation.
Complications during and immediately after endotracheal intubation and tracheostomy include cardiovascular compromise, bleeding, injury to the tracheal wall, damage to the vocal cords, pneumothorax, pneumomediastinum and subcutaneous emphysema. Late complications of tracheostomy include tracheal stenosis, tracheomalacia and tracheo-oesophageal fistula and infection. As noted earlier, damage to the trachea is exacerbated by high cuff pressures.55 PDT results in fewer wound infections, decreased incidence of bleeding and reduced mortality compared to ST.56
Tracheal Suction
Patients with an ETT or tracheostomy tube require tracheal suction to remove pulmonary secretions that can lead to atelectasis or airway obstruction and impair gas exchange.57 Suction should be performed as clinically indicated, with assessment of visible or audible secretions, rising inspiratory pressure, decreasing VT or increased work of breathing.58 A sawtooth pattern on the flow-volume waveform may also indicate the need for suction (discussed later in this chapter).59
Preoxygenation using a FiO2 of 1 for 60 seconds prior to performing suction minimises hypoxia and the potential for cardiac arrhythmias. Manual hyperinflation is discouraged due to the risk of barotrauma and lack of benefit. Similarly, installation of saline is not supported due to increased risk of flushing pathogens into distal lung regions.60
Methods
The three methods of suctioning are:
• open suction: a suction catheter is passed under aseptic technique directly into the ETT/tracheostomy after disconnection from the ventilator circuit. Disadvantages include loss of PEEP resulting in loss of alveolar recruitment and increased risk of transmission of infective organisms. A surgical mask and protective eyewear should be worn.61
• semi-closed suction: a suction catheter is passed through a swivel connector with a self-sealing rubber flange.
• closed suction: in-line system is attached between the ETT/tracheostomy tube and the ventilator circuit where the suction catheter is contained in an integrated plastic sleeve. Alveolar derecruitment occurs to a lesser degree than with open suction.
Adverse Effects
Adverse effects of suction can include hypoxaemia, introduction of infective organisms, tracheal trauma, bradycardia, hypertension and increased intracranial pressure. Tracheal suctioning causes discomfort, and should therefore be performed only when clinically indicated, such as audible presence of secretions and desaturation.58
Extubation
Following successful weaning from mechanical ventilation (see later in this chapter), assessment of the patient prior to extubation should include adequate gas exchange, respiratory rate and work of breathing on minimal support for prolonged periods, respiratory muscle strength, the ability to cough and clear secretions spontaneously and a stable haemodynamic status and mental status.62 Serious post-extubation complications of laryngospasm and stridor cannot be reliably predicted,63 so the ease/grade of intubation should be considered prior to extubation and provision made for immediate re-intubation.64
Mechanical Ventilation
As stated in the introduction, 41% of patients in Australian and New Zealand ICUs received invasive mechanical ventilation and 8% received non-invasive ventilation (NIV) in 2007–08.1 The median duration of invasive mechanical ventilation for these patients was 2.5 days. In the most recent international study of mechanical-ventilation practices, reporting data from 4968 patients in 349 ICUs and 23 countries found the median duration of ventilation to be 4 days (interquartile range 2–8 days).65 In this patient cohort the three most common reasons for mechanical ventilation were postoperative respiratory failure, coma and pneumonia. This international report did not include data from Australia and New Zealand. A study describing ventilation and weaning practices of 55 ICUs in Australia and New Zealand in 2005 reported a similar profile for the most common indications for mechanical ventilation.66
Principles of Mechanical Ventilation
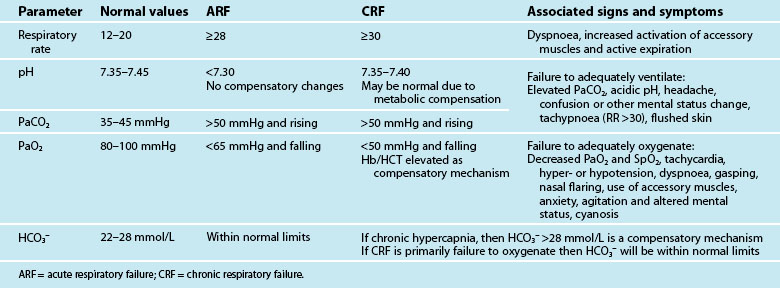
Mechanical ventilation describes the application of positive or negative pressure breaths using non-invasive or invasive techniques. Indications for initiation of mechanical ventilation are discussed below. Table 15.1 lists the patient parameters typically observed in acute and chronic respiratory failure that may be influential in the decision to ventilate. During positive pressure ventilation, the type of ventilation used most commonly in critical care, the ventilator delivers a flow of gas into the lungs during inspiration using a pneumatic system. Expiration is passive.
The Equation of Motion
The equation of motion for the respiratory system is a mathematical model that relates pressure volume and flow during the delivery of a breath, with the pressure required to deliver a volume of gas to the lungs determined by the elastic and resistive properties of the respiratory system67 (see Table 15.2).
| Equation: PT (Pairway + Pmuscle) = VT/Cr + VT/TI × R + PEEPT | |
|---|---|
| Abbreviations: | |
Compliance and Elastance
Lung tissue and the surrounding thoracic structures contribute to respiratory compliance. Normal compliance for a mechanically ventilated patient ranges from 35–50 mL/cmH2O.68
Resistance
Resistance refers to the forces that oppose airflow. Resistance in the airways is affected by the diameter and length of the airways, including the artificial airway, the gas flow rate and the density and viscosity of the inspired gas. During mechanical ventilation, bronchospasm, airway oedema, endotracheal tube lumen size, increased secretions, and inappropriate setting of flow rates can influence airway resistance. Normal resistance for intubated patients is 6 cmH2O/(L/sec).68
Ventilator Circuits
Delivery of mechanical ventilation requires a ventilator circuit to transport gas flow to the patient. To prevent condensation from cooling of warm humidified gas, inspired gas is heated via a wire inside the wall of the circuit in either the inspiratory limb alone or both the inspiratory and expiratory limbs.69 Historically ventilator circuits were changed frequently (48–72 hours) to decrease the risk of VAP.70 Current guidelines for prevention of VAP found evidence that the frequency of ventilator circuit changes had no relationship to the incidence of VAP and therefore recommended routine circuit changes were not necessary and circuits should only be changed when soiled or damaged.71
Humidification
Humidification techniques warm and moisten gas to facilitate cilia action and mucus removal as well as to prevent drying and irritation of respiratory mucosa and solidification of secretions. During endotracheal intubation and mechanical ventilation, the normal humidification processes of the nasopharynx are bypassed. This, in combination with the use of dry medical gas at high flow rates, means alternative methods of humidification are required. The best conditions for mucosal health and function over prolonged periods are when inspired gas is warmed to core body temperature and is fully saturated with water.72
Absolute and Relative Humidity
1. the inspired gas delivered into the trachea is at 37°C with a water content of 30–43 g/m3 (relative humidity is 100% at 37°C in the bronchi)
2. the set temperature remains constant without fluctuation
3. humidification and temperature are unaffected by a large or differing types of gas flow
4. the device is simple to use
5. the humidifier can be used with spontaneously breathing and ventilated patients
6. safety alarms prevent overheating, overhydration and electrocution
7. the resistance, compliance and dead space characteristics do not adversely affect spontaneous breathing modes
Heat–moisture Exchanger
Heat–moisture exchangers conserve heat and moisture during expiration, and enable inspired gas to be heated and humidified. Two types of HMEs exist: hygroscopic and hydrophobic. Hygroscopic HMEs absorb moisture onto a chemically impregnated foam or paper material and have been shown to be more effective than hydrophobic HMEs.74 HMEs are placed distally to the circuit Y-piece in line with the endotracheal tube and increase dead space by an amount equal to their internal volume.75 HMEs should be changed every 24 hours or when soiled with secretions and are usually reserved for short term humidification.
Heated Humidification
Generally, heated humidification (HH) is used for patients requiring greater than 24 hours of mechanical ventilation. Various models of heater bases and circuits are on the market and we recommend their use in accordance with manufacturer instructions. A recent systematic review and meta-analysis reported no overall effect on artificial airway occlusion, mortality, pneumonia, or respiratory complications when HMEs were compared to HHs, although it noted that PaCO2 and minute ventilation were increased and body temperature was lower with the use of HMEs.76
Non-Invasive Ventilation
Terminology
Positive pressure NIV can be further categorised as non-invasive positive pressure ventilation (NIPPV) or continuous positive airway pressure (CPAP). NIPPV is the provision of inspiratory pressure support (also referred to as inspiratory positive airway pressure [IPAP]) usually in combination with positive end expiratory pressure (PEEP) (also referred to as expiratory positive airway pressure [EPAP]). CPAP does not actively assist inspiration but provides a constant positive airway pressure throughout inspiration and expiration.77
The terms Biphasic (or bilevel) positive airway pressure (BiPAP®) and non-invasive pressure support ventilation (NIPSV) are also used to refer to NIPPV.78 The acronym BiPAP® is registered to Respironics (Murrayville, PA), a company that produces a number of non-invasive ventilators including the BIPAP Vision, a NIV ventilator commonly used in the ICU. The acronym NIPSV is primarily used in European descriptions of NIPPV.
Physiological Benefits
The efficacy of NIV in patients with acute respiratory failure is, at least in part, related to avoidance of inspiratory muscle fatigue through the addition of inspiratory positive pressure thus reducing inspiratory muscle work.79 Application of positive pressure during inspiration increases transpulmonary pressure, inflates the lungs, augments alveolar ventilation and unloads the inspiratory muscles.80 Augmentation of alveolar ventilation, demonstrated by an increase in tidal volume, increases CO2 elimination and reverses acidaemia. High levels of inspiratory pressure may also relieve dyspnoea.81
The main physiological benefit in patients with congestive heart failure (CHF) is attributed to the increase in functional residual capacity associated with the use of PEEP that reopens collapsed alveoli and improves oxygenation.82 Increased intrathoracic pressure associated with the application of positive pressure also may improve cardiac performance by reducing myocardial work and oxygen consumption through reductions to ventricular preload and left ventricular afterload.82–84 NIV also preserves the ability to speak, swallow, cough and clear secretions, and decreases risks associated with endotracheal intubation.85
Indications for NIV
The success of NIV treatment is dependent on appropriate patient selection.86 Table 15.3 outlines indications and contraindications to NIV.
TABLE 15.3 Indications and contraindications for non-invasive ventilation77
| Indications | |
| Bedside observations | Increased dyspnoea: moderate to severeTachypnoea: >24 breaths per min [obstructive] >30 breaths per min [restrictive] Signs of increased work of breathing, accessory muscle use and abdominal paradox |
| Gas exchange | Acute or acute-on-chronic ventilatory failure (best indication), PaCO2 >45 mm Hg, pH <7.35 Hypoxaemia (use with caution), PaO2/FIO2 ratio <200 |
| Contraindications | |
| Absolute | Respiratory arrest Unable to fit mask |
| Relative | Medically unstable: hypotensive shock, uncontrolled cardiac ischaemia or arrhythmia, uncontrolled upper gastrointestinal bleeding Agitated, uncooperative Unable to protect airway Swallowing impairment Excessive secretions not managed by secretion clearance techniques Multiple (i.e. two or more) organ failure Recent upper airway or upper gastrointestinal surgery |
PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; PaO2/FIO2: ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen.
Acute Respiratory Failure
Evidence supporting the role of NIV in patients with hypoxaemic respiratory failure is limited and conflicting.82 For patients with community-acquired pneumonia, NIV has been shown to reduce intubation rates, ICU length of stay and 2-month mortality but only in the subgroup of patients with COPD.87 Pneumonia also has been identified as a risk factor for NIV failure.88
Acute Exacerbation of COPD and CHF
Strong evidence exists to support the use of NIV for patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF). Three meta-analyses have shown a reduction in intubation rates, hospital length of stay and mortality for COPD patients managed with NIPPV compared to standard medical treatment.89–91 COPD patients most likely to respond favourably to NIPPV include those with an unimpaired level of consciousness, moderate acidaemia, a respiratory rate of <30 breaths/minute and who demonstrate an improvement in respiratory parameters within two hours of commencing NIV.79,92
Early use of NIV in combination with standard therapy for patients with CHF has also been shown to reduce intubation rates and mortality when compared to standard therapy alone.93–95 A recent meta-analysis found CPAP reduced hospital mortality whereas NIPPV did not have an effect on mortality.94 Both NIV modes were shown in this meta-analysis to reduce the need for intubation. An early study comparing NIPPV to CPAP in patients with CHF reported a higher incidence of myocardial infarction.96 Based on this finding, practice guidelines from the British Thoracic Society recommend NIPPV should only be used for patients with CHF when CPAP has been unsuccessful.97 More recently several studies have found no difference in myocardial infarction rates when comparing the two modes.98–101 A recent large multicentre randomised controlled trial found NIV delivered by either CPAP or NIPPV resulted in symptomatic improvements, but failed to demonstrate a mortality benefit.102 Practice surveys indicate CPAP may be the preferred method of NIV for patients with CHF in Australia and internationally.103,104
NIV in Weaning
NIV may be used as an adjunct to weaning to reduce the duration of invasive ventilation and associated complications.105 Patients are extubated directly to NIV and then weaned to standard oxygen therapy. This use of NIV differs from its role in preventing reintubation in patients that develop, or who are at high risk of, postextubation respiratory failure.106 A recent systematic review and meta-analysis of 12 trials of NIV as a weaning adjunct found reductions in mortality, ICU and hospital lengths of stay, duration of ventilation and rates of VAP.107 Conversely the largest study of NIV use in postextubation respiratory failure reported worsened survival rates hypothesised as a result of delayed reintubation.108 A subsequent meta-analysis suggested NIV may have a role in preventing the development of respiratory failure postextubation for those at risk, but should be used with caution once respiratory failure has developed and should not delay the decision to reintubate.106
Interfaces and Settings
NIV requires an interface that connects the patient to either a ventilator, portable compressor or flow generator with a CPAP valve. The selection of an appropriate interface can influence NIV success or failure. Oronasal masks cover both the mouth and nose and are the preferred mask type for the management of acute respiratory failure.110 Nasal masks enable speech, eating and drinking, and therefore are used more frequently for long-term NIV use. An oronasal mask enables delivery of higher ventilation pressures with less leak and greater comfort for the patient.111 Other interfaces include full-face masks111 that seal around the perimeter of the face and cover the eyes as well as the nose and mouth, nasal pillows, mouthpieces that are placed between the patient’s lips, and helmets that cover the whole head and consist of a transparent plastic hood attached to a soft neck collar.112,113 These alternative interfaces may increase patient tolerance by reducing pressure ulceration, air leaks and patient discomfort.114
Initiation and Monitoring Priorities
Successful initiation of NIV is dependent on patient acceptance and tolerance. Patient acceptance of NIV may be aided by a brief explanation of the procedure and its benefits. Strategies to enhance patient tolerance include: use of an interface that fits the patient’s facial features, commencing with low pressure levels, holding the mask gently in position prior to securing with the straps/headgear, and ensuring straps prevent major leaks but are not so tight they increase discomfort. Once NIV is commenced, the patient should be monitored for respiratory and haemodynamic stability, response to NIV treatment, ongoing tolerance, and presence of air leaks (Table 15.4). Arterial blood gas analysis should be performed at baseline and within the first one to two hours of commencement.97 During the initiation and stabilisation period, patients should be monitored using a nurse-to-patient ratio of 1 : 1 with ongoing coaching to promote NIV tolerance throughout the early stabilisation period.
