Vector-Borne Diseases
At the conclusion of this chapter, the reader should be able to:
• Describe the etiology, epidemiology, and signs and symptoms of Lyme disease.
• Analyze the immunologic manifestations and diagnostic evaluation of Lyme disease.
• Explain the principle, interpretation, and limitations of an antibody detection assay.
• Describe prevention strategies of Lyme disease.
• Summarize the etiology, epidemiology, and signs and symptoms of ehrlichiosis.
• Analyze the immunologic manifestations and diagnostic evaluation of ehrlichiosis.
• Explain the prevention of ehrlichiosis.
• Summarize the etiology, epidemiology, and signs and symptoms of Rocky Mountain spotted fever.
• Analyze the immunologic manifestations and diagnostic evaluation of Rocky Mountain spotted fever.
• Explain the prevention of Rocky Mountain spotted fever.
• Summarize the etiology, epidemiology, and signs and symptoms of babesiosis.
• Analyze the immunologic manifestations and diagnostic evaluation of babesiosis.
• Explain the prevention of babesiosis.
• Briefly discuss the etiology and laboratory diagnosis of West Nile virus infection.
• Analyze case studies related to the immune response in Lyme disease, Ehrlichiosis, and Babesiosis.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principle, limitations, and clinical applications of the rapid Borrelia burgdorferi antibody detection assay.
Globalization has made the world a more connected place. Bacterial and viral diseases transmitted by mosquitoes, ticks, and fleas continue to be an ever-present threat worldwide (Table 19-1). Some of these diseases have been present in the United States for a long time but others have emerged recently. These include some of the world’s most destructive diseases, many of which are increasing threats to human health as the environment changes and globalization increases.
Table 19-1
Examples of Vector-Borne Diseases
| Vector | Disease | Pathogen | Distribution |
| Mosquitoes | |||
| Aedes triseriatus | California encephalitis | Virus | United States: Upper Midwest, Appalachian region |
| Aedes aegypti | Dengue fever West Nile encephalitis West Nile fever |
Virus Virus |
Worldwide: tropical regions United States; spreading nationwide Africa, Asia |
| Culiseta melanura | Eastern equine encephalitis | Virus | Eastern United States Central and South America, Caribbean |
| Culex spp. | St. Louis encephalitis Western equine encephalitis |
Virus Virus |
Eastern United States Central and South America Western United States Central and South America |
| Ticks | |||
| Deer tick, Ixodes spp. | Anaplasmosis (formerly human granulocytic ehrlichiosis) | Bacteria | Worldwide; Europe United States—Northeast, Upper Midwest, northern California |
