153 Venous Thromboembolism in Medical-Surgical Critically Ill Patients
Venous thromboembolism (VTE) is a common complication of serious illness, conferring considerable morbidity and mortality in hospitalized patients. Patients with deep vein thrombosis (DVT) are at risk of subsequently developing pulmonary embolism, which may be fatal if untreated. Approximately 90% of cases of pulmonary embolism are believed to arise in the lower limbs,1 so DVT can be viewed as an important precursor to more serious disease. Most clinical research on VTE in the intensive care unit (ICU) is focused on DVT, and it will be the major focus of this chapter.
In the ICU, patients with DVT are significantly more likely to have pulmonary embolism2 and a longer duration of mechanical ventilation (P = .02), ICU stay (P = .005), and hospitalization (P < .001) than patients without DVT.3 Clinically unsuspected DVT and pulmonary embolism are found frequently at autopsy in critically ill patients.4–6
Prophylaxis against VTE was rated the number-one patient safety initiative for hospitalized patients in the U.S. Agency for Health Care Policy Research Evidence Report and Technology Assessment document.12 Juxtaposed against the foregoing is the invisibility of medical-surgical critically ill patients in publications such as the National Institutes of Health Consensus Conference on Prevention of Venous Thrombosis and Pulmonary Embolism,13 the European Consensus Statement on Prevention of Venous Thromboembolism,14 the Thromboembolic Risk Factors Consensus Conference,15 the Fifth American College of Chest Physicians Antithrombotic Consensus Conference,16 and the American Thoracic Society Clinical Practice Guideline on Diagnosis of Venous Thromboembolism.17 An editorial in 1998 stated that the medical-surgical ICU was “the last frontier for prophylaxis.”18
 Risk Factors for Venous Thromboembolism in Medical-Surgical ICU Patients
Risk Factors for Venous Thromboembolism in Medical-Surgical ICU Patients
Clinical Risk Factors
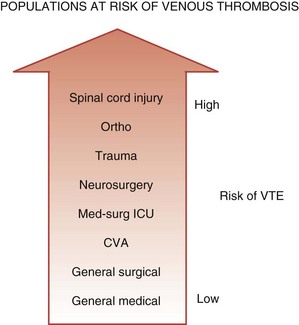
One conceptualization of risk factors for VTE in the ICU is to consider ICU admitting diagnosis as a risk factor. Medical-surgical ICU patients are at higher risk of VTE than general medical or surgical patients cared for on the ward, but at lower risk than other subgroups of critically ill patients such as trauma victims or neurosurgical patients (Figure 153-1). In the largest prospective cohort study using venographic diagnosis, of 716 trauma patients who did not receive prophylaxis, 201 (58%) had DVT between days 14 and 21, one-third of which were in the proximal venous circulation (and likely of clinical significance).19 Of these 201 patients, only 3 patients had symptoms of DVT. Among neurosurgical patients in three cohort studies using radioactive iodine leg scanning, the DVT rate was 35% without prophylaxis; in 7 randomized clinical trials that included a nonprophylaxis arm, the pooled incidence of DVT was 22%.7 Patients with acute spinal cord injury have been evaluated in 4 randomized trials and 6 cohort studies, 5 of which did not use prophylaxis.7 Four studies using either radioactive iodine fibrinogen or impedance plethysmography identified DVT in 39% to 90% of patients. In the single study using the reference standard for the diagnosis of DVT, which is ascending venography, 81% of the subgroup of trauma patients with spinal cord injury had DVT.19
Another conceptualization of risk factors for VTE in the ICU is to consider patient characteristics, events, and exposures that increase the risk of VTE. Critically ill patients have an increased risk of VTE due acute and chronic illnesses, immobility propagated by sedatives and paralytic drugs, and thrombin-generating invasive procedures. Observational studies in medical-surgical ICU patients have identified VTE risk factors11,20 including patient demographics (e.g., female sex), prior VTE events (i.e., personal history of VTE), morbidity (e.g., malignancy), ICU procedures (e.g., central venous catheters), treatments (e.g., mechanical ventilation), and VTE prophylaxis (i.e., decreasing risk). Inferences about many of these risk factors are limited by small sample sizes and infrequent use of multiple logistic regression to rigorously evaluate baseline and time-dependent risk factors.
Studies large enough to perform multivariate analysis are most helpful.2,3,21,22 In a prospective cohort study of patients ventilated for at least 1 week, the only independent risk factor for VTE was central venous catheterization; each day the catheter was in place was associated with a relative risk (RR) increase of 1.04.2 In another prospective cohort study,3 we enrolled consecutive medical-surgical patients 18 years of age or older expected to be in the ICU for 72 hours or more. Exclusion criteria were an admitting diagnosis of trauma, orthopedic surgery, pregnancy, and life-support withdrawal. We performed bilateral lower extremity compression ultrasound within 48 hours of ICU admission, then twice weekly thereafter or if VTE was clinically suspected. Thromboprophylaxis was protocol-directed and universal using unfractionated heparin. We recorded DVT risk factors at baseline and daily, using multivariate regression analysis to determine independent predictors. Patients were followed to hospital discharge. Among 261 patients with a mean Acute Physiology, Age, and Chronic Health Evaluation (APACHE) II score of 26, we identified four independent risk factors for ICU-acquired DVT: personal or family history of VTE (hazard ratio, 3.9; 95% confidence interval [CI], 1.5-10), end-stage renal failure (hazard ratio, 3.7; 95% CI, 1.3-11.2), platelet transfusion (hazard ratio, 3.2; 95% CI 1.2-8.5), and vasopressor use (hazard ratio, 2.8; 95% CI 1.1-7.2).
