CHAPTER 75 Venous Anatomy of the Thorax
THE VENAE CAVAE
The Superior Vena Cava
The superior vena cava (SVC) is the primary means of blood return to the heart from the upper extremities, head, and neck. It forms from the convergence of the right and left brachiocephalic veins (also known as the innominate veins) and ends at its entrance into the right atrium. The lower half of the vessel lies within the pericardium.1 The SVC has a slightly convex vertical course, with its apex to the right, beginning immediately below the first costosternal junction and ending opposite the upper border of the third right costal cartilage. It measures approximately 7 cm in length, with its midpoint near the level of the carina on a frontal chest radiograph.2
In cross-sectional imaging, the beginning of the SVC can be recognized at the junction of the brachiocephalic veins in the upper mediastinum, posterior and slightly inferior to the right clavicular head and just above the prevascular space (Fig. 75-1). The course of the SVC lies slightly to the right of midline, adjacent to the ascending aorta, and passes anterior to the right pulmonary artery before entering the heart (Fig. 75-2). When CT imaging of the chest is performed after an upper extremity injection of intravenous contrast material, the SVC can often be recognized as the densely opacified structure draining into the heart and creating some streak artifact in this area.
Variant Anatomy
To understand the duplicated and left-sided SVC variants, one must first understand the venous embryologic development. The venous system begins its development in the earliest stages of intrauterine life as a symmetrical structure of paired cardinal and subcardinal veins. Early in the intrauterine period, the paired cardinal veins run the entire length of the embryo. There is a larger and more developed dorsally positioned caudal tract (the right and left posterior cardinal veins) and a more ventrally located cranial tract (the right and left anterior cardinal veins). The common cardinal veins originate from where the posterior and anterior cardinal veins converge and reach the primitive heart, the sinus venosus, from both the right and left sides. In a normal progression of development, the inferior portion of the left anterior cardinal vein regresses, and an anastomotic channel is formed between the right and left anterior cardinal veins, which becomes the left brachiocephalic vein. This vein allows drainage from the left side to the right. The superior portion of the left anterior cardinal vein becomes the superior intercostal vein, which drains the second and third left intercostal spaces. The normal right-sided SVC forms from the right common cardinal vein and the right anterior cardinal vein.3 When the left distal anterior cardinal vein persists, a left-sided vena cava forms (Fig. 75-3). The incidence of a left-sided vena cava is 0.3% to 0.5% in the general population and 4% in patients with congenital heart disease.4
A left-sided SVC may or may not be accompanied by regression of the right anterior cardinal vein and the right common cardinal vein, which form the normal right-sided SVC. Without regression, both a right and left SVC develop, resulting in a duplicated SVC system, with an absent left brachiocephalic vein and smaller right SVC in 65% of cases (Fig. 75-4). Duplicated SVC occurs in 0.3% to 0.5% of the population and in approximately 11% of patients with congenital heart disease.5
With regression of the cardinal veins that form the right SVC, blood from the right side of the embryo drains from the right brachiocephalic vein to the left SVC, which then drains most commonly into the coronary sinus and on into the right atrium. Cross-sectional imaging of a left-sided SVC shows it coursing caudally to the left of the aortic arch and left main pulmonary artery within the mediastinum, passing medially to the left superior pulmonary vein, before emptying into the coronary sinus (Fig. 75-5). Although it is most commonly asymptomatic, the resultant dilation of the coronary sinus can rarely cause conduction abnormalities. In the absence of other congenital cardiac anomalies, a left-sided SVC with absent right SVC and visceroatrial situs solitus occurs in fewer than 0.1% of the population.6 Finally, a left-sided SVC can drain directly into the left atrium, producing a right-to-left shunt. This anomaly is rare and is usually associated with congenital cardiac anomalies.