| I. scapularis | Babesiosis | Protozoan parasite | United States—primarily northeastern states, rarely Pacific states |
| Lone star tick, Amblyomma americanum | Human monocytic ehrlichiosis | Bacteria | United States—Southeast, south central states |
| Dog tick, Rhipicephalus sanguineus | Mediterranean spotted fever | Bacteria | Europe, Africa, Central Asia |
| Tickborne, airborne vector | Q fever | Rickettsiae | Worldwide |
| Dog tick, wood tick, Dermacentor spp. | Rocky Mountain spotted fever Tick-associated rash, illness |
Bacteria Bacteria |
North and South America Southern |
| Ticks, various | Tickborne relapsing fever | Bacteria | Western United States (endemic∗); Southern British Columbia; plateau regions of Mexico; Central and South America; Mediterranean, Central Asia, and much of Africa |
| Lice, Fleas, Mites | |||
| Human body louse; squirrel flea and louse | Epidemic typhus | Rickettsiae | United States, eastern |
| Rat flea, Xenopsylla cheopis | Murine typhus | Bacteria | Worldwide, where rats are abundant |
| Cat or dog fleas | Murine typhus–like febrile disease | Rickettsiae | Worldwide |
| Mites (chiggers) | Scrub typhus | Rickettsiae | South Asia to Australia, East Asia in recently disturbed habitat (e.g., forest clearings or other persisting mite foci infested with rats and other rodents) |
| Human body louse | Louse-borne relapsing fever Trench fever |
Bacteria Rickettsiae |
Africa Industrialized countries |
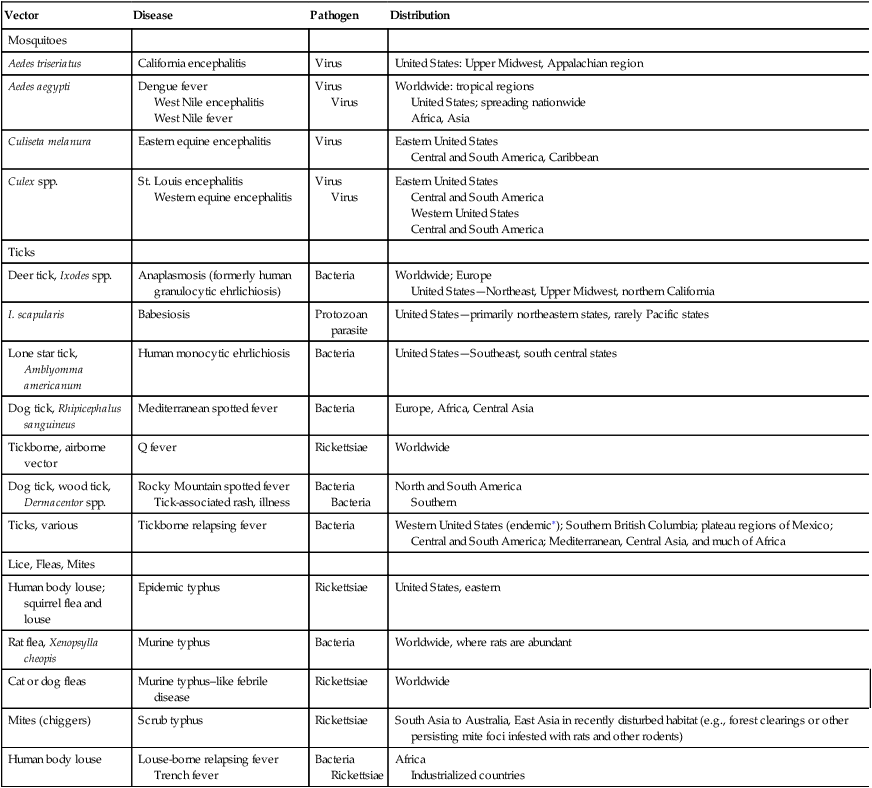
∗Most recent cases and outbreaks have occurred in rustic cabins at higher elevations (≥8000 ft) in coniferous forests in the western United States.
Some of the newly emerging infectious diseases in the United States include the following:
Lyme Disease
Etiology
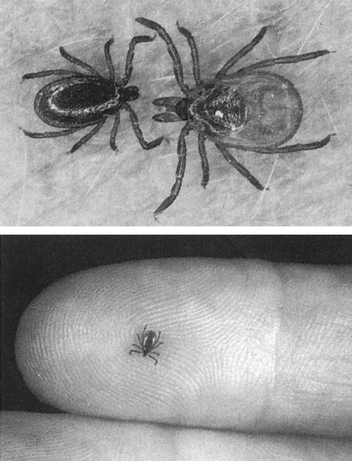
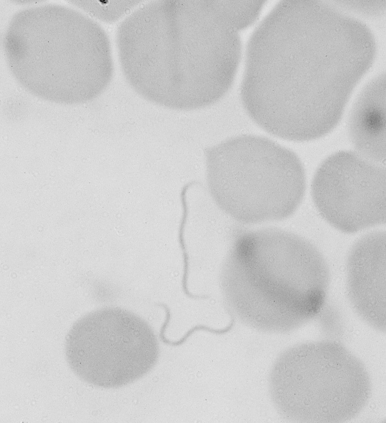
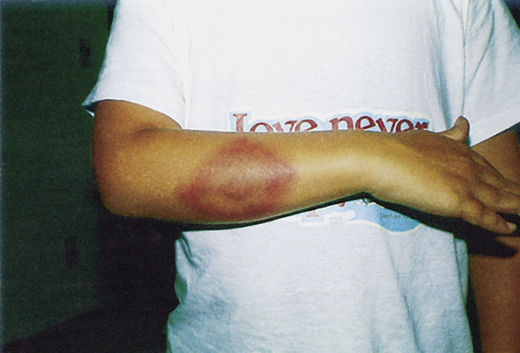
Lyme disease (Lyme borreliosis) is caused by a spirochete bacterium. It is a cutaneous systemic infection generally transmitted by a hard-bodied tick (Fig. 19-1) and caused by Borrelia burgdorferi (Fig. 19-2). The causative agent of Lyme borreliosis currently consists of three pathogenic species—B. burgdorferi, Borrelia afzelii, and Borrelia garinii. Only B. burgdorferi strains have been found in the United States. In contrast, most of the illness in Europe is caused by B. afzelii, which is associated with the chronic skin condition acrodermatitis chronica atrophicans (ACA), and B. garinii, which is associated with neurologic symptoms. Only these two species have been found in Asia. The complete genome of B. burgdorferi (strain B31) has now been sequenced.
