CHAPTER 76 Venous Anatomy of the Abdomen and Pelvis
ABDOMEN: PORTAL VENOUS CIRCULATION
General Anatomic Description
Detailed Description of Specific Areas
Portal Vein
The portal vein (PV) is approximately 7 to 8 cm long in adults and is formed by the union of the splenic vein and superior mesenteric vein at the level of the second lumbar vertebra.1 It lies posterior to the pancreatic head and anterior to the IVC (Figs. 76-1 to 76-3). The PV enters the liver at the porta hepatis, where it runs posterior and medial to the bile duct and posterior and lateral to the hepatic artery. At the porta hepatis, the PV divides into the right and left PVs. Of note, the adult PV and its tributaries are devoid of valves. However, in the fetus and for a brief postpartum period, valves can be found in portal tributaries.
Superior Mesenteric Vein
The superior mesenteric vein (SMV) drains the small intestine, cecum, appendix, and the ascending and transverse portions of the colon (see Fig. 76-1). It courses posterior to the head of the pancreas and horizontal segment of the duodenum, and lies anterior to the IVC. The SMV unites with the splenic vein to form the main PV.
Splenic Vein
The splenic vein (lienal vein) is the second largest tributary of the PV and measures about 1 cm in diameter. The main splenic vein is formed by the union of two (76%), three (20%), or four (4%) inflow veins.2
Normal Variants
In a study by Koc and colleagues, a total of 318 branching variants and anomalies of the portal venous system were observed in 307 (27.4%) of 1120 patients3; 72.6% of patients demonstrated conventional anatomy. The most frequent variation was trifurcation of the PV, detected in 12.4% of patients. In these individuals, the PV divided into a left portal branch, right anterior portal branch, and right posterior portal branch. The next most common variant was a right posterior portal branch as the first branch of the main portal vein, detected in 9.2% of cases. In 3.6% of patients, there were variants of PV origin in segments IV and VIII of the liver.
Another variant of the PV seen in very rare cases is infracardiac total anomalous pulmonary venous return (TAPVR), in which the pulmonary veins drain into the PV. The incidence of TAPVR is approximately 2% of all congenital heart defects and, in 15% of cases, the pulmonary veins drain into the portal venous system (type III).3,4
In most cases, the SMV is formed by its chief tributaries. However, in almost 13% of cases, the main trunk of the SMV is absent and large right and large left mesenteric branches join the splenic vein to form the portal vein.3,4
The inferior mesenteric vein demonstrates considerable anatomic variability. In a study by Graf, the inferior mesenteric vein drained into the splenic vein in 56% of patients, but in 18% it drained into the splenoportal angle, and in the remaining 26% drainage was observed into the superior mesenteric vein.5
Differential Considerations
Patients with portal hypertension may be asymptomatic, but most manifest with variceal bleeding, ascites, splenomegaly, or encephalopathy. In variceal bleeding, 85% of cases result from variceal hemorrhage at the gastroesophageal junction. Bleeding from gastric and esophageal varices account for 17% of cases of acute massive upper gastrointestinal hemorrhage.6
Treatment options for portal hypertension include medical management, surgical devascularization with portosystemic shunt formation, transjugular intrahepatic portosystemic shunt (TIPS), and liver transplantation.7
ABDOMEN: SYSTEMIC VENOUS CIRCULATION
Detailed Description of Specific Areas
The IVC is formed by the union of the common iliac veins at the level of the fifth lumbar vertebra (Fig. 76-5).1 It ascends to the right of the abdominal midline, anterior to the vertebral column, in the retroperitoneal space and traverses the tendinous portion of the diaphragm at the level of the eighth thoracic vertebra, draining into the right atrium. A semilunar valve is present at its atrial orifice (valve of the inferior vena cava), which in fetal life serves to direct blood through the foramen ovale into the left atrium, which becomes rudimentary in the adult.
Inflow to the Inferior Vena Cava
Inferior vena caval inflow comes from the lumbar, renal, right inferior phrenic, right testicular or ovarian, right suprarenal, and hepatic veins (Fig. 76-6).