The Inferior Vena Cava
The thoracic segment of the inferior vena cava (IVC), the suprahepatic segment, is very short; it crosses the diaphragm through the caval opening or foramen in the central tendon at approximately the level of T8. Branches of the phrenic nerve that are separated by fibrous pericardium accompany it.7 The drainage of the IVC into the right atrium is considered to be a reliable marker for determination of the atrial situs because the suprahepatic IVC almost never drains into a different cavity.8 The normal embryologic precursor to the suprahepatic IVC is the right supracardinal vein.
Variant Anatomy
Developmental anomalies of the IVC are rare, occurring in fewer than 1% of those with congenital heart disease.9 The embryogenesis of the IVC involves formation of several anastomoses of three paired embryonic veins. Variations in this development result in several important anomalous drainage patterns. The most common variation is absence of the suprahepatic IVC with azygos continuation of the IVC. It results from the involution of a segment of the supracardinal vein, leading to drainage of the infrahepatic IVC into the azygos vein; the hepatic veins continue to drain directly into the right atrium.8 The prevalence is 0.6%.10 An enlarged azygos vein will then join the SVC at the normal location in the right paratracheal space. Imaging findings include absence of the suprahepatic segment on the lateral view of a chest radiograph and a mass in the right paratracheal space on the anteroposterior view, in the expected region of the azygos vein. Cross-sectional imaging delineates the dilated azygos ascending along its normal course to drain into the SVC (Fig. 75-6).
VEINS THAT DRAIN THE UPPER EXTREMITIES AND HEAD INTO THE CHEST
General Anatomic Description
The venous drainage of the head and neck is provided primarily by a paired triple jugular system composed of both deep and superficial vessels: the internal, external, and anterior jugular veins.11 The jugular veins converge with the subclavian veins to form the brachiocephalic veins, also referred to as the innominate veins.
The subclavian veins drain the upper extremities, and after receiving the jugular drainage, they continue to the SVC as the brachiocephalic veins. The main tributaries of the brachiocephalic vein include the vertebral, internal mammary, and inferior thyroid veins, in that order from proximal to distal.7 These are discussed in subsequent sections. The right brachiocephalic vein is the shortest of the two and has an almost vertical course until it forms the SVC at its junction with the left brachiocephalic, which is longer and crosses midline, passing behind the sternal manubrium and anterior to the aortic branches.
Variant anatomy of the brachiocephalic veins is rare and is reported in 0.2% to 1% of all cardiac anomalies.12 These predominantly involve coursing of the left brachiocephalic vein under the aortic arch, superior to the pulmonary artery, and are associated with other congenital heart anomalies, most commonly tetralogy of Fallot.12 Cross-sectional imaging can clearly differentiate this from a persistent left-sided SVC or vertical vein and can be important in presurgical planning for congenital heart disease.
VEINS THAT DRAIN THE CHEST WALL
General Anatomic Description
The principal veins on the internal surface of the thorax are the internal thoracic veins, which ascend lateral to the sternum and medial to the internal thoracic (mammary) arteries. They mainly receive blood from the anterior intercostal veins, and they usually vary from one to two on each side. At the approximate level of the sternomanubrial joint, the thoracic veins will join into a common trunk that joins the right and left brachiocephalic veins.13 It is estimated that approximately half of the population will have two veins on each side of the sternum, 10% will have a single vein on each side, and the remaining population has a combination of one and two on either side.13 The internal thoracic veins run medial to the internal thoracic arteries.