Epidemiology
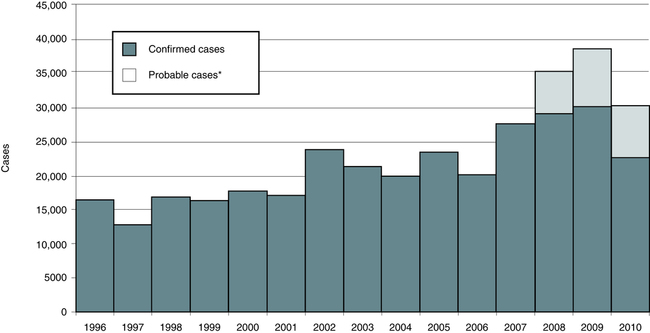
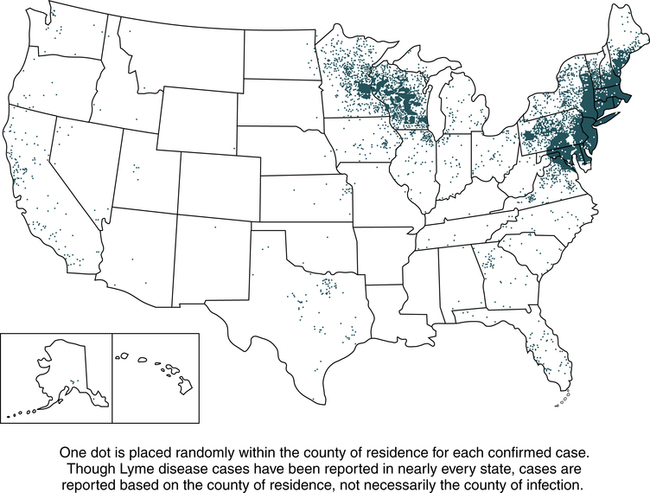
Lyme disease does not occur nationwide and is concentrated heavily in the northeast and upper midwest. The highest number of confirmed cases of Lyme disease to date was 29,959 in 2009 (Fig. 19-3). Persons of all ages and both genders are equally susceptible. In 2011, 96% of Lyme disease cases were reported from 13 states (Fig. 19-4):
Signs and Symptoms
Lyme borreliosis is a multisystem illness that primarily involves the skin, nervous system, heart, and joints (Table 19-2). Lyme disease usually begins during the summer months with EM and flulike symptoms and may be accompanied by right upper quadrant tenderness and a mild hepatitis (stage 1). This stage is followed weeks to months later by acute cardiac or neurologic disease in a minority of untreated individuals (stage 2) and then by arthritis and chronic neurologic disease (stage 3) in many untreated patients weeks to years after disease onset. There is considerable overlap of these stages, but Lyme disease is best characterized as an illness that evolves from early to late disease without reference to an arbitrary staging system. However, a patient may have one or all of the stages, and the infection may not become symptomatic until stage 2 or 3. Most affected patients have EM and 25% manifest arthritis; neurologic manifestations and cardiac involvement are uncommon.