TABLE 15.4 Monitoring priorities for non-invasive ventilation104
| Priority | Assessment |
|---|---|
| Patient comfort |
SpO2: saturation of peripheral oxygen; VT: tidal volume; PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood.
Potential Complications
Masks need to be tight-fitting to reduce air leaks; however, this contributes to pressure ulceration on the bridge of the nose or above the ears (due to mask straps/headgear). Air leaks may cause conjunctival irritation and the high flow of dry medical gas results in nasal congestion, oral or nasal dryness and insufflation of air into the stomach. Claustrophobia associated with the NIV interface may also lead to agitation reducing the efficacy of NIV treatment due to poor coordination of respiratory cycling between the patient and NIV unit.79 More serious, yet infrequent, complications include aspiration pneumonia, haemodynamic compromise associated with increased intrathoracic pressures and pneumothorax.80
Detecting NIV Failure
Failure to respond to NIV within 1–2 hours of commencement is demonstrated by unchanged or worsening gas exchange, as well as ongoing or new onset of rapid shallow breathing and increased haemodynamic instability.111 A decreased level of consciousness may be indicative of imminent respiratory arrest.
Invasive Mechanical Ventilation
Critically ill patients with persistent respiratory insufficiency (hypoxaemia and/or hypercapnia), due to drugs, disease or other conditions, may require intubation and mechanical ventilation to support oxygenation and ventilatory demands.115,116 Clinical criteria for intubation and ventilation should be based on individual patient assessment and patient response to measures aimed at reversing hypoxaemia.
Indications
Indications for intubation and mechanical ventilation include:
• inability to protect airway; e.g. loss of gag/cough reflex; decreased Glasgow Coma Scale (GCS) score
• clinical signs indicating respiratory distress; e.g. tachypnoea,117 activation of accessory and expiratory muscles, abnormal chest wall movements,118 tachycardia and hypertension
• inability to sustain adequate oxygenation for metabolic demands; e.g. cyanosis, SpO2 <88%, with supplemental FiO2 ≥0.5
• respiratory acidosis (e.g. acute decrease in pH <7.25)
Mechanical Ventilators
Contemporary ventilators use sophisticated microprocessor controls with sensitive detection, response and control of pressure and gas flow characteristics. These mechanical ventilators are more sensitive to patient ventilatory demands, enabling improved patient–ventilator synchrony during both inspiratory and expiratory breath phases. Parameters commonly manipulated during mechanical ventilation are detailed in Table 15.5. Parameters often observed and documented are discussed below.
| Parameter | Description |
|---|---|
| Fraction of inspired oxygen (FiO2) | The fraction of inspired oxygen delivered on inspiration to the patient. |
| Tidal volume (VT) | Volume (mL) of each breath. |
| Set breath rate (f) | The clinician determined set rate of breaths delivered by the ventilator (bpm). |
| Inspiratory trigger or sensitivity | Mechanism by which the ventilator senses the patient’s inspiratory effort. May be measured in terms of a change in pressure or flow. |
| Inspiratory pressure (Pinsp, Phigh) | Clinician determined pressure that is targeted during inspiration. |
| Inspiratory time (Tinsp) | The duration of inspiration (sec). |
| Inspiratory : expiratory ratio (I : E) | The ratio of the inspiratory time to expiratory time. |
| Flow (V) | The speed gas travels during inspiration. (L/min). |
| Pressure support (PS) | The flow of gas that augments a patient’s spontaneously initiated breath to a clinician-determined pressure (cmH2O). |
| Positive end-expiratory pressure (PEEP) | Application of airway pressure above atmospheric pressure at the end of expiration (cmH2O). |
| Rise time | Time to achieve maximal flow at the onset of inspiration for pressure-targeted breaths. |
| Expiratory sensitivity | During a spontaneous breath, the ventilator cycles from inspiration to expiration once flow has decelerated to percentage of initial peak flow. |
| Minute volume (VE) | Generally not set directly but is determined by VT and f settings. Tidal volume multiplied by the respiratory rate over one minute (L/min). |
| Airway pressure (Paw) | The pressure measured in cmH2O by the ventilator in the proximal airway. |
| Plateau pressure (Pplat) | The pressure, measured in cmH2O, applied to the small airways and alveoli. Pplat is not set but can be measured by performing an inspiratory hold manoevre. |
Fraction of Inspired Oxygen
The fraction of inspired oxygen (FiO2) is expressed as a decimal, between 0.21 and 1, when supplemental oxygen is applied. Room air has an oxygen content of 0.21 (21%). Ventilation is commonly commenced on a high FiO2 setting, but as noted earlier, consideration is given to the risks of oxygen toxicity which include disruption to the alveolar-capillary membrane and fibrosis of the alveolar wall.119
Tidal Volume
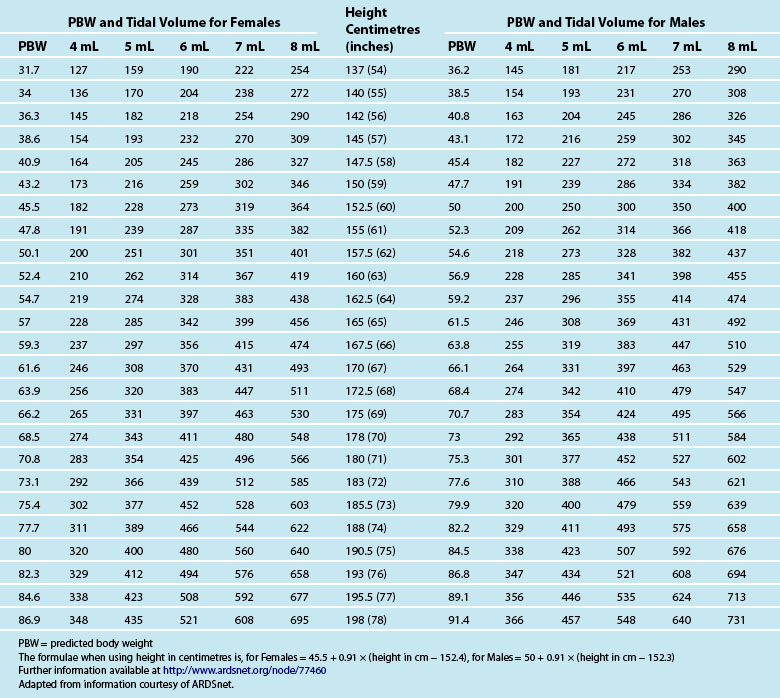
Tidal volume (VT) is the volume, measured in mL, of each breath. The VT is calculated using the patient’s ideal body weight using height and gender-specific tables120 to achieve 6–8 mL/kg (see Table 15.6). Strong evidence indicates a mortality benefit for using 6 mL/kg in patients with acute respiratory distress syndrome (ARDS).121 Some evidence also indicates 6 mL/kg as a target for patients without ARDS or acute lung injury (ALI).122,123 While further studies are required, clinicians should consider aiming for 6–8 mL/kg in all ventilated patients.
Respiratory Rate
Mandatory frequency or respiratory rate (f, RR) is set with consideration of the patient’s own respiratory effort, anticipated ventilatory requirements and the effect on the I : E ratio. Use of high doses of sedation with or without neuromuscular blockade requires setting a mandatory rate that facilitates adequate gas exchange and meets oxygenation requirements. A lower frequency can be set for a patient able to breathe spontaneously in modes such as synchronised intermittent mandatory ventilation (SIMV) and assist control (A/C) (see below) to enable spontaneous triggering. Physiologically normal respiratory rates are 12–20 breaths per minute. Patients with hypoxaemic respiratory failure generally breathe 20–30 breaths per minute.124
Triggering of Inspiration
Depending on the mode of ventilation, breaths are triggered by the ventilator or patient in various sequences. A breath may be triggered by the ventilator in response to time in modes with clinician-determined set frequency such as CMV, and in A/C and SIMV in the absence of spontaneous effort. Patient triggering requires the ventilator to sense the patient’s inspiratory effort. Most modern generation ventilators now use flow triggering, as evidence indicates that flow triggering may be more responsive to patient effort than pressure triggering.125 Pressure triggering requires the patient to create a negative pressure within the ventilator circuit for long enough to enable the ventilator to sense the effort and commence flow of gas. Flow triggering requires a predetermined flow of gas, usually 5–10 L/min, referred to as the bias (or base) flow, that travels continuously through the ventilator circuit. When the patient makes an inspiratory effort, they divert flow that is sensed by the ventilator. If the flow diversion reaches a clinician-determined set value, a breath is initiated.126 The flow trigger is usually set at 1–3 L/min (1 L/min represents less patient effort and 3 L/min represents greater patient effort). Despite advances in ventilator technology, various studies continue to identify missed patient triggers that contribute to patient–ventilator asynchrony.127 Conversely, ‘auto-triggering’ is triggering by the ventilator in the absence of spontaneous inspiratory effort.
Inspiratory Time and Inspiratory : expiratory Ratio
The total time available for each mandatory breath is determined by the set frequency. The total breath time comprises the inspiratory and expiratory time which can be expressed as a ratio (I : E). In normal spontaneous breathing, expiratory time is approximately twice as long as the inspiratory time (1 : 2 ratio). Gas flow also influences inspiratory time, with higher gas flows resulting in decreased time to achieve the target VT. The I : E ratio can be manipulated to create an inverse relationship (1 : 1, 2 : 1, 4 : 1) with the goal of increased mean airway pressure resulting in alveolar recruitment and improved oxygenation. Inverse ratios are more frequently applied with pressure control ventilation as application in volume control can result in increased risk of barotrauma due to peak and plateau airway pressure variation.128
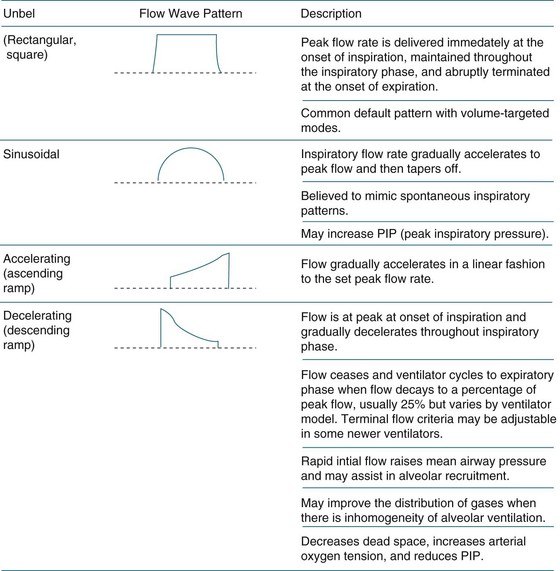
Inspiratory Flow and Flow Pattern
The flow rate refers to the speed of gas and is measured in litres per minute (L/min). Generally, inspiratory flow is delivered at speeds of 30–60 L/min. Higher flow rates cause gas to become more turbulent and result in increased peak airway pressures. Lower flow rates result in laminar flow, an increased inspiratory time, improved distribution of gas, and lower peak airway pressures.129 The flow of inspiratory gas can be delivered in three styles: constant or square wave, decelerating ramp and sinusoidal pattern (see Figure 15.4). In a constant flow pattern, the peak flow is achieved at the beginning of inspiration and is held constant throughout the inspiratory phase. This may result in higher peak airway pressures. Using a decelerating ramp, the gas flow is highest at the beginning of inspiration and tapers throughout the inspiratory phase. Sinusoidal gas flow resembles spontaneous ventilation.