Lumbar Veins
Four paired lumbar veins drain the lumbar musculature and vertebral plexus into the IVC.2 The lumbar veins are interconnected via longitudinal veins called the ascending lumbar veins, which allow for communication between the common iliac and iliolumbar veins. The ascending lumbar veins join the subcostal veins and form the azygos vein on the right and the hemiazygos vein on the left.
Normal Variants
In a large study by Koc and associates, 58.2% of patients demonstrated conventional hepatic vein anatomy.3 The most common hepatic vein variant (prevalence, 69.5% to 86%) is the presence of an inferior right hepatic vein (IRHV), which usually drains segment VI. Knowledge of the number of IRHVs and the distance from each IRHV to the IVC is important for surgical planning prior to liver transplantation. In cases of rare multiple IRHVs, some of these veins drain into the adrenal vein before joining the IVC (prevalence, 0.2%). Another common variant found in 65% to 85% of individuals is a common trunk for the middle and left hepatic veins proximal to their insertion into the IVC.
The presence of multiple renal veins is the most common renal vein variation, with a prevalence of 9% to 30%. A circumaortic renal vein is the most common variation of the left renal vein, with a prevalence of 8.7%. Less commonly, a retroaortic left renal vein is noted, with a prevalence of 2.1%.3
In studies of IVC development in the domestic cat, variations of vena caval anatomy were classified based on abnormal regression or persistence of the various embryonic veins.8 In total, 14 theoretical variations were suggested. We will discuss the most common variations of the IVC.
In 0.2% to 0.3% of the population, a double IVC forms when both supracardinal veins persist. The two vessels often demonstrate marked size discrepancy. This anomaly should be suspected in the setting of recurrent pulmonary embolism following single IVC filter placement. In most cases, the left-sided IVC joins the left renal vein, which drains in a conventional fashion into the right-sided IVC. In 0.6% of individuals, there is azygos continuation of the IVC, with absence of the hepatic segments of the IVC.8
Differential Considerations
Budd-Chiari Syndrome
First discussed in 1845 by George Budd and then further detailed by Hans Chiari in 1899, the Budd-Chiari syndrome (BCS) is marked by obstruction of the hepatic venous outflow system. Hepatic venous obstruction can occur at any level from the small hepatic veins to the caval-atrial junction. Although BCS is rare, it can be a life-threatening condition that can present relatively acutely or develop gradually. Causes of BCS can be primary (e.g., congenital membranous webs), or secondary (e.g., polycythemia vera, paroxysmal nocturnal hemoglobinuria, hypercoagulable states, oral contraceptive–induced, or pregnancy).9 In approximately two thirds of cases, no cause can be established.
Diagnosis may be achieved more invasively through hepatic venography or hepatic biopsy. Other noninvasive modalities such as magnetic resonance imaging (MRI), ultrasound, and CT serve as excellent diagnostic tools.10–12 With CT and MRI, signs of BCS include ascites, hepatomegaly, nonvisualization of the hepatic veins, and prominent enhancement of the central liver, with enhancement of the periphery only on delayed images. The caudate lobe is usually spared because it drains separately into the inferior vena cava.
Multiple intrahepatic and extrahepatic venous collateral pathways exist in the setting of BCS.13,14 The intrahepatic collateral veins serve to divert blood away from the occluded hepatic veins, draining into a patent hepatic or systemic vein. These veins usually are curvilinear and tortuous, often resembling a comma—hence, the “comma sign” of BCS (Fig. 76-7).
Nutcracker Syndrome
First described in 1950, the nutcracker syndrome results from compression of the left renal vein between the aorta and the superior mesenteric artery (SMA).15
Typically, the space in which the left renal vein courses anterior to the aorta and deep to the SMA is 4 to 5 mm and is maintained by retroperitoneal fat and the third segment of the duodenum. The pathophysiology of this syndrome is not completely understood; however, if the aortomesenteric angle is narrow (perhaps because of abnormal SMA branching), left renal vein compression occurs (Fig. 76-8). Increased left renal vein pressures and surrounding venous collateral formation may be appreciated.16 The syndrome typically occurs in young, previously healthy patients. The most common clinical presentation is hematuria in the absence of renal pathology and presence of gonadal varices. Hematuria may be caused by rupture of congested renal vein tributaries into the renal collecting system. Diagnostic modalities include CT, ultrasound, and venography, with pressure gradient measurements obtained between the left renal vein and IVC. Treatment options include surgical intervention or minimally invasive intravascular stent placement.17
PELVIS: SYSTEMIC VENOUS CIRCULATION
Detailed Description of Specific Areas
Common Iliac Veins
The common iliac veins are formed by the union of the internal (hypogastric) and external iliac veins anterior to the sacroiliac joint.1,2 Typically, the right common iliac vein is shorter than the left and is positioned posteriorly and laterally to the corresponding artery. The left common iliac vein is positioned at first on the medial aspect of the corresponding artery but then courses posterior to the artery, and can be compressed between the spine and the artery (see later, “May-Thurner Syndrome”).