Table 19-2
Clinical Features of Lyme Disease
| Stage | Duration | Signs and Symptoms |
| I | 4 wk (median) after injection | Cutaneous manifestations (erythema migrans) or other skin eruptions, flulike syndrome, neurologic symptoms |
| II | Follows a variable latent period | Target organs and systems include nervous system, heart, eyes, and skin, all of which can manifest abnormalities |
| III | Weeks to years after infection | Arthritis, late neurologic complications, acrodermatitis chronica atrophicans |
Cutaneous Manifestations
Cutaneous manifestations can be demonstrated as early ECM (Fig. 19-5), secondary lesions (disseminated lesions and lymphocytoma), and late lesions (ACA). Except for the late lesions, cutaneous manifestations generally resolve spontaneously over weeks to months. The red papule at the site of the tick bite is most often located on the thigh, groin, or axilla. Facial EM is seen more frequently in children.
Diagnostic Evaluation
In the United States, the diagnosis is usually based on the recognition of the characteristic clinical findings, a history of exposure in an area in which the disease is endemic and, except in patients with EM, an antibody response to B. burgdorferi. In more than 50% of cases, physicians are comfortable making the diagnosis based on symptoms and patient history. Testing becomes important when the telltale bull’s eye rash or other symptoms characteristic of Lyme disease do not appear (Table 19-3).
Table 19-3
Methods of Lyme Disease Detection
| Method | Comments |
| Isolation | Successful cultures have been obtained from ticks, skin biopsies, ear punches, CSF, blood, and synovial fluid; blood is not a reliable sample for culture. Isolation of spirochetes is highly variable. |
| Histology | Lyme spirochetes are rarely observed in blood smears; examination of tissue is usually performed in addition to an immunologic assay such as fluorescence microscopy. The process is labor-intensive; the test is of limited value. |
| Serology | FDA-approved IFA and EIA test systems |
| Molecular | DNA probe with patient DNA matched to Borrelia DNA |
IFA, Indirect fluorescent antibody; EIA, enzyme immunoassay; FDA, Food and Drug Administration.
Western Blot Analysis
Western blot analysis can verify reactivity of antibody to major surface or flagellar proteins of B. burgdorferi (Fig. 19-6). The Western blot test is helpful in determining borderline negative or weakly positive results obtained from other tests, but the values are not always reliable. This procedure is more definitive in later Lyme disease when multiple antibody bands specific for B. burgdorferi appear. Reported results from Western blot tests for Lyme disease in its late phase indicates reactive bands for IgM levels. The 41-kDa bands are the earliest to appear, but can cross-react with other spirochetes. The 18-, 23- to 25- (Osp C), 31- (Osp A), 34- (Osp B), 37-, 39-, 83-, and 93-kDa bands are the most specific, but may appear later or not appear at all.
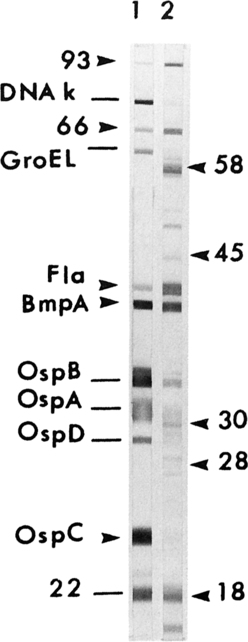
Lane 1, Monoclonal antibodies defining selected antigens to B. burgdorferi B31 separated in a linear SDS-PAGE gel Marblot (MarDx Diagnostics, Carlsbad, Calif). Lane 2, Human serum (IgG) reactive with the 10 antigens scored in recommended criteria for blot scoring; lines indicate other calibrating antibodies. Molecular masses are in kilodaltons. Osp, Outer surface protein. (From Detrick B et al, editors: Manual of molecular and clinical laboratory immunology, ed 7, Washington, DC, 2006, American Society for Microbiology Press, p 499.)