Congenital Hypercoagulable States
A growing number of epidemiologic studies have highlighted how inherited and acquired abnormalities in the coagulation system predispose to VTE. Activated protein C resistance due to factor V Leiden (found in 5% of the population) is the most common hereditary biochemical defect that predisposes to venous thrombosis, followed by the prothrombin 20210A regulatory sequence mutation, found in 2%.23–25 Although the impact of these prothrombotic states on the risk of VTE is confounded by the use of prophylactic anticoagulants, there is some evidence that these states increase the risk of first DVT in patients in high-risk clinical situations. Lowe and colleagues,26 in a large prospective cohort study of patients undergoing elective hip replacement, found in a univariate analysis that patients with the factor V Leiden mutation had an increased risk of postoperative venous thrombosis. No large-scale studies have yet reported on the incremental risk of DVT in high-risk situations for patients with factor V Leiden, but it is known that the prothrombin gene mutation predicts DVT in otherwise healthy outpatients (odds ratio [OR], 2.8).27 Additional but less common inherited hypercoagulable states include deficiencies of antithrombin, protein C, and protein S, each of which is a naturally occurring anticoagulant protein. The ORs for venous thrombosis are 8.1 to 13.7 for antithrombin deficiency, 7.3 to 11.9 for protein C deficiency, and 8.5 to 10 for protein S deficiency.28–30 Antiphospholipid antibodies including the lupus anticoagulant and anticardiolipin antibody are strong predictors of first and recurrent venous thrombosis. Elevations in the levels of homocysteine and coagulation factors VIII, IX, and XI also predispose to VTE in other settings.31–35
In the observational study described earlier, we evaluated the frequency and clinical importance of thrombophilia markers at the time of ICU admission and during the ICU stay.36 To examine whether baseline markers of activation of the coagulation system and known thrombophilic risk factors predicted the development of DVT, a comprehensive battery of tests was done at the time of enrollment, including activated protein C ratio (with confirmation of factor V Leiden where appropriate), protein C level, protein S level, antithrombin level, anticardiolipin antibody titer, and screening and confirmatory assays for the lupus anticoagulant. The receiver operating curves for four baseline coagulation tests at the time of ICU admission showed areas under the curve for each of the activated protein C ratio, antithrombin, protein C, and protein S tests that were not significantly different than 50%; that is, the presence of these abnormalities did predict the presence of DVT at the time of ICU admission. Tests with areas under the curve of 0.75 to 0.80 represent moderate diagnostic power. Baseline coagulation tests also were not useful predictors of DVT developing during the ICU stay.
Acquired Hypercoagulable States
Coagulation abnormalities acquired in the ICU have received considerable attention. Acquired thrombophilic markers associated with thrombosis include lupus anticoagulant, anticardiolipin antibody, and increased levels of homocysteine. In critically ill patients, acquired reductions in the levels of antithrombin, protein C, and protein S due to consumption may be common, and it is possible these deficiencies are associated with a high risk of VTE and other complications of ICU stay including death. The relationship between the inflammatory and coagulation cascades has been the focus of intense discussion in the sepsis literature.37 Longitudinal studies have shown that protein C levels in sepsis are inversely correlated with mortality.38 A randomized trial of recombinant activated protein C in 1690 patients with systematic inflammation and organ dysfunction showed a decrease in 28-day mortality from 30.8% to 24.7% (number needed to treat, 16).39 Approximately 80% of patients had protein C deficiency on entry into the trial, highlighting the prevalence of this acquired thrombophilic marker. The efficacy of recombinant activated protein C was the same, however, in patients with and without protein C deficiency. In another large randomized trial of antithrombin administration in patients with sepsis, antithrombin levels were less than 60% of normal functional levels in more than 50% of patients, but antithrombin administration did not decrease mortality.40
In the study of DVT incidence described earlier,3 we also evaluated whether quantitative D-dimer tests at the time of ICU admission and during ICU stay41 were associated with DVT. At the time of enrollment, twice weekly during the ICU stay, and at the time of any suspected venous thromboembolic events, patients had a battery of D-dimer tests, including whole-blood SimpliRed D-dimer tests, and five D-dimer assays performed using D-dimer Plus, IL test DD, MDA-DD, Sigma DD, and Biopool. For the five quantitative baseline D-dimer tests in relation to DVT detected at the time of ICU admission, the areas under the curve for each of D-dimer Plus (P = .01), MDA-DD (P = .002), and Sigma DD (P = .054) were significantly different from .50. The receiver operating curves for time-dependent quantitative D-dimer tests and DVT developing during the ICU stay did not differ from 50%, indicating that D-dimer tests are not useful for predicting the development of VTE in the ICU.