External Iliac Veins
The external iliac vein is the continuation of the common femoral vein at the level of the inguinal ligament. On the right, it is first medial and then posterior to the corresponding artery. On the left, the vein runs medial to the corresponding artery (Fig. 76-9). The tributaries of each external iliac vein include the inferior epigastric, deep circumflex iliac, and pubic veins.
Internal Iliac Veins
Group 1: Venous Origin Outside the Bony Pelvis
The superior gluteal veins receive tributaries from the buttock and enter the pelvis through the greater sciatic foramen. The inferior gluteal veins drain the upper portion of the posterior thigh and enter the pelvis through the greater sciatic foramen. The internal pudendal veins begin in the deep veins of the penis and prostatic venous plexus in the male and the uterovaginal plexus in the female (Figs. 76-10 and 76-11). These veins course alongside the corresponding artery to form a single vein draining into the internal iliac vein. The internal pudendal veins also receive tributaries from the urethral bulb, perineal, and inferior rectal veins.
Group 3: Venous Origin in Visceral Plexuses
The rectal venous plexus is positioned around the rectum and communicates with the vesical plexus (in males) and the uterovaginal plexus (in females; Fig. 76-10). The plexus consists of an internal submucosal portion and external muscular portion. At the anal canal, the plexus is composed of veins with longitudinal orientation, which are prone to become varicose, resulting in internal hemorrhoids. The internal portion of the plexus drains into the superior rectal vein and also communicates with the external portion.
Normal Variants
Developmental anomalies of the IVC may result in variability in pelvic venous outflow, although relatively rarely. On occasion, the left common iliac vein has been seen to ascend into the abdomen, receive the left renal vein, and subsequently cross anterior to the aorta to join the inferior vena cava.3
Differential Considerations
May-Thurner Syndrome
May-Thurner syndrome (also known as iliac vein compression syndrome, Cockett syndrome, iliocaval compression syndrome, or pelvic venous spur) is characterized by obstruction of the left common iliac vein related to compression by the overlying right common iliac artery.1 The mechanism of obstruction is caused by the physical entrapment of the vein between the artery and the spine or by extensive intimal hypertrophy of the vein resulting from the chronic pulsatile force of the anteriorly situated common iliac artery.
Following examination of 430 cadavers in 1957, May and Thurner described iliac compression syndrome and documented decreased venous flow resulting from intimal changes.18 They theorized that the intimal changes resulted from continual compression of the left common iliac vein by the right common iliac artery. The transmitted arterial pulsation may cause opposing venous walls to contact, resulting in endothelial irritation and subsequent proliferation. This may explain the formation of intraluminal webs or spurs within the iliac vein, sometimes visualized using intravascular ultrasound. Sequelae of venous compression include reduction of venous outflow and deep vein thrombosis of the left iliofemoral system (Fig. 76-13). Other symptoms include leg swelling, varicosities, chronic venous stasis ulcers, and symptomatic pulmonary emboli.
Pelvic Congestion Syndrome
PCS relates to ovarian and pelvic vein dilation, resulting in blood pooling and pain in the region of the uterus, ovaries, and vulva (Fig. 76-14). This condition is analogous to varicoceles in men.19 PCS is prevalent in women younger than 45 years of age (child-bearing age). The syndrome is uncommon in nulliparous women. It has been estimated that 15% of women between the ages of 20 and 50 years have pelvic varicose veins, although not all experience noncyclic pain. PCS has also been associated with polycystic ovaries and hormonal dysfunction. Symptoms of PCS include dull pain in the lower abdomen and lower back that increases on standing or with pregnancy, dyspareunia, dysmenorrhea, vaginal discharge, irritable bladder, and varicose veins involving the legs.