Positive End Expiratory Pressure
Positive end expiratory pressure (PEEP) is the pressure applied at the end of the expiratory cycle to prevent alveolar collapse. PEEP increases residual lung volume thereby recruiting collapsed alveoli, improving V/Q match and enhancing movement of fluid out of the alveoli.130,131 PEEP was originally introduced by Ashbaugh and colleagues132 in the 1960s as a technique for treating refractory hypoxaemia in patients with ARDS. Animal studies suggest ventilator-associated lung injury (VALI) may be prevented using PEEP by recruiting atelectic alveoli and bronchioles and preventing cyclic opening and closing of alveoli.133–136 PEEP may be beneficial, however, only if the lung has sufficient potential for recruitment which occurs in collapsed, as opposed to consolidated, lung.130 The setting of optimal PEEP remains controversial. Low PEEP levels have been shown to be associated with higher mortality for ARDS patients in a number of studies.137–140 Two recently published randomised, controlled trials comparing low tidal volume ventilation and conventional PEEP to low tidal volume ventilation and high PEEP, with and without additional recruitment manoeuvres (40 cmH2O applied for 40 sec),141,142 did not demonstrate a difference in hospital141 or 28-day142 mortality.
Expiratory Sensitivity
Expiratory sensitivity describes the percentage of decay in peak flow reached during the inspiratory phase that signals the ventilator to cycle to expiration for spontaneous breaths. In some ventilator models this is predetermined at 25%, whilst other ventilator models allow clinician selection. Premature termination of a breath will increase inspiratory muscle workload whereas delayed breath termination increases expiratory muscle load.143
Ventilator Modes
The mode of ventilation describes inspiratory phase variables; how the ventilator controls pressure, volume, and flow during a breath; as well as describing how breaths are sequenced. All breaths have trigger, limit and cycle inspiratory phase variables.144 Each breath is triggered (started) either by the patient or by the ventilator. During inspiration, the breath is limited to a set target of pressure, volume, or flow. This target cannot be exceeded during each breath. At the end of inspiration, the cycling variable determines the end of the inspiratory phase. Again this variable may be pressure, flow, volume, or time. Gas delivery during each breath is described by the control variable. There are five control variables: pressure, volume, flow, time and dual control (such as used in the mode pressure regulated volume control [PRVC]). Breath sequencing refers to the sequence of mandatory and spontaneous breath. A spontaneous breath is one during which inspiration is both started (triggered) and stopped (cycled) by the patient. Spontaneous breaths may be assisted, as with pressure support, or unassisted. Mandatory breaths are either triggered or cycled by the ventilator.145 A complete mode description should include: (1) the control variable; (2) the breath sequence; and (3) the targeting scheme (limit variable).
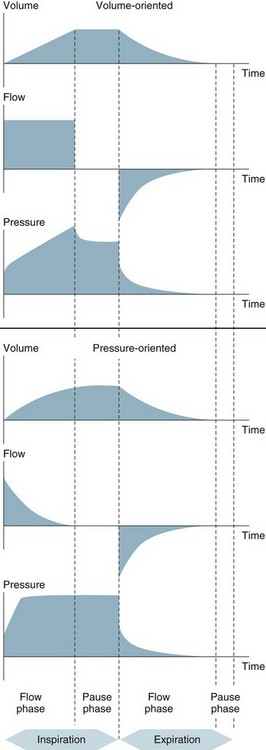
Pressure Control vs Volume Control
Traditionally, clinicians have favoured volume control due to the ability to regulate minute ventilation (VE) and carbon dioxide (CO2) elimination with straightforward manipulation of ventilation.146 Volume control provides consistent tidal volume delivery, independent of the patient’s lung mechanics. A disadvantage of volume control, however, is the lack of control over peak airway pressure that changes in response to altered compliance and resistance. Elevated peak airway pressures may cause alveolar overdistension, barotrauma and haemodynamic effects such as reduced venous return, cardiac output, hypotension and thus decreased organ perfusion.147 Clinicians need to carefully monitor ventilation to avoid injurious pressures. In volume control the peak airway pressure is achieved at the end of inspiration, and only for a short duration, therefore distribution of gas may not be optimised and shearing stress can occur.148
Pressure control allows ventilator control over the peak inspiratory pressure and inspiratory time. Clinicians are required to monitor minute ventilation and gas exchange due to the lack of a guaranteed tidal volume and possible changes in respiratory compliance and resistance. The variable and decelerating inspiratory gas flow pattern of pressure control enables rapid alveolar filling and more even gas distribution compared to the constant flow pattern that may be used with volume control. This decelerating flow pattern results in improved gas exchange, decreased work of breathing and prevention of overdistension in healthy alveoli.149–152 During pressure control, the set inspiratory pressure is achieved at the beginning of the inspiratory cycle and maintained for the set inspiratory time. This promotes recruitment of alveoli with high opening pressures and long time-constants.
Commonly Employed Modes of Ventilators
Contemporary ventilators now provide a range of modes to facilitate mechanical ventilation. Modes of mechanical ventilation are described in Table 15.7.
| Mode | Descriptor | Clinical implications |
|---|---|---|
| Controlled mechanical ventilation (CMV) | All breaths are mandatory, no patient triggering is enabled. Also called volume controlled ventilation (volume targeted) (VCV) and pressure controlled ventilation (pressure targeted) (PCV) | Patients with respiratory effort require sedation and neuromuscular blockade.Potential for respiratory muscle atrophy due to disuse. |
| Assist-control (A/C) | Breaths may be either machine or patient triggered but all are cycled by the ventilator. Assist control may be delivered as volume (AC-VC) or pressure (AC-PC) targeted. | Activation of the diaphragm with patient triggering.Potential for respiratory alkalosis If tachypnoea develops. |
| Synchronised intermittent mandatory ventilation (IMV) | Mandatory breaths are delivered using a set rate and volume (SIMV-VC) or pressure (SIMV-PC). Mandatory breaths are synchronised with patient triggers within a timing window. Between mandatory breaths the patient can breathe spontaneously. | Reduced need for sedation.Activation of the diaphragm with patient triggering. |
| Pressure support ventilation (PSV) | All breaths are patient triggered and cycled. Pressure applied by the ventilator during inspiration (pressure support) augments patient effort. | Reduced need for sedation.Facilitates ventilator weaning.Level of PS can be adjusted to achieve desired VT.Sustains respiratory muscle tone and decreases WOB. |
| Continuous positive airway pressure (CPAP) | All breaths are patient triggered and cycled. Positive pressure is applied throughout inspiratory and expiratory phases of the respiratory cycle. | Requires intact respiratory drive and patient ability to maintain adequate tidal volumes. |
| Volume support (VS) | Spontaneous mode with clinician preset target tidal volume delivery achieved with the lowest inspiratory pressure. | Requires intact respiratory drive |
| Pressure-regulated volume control (PRVC) | Mandatory rate and target tidal volume are set, and the ventilator then delivers the breaths using the lowest achievable pressure. | Dual control of volume and pressure enables guarantee of volume and pressure |
| Airway pressure release ventilation (APRV) | Ventilator cycles between 2 preset pressure levels for defined time periods. I : E ratio is inverse often with a prolonged Inspiratory time (4 sec) and shortened expiratory time (0.8 sec). Patient can breathe spontaneously at both pressure levels | Reduced need for sedation.Activation of the diaphragm with patient triggering.Promotes alveolar recruitment. Considered a rescue mode in ALI/ARDS when used with extreme inverse ratio. |
| Biphasic positive airway pressure (BiPAP/ BILEVEL/Bivent) | As with APRV, the ventilator cycles between 2 preset pressure levels for defined time periods and the patient can breathe spontaneously at both pressure levels. The inspiratory time is generally shorter than, or the same length, as the expiratory time. | Reduced need for sedation.Activation of the diaphragm with patient triggering.Promotes alveolar recruitment. |
| Mandatory minute ventilation (MMV) | The patient’s spontaneous minute ventilation is monitored by the ventilator. When the minute ventilation falls below the clinician determined target, the ventilator increases the mandatory rate or size of tidal volumes to regain the desired minute ventilation. | Guarantees minute ventilation for patients with fluctuating respiratory drive and muscle innervation such as patients awakening from anaesthesia and those with Guillain–Barré. |
| Proportional assist ventilation (PAV)269 | Delivers positive pressure throughout inspiration in proportion to patient generated effort, and dependent on the set levels of flow assist (offsets resistance) and volume assist (offsets elastance).268 | Requires intact respiratory drive.Patients with high respiratory drive as the ventilator may overassist and continue to apply support when the patient has stopped inspiration.269 |
| Proportional assist ventilation (PAV+™) | Clinician only sets a percentage of work for the ventilator. The ventilator assesses total work of breathing by randomly measuring compliance and resistance every 4–10 breaths. | Requires intact respiratory drive.Decreases work of breathing and improves patient ventilator synchrony.Potential for use as a weaning mode. |
| Adaptive support ventilation (ASV) | Automatic adaptation of respiratory rate and pressure levels based on a clinician-set desired percentage of minute ventilation.270 | Automatically sets all ventilator settings except PEEP and FiO2.Potential for use as a weaning mode. |
| Volume assured pressure support (VAPS) | The ventilator switches from pressure control to volume control, or pressure support to volume control during inspiration. | Enables maintenance of a preset minimum VT and reduces work of breathing. |
Controlled Mandatory Ventilation
Controlled mandatory ventilation (CMV) is a mandatory mode, and is the original and most basic mode of ventilation.153 CMV delivers all breaths at a clinician-determined set frequency (rate) and the patient’s spontaneous effort is not acknowledged by the ventilator.68 CMV may also be called volume-controlled ventilation (VCV) or pressure-controlled ventilation (PCV) depending on the target (volume or pressure) variable. VCV requires clinician selection of the frequency, PEEP, FiO2, tidal volume, flow waveform, peak inspiratory flow and either the inspiratory time or I : E ratio. PCV requires clinician selection of rate, PEEP, FiO2, inspiratory pressure, as opposed to tidal volume, and inspiratory time or I : E ratio depending on the ventilator type. Peak inspiratory flow and the flow waveform are manipulated by the ventilator, to achieve the clinician-selected inspiratory pressure within the set inspiratory time. The inability to breathe spontaneously during CMV contributes to diaphragm muscle dysfunction and atrophy which may result in difficulty weaning from the ventilator.154
Synchronised Intermittent Mandatory Ventilation
Synchronised intermittent mandatory ventilation (SIMV) delivers breaths at a set frequency (rate), and can be either pressure- or volume-targeted. Setting of the ventilator is similar to setting VCV or PCV. The availability of patient triggering with SIMV facilitates provision of gas flow in recognition of a patient’s spontaneous effort. SIMV uses a timing window to deliver mandatory breaths in synchrony with patient inspiratory effort.116 Additional spontaneous breaths occurring outside of the timing window may be assisted with pressure support to augment the patient’s spontaneous effort to a pre-set pressure level.
Airway Pressure Release Ventilation and Biphasic Positive Airway Pressure
Airway pressure release ventilation (APRV) and biphasic positive airway pressure (BiPAP) are ventilator modes that allow unrestricted spontaneous breathing independent of ventilator cycling, using an active expiratory valve that allows patients to exhale even in the inspiratory phase.147,148,155,156 Both modes are pressure-limited and time-cycled. In the absence of spontaneous breathing, these modes resemble conventional pressure limited, time-cycled ventilation.157 In North America the acronym BiPAP® is registered to Respironics non-invasive ventilators (Murrayville, PA). Therefore ventilator companies have developed brand names such as BiLevel (Puritan Bennett, Pleasanton, CA, GE Healthcare, Madison, WI) Bivent (Maquet, Solna, Sweden), DuoPaP (Hamilton Medical, Rhäzüns, Switzerland), PCV+ (Dräger Medical, Lübeck, Germany) or BiPhasic (Viasys, Conshocken, PA) to describe essentially equivalent modes. Ambiguity exists in the criteria that distinguish APRV and BiPAP. When applied with the same I : E ratio, no difference exists between the two modes. APRV as opposed to BiPAP, however, is more frequently described with an extreme inverse ratio and advocated as a method to improve oxygenation in refractory hypoxemia.158
Automatic Tube Compensation
Automatic tube compensation (ATC) is active during spontaneous breaths and compensates for the work of breathing associated with artificial airway tube resistance via closed-loop control of continuously calculated tracheal pressure.159,160 During spontaneous inspiration, a pressure gradient exists between the proximal and distal ends of the artificial airway due to resistance created by the tube. A reduced pressure at the proximal end of the tube means a patient needs to produce a greater inspiratory force (greater negative pressure) to generate an adequate tidal volume.161 Higher flow rates generate larger pressure gradients and greater resistance. ATC requires the airway type and size to be entered into the ventilator program as well as the percentage of automatic tube compensation (ATC) to be applied. It appears to have most use in reducing the work of breathing for patients with high respiratory drive who require high inspiratory flow.162
Neurally-adjusted Ventilatory Assist
Neurally-adjusted ventilatory assist (NAVA) is available on the Servo-I ventilator (Maquet, Solna, Sweden) and uses the electrical activity of the diaphragm to control patient–ventilator interaction.163 Electrical activity of the diaphragm, measured using an oesophageal catheter, should result in optimal patient–ventilator synchrony as it represents the endpoint of neural output from the respiratory centres and thus is the earliest signal of patient inspiratory trigger and expiratory cycling. Pressure delivered to the airways (Paw) is proportional to inspiratory diaphragmatic electrical activity using a clinician determined proportionality factor set on the ventilator.164 NAVA provides breath-by-breath assist in synchrony with, and in proportion to, respiratory demand.165 Although clinical data on NAVA is currently limited,164,166–168 this mode shows promise for improving patient–ventilator synchrony.