Summary
Venous thromboembolism is a multicausal disease.42 In considering clinical risk factors, it is useful to classify them into risk factors that are fixed, such as admitting diagnoses, and risk factors that are modifiable, such as invasive procedures. Modifiable risk factors can form the basis of VTE prevention strategies. Studies to analyze the relative contributions of congenital and acquired thrombophilia markers suggest that these markers are not useful for screening or diagnostic purposes in the ICU.
 Prevalence and Incidence
Prevalence and Incidence
The incidence of VTE in the ICU depends on whether the events are clinically diagnosed or detected by screening methods. Venous thromboembolism rates observed in usual clinical practice are much lower than rates observed during systematic screening, because the former primarily represent diagnoses prompted by signs or symptoms. For example, 10%43 to 100%11,44 of proximal DVTs found by ultrasound screening were clinically unsuspected. In this section, we report the incidence of VTE in critically ill patients based on studies using systematic screening methods for case identification.
Understanding DVT rates requires distinguishing events diagnosed at the time of ICU admission (prevalence at a point in time) from the events that develop over the course of critical illness (incidence over the ICU stay). Cross-sectional studies at the time of admission to a medical ICU45 and surgical ICU44 suggest a 10% prevalence of DVT diagnosed by screening compression ultrasonography. As mentioned earlier in the section on risk factors, however, the prevalence of DVT on admission to any ICU is influenced heavily by the case mix of patients.
The risk of DVT developing over the ICU stay was established in three longitudinal studies using systematic screening.11,43,46 Among ICU patients not receiving prophylaxis, 76% of whom were mechanically ventilated, radioactive iodine fibrinogen scanning for 3 to 6 days identified DVT in 3 of 34 (9%) patients.46 Using Doppler ultrasound twice weekly then at 1 week after ICU discharge in 100 medical patients expected to stay more than 48 hours—70% of whom were ventilated—DVT was diagnosed in 32% of 100 patients receiving no prophylaxis, in 40% of patients receiving unfractionated heparin, and in 33% of patients who received mechanical prophylaxis.11 In a third study of 102 medical-surgical ICU patients undergoing duplex ultrasound during days 4 to 7 and as clinically indicated,43 DVT rates were 25%, 19%, and 7% in patients receiving no prophylaxis, mechanical prevention, and unfractionated heparin.
Earlier studies suggest that the prevalence of proximal DVT on admission to a medical-surgical ICU is estimated to be 10%, and the incidence of DVT developing over the ICU stay based on systematic screening ranges from 9% to 40%. Two of these studies performed surveillance for approximately 1 week,43,46 however, and one study used radioactive iodine fibrinogen scanning for detection,46 which likely underestimated the risk of ICU-acquired DVT. No studies used systematic screening for pulmonary embolism, and the true incidence of pulmonary embolism is not known.
More recent studies suggest a lower rate of VTE in medical-surgical ICU studies, partly due to the administration of thromboprophylaxis. In a single-center cohort of 239 medical ICU patients who did not undergo systematic screening ultrasound, 44 (18.4%) patients had lower-extremity DVT.47 Ibrahim et al.,2 in a cohort study involving twice-weekly upper- and lower-extremity ultrasound screening, found a 26.6% incidence of DVT. Among 261 patients with a mean APACHE II score of 25.5 (±8.4), the prevalence of DVT was 2.7% (95% CI, 1.1-5.5) on ICU admission, and the incidence was 9.8% (95% CI, 6.5-14.2) over the ICU stay.3
 Diagnosis
Diagnosis
A helpful constellation of signs and symptoms in a mathematically derived and validated clinical model has been developed and validated for its prediction of DVT in outpatients.48 Diagnosing DVT in the ICU is more challenging, however. Symptoms rarely are elicited from mechanically ventilated patients, most of whom receive sedation and analgesia, rendering the notion of symptomatic DVT unhelpful in this setting. Compounding the problem is the fact that physical examination of the lower extremities may be devalued in the high-technology ICU environment compared with cardiopulmonary monitoring. In a survey of Canadian ICU directors, respondents stated that physical examination did not yield information that was helpful in the diagnosis of DVT.49
The reference standard for DVT remains ascending contrast lower limb venography, despite its widespread replacement by ultrasonography. Venography can reliably detect all clinically important forms of DVT—calf thrombosis, thrombosis in the pelvis, and thrombosis of the muscular veins of the thigh, for example—none of which are reliably detected by ultrasonography. Despite its utility, venography rarely is performed in practice in the ICU. In a Canadian ICU directors’ survey, the use of venography to detect thrombosis was reported rarely (56%) or never (9%).49 Concern about transporting potentially unstable patients to the radiology department,50 the invasive nature of the test, and the risk of contrast dye–induced nephropathy51 may contribute to the aversion to venography in this setting; however, it is also possible that many intensivists are unaware of the limitations of ultrasonography for diagnosing DVT. Studies conducted in the 1980s cited contrast nephropathy as