The posterior veins will drain to different systems, depending on their level; the lower eight drain to the azygos system on the right and the accessory hemiazygos and hemiazygos veins on the left. The first posterior intercostal veins drain into the respective right and left brachiocephalic vein. The second, third, and sometimes fourth intercostal veins will drain into the ipsilateral brachiocephalic vein through one of its tributaries, the superior intercostal vein.7 The left superior intercostal will form an arch on the left side of the mediastinum that is analogous to the azygos arch on the right, sometimes called the hemiazygos arch, which most of the time is diminutive (Fig. 75-7). About 75% of the time, this vein will connect to the accessory hemiazygos vein.14 This is the so-called aortic nipple sometimes seen on the frontal chest radiograph.15
THE AZYGOS AND HEMIAZYGOS SYSTEMS
General Anatomic Description
The azygos system is a paired paravertebral system. Interestingly, the azygos and hemiazygos veins are the only large veins in the thoracic cavity with valves.16
Embryologically, the azygos vein is formed from the right posterior cardinal vein inferiorly and the right supracardinal vein superiorly. It originates as the confluence of the lumbar venous plexus in the abdomen and extends cephalad, entering the thorax through the aortic hiatus or behind the lateral aspect of the right diaphragmatic crus. It continues to the right of the spine, and at the approximate level of T4, it creates the azygos arch where it joins the SVC (Fig. 75-8). This can sometimes be seen with aberrant SVC catheter positioning, with the tip directed over the SVC on the anteroposterior view and directed posteriorly on the lateral radiograph, indicating its position within the azygos vein (Fig. 75-9).
On the left, the hemiazygos and accessory hemiazygos veins are embryologically derived from the left supracardinal vein. Similar to the azygos on the right, the perilumbar veins usually form the hemiazygos vein. The hemiazygos vein ascends to the left of the spine until it reaches the level of T8, where it crosses over the midline to join the azygos vein (Fig. 75-10). From then on, the continuation of the venous system on the left is called the accessory hemiazygos vein, which has a variable amount of branches communicating with the azygos, hemiazygos, or left brachiocephalic vein.17
Normal Variants
The azygos lobe, a normal variation, is present in 0.4% to 1.0% of the population. When present, it may alter the shape of the SVC and the location of the azygos arch.18 This anomaly is formed by the indentation of the right upper lobe by an invagination of the azygos arch, which brings with it four layers of pleura (two layers of visceral pleura and two layers of parietal pleura). On plain film examinations of the chest, an azygos lobe is recognized as a curvilinear density with a distal teardrop shadow arising from the upper right mediastinum, coursing through the medial aspect of the apex of the lung. On cross-sectional examinations, an azygos fissure can be seen as a curved tubular vascular structure at the right upper thorax separated from the mediastinum by interposed lung parenchyma (Fig. 75-11). Embryologically, formation of an azygos lobe results from failure of the posterior cardinal vein to migrate over the apex of the lung, and the vein courses through lung parenchyma.
THE PULMONARY VEINS
General Anatomic Description
During embryonic development, two pulmonary veins from each lung join to form a common pulmonary vein. These common veins unite with a pulmonary bud from the primitive left atrium, which results, in most people, in four individual pulmonary veins, two for each lung (expected to occur in approximately 66% to 70% of the population). However, significant variations in the embryologic process can lead to variations in the number, site, and branching pattern of pulmonary veins. If there is underincorporation of the pulmonary veins into the left atrium, a common pulmonary vein will result. On the other hand, overincorporation of the common pulmonary vein into the dorsal left atrium results in supernumerary pulmonary veins.19 Most of the adult left atrium is also derived from this primitive common pulmonary vein, which becomes incorporated into the left atrial wall as it expands in size.7,20
Approximately 70% of patients have two separate ostia on the right side for upper and lower lobe pulmonary veins. The majority of the remaining individuals will have three to five pulmonary vein ostia; less than 2% have a common ostium on the right. On the left, it is expected that approximately 85% of patients will have two ostia, for the upper and lower lobe veins; virtually all the remainder have a single common ostium as a less common variant (Fig. 75-12).21 When present, accessory veins are named for the pulmonary lobe or segment that they drain. The superior pulmonary veins are found in front of the pulmonary arteries. In the left lung root, the superior pulmonary vein is in front of the left main bronchus, and the inferior pulmonary vein is below it. In the right lung root, the pulmonary veins are similarly distributed above and below the right bronchus.7
The pulmonary veins do not closely follow the bronchi, and they run in intersegmental septa (Fig. 75-13). These veins drain the lungs and enter the left atrium from superior and inferior to the oblique fissure on each side. The portion of the left atrium defined by the boundaries of the pulmonary veins is the anterior wall of the oblique pericardial sinus, which is separated from the esophagus by the fibrous pericardium.7
Detailed Description of Specific Areas
Normal Variants
Anomalous pulmonary venous anatomy occurs when pulmonary veins drain into a structure other than the left atrium. Partial anomalous pulmonary venous return occurs when at least one of the pulmonary veins drains into the left atrium.20 If no pulmonary veins drain into the left atrium, the resulting condition is total anomalous pulmonary venous return (TAPVR), also known as total anomalous pulmonary venous connection.