Treatment and Prevention
Treatment decisions after a tick bite are influenced by the following factors:
• Probability that the tick is a carrier of B. burgdorferi
• Length of time the tick was attached
• Chance that disease will develop without the telltale rash
• Risk and severity of short- and long-term sequelae
• Efficacy of antibiotics at various stages of the disease
• Risk of adverse reactions to the antibiotics
• Probability that the patient will comply with follow-up monitoring
• Cost of various strategies; presence of coinfections or immunodeficiencies; prior significant steroid use while infected; age and weight; gastrointestinal (GI) function; blood levels achieved
Human Ehrlichiosis
Diagnostic Evaluation
Laboratory studies have indicated that the hematologic, hepatic, and central nervous systems are usually involved in human ehrlichiosis. Definitive diagnosis is based on inclusion in leukocytes (Fig. 19-7). Ehrlichia spp. undergo three developmental stages, as follows:
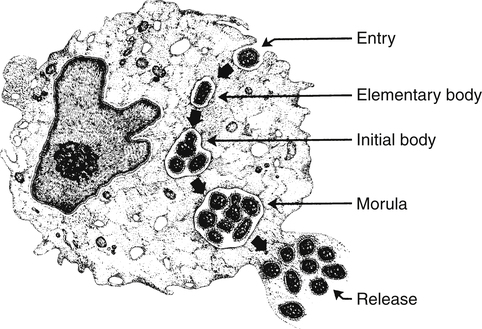
Elementary bodies (EBs; individual ehrlichiae) enter the leukocyte by phagocytosis and multiply. After 3 to 5 days, small numbers of tightly packed EBs are observable and are called initial bodies. During the next 7 to 12 days, additional growth and replication occur, and the initial bodies develop into mature inclusions, which appear by light microscopy as mulberry (morular) forms. This morula is a hallmark of ehrlichial infection. (From McDade J: Ehrlichiosis—a disease of animals and human beings. J Infect Dis 161:609–617, 1990.)
1. Elementary bodies enter a leukocyte by phagocytosis and multiply rapidly.
2. After 3 to 5 days, small numbers of tightly packed elementary bodies (initial bodies) are visible.
3. During the next 7 to 12 days, the initial bodies develop into morular, or mulberry, forms.
Babesiosis
Etiology
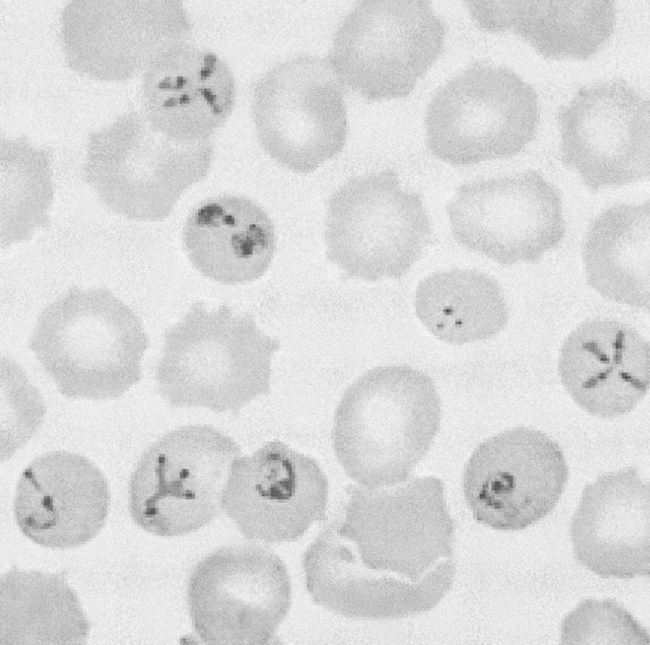
Babesiosis is a rare, severe, and sometimes fatal tickborne disease caused by various types of Babesia, a microscopic parasite that infects red blood cells (Fig. 19-8). The causative organism of babesiosis was first described by Babes in 1888. In New England and the eastern United States, the disease is caused by Babesia microti; in California, it is caused by Babesia equi. In Europe, the disease is caused by Babesia divergens and Babesia bovis. Babesia canis has been found to be responsible for several cases in Mexico and France.
West Nile Virus
Etiology
Epidemiology
The virus has been in the United States since at least the summer of 1999. Figure 19-9 shows the U.S. distribution of WNV in 2011. If WNV infection is reported to the CDC from any area of a state, the entire state is shaded.