the third leading cause of new-onset renal failure in hospitalized patients.52 Although currently employed nonionic contrast media are associated with a lower rate of nephrotoxicity than ionic contrast media,53 the volume of contrast administered remains an independent predictor of nephrotoxicity.54 Additional risk factors for acquired renal insufficiency in medical ICU patients include common problems such as sepsis, volume depletion, mechanical ventilation, and surgery.55 The high rate of renal dysfunction in critically ill patients with normal serum creatinine is concerning, and even mild renal insufficiency in these patients is associated with increased attributable mortality. For patients undergoing venography, intravenous fluid loading before and after the contrast dye and acetylcysteine, 600 mg twice daily by nasogastric tube the day before and the day after the procedure, reduce the rate of contrast-induced nephropathy, as shown in a randomized trial.56
The test properties of lower-extremity bilateral Doppler ultrasound in medical-surgical ICU patients have not been determined. A meta-analysis reported a pooled sensitivity of Doppler ultrasound for proximal DVT in symptomatic patients of 97% (95% CI, 96%-98%) and in asymptomatic patients of 62% (95% CI, 53%-71%).57 Ultrasound is more insensitive for distal DVT (pooled sensitivity for symptomatic patients, 73% [95% CI, 54%-93%] and asymptomatic patients, 53% [95% CI, 32%-74%]). Symptomatic outpatients with suspected DVT and serially negative screening ultrasound studies have a 1% likelihood of subsequently developing a DVT or pulmonary embolism, suggesting that serially negative ultrasound studies safely and effectively rule out clinically important DVT.58–60 It is unclear, however, to what extent serially negative ultrasound studies in medical-surgical ICU patients indicate the absence of DVT. Finally, ultrasound also inaccurately diagnoses some patients with DVT who do not have DVT by venogram, highlighting the false-positive rate of ultrasonography. Robinson and colleagues61 performed ultrasonography and contrast venography in a large group of asymptomatic patients at the time of hospital discharge after joint replacement surgery; in this study, 6 of 19 positive compression ultrasound studies were not confirmed by venography.
Despite the advantages of using ultrasonography to diagnose DVT in the ICU, it is associated with a false-positive and false-negative rate that is not yet clearly established in the critical care setting. Nevertheless, bilateral lower-extremity ultrasound is the most widely used diagnostic test for DVT, according to VTE researchers in the medical-surgical ICU11,43–46 and according to a survey of radiologists from the United Kingdom.62 A recent review referred to ultrasonography as the imaging procedure of choice for the diagnosis of DVT.63 The American College of Radiology cited bilateral lower-extremity ultrasound as the most appropriate test for DVT.64 Finally, bilateral lower-limb ultrasound is also the most feasible diagnostic test in the ICU. An ultrasound diagnosis of DVT requires non-compressibility of one or more lower-limb venous segments, including (1) the trifurcation of the deep calves, (2) distal popliteal, (3) proximal popliteal, (4) distal femoral, (5) mid-femoral, and (6) common femoral veins.
There is no diagnostic test for DVT that is highly accurate and feasible in the ICU population for daily practice. Nevertheless, Doppler ultrasound is the most widely accepted DVT diagnostic test. Because the likelihood of embolization from undiagnosed, untreated proximal DVT is high, strategies that screen for proximal DVT in these critically ill patients have the potential to reduce the risk of pulmonary embolism and its cardiopulmonary consequences through early treatment. Universal screening for DVT with ultrasonography cannot be recommended currently, however.44,65 Development of a reliable screening test for VTE in critically ill patients should be a high clinical priority because it is possible that the most widely used screening test today (ultrasonography) has an unacceptably high rate of false-positive tests. A false-positive ultrasound study is likely to lead to unneeded anticoagulant therapy (with its attendant risks).
 Thromboprophylaxis
Thromboprophylaxis
Only 4 published randomized trials have tested DVT prophylaxis in medical-surgical ICU patients.66,67 One double-blind, single-center trial allocated 119 medical-surgical ICU patients at least 40 years old to unfractionated heparin, 5000 units twice daily, or placebo subcutaneous injections.66 Using serial fibrinogen leg scanning for 5 days, the rate of DVT was 13% in the unfractionated heparin group and 29% in the placebo group (RR, 0.45; P < .05). Rates of bleeding and pulmonary embolism were not reported. In a more recent multicenter trial by Fraisse et al.,67 223 patients with an acute exacerbation of chronic obstructive pulmonary disease requiring mechanical ventilation for at least 2 days were allocated to the LMWH, nadroparin, 3800 or 5700 International Units once daily, or placebo. Patients were screened with weekly duplex ultrasound studies and on clinical suspicion of DVT; venography was attempted in all patients. The rate of DVT was 16% in the nadroparin group and 28% in the placebo group (RR, 0.67; P < .05). A similar number of patients bled in each group (25 versus 18 patients, P = .18). Although patients were not screened for pulmonary embolism, no patients developed pulmonary embolism during the trial.