Premature atresia of the right or left portion of the primordial pulmonary vein while primitive pulmonary-systemic connections are still present results in a partial anomalous pulmonary venous connection.22 This anomalous pulmonary venous connection creates a system whereby oxygenated blood is returned to the right atrium or its tributaries instead of to the left atrium, creating a left-to-right shunt.23–25 The described prevalence of a partial anomalous pulmonary venous connection is 0.4% to 0.7%, with 90% of them being right sided (Fig. 75-14).26–28 Between 80% and 90% of patients with this condition will have an associated atrial septal defect of either the sinus venosus or secundum type.22,29
TAPVR occurs when all the pulmonary veins fail to connect to the left atrium and instead connect to a systemic vein or veins. The location of this connection is used to categorize this anomaly, and there are four types: above the heart (supracardiac, type I), below the heart (infracardiac, type II; Fig. 75-15), to the right atrium or coronary sinus (cardiac, type III), or some combination of these types (mixed, type IV). Because the anomalous pulmonary venous return results in a left-to-right shunt, with mixing of the systemic venous and pulmonary venous blood, there is resultant cyanosis, necessitating a patent foramen ovale or ductus arteriosus for compatibility with life. Accurate delineation of the different subtypes is crucial in determining the appropriate surgical management.
The incidence of TAPVR is 0.008% in the general population and 2.2% in patients with congenital heart disease.30 The supracardiac form is the most common, representing 45%.31 Most commonly, with the supracardiac type of TAPVR, the pulmonary veins empty into the vertical vein, which empties into the left brachiocephalic vein and then into the SVC. The infracardiac form occurs in approximately 26% of patients with TAPVR.31 Because pulmonary venous obstruction is virtually invariably seen with this form, infracardiac TAPVR is the most severe form, presenting with cyanosis in the first day of life. In this form, the pulmonary veins empty into the portal vein, IVC, or hepatic veins. The cardiac form of TAPVR represents 13%, with the pulmonary veins emptying into the right atrium or coronary sinus. The mixed type is the rarest, representing 5% of this anomaly.31
1 Gray H Anatomy of the Human Body. 1918. Lea & Febiger. Philadelphia. Bartleby.com.
2 Mahlon M, Yoon H. CT angiography of the superior vena cava: normative values and implications for central venous catheter position. J Vasc Interv Radiol. 2007;18:1106-1110.
3 Minniti S, Visentini S, Procacci C. Congenital anomalies of the venae cavae: embryological origin, imaging features and report of three new variants. Eur Radiol. 2002;12:2040-2055.
4 Cha EM, Khoury GH. Persistent left superior vena cava: radiologic and clinical significance. Radiology. 1972;103:375-381.
5 Demos TC, Posniak HV, Pierce KL, et al. Venous anomalies of the thorax. AJR Am J Roentgenol. 2004;182:1139-1150.
6 Bartram U, Van Praagh S, Levine JC, et al. Absent right superior vena cava in visceroatrial situs solitus. Am J Cardiol. 1997;80:175-183.
7 Lawler L, Fishman EK. Thoracic venous anatomy multidetector row CT evaluation. Radiol Clin North Am. 2003;41:545-560.
8 Kirsch J, Araoz PA, Breen JF, Chareonthaitawee P. Isolated total anomalous connection of the hepatic veins to the left atrium. J Cardiovasc Comput Tomogr. 2007;1:55-57.
9 Peterson RW. Infrahepatic interruption of the inferior vena cava with azygos continuation (persistent right cardinal vein). Radiology. 1965;84:304-307.