Ventilator Graphics
Scalars: Pressure/time, Flow/time, Volume/time
Many mechanical ventilators now offer integrated graphic displays as waveforms that plot one of three parameters, pressure, flow or volume, on the vertical (y) axis against time, measured in seconds, on the horizontal (x) axis referred to as scalars. Examination of scalars can assist with assessment of patient–ventilator synchrony, patient triggering, appropriateness of inspiratory/expiratory times, presence of gas trapping, appropriateness and adequacy of flow, lung compliance and airway resistance and circuit leaks.169,170
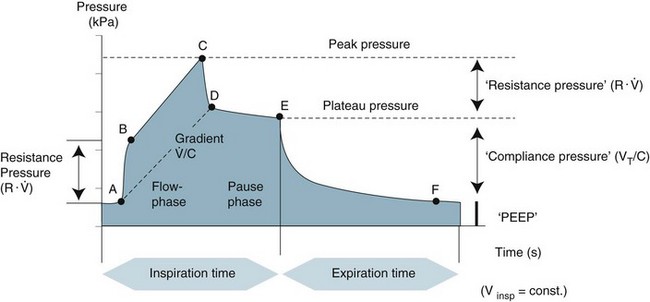
Pressure vs time scalar
The morphology of this waveform depends on the breath target (volume or pressure) and the breath type (mandatory or spontaneous).171 Pressure–time waveforms reflect airway pressure (Paw) during inspiration and expiration and can be used to evaluate peak, plateau and end inspiratory pressures as well as inspiratory and expiratory times and appropriateness of flow (see Figure 15.5). Pressure–time scalars vary in appearance depending on the control variable (volume vs pressure). In volume-control breaths, the inspiratory waveform continues to rise until peak airway pressure is achieved at the end of inspiration. In pressure control breaths, the inspiratory waveform reaches its peak at the beginning of inspiration and remains at this elevation until cycling to expiration. Spontaneous triggering of ventilation can be identified by examination of the pressure–time scalar at the beginning of inspiration. A small negative deflection indicates patient effort. When pressure-triggering is used, a breath is triggered when the pressure drops below baseline. The depth of the deflection is proportional to patient effort required to trigger inspiration. A flow-triggered breath occurs when the flow rises above baseline, although this is frequently accompanied by a small negative deflection in the pressure-time scalar. Patient inspiratory attempts that fail to trigger the ventilator can also be identified as negative deflections in the pressure waveform without corresponding responses from the ventilator.172 Appropriateness of flow can be detected from the pressure–time scalar. If the flow is set too high or the rise time too short this can be seen as a sharp peak in the waveform. Conversely if flow is inadequate or the rise time too long, the incline of the inspiratory portion of the pressure waveform may be dampened.169
Flow vs time scalar
The flow–time scalar presents the inspiratory phase above the horizontal axis and the expiratory phase below (see Figure 15.6). The shape of the inspiratory flow waveform is influenced by the selection of flow pattern (constant, decelerating, sinusoidal) in volume-control breaths or the variable and decelerating flow waveform associated with pressure-control breaths. The inspiratory flow waveform of spontaneous breaths, those triggered and cycled by the patient, is influenced by the presence or absence of pressure support and the expiratory sensitivity.169
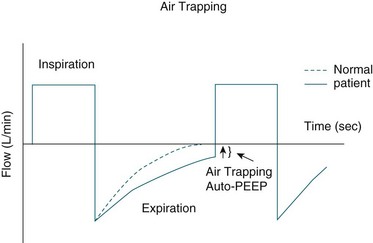
Evaluation of the expiratory limb of the flow-time scalar assists with detection of gas trapping as well as the patient’s response to bronchodilators. In the absence of gas trapping, the expiratory limb drops sharply below baseline then gradually returns to zero before the next breath. Failure to return to baseline indicates gas trapping whereby the gas inspired is not totally expired. Gas trapping results in development of intrinsic or ‘auto-PEEP’. This can adversely affect a patient’s haemodynamic status and cause patient–ventilator asynchrony.173 Gas trapping may occur in patients with airflow limitation such as those with COPD and asthma. Consequences of gas trapping include dynamic hyperinflation, reduced respiratory compliance and respiratory muscle fatigue.174 Evaluation of the expiratory flow waveform also enables evaluation of the effects of bronchodilator therapy as, if efficacious, improvements should be seen in the return to baseline of the expiratory flow waveform (see Figure 15.7).173,175 Patient–ventilator asynchrony can be detected in the flow waveform as abrupt decreases in expiratory flow in the expiratory limb and abrupt increases in flow in the inspiratory limb of the flow waveform.172
Volume vs time scalar
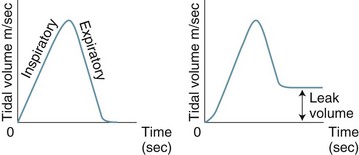
The volume waveform originates from the functional residual capacity (baseline), rises as inspiratory flow is delivered to reach the maximum inspiratory tidal volume, then returns to baseline during expiration. The volume waveform is useful in troubleshooting circuit leaks (see Figure 15.8) as it will fail to return to baseline if a leak in the circuit–patient interface is present.
Loops: Pressure/volume, Flow/volume
Pressure–volume loops
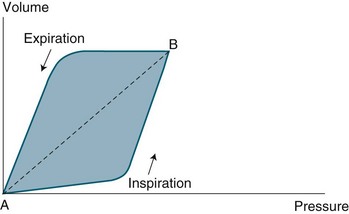
The two parameters, Paw and VT are plotted against each other, with Paw on the x axis. For mandatory breaths, the loop is drawn counter clockwise (see Figure 15.9). Spontaneous (triggered and cycled) breaths are drawn in a clockwise fashion. At the beginning of inspiration, the Paw starts to rise with little change in VT. As Paw continues to rise, the VT increases exponentially as alveoli are recruited, resulting in a marked increase in the slope of the inspiratory limb. This point represents alveolar recruitment and is referred to as the lower inflection point, and may be used to guide PEEP selection.176,177 The inspiratory limb continues until peak inspiratory pressure and maximal VT are achieved. The bend in the inspiratory limb towards the end of inspiration is referred to as the upper inflection point, and denotes the point at which small volume increases produce large pressure increases indicating lung overdistension.176 The expiratory limb represents lung derecruitment and is also useful in guiding PEEP selection.178,179
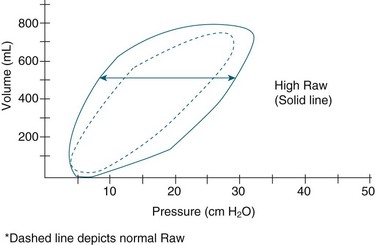
For patient-triggered mandatory breaths, the initial part of the loop occurs to the left of the y axis and flows in a clockwise fashion, reflecting patient effort. The loop then shifts to the right of the y axis and moves in a counter- clockwise fashion as the ventilator assumes the work of breathing.68 P–V loops reflect dynamic compliance between the lungs and the ventilator circuit. Decreased compliance requires greater pressure to achieve VT and is reflected in a flattened P–V loop.180 The area between the loops represents the resistance to inspiration and expiration, known as hysteresis. As resistance increases, less VT is delivered resulting in a shorter and wider loop; conversely, as resistance decreases, a longer, wider loop is generated (see Figure 15.10).181
Management of Refractory HypoxAemia
Refractory hypoxaemia may require strategies in addition to conventional lung-protective mechanical ventilation.121 These include recruitment manoeuvres, high frequency oscillatory ventilation, extracorporeal membrane oxygenation and nitric oxide.
Recruitment Manoeuvres
Recruitment manoeuvres (RMs) refer to brief application of high levels of PEEP to raise the transpulmonary pressure to levels higher than achieved during tidal ventilation with the goals of opening collapsed alveoli, recruiting slow opening alveoli, preventing alveolar derecruitment, and reducing shearing stress.182–184 The most common RM is elevation of PEEP to achieve a peak pressure of 40 cmH2O for a sustained period of 40 sec, although studies report peak pressure elevations ranging from 25–50 cmH2O for durations ranging from 20–40 sec.185 The best method in terms of pressure, duration and frequency have yet to be determined.186 Recruitment manoeuvres in humans have not produced consistent results in clinical studies,184,187 with a recent systematic review demonstrating no mortality benefit despite transient increases in oxygenation.185 Effective recruitment may be difficult to assess with the potential for either overdistension of alveoli or failure to recruit.140 Once the recruitment manoeuvre is terminated, derecruitment may occur rapidly. Serious adverse effects have been noted during the use of RMs due to increased intrathoracic and intrapulmonary pressures resulting in reductions in venous return and cardiac output, and cardiac arrest and increased risk of barotrauma.184,188
High Frequency Oscillatory Ventilation
High frequency oscillatory ventilation (HFOV) requires a specialised ventilator and manipulation of four variables: mean airway pressure (cmH2O), frequency (Hz), inspiratory time, and amplitude (or power [ΔP]).189 Alveolar overdistension is limited through the use of sub-deadspace tidal volumes whereas cyclic collapse of alveoli is prevented by maintenance of high end-expiratory lung pressures.190,191 High frequency (between 3 and 15 Hz) oscillations at extremely fast rates (300–420 breaths/min) create pressure waves enabling CO2 elimination.133,192 Oxygenation is facilitated through application of a constant mean airway pressure via the bias flow (rate of fresh gas).192,193 In adults, recommendations for the initiation of HFOV state mean airway pressure should be set 5 cmH2O above the peak airway pressure achieved with conventional ventilation.194 The recommended frequency range is 3–10 Hz with 5 Hz conventionally used to initiate HFOV. Inspiratory time is set at 33% and the amplitude setting is determined by adequate CO2 elimination.133 Increased CO2 elimination is achieved by lowering the frequency and increasing the amplitude.
Until recently, HFOV was considered a rescue mode for adult patients with acute respiratory distress syndrome (ARDS) experiencing refractory hypoxaemia and failing conventional ventilation.195,196 HFOV has been evaluated in patients in early-onset ARDS and has been found to improve oxygenation and to be well tolerated.197 While further studies are required, these data suggest HFOV can be implemented in early ARDS.
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) improves total body oxygenation using an external (extracorporeal) oxygenator, while allowing intrinsic recovery of lung pathophysiology. Indications for ECMO include acute severe cardiac or respiratory failure such as severe ARDS and refractory shock.198 Bleeding as a complication of anticoagulation is a major risk of ECMO, with cerebral bleeds being the most catastrophic.199 Another serious complication is limb ischaemia when the femoral artery is used.
ECMO consists of three key components:
1. a blood pump (either a simple roller or centrifugal force pump)
2. a membrane oxygenator (bubble, membrane or hollow fibre)
3. a countercurrent heat exchanger, where the blood is exposed to warmed water circulating within metal tubes.
In addition, essential safety features include bubble detectors that detect gas in the arterial line and shut the pump off; arterial line filters between the heat exchanger and arterial cannula, to trap air thrombi and emboli; pressure monitors placed before and after the oxygenator, that measure the pressure within the circuit and detect rising circuit pressures commonly caused by thrombus or circuit or cannulae occlusion; and continuous venous oxygen saturation and temperature monitoring. On commencement of ECMO the circuit is primed with fresh blood. The acid–base balance and blood gas of the primer is adjusted to ensure that the pH is within the normal range (7.35–7.45) and PaO2 is adequate. ECMO can be delivered via veno-arterial access which requires cannulation of an artery. This method bypasses the pulmonary circulation while providing cardiac support to the systemic circulation and achieves a higher PaO2 with lower perfusion rates. The alternative is veno-venous access, used for patients in respiratory failure with adequate cardiac function as there is no support of systemic circulation. Perfusion rates are higher, the mixed venous PO2 is elevated and the PaO2 is lower.199
Nitric Oxide
Nitric oxide (NO) is an endothelial smooth muscle relaxant. Inhaled NO is effective in the dilation of pulmonary arteries resulting in reduced pulmonary shunting and reduced right ventricular afterload due to reduced pulmonary artery tone. Pulmonary shunting refers to failure of uptake of alveolar gas by the pulmonary vascular bed due to vascular constriction or interstitial oedema. Inhaled NO has a role in the management of pulmonary hypertension and was previously thought to have a role in management of refractory hypoxaemia for patients with ARDS. However, the most recent systematic review and meta-analysis of NO in ARDS comprising 14 RCTs and 1303 participants reported no effect on overall mortality despite a statistically significant improvement in oxygenation in the first 24 hours, and some risk of renal impairment among adults.200
Positioning
Regular repositioning of critically ill patients is essential for lung recruitment, prevention of atelectasis and maintenance of skin integrity (see Chapter 6).
Head of Bed Elevation
Supine positioning has been associated with aspiration of abnormally-colonised oropharyngeal and gastric contents201–203 and increased incidence of VAP compared to a semirecumbent position, defined as backrest elevation at 45 degrees.204 Guidelines and care bundles for the prevention of VAP recommend semirecumbent positioning for all mechanically-ventilated patients.71,205,206 A more recent trial has however questioned the feasibility of 45 degree semirecumbent positioning as this backrest elevation was only achieved for 15% of study observations.207 There was also no differences in VAP incidence between the supine and semirecumbent group. Contraindications to backrest elevation include:
• suspected or existing spinal injury
• intracranial hypertension (for 45 degree elevation)
• haemodynamic support devices (IABP, LVAD, ECMO)
• femoral catheterisation for continuous renal replacement therapy
Clinical practice audits conducted internationally and in Australia and New Zealand indicate that compliance with a 45 degree semirecumbent position rarely occurs, even when taking into consideration contraindications.208–212 Similarly, interventions to improve compliance failed to demonstrate adherence to the 45 degree backrest position that can be sustained by the patient over time.213,214 Due to uncertainty over compliance with 45 degree semirecumbency in the original trial conducted by Drakulovic,204 and the lack of difference in VAP rates despite difficulty achieving compliance with semirecumbency in the van Niewenhoven study,207 new studies are required to confirm the equivalence or lack of inferiority of lower degrees of backrest elevation to the strict 45 degree semirecumbent position.
Lateral Positioning
Patients with unilateral lung disease experience a mismatch of ventilation to perfusion if the consolidated (pneumonic) or atelectic lung is placed in the dependent position.215 Continuous lateral rotational therapy is a positioning therapy advocated for the prevention and management of respiratory complications associated with immobility.216 The most recently reported multicentre randomised controlled trial found a significant reduction in VAP and shorter durations of ventilation and ICU stay.217 Continuous lateral rotation therapy requires a special bed system enabling rotation of the upper part of the body to a maximum angle of 90 degrees.