A third trial among critically ill patients scheduled to undergo major elective surgery compared unfractionated heparin, 5000 International Units twice daily, with LMWH enoxaparin, 40 mg once daily. Each patient was evaluated postoperatively clinically and confirmed by Doppler study for development of DVT. Among 156 patients completing the protocol, there was similar efficacy of unfractionated heparin as compared with LMWH in the prevention of DVT (2 patients [2.66%] versus 1 [1.23%], P = 0.51). There was no difference in the incidence of major complications between groups. However, minor hemorrhagic complications such as wound hematoma and surgical site bleeding were significantly more in the heparin group as compared with the LMWH group. Overall, 18 patients (24%) had bleeding either from the gastrointestinal tract or from incision site or tracheostomy site in the unfractionated heparin group, whereas 8 patients (9.87%) developed wound hematoma or gastrointestinal bleeding in the LMWH group (P = 0.01).68
A fourth study, Xigris and Prophylactic Heparin Evaluation in Severe Sepsis (XPRESS), was a randomized, double-blind, placebo-controlled trial of prophylactic heparin in patients with severe sepsis and higher disease severity who were treated with drotrecogin alfa (activated; DAA).69 A recent report focused on how patients were randomized to unfractionated heparin, LMWH, or placebo during the DAA infusion period. All patients underwent ultrasonography between days 4 and 6; 1935 patients were included, and before enrollment approximately half were given no form of prophylaxis. By day 6, 5% of patients developed a VTE, and the rate of VTE did not vary based on type of heparin administered. The vast majority of VTE detected by day 6 were clinically silent. Of factors analyzed, history of VTE was the only variable independently associated with development of a VTE (OR, 3.66; 95% CI, 1.77-7.56; P = 0.005).70
A fifth trial outside the ICU setting but of some relevance to the ICU enrolled acutely ill medical patients hospitalized with heart failure, respiratory failure not requiring mechanical ventilation, or one of the following if associated with an additional VTE risk factor: infection without septic shock, musculoskeletal disorder, or inflammatory bowel disease.71 Patients were excluded if they required intubation, had a coagulopathy, or had serum creatinine greater than 150 µmol/L. Patients were randomized to receive daily subcutaneous LMWH enoxaparin, 40 mg or 20 mg, or placebo for 6 to 14 days. Patients had venography between days 6 and 14 or as clinically indicated. Ultrasonography was performed if venography was not feasible. Of 1102 randomized patients, 236 were not included in the main analysis (because the venogram could not be evaluated [n = 72], was technically unfeasible [n = 12], was not performed [n = 4], was not performed at the investigators’ discretion [n = 58]; the patient refused [n = 62]; or the patient died [n = 28]). Among the remaining 866 patients, the DVT rate was 6% in patients receiving enoxaparin, 40 mg, compared with 15% among patients receiving either enoxaparin, 20 mg, or placebo (RR, 0.37). Major hemorrhage developed in 12, 4, and 7 patients (P = not significant). Clinically suspected and objectively confirmed pulmonary embolism developed in one patient in the low-dose enoxaparin group and three patients in the placebo group, although pulmonary embolism events were not evaluated per protocol. The fact that these patients, although requiring medical admission to the hospital, were not critically ill limits the generalizability of these findings to the critical care setting. Nonpharmacologic approaches such as pneumatic compression devices and antiembolic stockings, although widely used, have not been evaluated in medical-surgical ICU patients, and their effectiveness must be extrapolated from other settings.
Summary
Only four randomized trials evaluating VTE prophylaxis in the ICU have been published. One trial of medical-surgical patients showed that unfractionated heparin is better than no prevention (the number of patients who needed to receive prophylaxis with 5000 units twice daily of subcutaneous unfractionated heparin to prevent one DVT was four).66 The second trial of exclusively ventilated chronic obstructive pulmonary disease patients showed that nadroparin is better than no prevention (the number of patients who needed to receive prophylaxis with weight-adjusted LMWH to prevent one DVT was eight).71 Two trials compared unfractionated heparin with LMWH for VTE prophylaxis in medical-surgical ICU patients, but they were underpowered and inconclusive. 68,70 In contrast, in trauma patients, LMWH is clearly superior to unfractionated heparin based on randomized trials.72
 Thromboprophylaxis Compliance
Thromboprophylaxis Compliance
Several prospective single-center usage reviews of VTE prophylaxis provide evidence about practice patterns. Prophylaxis was prescribed in 33% of 152 medical ICU patients in one study73 and 61% of 100 medical ICU patients in another.11 In contrast, in a medical-surgical ICU in which a clinical practice guideline was in place, VTE prophylaxis was prescribed for 86% of 209 patients.74 In another study of medical-surgical ICU patients, after excluding patients receiving therapeutic anticoagulation and for whom heparin was contraindicated, 63% of 96 patients received unfractionated heparin thromboprophylaxis.20
In a 1-day cross-sectional multicenter usage review of Canadian surgical ICU patients whose procedure was no more than 1 week earlier, unfractionated heparin was used predominantly.75 We considered a range of patients, including those with an admission diagnosis of hemorrhage and the potential for immediate postoperative bleeding, to highlight the dual risks of thrombosis and bleeding. Two methods of VTE prophylaxis were prescribed for 20 of 89 (22.5%) patients. Prophylaxis with unfractionated heparin or LMWH was significantly less likely for postoperative ICU patients requiring mechanical ventilation compared with patients weaned from mechanical ventilation later in their ICU course (OR, 0.36; P = .03). Use of intermittent pneumatic compression devices was significantly associated with current hemorrhage (OR, 13.5; P = .021) and risk of future hemorrhage (OR, 19.3; P = .001).