10 Bass JE, Redwine MD, Kramer LA, et al. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000;20:639-652.
11 Schummer W, Schummer C, Bredle D, Fröber R. The anterior jugular venous system: variability and clinical impact. Anesth Analg. 2004;99:1625-1629.
12 Chen SJ, Liu KL, Chen HY, et al. Anomalous brachiocephalic vein: CT, embryology, and clinical implications. AJR Am J Roentgenol. 2005;184:1235-1240.
13 Loukas M, Tobola MS, Tubbs RS, et al. The clinical anatomy of the internal thoracic veins. Folia Morphol (Warsz). 2007;66:25-32.
14 Godwin JD, Chen JT. Thoracic venous anatomy. AJR Am J Roentgenol. 1986;147:674-684.
15 Ball JBJr, Proto AV. The variable appearance of the left superior intercostal vein. Radiology. 1982;144:445-452.
16 Yeh BM, Coakley FV, Sanchez HC, et al. Azygos arch valves: prevalence and appearance at contrast-enhanced CT. Radiology. 2004;230:111-115.
17 Dudiak CM, Olson MC, Posniak HV. CT evaluation of congenital and acquired abnormalities of the azygos system. Radiographics. 1991;11:233-246.
18 Smathers RL, Buschi AJ, Pope TLJr, et al. The azygous arch: normal and pathologic CT appearance. AJR Am J Roentgenol. 1982;139:477-483.
19 Konin GP, Jain VR, Fisher JD, Haramati LB. The ambiguous pulmonary venoatrial junction: a new perspective (review). Int J Cardiovasc Imaging. 2008;24:433-443. Epub 2007 Oct 2
20 Lacomis JM, Wigginton W, Fuhrman C, et al. Multi-detector row CT of the left atrium and pulmonary veins before radio-frequency catheter ablation for atrial fibrillation. Radiographics. 2003;23(Spec No):S35-S48.
21 Marom EM, Herndon JE, Kim YH, et al. D variations in pulmonary venous drainage to the left atrium: implications for radiofrequency ablation. Radiology. 2004;230:824-829.
22 Senocak F, Ozme S, Bilgic A, et al. Partial anomalous pulmonary venous return. Evaluation of 51 cases. Jpn Heart J. 1994;35:43-50.
23 Herlong JR, Jaggers JJ, Ungerleider RM. Congenital Heart Surgery Nomenclature and Database Project: pulmonary venous anomalies. Ann Thorac Surg. 2000;69(Suppl):S56-S69.
24 Spindola-Franco H, Fish BG. Radiology of the heart. In: Radiology of the Heart: Cardiac Imaging in Infants, Children, and Adults. New York: Springer-Verlag; 1985:317-322.
25 White CS, Baffa JM, Haney PJ, et al. MR imaging of congenital anomalies of the thoracic veins. Radiographics. 1997;17:595-608.
26 Brody H. Drainage of the pulmonary veins into the right side of the heart. Arch Pathol. 1942;33:221-240.
27 Healy JE. Anatomic survey of anomalous pulmonary veins: their clinical significance. J Thorac Surg. 1952;23:433-443.
28 Van Meter CJr, LeBlanc JG, Culpepper WSIII, et al. Partial anomalous pulmonary venous return. Circulation. 1990;82:IV195-IV198.
29 Toyoshima M, Sato A, Fukumoto Y, et al. Partial anomalous pulmonary venous return showing anomalous venous return to the azygos vein. Intern Med. 1992;31:1112-1116.
30 Ferencz C, Rubin JD, McCarter RJ, et al. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol. 1985;121:31-36.
31 Delisle G, Ando M, Calder AL, et al. Total anomalous pulmonary venous connection: report of 93 autopsied cases with emphasis on diagnostic and surgical considerations. Am Heart J. 1976;91:99-122.