The diagnosis of PCS is challenging because other causes of CPP must be excluded. Useful modalities for the diagnosis of PCS include pelvic venography, MRI, ultrasound, and CT.20 On ultrasound, the ovarian and pelvic varicoceles are dilated and often tortuous, with surrounding dilated pelvic venous plexuses demonstrating reversed (caudal) flow within the ovarian vein. CT may demonstrate varicose veins surrounding the uterus and ovaries, with retrograde filling of dilated ovarian veins from the left renal vein in the arterial phase. Of note, dilated pelvic veins larger than 5 mm in diameter is consistent with varices and larger than 8 mm in diameter is suggestive of PCS, given the appropriate clinical symptomatology.
Numerous treatment options are available for PCS.21 Medical management with analgesics and hormones can be effective, but does not cure the underlying cause of pain. Surgical options including hysterectomy and bilateral salpingo-oophorectomy have been performed. However, minimally invasive options include endovascular embolization of the varicose ovarian veins using metallic coils (Fig. 76-15). This procedure is reportedly successful in terms of symptom relief in approximately 85% to 95% of patients.
Pertinent Imaging Considerations
Cross-sectional portal venous imaging is commonly performed during multiphase CT or MR abdominal studies. The optimal portal venous scan delay from the time of peripheral venous contrast injection is routinely calculated using a timing bolus, in which peak aortic enhancement is determined. When additional scanning parameters such as contrast injection rates are taken into consideration, the results are often spectacular, with minimal contamination from nonportal vascular opacification (Fig. 76-16). CT portography combining catheter angiography with direct superior mesenteric arterial injections has emerged as a superior means to evaluate the portal venous system. In particular, when there is clinical concern for PV thrombosis or portal hypertension, CT portography may be invaluable for surgical planning.
Gallego C, Velasco M, Marcuello P, et al. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002;22:141-159.
Kang HK, Jeong YY, Choi JH, et al. Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics. 2002;22:1053-1061.
Koc Z, Ulusana S, Oguzkurta L, Tokmaka N. Venous variants and anomalies on routine abdominal multi-detector row CT. Eur J Radiol. 2007;61:267-278.
Minniti S, Visentini S, Procacci C. Congenital anomalies of the venae cavae: embryological origin, imaging features and report of three new variants. Eur Radiol. 2002;12:2040-2055.
1 LaBerge J, Gordon RL, Kerlan RKJr, Wilson MW. Interventional Radiology Essentials. Philadelphia: Lippincott Williams & Wilkins; 2000.
2 Uflacker R. Atlas of Vascular Anatomy: An Angiographic Approach. Philadelphia: Lippincott Williams & Wilkins; 1997.
3 Koc Z, Ulusan S, Oguzkurt L, Tokmak N. Venous variants and anomalies on routine abdominal multi-detector row CT. Eur J Radiol. 2007;61:267-278.
4 Bharati S, Lev M. Congenital anomalies of the pulmonary veins. Cardiovasc Clin. 1973;5:23-41.
5 Graf O, Boland GW, Kaufman JA, et al. Anatomic variants of mesenteric veins: Depiction with helical CT venography. AJR Am J Roentgenol. 1997;168:1209-1213.
6 Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-1654.
7 Thompson ABR, Shaffer EA. First Principles of Gastroenterology: The Basis of Disease and an Approach to Management. Mississauga, Ontario, Canada: Astra Pharma; 1994.
8 Bass JE, Redwine MD, Kramer LA, et al. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000;20:639-652.
9 Fitzgerald O, Fitzgerald P, Cantwell D, Mehigan JA. Diagnosis and treatment of the Budd-Chiari syndrome in polycythaemia vera. Br Med J. 1956;2:1343-1345.
10 Vogelzang RL, Anschuetz SL, Gore RM. Budd-Chiari syndrome: CT observations. Radiology. 1987;163:329-333.
11 Erden A, Erden I, Karayalçin S, Yurdaydin C. Budd-Chiari syndrome: evaluation with multiphase contrast-enhanced three-dimensional MR angiography. AJR Am J Roentgenol. 2002;179:1287-1292.