In a 1-day bi-national cross-sectional usage review of medical ICU patients in France and Canada,76 we found that among 1222 patients (65% of whom were mechanically ventilated), heparin VTE prophylaxis was administered similarly to 63.9% of patients between the two countries. Excluding patients with contraindications to heparin and patients receiving therapeutic anticoagulation, 91.7% of medical ICU patients appropriately received either unfractionated heparin or LMWH prophylaxis. Independent predictors of any type of heparin prophylaxis were invasive mechanical ventilation (OR, 2.4; 95% CI, 1.4-4.3]) and obesity (OR, 3.1; 95% CI, 1.1-8.8). LMWH was less likely to be prescribed for patients with renal failure (OR, 0.1; 95% CI, 0.0009-0.9) or receiving antiembolic stockings (OR, 0.4; 95% CI, 0.1-0.9) and much more likely to be prescribed in French ICUs (OR, 9.2; 95% CI, 5-16.9). However, among patients receiving LMWH, high doses were more likely to be prescribed in Canadian ICUs (OR, 8.7; 95% CI, 2-37.6). Patients who were pregnant or postpartum (OR, 7.7; 95% CI, 1.3-44.3), had neurologic failure (OR, 2.1; 95% CI, 1.3-3.4), or were Canadian (OR, 3; 95% CI, 2.1-4.4) were most likely to receive mechanical VTE prophylaxis (with antiembolic stockings or pneumatic compression devices), whereas patients who already were receiving heparin were less likely to receive mechanical prophylaxis (OR, 0.5; 95% CI, 0.3-0.7).
Summary
Use of effective VTE prophylaxis ranges widely. One inference from the health services research describing practice patterns is that insufficient attention is paid to VTE prevention in the critical care setting. When deciding on the type and intensity of prophylaxis, clinicians seem to risk-stratify such that patients with a greater number of VTE risk factors are more likely to receive more intensive prophylaxis than patients with fewer risk factors. The variety of prophylactic approaches used highlights the diverse and dynamic competing risks of bleeding and thrombosis in heterogeneous ICU patients, underscoring population-based and individual risk-to-benefit ratios and delineating the need for large definitive studies to guide prophylaxis. VTE prevention methods should be individualized based on current and potential risks of bleeding and thrombosis. More randomized trials of VTE prophylaxis in medical-surgical critically ill medical patients would better inform practice. These trials should be followed up with effective implementation strategies designed to change clinician behavior and improve patient outcomes.77
Key Points
2002 AHCRQ evidence report/technology assessment: prevention of venous thromboembolism after injury. Rockville, MD: Agency for Health Care Research and Quality, 2002.
Attia J, Ray JG, Cook DJ, et al. Deep vein thrombosis and its prevention in critically ill patients. Arch Intern Med. 2001;161:1268-1279.
Ibrahim EH, Iregui M, Prentice D, et al. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med. 2002;30:771-774.
Kearon CJ, Julian JA, Newman TE, et al. Noninvasive diagnosis of deep vein thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann Intern Med. 1998;128:663-667.
This is a systematic review of the properties of ultrasonography for the diagnosis of DVT.
Lacherade JC, Cook DJ, Heyland DK, et al. French and Canadian ICU Directors Groups. Prevention of venous thromboembolism (VTE) in critically ill medical patients: a Franco-Canadian cross-sectional study. J Crit Care. 2003;18:228-237.
This is a Franco-Canadian survey of thromboprophylaxis patterns in medical ICU patients.
1 Saeger W, Genzkow M. Venous thromboses and pulmonary emboli in post-mortem series: Probable causes by correlations of clinical data and basic diseases. Pathol Res Pract. 1994;190:394-399.
2 Ibrahim EH, Iregui M, Prentice D, et al. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med. 2002;30:771-774.
3 Cook DJ, Crowther M, Meade M, et al. Deep venous thrombosis in medical-surgical ICU patients: Prevalence, incidence and risk factors [abstract]. Crit Care. 2003;7(Suppl 2):S54.
4 Karwinski B, Svendsen E. Comparison of clinical and postmortem diagnosis of pulmonary embolism. J Clin Pathol. 1989;42:135-139.
5 Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108:978-981.
6 Twigg SJ, McCrirrick A, Sanderson PM. A comparison of post mortem findings with post hoc estimated clinical diagnoses of patients who die in an intensive care unit. Intensive Care Med. 2001;27:706-710.
7 Attia J, Ray JG, Cook DJ, et al. Deep vein thrombosis and its prevention in critically ill patients. Arch Intern Med. 2001;161:1268-1279.
8 Joynt GM, Kew J, Gomersall CD, et al. Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000;117:178-183.
9 Durbec O, Viviand X, Potie F, et al. Lower extremity deep vein thrombosis: A prospective randomized controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997;25:1982-1985.