Prone Positioning
Prone positioning has been shown to improve oxygenation and intrapulmonary shunt fraction when compared with rotational turning during the first 72 hours of ALI218 and in patients with multiorgan failure.219 Prone positioning may also decrease the risk of VAP due to improved bronchial secretion drainage, limitation of colonisation of distal lung, decreased atelectasis and increased alveolar recruitment but may increase spread of pathogens in the lung and may increase the risk of aspiration.220–223
Prone positioning results in changes to the distribution of ventilation and pulmonary blood flow. Pleural pressures are lower in non-dependent regions and higher in dependent regions due to gravitational forces, the weight of the overlying lung and mismatch between the local physical structures of the lung and chest wall.224 The weight of the overlying lung increases in ARDS due to parenchymal oedema and fluid within the alveoli.225 This gradient in pleural pressures means transpulmonary pressure is higher in non-dependent lung regions, compared to dependent regions.225 Perfusion also increases from previously nondependent to dependent lung regions resulting in optimal matching of ventilation and perfusion to promote gas exchange.
Pleural pressure in the dependent dorsal regions in the supine position can result in airway closure, atelectasis and hypoxaemia.224 The difference in pleural pressures from non-dependent and dependent lung regions is greater in the supine compared to the prone position. In the supine position, the heart and abdominal contents also compress lung bases and decrease FRC, whereas in prone positioning, the weight of these structures are lifted from the lung.
The benefits of prone positioning continue to be debated. Although oxygenation improves in 70–80% of patients turned from supine to prone,226 a mortality benefit has not been shown in all trials. The most recent systematic review and meta-analysis227 confirms a reduction in mortality in patients with severe baseline hypoxaemia (PaO2/FiO2 ratio <100 mmHg), but reported this benefit was not present in patients with less-severe hypoxaemia. Other benefits of prone positioning demonstrated were improved oxygenation and decreased rates of VAP. Adverse events related to prone positioning were increased risk of decubitus ulcer formation, endotracheal obstruction and accidental line or tube dislodgement.
Implementing prone positioning requires forward planning to ensure eye care and protection, mouth care, wound dressings, and tracheal suction are attended to before positioning the patient prone. Intravenous lines, electrocardiogram leads, urinary catheter drainage, chest drains and ostomy bags need to be secured and repositioned appropriately once the patient is positioned.228 Prone positioning can be achieved by manual handling of the patient, requiring up to five staff, although commercial devices are available that facilitate the turning and positioning of the patient.228
Weaning From the Ventilator
Current Recommendations
No ventilation strategy is more lung-protective than the timely and appropriate discontinuation of mechanical ventilation. Weaning refers to the transition from ventilatory support to spontaneous breathing.229 Evidence based consensus guidelines published for weaning in 2001116 and 2007230 emphasise the importance of preventing unnecessary delays in the weaning process, early recognition of a patient’s ability for spontaneous breathing and the use of a systematic method to identify the potential for extubation.
Weaning Predictors
Clinician judgement regarding prediction of weaning readiness is known to be imperfect, with unnecessary prolongation of ventilation231 or high rates of reintubation as resultant consequences, both of which are associated with adverse outcomes.232,233 An evidence based review evaluating over 50 objective physiological measurements for determining readiness for weaning and extubation found most had only a modest relationship with weaning outcome; no single factor or combination of factors demonstrating superior accuracy.234 Of all predictors studied, the respiratory frequency to tidal volume ratio (f/VT) appears to be most accurate.235 However inclusion of the f/VT as part of a weaning protocol was found in one randomised study to increase, as opposed to decrease, the duration of weaning.236 At present, consensus guidelines230 do not recommend routine inclusion of weaning predictors.
Weaning Methods
Various studies have attempted to identify the best weaning method. Two of the most frequently-cited studies have produced conflicting results. Brochard and colleagues237 compared PSV, T piece trial and SIMV, and concluded that PSV reduced the duration of mechanical ventilation compared with the other methods. Esteban and colleagues238 compared PSV, T piece trials, CPAP and progressive reduction of SIMV support, and found a once-daily T piece trial led to extubation three times more quickly than SIMV and nearly twice as quickly as PSV. Failure to produce consistent results favouring a single weaning style suggests it is not the mode that is important but rather the application of a systematic process.239
Spontaneous breathing trials
Spontaneous breathing trials (SBTs) incorporate a focused assessment of a patient’s capacity to breathe prior to extubation240 and are recommended as the major diagnostic test to determine extubation readiness.230 SBTs can be conducted using either a T piece or low levels of pressure support241 and should need to last only 30 minutes.242 This method of weaning is uncommon in the ANZ setting, in contrast to international findings.65,66
Protocols
Implementation of various organisational strategies such as weaning teams and non-physician-led weaning protocols may assist in the timely recognition of weaning and extubation readiness.243–247 Recently, coupling of a sedation and weaning protocol was found to result in a three-day reduction in the duration of ventilation compared to standard care in four North American hospitals.248 A recent systematic review and meta-analysis of 11 weaning protocol trials including 1971 patients demonstrated a reduction in the duration of mechanical ventilation.249 However, the authors cautioned that the effect of weaning protocols may vary according to the ICU organisational characteristics such as an intensivist-led ICU model, high levels of physician staffing, structured ward rounds, collaborative discussion and more frequent medical review; all characteristics reported for ICUs in Australia and New Zealand.250,251
Automated weaning
Automated computerised systems potentially enable more efficient weaning by providing improved adaptation of ventilatory support through continuous monitoring and real-time intervention.252 One such system, SmartCare™/PS, monitors three respiratory parameters, frequency, VT and end-tidal carbon dioxide (ETCO2) concentration, every two or five minutes and periodically adapts pressure support (PS).252,253 SmartCare/PS establishes a respiratory status diagnosis, based on evaluation of the three parameters, and may either decrease or increase PS, or leave it unchanged to maintain the patient in a defined ‘respiratory zone of comfort’.254,255 Once SmartCare/PS has successfully minimised the level of PS, a one-hour observation period occurs. For patients who remain within the respiratory zone of comfort throughout the observation period, SmartCare/PS recommends to ‘consider separation’, indicating the patient’s respiratory status now suggests the patient will tolerate extubation.
SmartCare/PS substantially reduced the duration of ventilation and ICU length of stay when compared to physician-controlled weaning using local guidelines in five European ICUs.256 These effects were not confirmed when the SmartCare/PS system was compared to weaning managed by experienced critical care nurses in a single Australian setting.257
The difficult-to-wean patient
International reports indicate patients that require mechanical ventilation for ≥21 days account for less than 10% of all mechanically-ventilated patients, but occupy 40% of ICU bed days and accrue 50% of ICU costs.258,259 A recommendation from the National Association for Medical Direction of Respiratory Care (NAMDRC) states that prolonged mechanical ventilation should be defined as ‘≥21 consecutive days of ventilation required for ≥6 hours per day’.230 Prolonged weaning has been defined as greater than 7 days of weaning after the first SBT or more than three SBTs.230 Little evidence defines the optimal method for managing the difficult-to-wean patient. One trial found no difference in weaning duration or success when comparing tracheostomy trials to low-level pressure support in patients with COPD experiencing weaning difficulty.260 These patients are most likely to benefit from an individualised and structured approach to weaning using progressive lengthening of tracheostomy trials with supportive ventilation in between in combination with early physical therapy.
Complications of Mechanical Ventilation
Physiological complications associated with mechanical ventilation include ventilator-associated lung injury (VALI) and nosocomial infection (VAP).116,122 VALI occurs through alveolar over-distension and cyclic opening and closing of alveoli resulting in diffuse alveolar damage, increased permeability, pulmonary oedema, cell contraction and cytokine production.122,130,136,261–263 VAP substantially increases the duration of ICU stay and is associated with an attributable mortality of 5.8–8.5%.264–266 Additional complications associated with mechanical ventilation are listed in Table 15.8. Complications can occur due to inappropriate application of mechanical ventilation. This may result in extra-alveolar gas causing pneumothoraces or subcutaneous emphysema due to high peak inspiratory pressures, and alveolar stretch and oedema formation as the result of large tidal volumes.68
| Item | Complication |
|---|---|
| Barotrauma |
Summary
• ETT placement should be confirmed with end-tidal CO2 monitoring
• the aim of endotracheal cuff management is to prevent airway contamination and enable positive pressure ventilation
• closed suctioning reduces alveolar derecruitment compared to opening suctioning
• instillation of normal saline is not recommended during routine tracheal suctioning.
• despite its life-saving potential, mechanical ventilation carries the risk of serious physical and psychological complications
• humidification of dry medical gas is required during mechanical ventilation to prevent drying of secretions, mucous plugging and airway occlusion
• the pressure required to deliver a volume of gas into the lungs is determined by elastic and resistive forces
• contemporary ventilators now provide a range of modes to facilitate mechanical ventilation
• analysis of ventilator graphics provides clinicians with the ability to assess patient–ventilator interaction, appropriateness of ventilator settings and lung function
• semirecumbent positioning at 45 degree elevation has been shown to reduce VAP but compliance is poor
• recruitment manoeuvres, HFOV, ECMO and prone positioning are strategies that may facilitate management of refractory hypoxaemia
• timely recognition of a patients readiness for weaning and extubation is imperative. Strategies such as weaning protocols, teams and automatic weaning are all aimed at optimising this process.
Research vignette
Learning activities
1. What is the rationale for using oxygen therapy in patients with COPD and low SpO2?
2. Why is it important to consider the patient’s respiratory rate and tidal volume when using a low flow (variable flow) oxygen delivery device?
3. How should ETT placement be confirmed?
4. Describe assessment priorities and interventions before and after extubation.
5. What are the indications and relative and absolute contraindications for NIV?
6. Familiarise yourself with the ventilators in your unit. Confirm you have a thorough understanding of the function and purpose of all ventilator settings.
7. Ensure you have a clear understanding of ventilator mode terminology.
8. Identify the clinical applications of pressure-volume and flow-volume loops.
9. What are the potential risks of mechanical ventilation as well as those related to premature discontinuation of ventilation?
Activities 10–15 relate to the case study:
10. What were the rationales for switching from SIMV-VC to SIMV-PC for Mr Smith on day 2?
11. What is the current research evidence on the benefits and disadvantages of volume versus pressure ventilation?
12. Why was semirecumbent positioning a priority for Mr Smith?
13. Why do you think FiO2 was weaned before PEEP?
14. What is the current research evidence for the use, benefits and disadvantages of muscle relaxants in the critically ill?
American Association for Respiratory Care. http://www.aarc.org/resources/.
Anaesthesia UK. http://www.frca.co.uk/default.aspx.
Australian and New Zealand Intensive Care Society. www.anzics.com.au/safety-quality?start=2.
ARDS network. http://www.ardsnet.org/.
Canadian Society of Respiratory Therapists. Respiratory Resource http://www.respiratoryresource.ca/
College of Intensive Care Medicine of Australia and New Zealand. www.cicm.org.au/policydocs.php.
Covidien education resources http://www.nellcor.com/educ/OnlineEd.aspx
Critical Care Medicine Tutorials. http://www.ccmtutorials.com/.
Fisher and Paykel Resource Centre. http://www.fphcare.com/respiratory-acute-care/resource-library.html.
Intensive Care Coordination and Monitoring Unit. http://intensivecare.hsnet.nsw.gov.au/.
NHS Institute for Innovation and Improvement. http://www.institute.nhs.uk/safer_care/general/human_factors.html.
Thoracic Society of Australia and New Zealand. http://www.thoracic.org.au/.
Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183(3):E195–E214.
Esan A, Hess DR, Raoof S, George L, Sessler CN. Severe hypoxemic respiratory failure. Part 1: ventilatory strategies. Chest. 2010;137(6):1203–1216.
Lacherade JC, De Jonghe B, Guezennec P, Debbat K, Hayon J, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med. 2010;182(7):910–917.
Pelosi P, Gama de Abreu M, Rocco PR. New and conventional strategies for lung recruitment in ARDS. Critical Care. 2010;14(2):210.
Raoof S, Goulet K, Esan A, Hess DR, Sessler CN. Severe hypoxemic respiratory failure. Part 2: nonventilatory strategies. Chest. 2010;137(6):1437–1448.
Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408–413.
1 Australian and New Zealand Intensive Care Society Centre for Outcome and Resource Evaluation (ANZICS CORE). 2008 Annual Report. Melbourne: Australian and New Zealand Intensive Care Society; 2010.
2 Celli B, MacNee W, ATS/ERS Taskforce. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946.
3 Naughton M, Tuxen D. Acute respiratory failure in chronic obstructive pulmonary disease. In: Bersten A, Soni N. Oh’s intensive care manual. Philadelphia: Butterworth-Heineman/Elsevier; 2009:343–354.
4 Wagstaff A. Oxygen therapy. In: Bersten A, Soni N. Oh’s intensive care manual. Philadelphia: Butterworth-Heineman/Elsevier; 2009:316–326.
5 Wagstaff T, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia. 2007;62(5):492–503.
6 Sim M, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008;63(9):938–940.
7 Groves N, Tobin A. High flow nasal oxygen generates positive airway pressures in adult volunteers. Aust Crit Care. 2007;20(4):126–131.
8 Kernick J, Magary J. What is the evidence for the use of high flow nasal cannula oxygen in adult patients admitted to critical care units? A systematic review. Aust Crit Care. 2010;23(2):53–70.