12 Mathieu D, Vasile N, Menu Y, et al. Budd-Chiari syndrome: dynamic CT. Radiology. 1987;165:409-413.
13 Cho O-K, Koo J-H, Kim Y-S, et al. Collateral pathways in Budd-Chiari syndrome: CT and venographic correlation. AJR Am J Roentgenol. 1996;167:1163-1167.
14 Takayasu K, Mizuguchi Y, Muramatsu Y, et al. Neovasculature of benign thrombus of the inferior vena cava demonstrated by computed tomography during hepatic arteriography, mimicking a small hepatocellular carcinoma. Jpn J Clin Oncol. 2003;33:44-46.
15 Takebayashi S, Ueki T, Ikeda N, Fujikawa A. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol. 1999;172:39-43.
16 Kim SH, Cho SW, Kim HD, et al. Nutcracker syndrome: diagnosis with Doppler US. Radiology. 1996;198:93-97.
17 Park YB, Lim SH, Ahn JH, et al. Nutcracker syndrome: intravascular stenting approach. Nephrol Dial Transplant. 2000;15:99-101.
18 Cil BE, Akpinar E, Karcaaltincaba M, Akinci D. Case 76: May-Thurner syndrome. Radiology. 2004;233:361-365.
19 Koc Z, Ulusan S, Tokmak N, et al. Double retroaortic left renal veins as a possible cause of pelvic congestion syndrome: imaging findings in two patients. Br J Radiol. 2006;79:152-155.
20 Park SJ, Lim JW, Ko YT, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182:683-688.
21 Ganeshan A, Upponi S, Hon LQ, et al. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol. 2007;30:1105-1111.

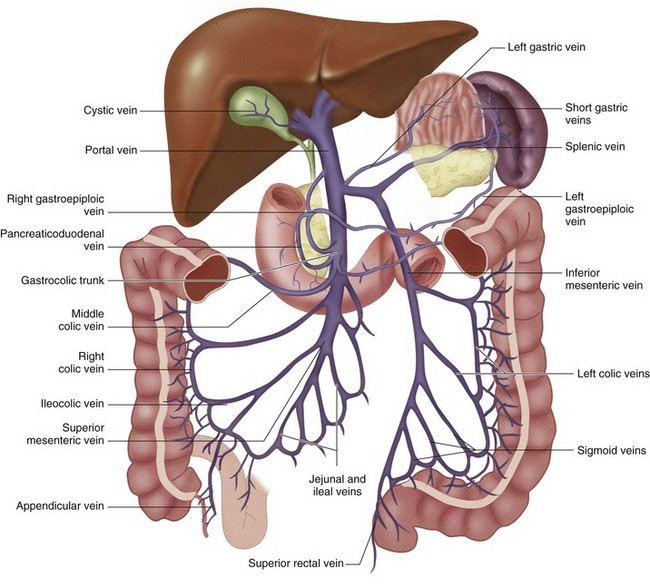
 FIGURE 76-1
FIGURE 76-1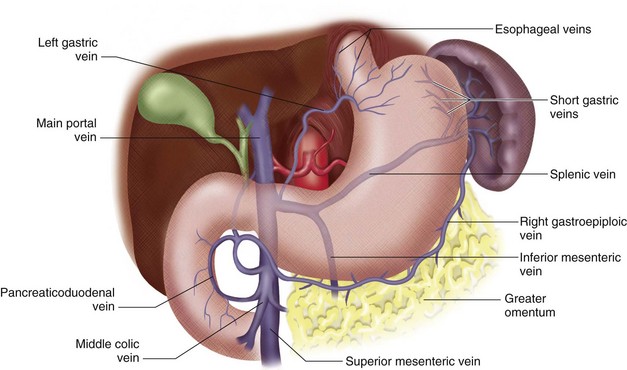
 FIGURE 76-2
FIGURE 76-2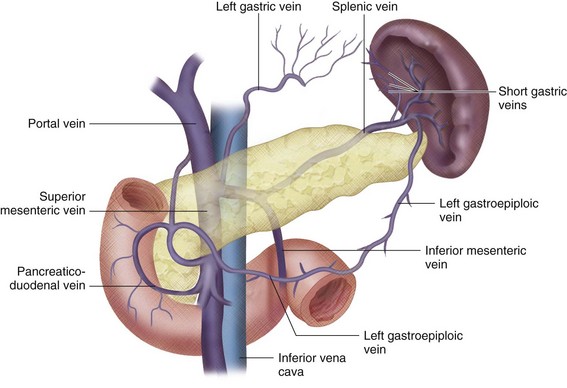
 FIGURE 76-3
FIGURE 76-3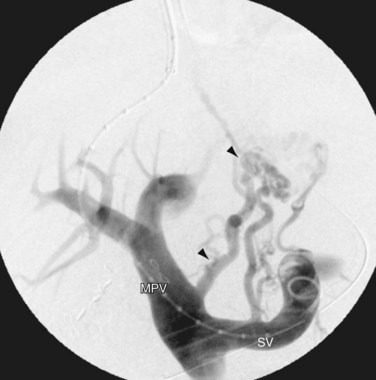