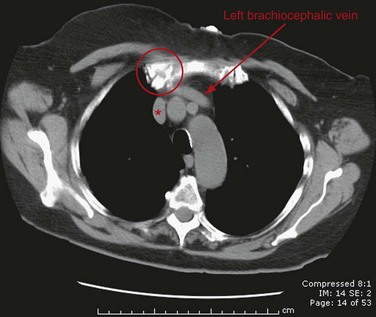
 FIGURE 75-1
FIGURE 75-1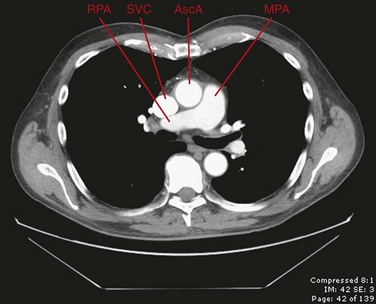
 FIGURE 75-2
FIGURE 75-2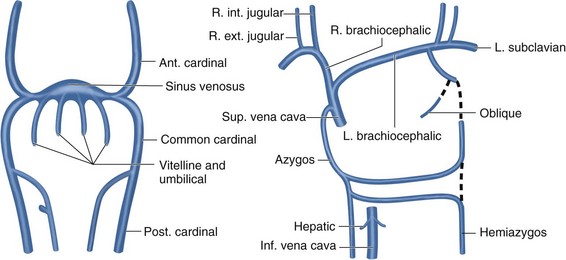
 FIGURE 75-3
FIGURE 75-3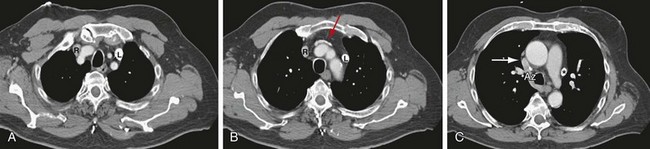
 FIGURE 75-4
FIGURE 75-4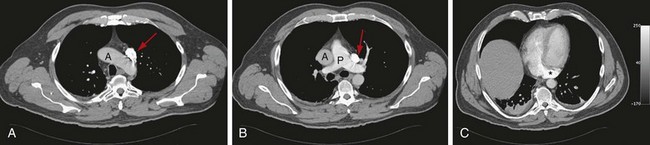
 FIGURE 75-5
FIGURE 75-5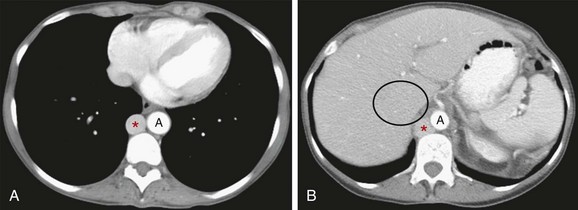
 FIGURE 75-6
FIGURE 75-6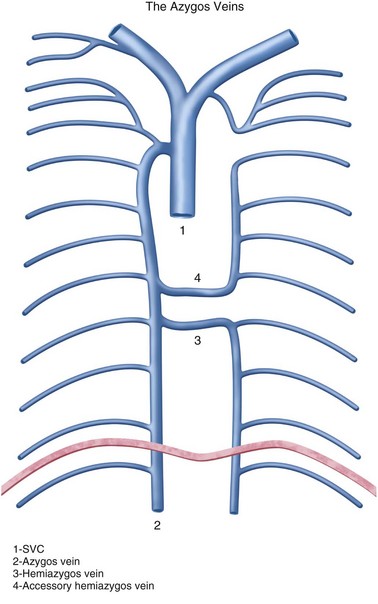
 FIGURE 75-7
FIGURE 75-7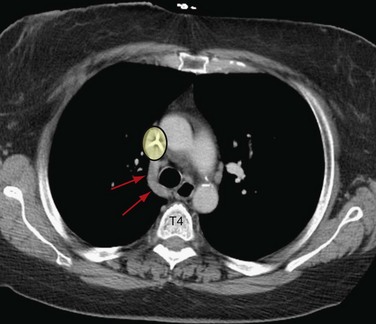