9 Fisher and Paykel Healthcare New Zealand. Respiratory and acute care, nasal high flow. http://www.fphcare.com/rsc/adult-care/nasal-high-flow.html, 2010. [Cited September 2010]. Available from
10 Dysart K, Miller T, Wolfson M, Shaffer T. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400–1405.
11 Hnatiuk O, Moores L, Thompson J, Jones M. Delivery of high concentrations of inspired oxygen via tusk mask. Crit Care Med. 1998;26(6):1032–1035.
12 Peruzzi W, Smith B. Oxygen delivery: tusks versus flow. Crit Care Med. 1998;26(6):986.
13 Boumphrey S, Morris E, Kinsella S. 100% Inspired oxygen from a Hudson mask – a realistic goal? Resuscitation. 2003;57(1):69–72.
14 Anesthesiology Rotation and Elective. Understanding equipment. http://www.healthsystem.virginia.edu/Internet/Anesthesiology-Elective/airway/equipment.cfm, 2004. [Cited February 2011]. Available from
15 Cook T, Hommers C. New airways for resuscitation? Resuscitation. 2006;69(3):371–387.
16 Joynt G. Airway management and acute upper-airway obstruction. In: Bersten A, Soni N. Oh’s intensive care manual. Philadelphia: Butterworth-Heineman/Elsevier; 2009:327–341.
17 Lavery G, McCloskey B. The difficult airway in adult critical care. Crit Care Med. 2008;36(7):2163–2173.
18 Nolan J, Hazinski M, Billi J, Boettiger B, Bossaert L, et al. Part 1: Executive summary: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(1):e1–25.
19 Wahlen B, Roewer N, Lange M, Kranke P. Tracheal intubation and alternative airway management devices used by healthcare professionals with different level of pre-existing skills: a manikin study. Anaesthesia. 2009;64(5):549–554.
20 Vézina M-C, Trépanier C, Nicole P, Lessard M. Complications associated with the Esophageal-Tracheal Combitube® in the pre-hospital setting. Can J Anesth. 2007;54(2):124–128.
21 Ball J, Platt S. Obstruction of a reinforced oral tracheal tube. Brit J Anaesthesia. 2010;105(5):699–700.
22 Davies R. The importance of a Murphy eye. Anaesthesia. 2001;56(9):906–924.
23 Jaber S, Amraoui J, Lefrant J-Y, Arich C, Cohendy R, Landreau L. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Crit Care Med. 2006;34(9):2355–2361.
24 Weingart S. Preoxygenation, reoxygenation, and delayed sequence intubation in the emergency department. J Emerg Med. 2010. DOI 10.1016/j.jemermed.2010.02.014
25 Holzapfel L, Chastang C, Demingeon G, Bohe J, Piralla B, Coupry A. A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am J Resp Crit Care Med. 1999;159(3):695–701.
26 Beavers R, Moos D, Cuddeford J. Analysis of the application of cricoid pressure: implications for the clinician. J PeriAnesth Nurs. 2009;24(2):92–102.
27 Brisson P, Brisson M. Variable application and misapplication of cricoid pressure. Trauma. 2010;69(5):1182–1184.
28 Priebe H. Cricoid pressure: an expert’s opinion. Minerva Anestesiol. 2009;75(12):710–714.
29 Knill R. Difficult laryngoscopy made easy with a “BURP”. Can J Anaesth. 1993;40(3):279–282.
30 Takahata O, Kubota M, Mamiya K, Akama Y, Nozaka T, Matsumoto H, Ogawa H. The efficacy of the “BURP” maneuver during a difficult laryngoscopy. Anesth Analg. 1997;84(2):419–421.
31 Bernhard W, Yost L, Joynes D, Cothalis S, Turndorf H. Intracuff pressures in endotracheal and tracheostomy tubes: related cuff physical characteristics. Chest. 1985;87(6):720–725.
32 Seegobin R, van Hasselt G. Endotracheal cuff pressures and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. BMJ. 1984;288(6422):965–968.
33 Rello J, Sonara R, Jubert P, Artigas A, Rue M, Valles J. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med. 1996;154(1):111–115.
34 Rose L, Redl L. Survey of cuff management practices within Australia and New Zealand. Am J Crit Care. 2008;17(5):428–435.
35 Vyas D, Inweregbu K, Pittard A. Measurement of tracheal tube cuff pressure in critical care. Anaesthesia. 2002;57(3):275–277.
36 Sole M, Byers J, Ludy J, Zhang Y, Banta C, Brummel K. A multisite survey of suctioning techniques and airway management practices. Am J Crit Care. 2003;12(3):220–230.
37 Sole M, Penoyer D, Su X, Jimenez E, Kalita S, Poalillo E, Byers J, Bennett M, Ludy J. Assessment of endotracheal cuff pressure by continuous monitoring: a pilot study. Am J Crit Care. 2009;18(2):133–143.
38 Intensive Care Coordination and Monitoring Unit. Stabilisation of an endotracheal tube for the adult intensive care patient. . NSW Health statewide guidelines for intensive care. 2007. [Cited January 2011]. Available from http://intensivecare.hsnet.nsw.gov.au/state-wide-guidelines
39 Gardner A, Hughes D, Cook R, Osborne S, Gardner G. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Aust Crit Care. 2005;18(4):158–165.
40 Sitzwohl C, Langheinrich A, Schober A, Krafft P, Sessler D, et al. Endobronchial intubation detected by insertion depth of endotracheal tube, bilateral auscultation, or observation of chest movements: randomised trial. BMJ. 2010;341:c5943.
41 Rudraraju P, Eisen L. Confirmation of endotracheal tube position: a narrative review. J Intens Care Med. 2009;24(5):283–292.
42 College of Intensive Care Medicine of Australia and New Zealand. IC-1 Minimum standards for intensive care units. www.cicm.org.au/policydocs.php, 2010. [Cited February 2011]. Available from
43 Mallick A, Bodenham A. Tracheostomy in critically ill patients. Eur J Anaesthesiol. 2010;27(8):676–682.
44 De Leyn P, Bedert L, Delcroix M, Depuydt P, Lauwers G, et al. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32(3):412–421.
45 Australian and New Zealand Intensive Care Society. Percutaneous dilatational tracheostomy consensus statement. www.anzics.com.au/safety-quality?start=2, 2010. [Cited January 2011]. Available from
46 Russell C. Providing the nurse with a guide to tracheostomy care and management. Brit J Nur. 2005;14(8):428–433.
47 Dennis-Rouse M, Davidson J. An evidence-based evaluation of tracheostomy care practices. Crit Care Nurs Q. 2008;31:150–160.
48 Thomas A, McGrath B. Patient safety incidents associated with airway devices in critical care: a review of reports to the UK National Patient Safety Agency. Anaesthesia. 2009;64(4):358–365.
49 Happ MB. Treatment interference in acutely and critically ill adults. Am J Crit Care. 1998;7(3):224–235.
50 Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care. 2008;17(1):45–51.
51 Mion LC, Minnick AF, Leipzig R, Catrambone CD, Johnson ME. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med. 2007;35(12):2714–2720.
52 Birkett KM, Southerland KA, Leslie GD. Reporting unplanned extubation. Intens Crit Care Nurs. 2005;21(2):65–75.
53 Tung A, Tadimeti L, Caruana-Montaldo B, Atkins PM, Mion LC, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth. 2001;13(1):24–29.
54 Atkins PM, Mion LC, Mendelson W, Palmer RM, Slomka J, Franko T. Characteristics and outcomes of patients who self-extubate from ventilatory support: a case-control study. Chest. 1997;112(5):1317–1323.
55 Engels P, Bagshaw S, Meier M, Brindley P. Tracheostomy: from insertion to decannulation. Can J Surg. 2009;52(5):427–433.
56 Delaney A, Bagshaw S, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care. 2006;10(2):R55.
57 Fernandez M, Piacentini E, Blanch L, Fernandez R. Changes in lung volume with three systems of endotracheal suctioning with and without pre-oxygenation in patients with mild-to-moderate lung failure. Intens Care Med. 2004;30(12):2210–2215.
58 Intensive Care Coordination and Monitoring Unit. Suctioning an adult with a tracheal tube. NSWHealth Statewide Guidelines for Intensive Care. http://intensivecare.hsnet.nsw.gov.au/state-wide-guidelines, 2007. [Cited January 2011]. Available from
59 Overend T, Anderson C, Brooks D, Cicutto L, Keim M, et al. Updating the evidence base for suctioning adult patients: A systematic review. Can Respir J. 2009;16(3):e6–17.
60 AARC. AARC Clinical Practice Guidelines. Endotracheal suctioning of mechanically ventilated patients with artificial airways. Respir Care. 2010;55(6):758–764.
61 National Health and Medical Research Council (NHMRC). Australian guidelines for the prevention and control of infection in healthcare. Canberra: NHMRC; 2010.
62 AARC. AARC clinical practice guideline: removal of the endotracheal tube 2007 revision & update. Respir Care. 2007;52(6):81–93.
63 Antonaglia V, Vergolini A, Pascotto S, Bonini P, Renco M, et al. Cuff-leak test predicts the severity of post extubation acute laryngeal lesions: a preliminary study. Eur J Anaesthesiol. 2010;27(6):534–541.
64 Cormack R, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105–1111.
65 Esteban A, Ferguson N, Meade M, Frutos-Vivar F, Apezteguia C, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008;177(2):170–177.
66 Rose L, Presneill J, Johnston L, Nelson S, Cade J. Ventilation and weaning practices in Australia and New Zealand. Anaeth Intensive Care. 2009;37(1):99–107.
67 Hess D. Ventilator waveforms and the physiology of pressure support ventilation. Respir Care. 2005;50(2):166–186.
68 Pilbeam S, Cairo J. Mechanical ventilation: physiological and clinical applications, 4th edn. St Louis: Mosby Elsevier; 2006.
69 Nishida T, Nishimura M, Fujino Y, Mashimo T. Performance of heated humidifiers with a heated wire according to ventilatory settings. J Aerosol Med. 2001;14(1):43–51.
70 Branson R. The ventilator circuit and ventilator-associated pneumonia. Respir Care. 2005;50(6):774–785.
71 Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23(1):126–137.
72 Kilgour E, Rankin N, Ryan S, Pack R. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intens Care Med. 2004;30(7):1491–1494.
73 Bersten A. Humidication and inhalation therapy. In: Bersten A, Soni N, Oh T. Oh’s intensive care manual. Oxford: Butterworth-Heinemann, 2003.
74 Branson R, Davis K. Evaluation of 21 passive humidifiers according to the ISO 9360 standard: moisture output, dead space, and flow resistance. Respir Care. 1996;41:736–743.
75 Iotti G, Olivei M, Braschi A. Mechanical effects of heat-moisture exchangers in ventilated patients. Crit Care. 1999;3(5):R77–R82.
76 Kelly M, Gillies D, Todd D, Lockwood C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Cochrane Database Syst Rev 2010; CD004711.
77 Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet. 2009;374(9685):250–259.
78 Rose L, Gerdtz M. Review of non-invasive ventilation in the emergency department: clinical considerations and management priorities. J Clin Nurs. 2009;18(23):3216–3224.
79 Mehta S, Hill N. Noninvasive ventilation: state of the art. Am J Respir Crit Care Med. 2001;163(2):540–577.
80 Hill N. Noninvasive positive pressure ventilation. In: Tobin M, ed. Principles and practice of mechanical ventilation. New York: McGraw-Hill, 2006.
81 L’Her E, Deye N, Lellouche F, Taille S, Demoule A, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med. 2005;172(9):1112–1118.
82 Hill N, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med. 2007;35(10):2402–2407.
83 Naughton M, Rahman M, Hara K, Floras J, Bradley T. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91(6):1725–1731.
84 Kaye D, Mansfield D, Naughton MT. Continuous positive airway pressure decreases myocardial oxygen consumption in heart failure. Clin Sci. 2004;106(6):599–603.
85 Pladeck T, Hader C, Von Orde A, Rasche K, Wiechmann H. Non-invasive ventilation: comparison of effectiveness, safety, and management of acute heart failure syndromes and acute exacerbations of chronic obstructive pulmonary disease. J Physiol Pharmacol. 2007;58(5Suppl Pt2):539–549.
86 Caples S, Gay P. Noninvasive positive pressure ventilation in the intensive care unit: a concise review. Crit Care Med. 2005;33(11):2651–2658.
87 Confalonieri M, Potena A, Carbone G, Della Porta R, Tolley E, Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia. Am J Respir Crit Care Med. 1999;160(5 Pt1):1585–1591.
88 Antonelli M, Conti G, Moro M, Esquinas A, Gonzalez-Diaz G, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure; a multi-center study. Intens Care Med. 2001;27(11):1718–1728.
89 Keenan S, Sinuff T, Cook D, Hill N. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive pressure ventilation? A systematic review of the literature. Ann Int Med. 2003;138(11):861–870.
90 Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185.
91 Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004; CD004104.
92 Confalonieri M, Garuti G, Cattaruzza M, Osborn J, Antonelli M, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25(2):348–355.
93 Masip J, Roque M, Sanchez B, Ferandez R, Subirana M, Exposito J. Noninvasive ventilation in acute cardiogenic pulmonary edema. JAMA. 2005;294(24):3124–3130.
94 Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367(9517):1155–1163.
95 Winck JC, Azevedo LF, Costa-Pereira A, Antonelli M, Wyatt JC. Efficacy and safety of non-invasive ventilation in the treatment of acute cardiogenic pulmonary edema – a systematic review and meta-analysis. Crit Care. 2006;10(2):R69.
96 Mehta S, Jay GD, Woolard RH, Hipona R, Connolly E, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25(4):620–628.
97 British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57(3):192–211.
98 Park M, Sangean M, Volpe M, Feltrim M, Nozawa E, et al. Randomized, prospective trial of oxygen, continuous positive airway pressure, and bilevel positive airway pressure by face mask in acute cardiogenic pulmonary edema. Crit Care Med. 2004;32(12):2407–2415.