10 Douketis JD. Prognosis in pulmonary embolism. Curr Opin Pulm Med. 2001;7:354-359.
11 Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274:335-337.
12 AHCRQ evidence report/technology assessment: prevention of venous thromboembolism after injury. Rockville, MD: Agency for Health Care Research and Quality, 2002.
13 NIH Consensus conference report: prevention of venous thrombosis and pulmonary embolism. JAMA. 1986;256:744-749.
14 European Consensus statement: prevention of venous thromboembolism. Int Angiol. 1992;11:151-159.
15 Thromboembolic Risk Factors (THRIFT) Consensus Group: risk and prophylaxis for venous thromboembolism in hospital patients. BMJ. 1992;305:567-574.
16 Clagett GP, Anderson FA, Geerts W, et al. Prevention of venous thromboembolism. Chest. 1998;114(Suppl):531S-560S.
17 American Thoracic Society Clinical practice guideline: the diagnostic approach to acute venous thromboembolism. Am Rev Respir Crit Care Med. 1999;160:1043-1066.
18 Goldhaber SZ. Venous thromboembolism in the intensive care unit: the last frontier for prophylaxis. Chest. 1998;113:5-7.
19 Geerts WH, Code KI, Jay RM, et al. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601-1606.
20 Cook DJ, Attia J, Weaver B, et al. Venous thromboembolic disease: an observational study in medical-surgical ICU patients. J Crit Care. 2000;15:127-132.
21 Merrer J, De Jonghe B, Golliot F, et al. French Catheter Study Group in Intensive Care. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700-707.
22 Kollef MH, Eisenberg PR, Shannon W. A rapid assay for the detection of circulating D-dimer is associated with clinical outcomes among critically ill patients. Crit Care Med. 1998;26:1054-1060.
23 Lee DH, Henderson P, Blajchman M. Prevalence of factor V Leiden in a Canadian blood donor population. Can Med Assoc J. 1996;155:285-289.
24 Rodeghiero F, Tosetto A. Activated protein C resistance and factor V Leiden mutation are independent risk factors for venous thromboembolism. Ann Intern Med. 1999;130:643-650.
25 de Moerloose P, Reber G, Perrier A, et al. Prevalence of factor V Leiden and prothrombin G20210A mutations in unselected patients with venous thromboembolism. Br J Haematol. 2000;110:125-129.
26 Lowe GD, Haverkate F, Thompson SG, et al. Prediction of deep vein thrombosis after elective hip replacement surgery by preoperative clinical and haemostatic variables: The ECAT DVT Study. European Concerted Action on Thrombosis. Thromb Haemost. 1999;81:879-886.
27 Poort SR, Rosendaal FR, Reitsma PH, et al. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698-3703.
28 van den Belt AG, Prins MH, Huisman MV, et al. Familial thrombophilia: A review analysis. Clin Appl Thromb Hemost. 1996;2:227-236.
29 Martinelli I, Mannucci PM, Stefano De V, et al. Different risks of thrombosis in four coagulation defects associated with inherited thrombophilia: A study of 150 families. Blood. 1998;92:2353-2358.
30 Crowther MA, Kelton JG. Congenital thrombophilic states associated with venous thrombosis: a qualitative overview and proposed classification system. Ann Intern Med. 2003;138:128-134.
31 Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET Study). Thromb Haemost. 1997;77:444-451.
32 Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. 2000;83:5-9.
33 Meijers JC, Tekelenburg WL, Bouma BN, et al. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696-701.
34 van Hylckama V, van der Linden I, Bertina RM, et al. High levels of factor IX increase the risk of venous thrombosis. Blood. 2000;95:3678-3682.
35 Ray JG. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch Intern Med. 1998;158:2101-2106.
36 Crowther MA, Cook DJ, Meade M, et al. Baseline thrombophilic markers do not predict venous thrombosis in medical-surgical ICU patients [abstract]. J Thromb Haemost. 1, 2003. P0925
37 Dhainaut JF. Introduction to the Margaux Conference on Critical Illness: Activation of the coagulation system in critical illnesses. Crit Care Med. 2000;28:S1-S3.
38 Fourrier F, Chopin C, Goudemand J, et al. Septic shock, multiple organ failure, and disseminated intravascular coagulation: compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest. 1992;101:816-823.
39 Bernard G, Vincent JL, Laterre PF, et al. Recombinant Human Activated Protein C Worldwide Evaluation in Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
40 Warren B, Eid A, Singer P, et al. KyberSept Trial Study Group. High-dose antithrombin III in severe sepsis: A randomized controlled trial. JAMA. 2001;286:1869-1878.
41 Crowther MA, Cook DJ, Meade M, et al. Levels of D-dimer do not reliably predict deep vein thrombosis in medical-surgical ICU patients [abstract]. J Thromb Haemost. 1, 2003. P1402
42 Rosendaal FR. Venous thrombosis: A multicausal disease. Lancet. 1999;353:1167-1173.
43 Marik PE, Andrews L, Maini B. The incidence of deep venous thrombosis in ICU patients. Chest. 1997;111:661-664.
44 Harris LM, Curl GR, Booth FV, et al. Screening for asymptomatic deep vein thrombosis in surgical intensive care patients. J Vasc Surg. 1997;26:764-769.