 FIGURE 75-8
FIGURE 75-8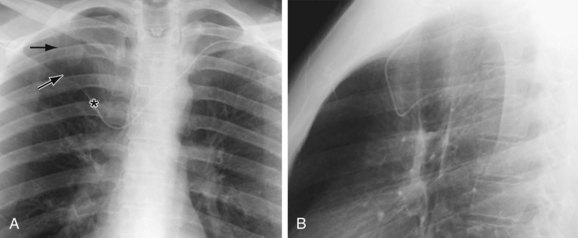
 FIGURE 75-9
FIGURE 75-9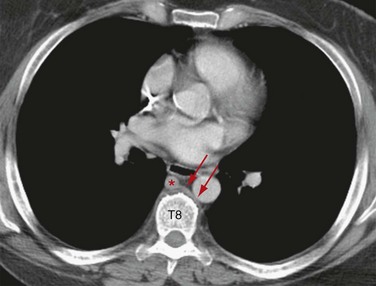
 FIGURE 75-10
FIGURE 75-10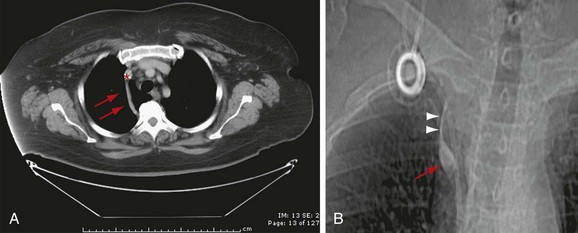
 FIGURE 75-11
FIGURE 75-11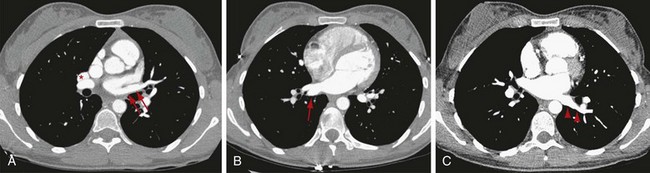
 FIGURE 75-12
FIGURE 75-12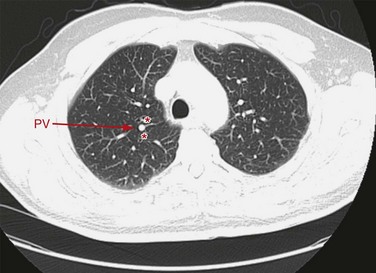
 FIGURE 75-13
FIGURE 75-13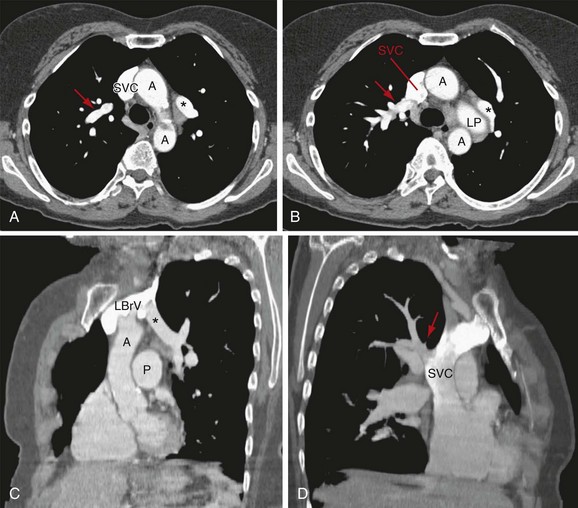
 FIGURE 75-14
FIGURE 75-14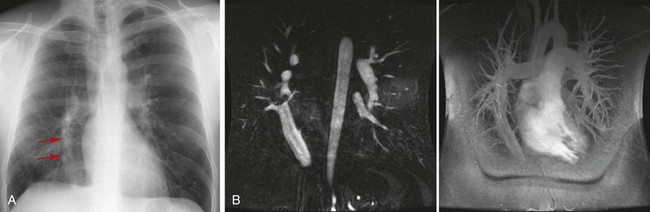
 FIGURE 75-15
FIGURE 75-15