99 Bellone A, Monari A, Cortellaro F, Vettorello M, Arlati S, Coen D. Myocardial infarction rate in acute pulmonary edema: noninvasive pressure support ventilation versus continuous positive airway pressure. Crit Care Med. 2004;32(9):1860–1865.
100 Moritz F, Brousse B, Gellee B, Chajara A, L’Her E, et al. Continuous positive airway pressure versus bilevel noninvasive ventilation in acute cardiogenic pulmonary edema: a randomized multicenter trial. Ann Emerg Med. 2007;50(6):666–675.
101 Crane SD, Elliott MW, Gilligan P, Richards K, Gray AJ. Randomised controlled comparison of continuous positive airways pressure, bilevel non-invasive ventilation, and standard treatment in emergency department patients with acute cardiogenic pulmonary oedema. Emerg Med J. 2004;21(2):155–161.
102 Gray A, Goodacre S, Newby D, Masson M, Sampson F, et al. A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial. Health Technol Assess. 2009;13(33):1–106.
103 Browning J, Atwood B, Gray A. Use of non-invasive ventilation in UK emergency departments. Emerg Med J. 2006;23(12):920–921.
104 Rose L, Gerdtz M. Use of non-invasive ventilation in Australian emergency departments. Int J Nurs Stud. 2009;46(5):617–623.
105 Udwadia Z, Santis G, Steven M, Simonds A. Nasal ventilation to facilitate weaning in patients with chronic respiratory insufficiency. Thorax. 1992;47(9):715–718.
106 Agarwal R, Aggarwal A, Gupta D, Jindal S. Role of noninvasive positive-pressure ventilation in postextubation respiratory failure: a meta-analysis. Respir Care. 2007;52(11):1472–1479.
107 Burns K, Adhikari N, Keenan S, Meade M. Use of non-invasive ventilation to wean critically ill adults off invasive ventilation: meta-analysis and systematic review. BMJ. 2009;338:1574.
108 Esteban A, Frutos F, Ferguson ND, Arabi Y, Apezteguia C, et al. Noninvasive positive pressure ventilation for respiratory failure after extubation. New Engl J Med. 2004;350(24):2452–2460.
109 Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005; CD004360.
110 Maheshwari V, Paioli D, Rothaar R, Hill N. Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest. 2006;129(5):1226–1233.
111 Evans TW. International Consensus Conferences in Intensive Care Medicine: non-invasive positive pressure ventilation in acute respiratory failure. Intens Care Med. 2001;27(1):166–178.
112 Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1):71–84.
113 Pelosi P, Severgnini P, Aspesi M, Gamberoni C, Chiumello D, et al. Non-invasive ventilation delivered by conventional interfaces and helmet in the emergency department. Eur J Emerg Med. 2003;10(2):79–86.
114 Chiumello D. Is the helmet different than the face mask in delivering noninvasive ventilation. Chest. 2006;129(6):1402–1403.
115 Luce JM. Reducing the use of mechanical ventilation. New Engl J Med. 1996;335(25):1916–1917.
116 MacIntyre NR. Evidence-based guidelines for weaning and discontinuing ventilatory support. Chest. 2001;120(6Suppl):S375–S395.
117 Laghi F, Tobin M. Indications for mechanical ventilation. In: Tobin M, ed. Principles and practice of mechanical ventilation. New York: McGraw-Hill, 2006.
118 Tobin M, Guenther S, Perez W, Lodato R, Mador M, et al. Konno-Mead analysis of ribcage-abdominal motion during successful and unsuccessful trials of weaning from mechanical ventilation. Am Rev Respir Dis. 1987;135(8):1320–1328.
119 Davis W, Rennard S, Bitterman P, Crystal R. Pulmonary oxygen toxicity – early reversible changes in human alveolar structures induced by hyperoxia. New Engl J Med. 1983;309(15):878–883.
120 Diacon A, Koegelenberg C, Klüsmann K, Bolliger C. Challenges in the estimation of tidal volume settings in critical care units. Intens Care Med. 2006;32(10):1670–1671.
121 ARDSnet. Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342(18):1301–1308.
122 Gajic O, Saqib I, Mendez J, Adesanya A, Festic E, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817–1824.
123 Determann R, Royakkers A, Wolthuis E, Vlaar A, Choi G, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care Med. 2010;14(1):R1.
124 Holets S, Hubmayr R. Setting the ventilator. In: Tobin M, ed. Principles and practice of mechanical ventilation. New York: McGraw-Hill, 2006.
125 Goulet R, Hess D, Kacmarek R. Pressure vs flow triggering during pressure support ventilation. Chest. 1997;111(6):1649–1653.
126 Hill L, Pearl R. Flow triggering, pressure triggering, and autotriggering during mechanical ventilation. Crit Care Med. 2000;28(2):579–581.
127 Kondili E, Akoumianaki E, Alexopoulou C, Georgopoulos D. Identifying and relieving asynchrony during mechanical ventilation. Expert Rev. Respir Med. 2009;3(3):231–243.
128 Amato M, Marini J. Pressure-controlled and inverse-ratio ventilation. In: Tobin M, ed. Principles and practice of mechanical ventilation. New York: McGraw-Hill; 2006:251–272.
129 Pierce L. Management of the mechanically ventilated patient, 2nd edn. St Louis: Saunders: Elsevier; 2007.
130 Gattinoni L, Caironi P, Carlesso E. How to ventilate patients with acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care. 2005;11(1):69–76.
131 Kallet R. Evidence-based management of acute lung injury and acute respiratory distress syndrome. Respir Care. 2004;49(7):793–809.
132 Ashbaugh D, Bigelow D, Petty T, Levine B. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323.
133 Hemmila M, Napolitano LM. Severe respiratory failure: advanced treatment options. Crit Care Med. 2006;34(9Suppl):S278–S290.
134 Ferguson ND, Frutos-Vivar F, Esteban A, Anzueto A, Alia I, et al. Airway pressures, tidal volumes and mortality in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(1):21–30.
135 Muscedere J, Mullen J, Gan K, Slutsky A. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327–1334.
136 Tremblay L, Valenza F, Ribeiro S, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos, – RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952.
137 Amato M, Barbas C, Medeiros D, Magaldi R, Schettino G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. New Engl J Med. 1998;338(6):347–354.
138 Grasso S, Fanelli V, Cafarelli A, Anaclerio R, Amabile M, Ancona G, Fiore T. Effects of high versus low positive end-expiratory pressure in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(9):1002–1008.
139 Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, et al. Mechanical ventilation with higher versus lower positive end-expiratory pressures in patients with acute lung injury and acute respiratory distress syndrome. New Engl J Med. 2004;351(4):327–336.
140 Suarez-Sipmann F, Bohm S, Tusman G, Pesch T, Thamm O, et al. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med. 2007;35(1):214–221.
141 Meade M, Cook D, Guyatt G, Slutsky A, Arabi Y, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
142 Mercat A, Richard J-CM, Vielle B, Jaber S, Osman D, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
143 Hess D, Kacmarek R. Essentials of mechanical ventilation, 2nd edn. New York: McGraw-Hill; 2002.
144 Chatburn R. Classification of ventilator modes: update and proposal for implementation. Respir Care. 2007;52(3):301–323.
145 Chatburn R. Understanding mechanical ventilators. Expert Rev. Respir Med. 2010;4(6):809–819.
146 Rose L. Advanced modes of mechanical ventilation: implications for practice. AACN Adv Crit Care. 2006;17(2):145–158.
147 Kuhlen R, Rossaint R. The role of spontaneous breathing during mechanical ventilation. Respir Care. 2002;47(3):296–303.
148 Habashi N. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med. 2005;33(3Suppl):S228–S240.
149 Marik P, Krikorian J. Pressure-controlled ventilation in ARDS: a practical approach. Chest. 1997;112(4):1102–1106.
150 Esteban A, Alia I, Gordo F, de Pablo R, Suarez J, et al. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. Chest. 2000;117(6):1690–1696.
151 Campbell R, Davis B. Pressure-controlled versus volume-controlled ventilation: does it matter? Respir Care. 2002;47(4):416–426.
152 Brochard L, Pluskwa F, Lemaire F. Improved efficacy of spontaneous breathing with inspiratory pressure support. Am Rev Respir Dis. 1987;32(2):1110–1116.
153 Chen K, Sternbach G, Fromm R, Varon J. Mechanical ventilation: past and present. J Emerg Med. 1998;16(3):435–460.
154 Haitsma J. Diaphragmatic dysfunction in mechanical ventilation. Curr Opin Anaesthesiol. 2011;24(2):214–218.
155 Hormann C, Baum M, Putensen C, Mutz N, Benzer H. Biphasic positive airway pressure (BIPAP) – a new mode of ventilatory support. Eur J Anaesthesiol. 1994;11(1):37–42.
156 McCunn M, Habashi NM. Airway pressure release ventilation in the acute respiratory distress syndrome following traumatic injury. Int Anesthesiol Clin. 2002;40(3):89–102.
157 Putensen C, Wrigge H. Airway pressure-release ventilation. In: Tobin M, ed. Principles and practice of mechanical ventilation. New York: McGraw-Hill, 2006.
158 Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intens Care Med. 2008;34(10):1766–1773.
159 Mols G, von Ungern-Sternberg B, Rohr E, Haberthur C, Geiger K, Guttman J. Respiratory comfort and breathing pattern during volume proportional assist ventilation and pressure support ventilation: a study on volunteers with artificially reduced compliance. Crit Care Med. 2000;28(6):1940–1946.
160 Guttmann J, Bernhard H, Mols G, Benzing A, Hofmann P, et al. Respiratory comfort of automatic tube compensation and inspiratory pressure support in conscious humans. Intens Care Med. 1997;23(11):1119–1124.
161 Unoki T, Serita A, Grap M. Automatic tube compensation during weaning from mechanical ventilation: evidence and clinical implications. Crit Care Nurse. 2008;28(4):34–42.
162 Fabry B, Haberthur C, Zappe D, Guttmann J, Kuhlen R, Stocker R. Breathing pattern and additional work of breathing in spontaneously breathing patients with different ventilatory demands during inspiratory pressure support and automatic tube compensation. Intens Care Med. 1997;23(5):545–552.
163 Branson R, Johannigman J. Innovations in mechanical ventilation. Respir Care. 2009;54(7):933–947.
164 Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5(12):1433–1436.
165 Brander L, Sinderby C, Lecomte F, Leong-Poi H, Bell D, et al. Neurally adjusted ventilatory assist decreases ventilator-induced lung injury and non-pulmonary organ dysfunction in rabbits with acute lung injury. Intens Care Med. 2009;35(11):1979–1989.
166 Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, et al. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010;38(2):518–526.
167 Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, et al. Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intens Care Med. 2011;37(2):263–271.
168 Coisel Y, Chanques G, Jung B, Constantin J, Capdevila X, et al. Neurally adjusted ventilatory assist in critically ill postoperative patients: a crossover randomized study. Anesthesiol. 2010;113(4):925–935.
169 Burns S. Working with respiratory waveforms: how to use bedside graphics. AACN Clin Issues. 2003;14(2):133–144.
170 Rittner F, Doring M. Curves and loops in mechanical ventilation. Hong Kong: Draeger Medical Asia Pacific; n.d.
171 Tobin M. Monitoring of pressure, flow, and volume during mechanical ventilation. Respir Care. 1992;37(9):1081–1096.
172 Nilsestuen J, Hargett K. Using ventilator graphics to identify patient-ventilator asynchrony. Respir Care. 2005;50(2):202–234.
173 Yang SC, Yang SP. Effects of inspiratory flow waveforms on lung mechanics, gas exchange, and respiratory metabolism in COPD patients during mechanical ventilation. Chest. 2002;122(6):2096–2104.
174 Blanch L, Bernabé F, Lucangelo U. Measurement of air trapping, intrinsic positive end-expiratory pressure, and dynamic hyperinflation in mechanically ventilated patients. Respir Care. 2005;50(1):110–123.
175 Koh Y. Ventilatory management of patients with severe asthma. Int Anesthesiol Clin. 2001;39(1):63–73.
176 Lu Q, Rouby J-J. Measurement of pressure-volume curves in patients on mechanical ventilation: methods and significance. Crit Care. 2000;4(2):91–100.
177 Bonetto C, Calo M, Delgado M, Mancebo J. Modes of pressure delivery and patient–ventilator interaction. Respir Care Clin N Am. 2005;11(2):247–263.
178 Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L. Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med. 2001;164(5):795–801.
179 Hickling K. Best compliance during a decremental, but not incremental, positive end-expiratory pressure trial is related to open-lung positive end-expiratory pressure: a mathematical model of acute respiratory distress syndrome lungs. Am J Respir Crit Care Med. 2001;163(1):69–78.
180 Banner MJ, Jaeger MJ, Kirby RR. Components of the work of breathing and implications for monitoring ventilator-dependent patients. Crit Care Med. 1994;22(3):515–523.
181 Lucangelo U, Bernabé F, Blanch L. Respiratory mechanics derived from signals in the ventilator circuit. Respir Care. 2005;50(1):55–65.
182 Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri V, et al. Lung recruitment in patients with the acute respiratory distress syndrome. New Engl J Med. 2006;354(17):1775–1786.
183 Hinz J, Moerer O, Neumann P, Dudykevych T, Hellige G, Quintel M. Effect of positive end-expiratory-pressure on regional ventilation in patients with acute lung injury evaluated by electrical impedance tomography. Eur J Anaesthesiol. 2005;22(11):817–825.
184 Brower R, Morris A, MacIntyre N, Matthay M, Hayden D, et al. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end expiratory pressure. Crit Care Med. 2003;31(11):2592–2597.