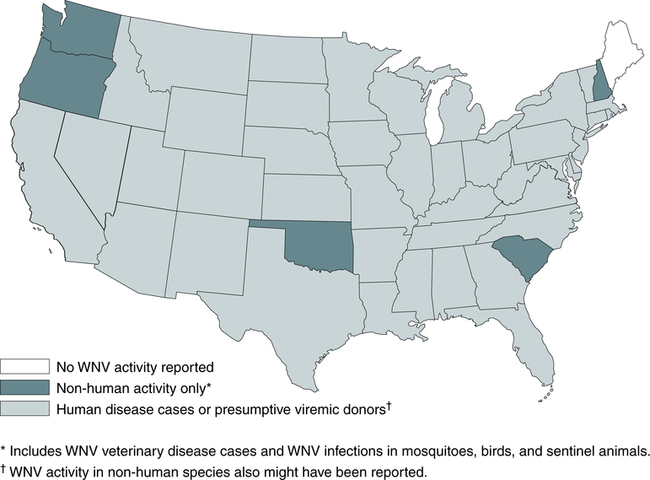
The map shows the distribution of nonhuman activity (light green) and human infections, including PVDs (dark green). If WNV infection is reported from any area of a state, that entire state is shaded. (From Centers for Disease Control and Prevention, Atlanta [http://www.cdc.gov/ncidod/dvbid/westnile/Mapsactivity/surv&control11MapsAnybyState.htm].)
Chapter Highlights
• Lyme disease (borreliosis) is caused by the tick-borne spirochete Borrelia burgdorferi and is a major health hazard for human beings and domestic animals.
• Lyme disease has been considered an emerging infectious disease because of the impact of changing environmental and socioeconomic factors (e.g., transformation of farmland into suburban woodlots favorable for deer and deer ticks).
• The basic features of Lyme disease are similar worldwide. In at least 60% to 80% of U.S. patients, it begins with a slowly expanding skin lesion, EM, at the site of the tick bite.
• Lyme borreliosis is a multisystem illness that primarily involves the skin, nervous system, heart, and joints. It usually begins during the summer months with EM and flulike symptoms.
• Cellular immune responses to B. burgdorferi antigens begin concurrently with early clinical illness, with increased spontaneous suppressor cell and reduced NK cell activity. Mononuclear cell, antigen-specific responses develop during spirochetal dissemination, and humoral (antibody) immune responses soon follow.
• Serodiagnostic tests are insensitive during the first several weeks of Borrelia infection. About 20% to 30% of U.S. patients have positive responses, usually of the IgM isotype, during this period, but by convalescence 2 to 4 weeks later, about 70% to 80% have seroreactivity even after antibiotic treatment. After about 1 month, most patients with active infection have IgG antibody responses. After antibiotic treatment, antibody titers fall slowly, but IgG and IgM responses may persist for years.
• Specific IgM or IgG antibodies against B. burgdorferi are usually not detectable in a patient’s serum unless symptoms have been present for at least 2 to 4 weeks. In Lyme arthritis, test results (ANAs, RF, VDRL) are generally negative, and anti–B. burgdorferi antibodies (IgG) should be present.
• The most common laboratory assays for B. burgdorferi antibody detection include IFA, ELISA, and PreVue. Immunoblotting techniques can be used with ELISA. PreVue is the first presumptive step in testing individuals with suspected Lyme disease. Positive results must be confirmed by Western blot testing.
• Described first in the United States in 1986, tickborne rickettsiae of the genus Ehrlichia cause human illness. Ehrlichiosis is a general term for anaplasmosis and HME.
• Anaplasmosis diagnosis is confirmed by seroconversion (fourfold rise in acute/convalescent sera titer) or single serologic titer greater than 1:80 in patients with a history and symptoms. HME diagnosis is confirmed by seroconversion or serologic titer greater than 1:128.
• Babesiosis is a rare, severe, possibly fatal tickborne disease caused by Babesia, which infects RBCs.
• Babesia spp. are visualized as intraerythrocytic organisms in thick peripheral (rapid Field’s test) or thin blood films. Acute and convalescent antibody titers may be useful; a titer higher than 1:256 is diagnostic of acute infection. Only IgG antibody determinations are performed. PCR amplification can be used for diagnosis.
• West Nile virus, a mosquito-borne virus present in the United States since at least 1999, causes febrile illness and encephalitis in human beings.
• In WNV, IgM antibody is evident in most infected patients 7 to 8 days after the onset of symptoms, persisting for more than 500 days in 60% of cases. Most patients demonstrate IgG antibody in 3 to 4 weeks after infection.