 FIGURE 76-4
FIGURE 76-4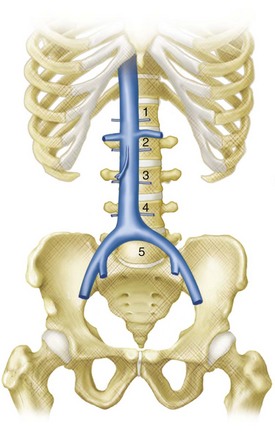
 FIGURE 76-5
FIGURE 76-5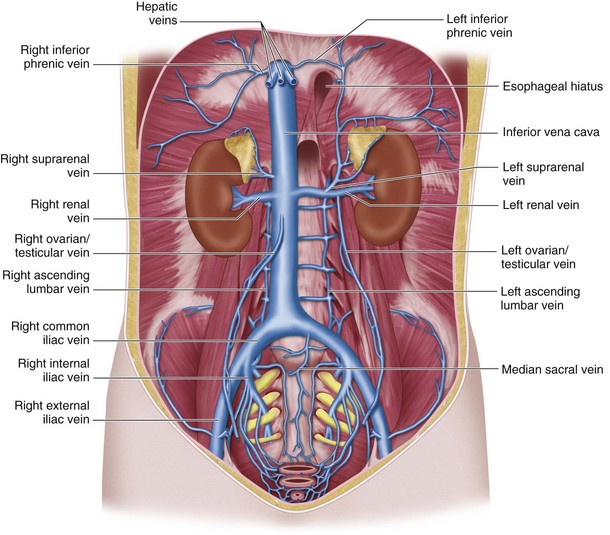
 FIGURE 76-6
FIGURE 76-6
 FIGURE 76-7
FIGURE 76-7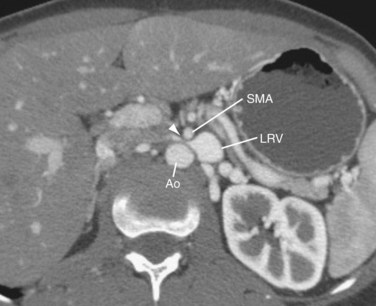
 FIGURE 76-8
FIGURE 76-8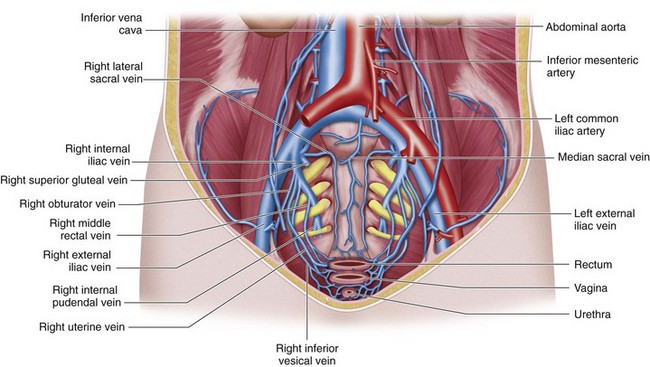
 FIGURE 76-9
FIGURE 76-9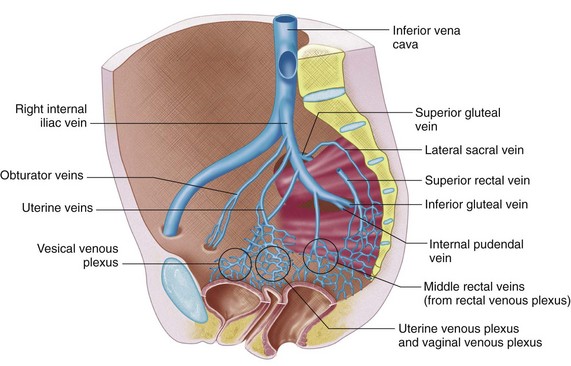
 FIGURE 76-10
FIGURE 76-10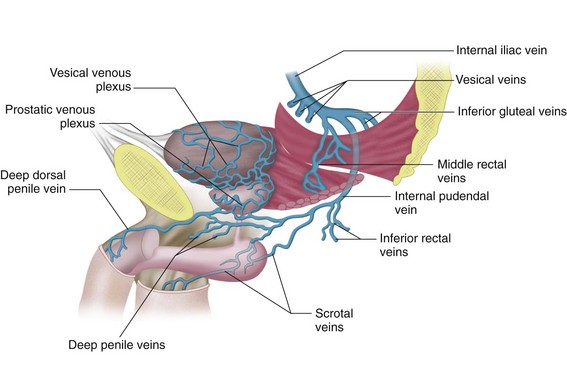
 FIGURE 76-11
FIGURE 76-11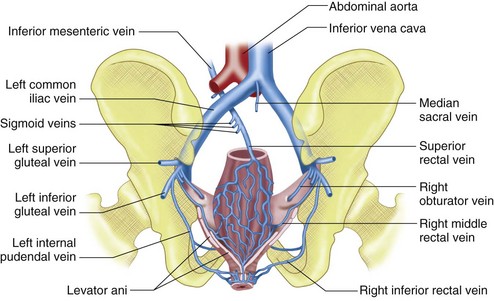
 FIGURE 76-12
FIGURE 76-12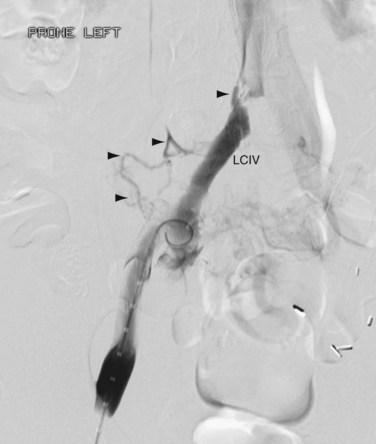
 FIGURE 76-13
FIGURE 76-13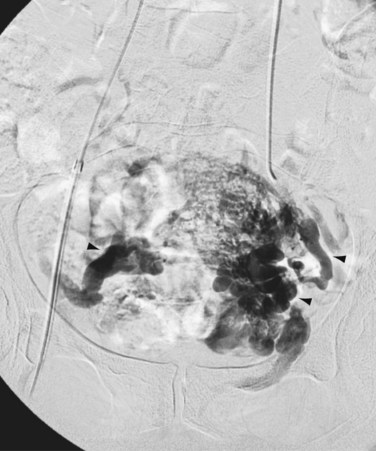
 FIGURE 76-14
FIGURE 76-14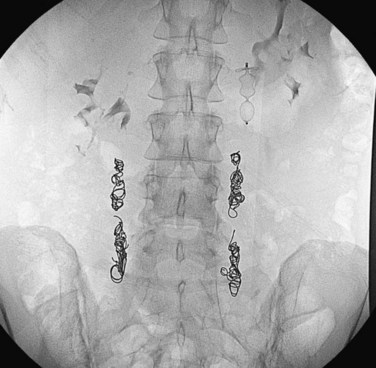
 FIGURE 76-15
FIGURE 76-15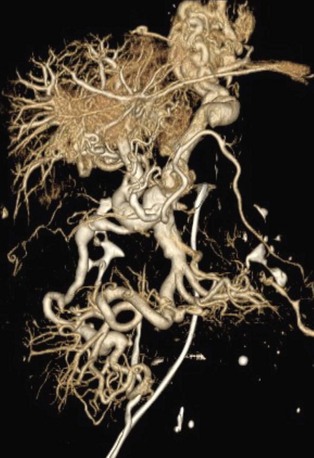
 FIGURE 76-16
FIGURE 76-16