185 Hodgson C, Keating J, Holland A, Davies A, Smirneos L, Bradley S. Recruitment manoeuvres for adults with acute lung injury receiving mechanical ventilation. Cochrane Database Syst Rev 2009; CD006667.
186 Fan E, Needham D, Stewart T. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005;294(22):2889–2896.
187 Foti G, Cereda M, Sparacini M, de Marchi L, Villa F, Pesenti A. Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intens Care Med. 2000;26(5):501–507.
188 Odenstedt H, Aneman A, Kárason S, Stenqvist O, Lurdin S. Acute hemodynamic changes during lung recruitment in lavage and endotoxin-induced ALI. Intens Care Med. 2005;31(1):112–120.
189 Rose L. Clinical application of ventilation modes: ventilatory strategies for lung protection. Aus Crit Care. 2010;23(2):71–80.
190 Mehta S, Grabton J, MacDonald R, Bowman D, Meatte-Martyn A, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest. 2004;126(2):518–527.
191 Singh J, Stewart T. High-frequency mechanical ventilation principles and practices in the era of lung-protective ventilation strategies. Respir Care Clin N Am. 2002;8(2):247–260.
192 Singh J, Stewart T. High-frequency oscillation ventilation in adults with acute respiratory distress syndrome. Curr Opin Crit Care. 2003;9(1):28–32.
193 Krishnan J, Brower R. High-frequency ventilation for acute lung injury and ARDS. Chest. 2000;118(3):795–807.
194 Mehta S, Lapinsky SE, Hallett DC, Merker D, Groll R, et al. A prospective trial of high frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2001;29(7):1360–1369.
195 Mehta S, MacDonald R. Implementing and troubleshooting high-frequency oscillatory ventilation in adults in the intensive care unit. Respir Care Clin N Am. 2001;7(4):683–695.
196 Higgins J, Estetter B, Holland D, Smith B, Derdak S. High-frequency oscillatory ventilation in adults: respiratory therapy issues. Crit Care Med. 2005;33(3Suppl):S196–S203.
197 Ferguson ND, Chiche J, Kacmarek R, Hallett DC, Mehta S, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the treatment with oscillation and an open lung strategy (TOOLS) trial pilot study. Crit Care Med. 2005;33(3):479–486.
198 Rossaint R, Slama K, Lewandowski StreichR, Henin P, et al. Extracorporeal lung assist with heparin-coated systems. Int J Artificial Organs. 1992;15(1):29–34.
199 Iwahashi H, Yuri K. Development of the oxygenator: past, present, and future. Artificial Organs. 2004;7(3):111–120.
200 Afshari A, Brok J, Møller A, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev 2010; CD002787.
201 Orozco-Levi M, Torres A, Ferrer M, Piera C, el-Ebiary M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care. 1995;152(4Pt1):1387–1390.
202 Torres A, Serra-Battlles J, Ros E, Piera C, Puig de la Bellacasa J, et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Int Med. 1992;116(7):540–543.
203 Ibanez J, Penafiel A, Raurich J, Marse P, Jorda R, Mata F. Gastroesophageal reflux in intubated patients receiving enteral nutrition: effect of supine and semirecumbent positions. J Parenter Enteral Nutr. 1992;16(5):419–422.
204 Drakulovic M, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851–1858.
205 American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator associated, and healthcare associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
206 Tablan O, Anderson L, Besser R, Bridges C, Hajjeh R. Guidelines for prevention of health care-associated pneumonia, 2003: recommendations of the CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(3):1–36.
207 van Nieuwenhoven C, Vandenbroucke-Grauls C, van Tiel F, Joore H, Strack van Schijndel R, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006;34(2):396–402.
208 Evans D. The use of position during critical illness: current practice and review of the literature. Aust Crit Care. 1994;7(3):16–21.
209 Reeve B, Cook D. Semirecumbency among mechanically ventilated ICU patients: a multicentre observational study. Clin Intensive Care. 1999;10(6):241–244.
210 Heyland D, Cook D, Dodek P. Prevention of ventilator-associated pneumonia: current practice in Canadian intensive care units. J Crit Care. 2002;17(3):161–167.
211 Grap M, Munro C, Bryant S, Ashanti B. Predictors of backrest elevation in critical care. Intens Crit Care Nurs. 2003;19(2):68–74.
212 Rose L, Baldwin I, Crawford T, Parke R. Semirecumbent positioning in ventilator-dependent patients: a multicenter, observational study. Am J Crit Care. 2010;19(6):e100–e108.
213 Helman D, Sherner J, Fitzpatrick T, Callendar M, Shorr A. Effect of standardized orders and provider education on head-of-bed positioning in mechanically ventilated patients. Crit Care Med. 2003;31(9):2285–2290.
214 Rose L, Baldwin I, Crawford T. The use of bed-dials to maintain recumbent positioning for critically ill mechanically ventilated patients (The RECUMBENT study): Multicentre before and after observational study. Int J Nurs Studies. 2010;47(11):1425–1431.
215 Remolina C, Khan A, Santiago T, Edelman N. Positional hypoxemia in unilateral lung disease. New Engl J Med. 1981;304(9):523–525.
216 Choi S, Nelson L. Kinetic therapy in critically ill patients: combined results based on meta-analysis. J Crit Care. 1992;7(1):57–62.
217 Staudinger T, Bojic A, Holzinger U, Meyer B, Rohwer M, et al. Continuous lateral rotation therapy to prevent ventilator-associated pneumonia. Crit Care Med. 2010;38(2):486–490.
218 Staudinger T, Kofler J, Müllner M, Locker G, Laczika K, et al. Comparison of prone positioning and continuous rotation of patients with adult respiratory distress syndrome: results of a pilot study. Crit Care Med. 2001;29(1):51–56.
219 Hering R, Wrigge H, Vorwerk R, Brensing K, Schröder S, et al. The effects of prone positioning on intraabdominal pressure and cardiovascular and renal function in patients with acute lung injury. Anesth Analg. 2001;92(5):1226–1231.
220 Guerin C, Badet M, Rosselli S, Heyer L, Sab J, Langevin B, Philit F, Fournier G, Robert D. Effects of prone position on alveolar recruitment and oxygenation in acute lung injury. Intens Care Med. 1999;25(11):1222–1230.
221 van Kaam A, Lachmann R, Herting E, De Jaegere A, van Iwaarden F, et al. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med. 2004;169(9):1046–1053.
222 Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. New Engl J Med. 2001;345(8):568–573.
223 Schortgen F, Bouadma L, Joly-Guillou M, Ricard J, Dreyfuss D, Saumon G. Infectious and inflammatory dissemination are affected by ventilation strategy in rats with unilateral pneumonia. Intens Care Med. 2004;30(4):693–701.
224 Fessler H, Talmor D. Should prone positioning be routinely used for lung protection during mechanical ventilation? Respir Care. 2010;55(1):88–99.
225 Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20(4):1017–1028.
226 Gattinoni L, Carlesso E, Taccone P, Polli F, Guérin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010;76(6):448–454.
227 Sud S, Friedrich J, Taccone P, Polli F, Adhikari N, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intens Care Med. 2010;36(4):585–599.
228 Vollman K. Prone positioning in the patient who has acute respiratory distress syndrome: the art and science. Crit Care Nurs Clin North Am. 2004;16(3):319–336.
229 Mancebo J. Weaning from mechanical ventilation. Eur Respir J. 1996;9(9):1923–1931.
230 Boles J-M, Bion J, Connors A, Herridge M, Marsh B, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–1056.
231 Stroetz RW, Hubmayr RD. Tidal volume maintenance during weaning with pressure support. Am J Respir Crit Care Med. 1995;152(3):1034–1040.
232 Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186–192.
233 Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156(2Pt1):459–465.
234 Meade M, Guyatt G, Cook D, Griffith L, Sinuff T, et al. Predicting success in weaning from mechanical ventilation. Chest. 2001;120(Suppl 6):S400–S424.
235 Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. New Engl J Med. 1991;324(21):1445–1450.
236 Tanios M, Nevins M, Hendra K, Cardinal P, Allan J, et al. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit Care Med. 2006;34(10):2530–2535.
237 Brochard L, Rauss A, Benito S, Conti G, Mancebo J, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150(4):896–903.
238 Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. New Engl J Med. 1995;332(6):345–350.
239 Kollef MH, Horst HM, Prang L, Brock WA. Reducing the duration of mechanical ventilation: three examples of change in the intensive care unit. New Horizons. 1998;6(1):52–60.
240 Robertson T, Mann H, Hyzy R, Rogers A, Douglas I, et al. Multicenter implementation of a consensus-developed, evidence-based, spontaneous breathing trial protocol. Crit Care Med. 2009;36(10):2753–2762.
241 Matic I, Majeric-Kogler V. Comparison of pressure support and T-tube weaning from mechanical ventilation: randomized prospective study. Croat Med J. 2004;45(2):162–166.
242 Esteban A, Alia I, Tobin MJ, Gil A, Gordo F, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999;159(2):512–518.
243 Hughes MR, Smith CD, Tecklenburg FW, Habib DM, Hulsey TC, Ebeling M. Effects of a weaning protocol on ventilated pediatric intensive care unit (PICU) patients. Topics Health Inform Management. 2001;22(2):35–43.
244 Burns SM, Earven S. Improving outcomes for mechanically ventilated medical intensive care unit patients using advanced practice nurses: a 6 year experience. Crit Care Nurs Clin N Am. 2002;14(3):231–243.
245 Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118(2):459–467.
246 Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. New Engl J Med. 1996;335(25):1864–1869.
247 Kollef MH, Shapiro SD, Silver P, St John RE, Prentice D, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25(4):567–574.
248 Girard T, Kress J, Fuchs B, Thomason J, Schweickert W, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134.
249 Blackwood B, Alderdice F, Burns K, Cardwell C, Lavery G, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2010; CD006904.
250 Bellomo R, Stow P, Hart G. Why is there such a difference in outcome between Australian intensive care units and others? Curr Opin Anaesthesiol. 2007;20(2):100–105.
251 Rose L, Nelson S, Johnston L, Presneill J. Workforce profile, organisation structure and role responsibility for ventilation and weaning practices in Australia and New Zealand intensive care units. J Clin Nurs. 2008;17(8):1035–1043.
252 Dojat M, Harf A, Touchard D, Laforest M, Lemaire F, Brochard L. Evaluation of a knowledge-based system providing ventilatory management and decision for extubation. Am J Respir Crit Care Med. 1996;153(3):997–1004.
253 Rose L, Presneill J, Cade J. Update in computer-driven weaning from mechanical ventilation. Anaesth Intens Care. 2007;35(2):213–221.
254 Dojat M, Brochard L, Lemaire F, Harf A. A knowledge-based system for assisted ventilation of patients in intensive care units. Int J Clin Monit Comput. 1992;9(4):239–250.
255 Dojat M, Harf A, Touchard D, Lemaire F, Brochard L. Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med. 2000;161(4Pt1):1161–1166.
256 Lellouche F, Mancebo J, Jolliet P, Roeseler J, Schortgen F, et al. A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. 2006;174(8):894–900.
257 Rose L, Presneill J, Johnston L, Cade J. A randomised, controlled trial of conventional weaning versus an automated system (SmartCare™/PS) in mechanically ventilated critically-ill patients. Intens Care Med. 2008;34(10):1788–1795.
258 Carson SS. Outcomes of prolonged mechanical ventilation. Curr Opin Crit Care. 2006;12(5):405–411.
259 Iregui M, Malen J, Tutleur P, Lynch J, Holtzman M, Kollef M. Determinants of outcome for patients admitted to a long-term ventilator unit. South Med J. 2002;95(3):310–317.
260 Vitacca M, Vianello A, Colombo D, Clini E, Porta R, et al. Comparison of two methods for weaning patients with chronic obstructive pulmonary disease requiring mechanical ventilation for more than 15 days. Am J Respir Crit Care Med. 2001;164(2):225–230.
261 Burns SM, Ryan B, Burns JE. The weaning continuum use of Acute Physiology and Chronic Health Evaluation III, Burns Wean Assessment Program, Therapeutic Intervention Scoring System, and Wean Index scores to establish stages of weaning. Crit Care Med. 2000;28(7):2259–2267.
262 Gajic O, Dara SI, Mendez J, Adesanya A, Festic E, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817–1824.
263 Ranieri VM, Suter P, Tortorella C, deTullio R, Dayer J, Brienza A, Bruno F, Slutsky A. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome. JAMA. 1999;282(1):54–61.
264 Heyland D, Cook D, Griffith L, Keenan S, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am J Respir Crit Care Med. 1999;159(4Pt1):1249–1256.
265 Vallés J, Pobo A, García-Esquirol O, Mariscal D, Real J, Fernández R. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intens Care Med. 2007;33(8):1363–1368.
266 Muscedere J, Martin C, Heyland D. The impact of ventilator-associated pneumonia on the Canadian health care system. J Crit Care. 2008;23(1):5–10.
267 Younes M. Proportional assist ventilation, a new approach to ventilatory support: theory. Am Rev Respir Dis. 1992;145(1):114–120.
268 Navalesi P, Costa R. New modes of mechanical ventilation: proportional assist ventilation, neurally adjusted ventilatory assist, and fractal ventilation. Curr Opin Crit Care. 2003;9(1):51–58.
269 Passam F, Hoing S, Prinianakis G, Siafakas N, Milic-Emili J. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration. 2003;70(4):355–361.
270 Petter A, Chiolero R, Cassina T, Chassot PG, Muller XM, Revelly JP. Automatic respirator weaning with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analgesia. 2003;97(6):1743–1750.
271 Dhand R. Ventilator graphics and respiratory mechanics in the patient with obstructive lung disease. Respir Care. 2006;50(2):246–261.
272 Davey A, Diba A. Ward’s anaesthetic equipment, 5th edn. London: Elsevier Saunders; 2005.