45 Schonhofer B, Kohler D. Prevalence of deep-venous thrombosis of the leg in patients with acute exacerbations of chronic obstructive pulmonary disease. Respiration. 1998;65:173-177.
46 Moser KM, LeMoine JR, Nachteway FJ, et al. Deep venous thrombosis and pulmonary embolism: Frequency in a respiratory intensive care unit. JAMA. 1981;246:1422-1424.
47 Kollef MH, Zahid M, Eisenberg PR. Predictive value of a rapid semi-quantitative D-dimer assay in critically ill patients with suspected venous thromboembolic disease. Crit Care Med. 2000;28:414-420.
48 Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326-1330.
49 Cook DJ, McMullin J, Hodder R, et al. Canadian ICU Directors Group. Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey. Crit Care. 2001;5:336-342.
50 Wallace PG, Ridley S. ABC of intensive care: transport of critically ill patients. BMJ. 1999;319:368-371.
51 Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000;11:177-182.
52 Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: A prospective study. Am J Med. 1983;74:243-248.
53 Rudnick MR, Goldfarb S, Wexler L, et al. Iohexol Cooperative Study: Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: A randomized trial. Kidney Int. 1995;47:254-261.
54 McCullough PA, Wolyn R, Rocher L, et al. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368-375.
55 Jochimsen F, Schafer JH, Maurer A, et al. Impairment of renal function in medical intensive care: Predictability of acute renal failure. Crit Care Med. 1990;18:480-485. 2
56 Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-184.
57 Kearon CJ, Julian JA, Newman TE, et al. Noninvasive diagnosis of deep venous thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann Intern Med. 1998;128:663-677.
58 Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: Prospective cohort study. BMJ. 1998;316:17-20.
59 Heijboer H, Ginsberg JS, Buller HR, et al. The use of the D-dimer test in combination with serial non-invasive testing versus serial non-invasive testing alone for the diagnosis of deep-vein thrombosis. Thromb Haemost. 1992;67:510-513.
60 Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
61 Robinson KS, Anderson DR, Gross M, et al. Accuracy of screening compression ultrasonography and clinical examination for the diagnosis of deep vein thrombosis after total hip or knee arthroplasty. Can J Surg. 1998;41:368-373.
62 Burn PR, Blunt DM, Sansom HE, et al. The radiological investigation of suspected lower limb deep vein thrombosis. Clin Radiol. 1997;52:625-628.
63 Fraser JD, Anderson DR. Deep venous thrombosis: Recent advances and optimal investigation with ultrasound. Radiology. 1999;211:9-24.
64 American College of Radiology. Appropriateness criteria. Available at. www.acr.org/dyna/?id=appropriateness_criteria.
65 Goldhaber SZ, Dunn K, MacDougall RC. New onset of venous thromboembolism among hospitalized patients at Brigham and Women’s Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118:1680-1684.
66 Cade JF. High risk of the critically ill for venous thromboembolism. Crit Care Med. 1982;10:448-450.
67 Fraisse F, Holzapfel L, Couland JM, et al. and the Association of Non-University affiliated Intensive Care Specialist Physicians of France: Nadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. Am J Respir Crit Care Med. 2000;161:1109-1114.
68 De A, Roy P, Garg VK, Pandey NK. Low-molecular-weight heparin and unfractionated heparin in prophylaxis against deep vein thrombosis in critically ill patients undergoing major surgery. Blood Coagul Fibrinolysis. 2009;21:57-61.
69 Levi M, Levy M, Williams MD, et al. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). Am J Respir Crit Care Med. 2007;176:483-490.
70 Shorr AF, Williams MD. Venous thromboembolism in critically ill patients Observations from a randomized trial in sepsis. Thromb Haemost. 2009;101:139-144.
71 Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin versus placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Group. N Engl J Med. 1999;341:793-800.
72 Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335:701-707.
73 Keane MG, Ingenito EP, Goldhaber SZ. Utilization of venous thromboembolism prophylaxis in the medical intensive care unit. Chest. 1994;106:13-22.
74 Ryskamp RP, Trottier SJ. Utilization of venous thromboembolism prophylaxis in a medical-surgical ICU. Chest. 1998;113:162-164.
75 Cook DJ, Laporta D, Skrobik Y, et al. Canadian ICU Directors Group: Prevention of venous thromboembolism in critically ill surgery patients: A cross-sectional study. J Crit Care. 2001;16:161-166.
76 Lacherade JC, Cook DJ, Heyland DK, et al. French and Canadian ICU Directors Groups: Prevention of venous thromboembolism (vte) in critically ill medical patients: a Franco-Canadian cross-sectional study. J Crit Care. 2003;18:228-237.
77 Bero LA, Grilli R, Grimshaw JM, et al. Cochrane Effective Practice and Organization of Care Review Group: Closing the gap between research and practice: An overview of systematic reviews of interventions to promote the implementation of research findings. BMJ. 1998;317:465-468.