Chapter 48 Vasopneumatic Compression Devices
(Intermittent Compression Pumps)
OVERVIEW.
Vasopneumatic compression devices (VCD) are a form of compression therapy that uses mechanical force from a pump to intermittently exerts external pressure on a body part (i.e., increasing hydrostatic pressure, milking effects on fluid). The device typically includes a chambered sleeve wrapped around the involved limb, a connecting elastic tube, and an electric pump.1
Vasopneumatic compression devices are used to treat lymphedema, reduce edema (due to venous insufficiency), help heal venous stasis ulcers (due to impaired circulation), prophylactically reduce the incidence of DVTs in postoperative patients, reshape residual limbs following amputation, and control hypertrophic scarring. Note: Because ICP can be applied at pressures that act as a tourniquet or induce fluid overload in some patients who have compromised cardiovascular systems, proper screening and monitoring are required.2
SUMMARY: ADVERSE EVENTS.
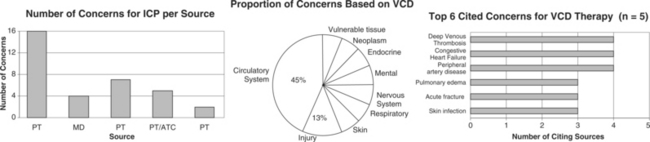
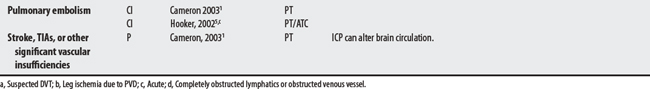
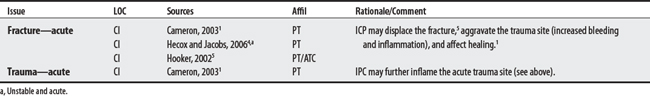
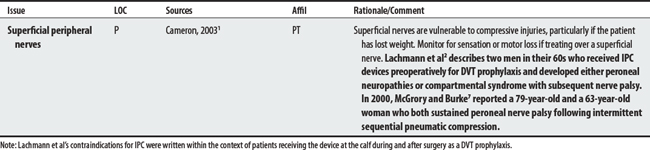
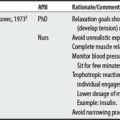
Many of the reported compression device complications3 (embolism-related deaths; palsies, pressure ulcers, compartment syndromes) stem from its use in DVT prophylaxis with surgical patients. In this protocol (compression lasts 12 seconds per minute, 40 mm Hg), calf compressions serve to reproduce leg contractions, promote venous return, and stimulate fibrinolysis for the prevention of thrombus formation.2
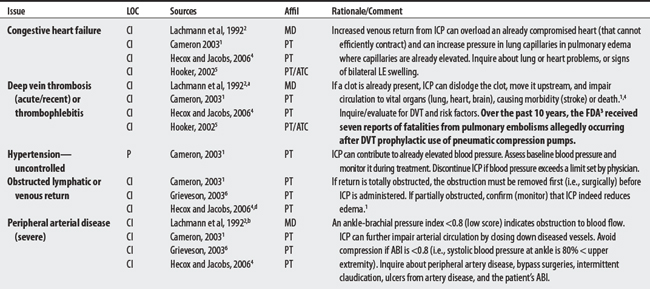
CONTRAINDICATIONS AND PRECAUTIONS
E00-E90 ENDOCRINE, NUTRITIONAL AND METABOLIC DISEASES
F00-F99 MENTAL AND BEHAVIORAL DISORDERS
G00-G99 DISEASES OF THE NERVOUS SYSTEM
I00-I99 DISEASES OF THE CIRCULATORY SYSTEM
J00-J99 DISEASES OF THE RESPIRATORY SYSTEM
L00-L99 DISEASES OF THE SKIN AND SUBCUTANEOUS TISSUE
S00-T98 INJURY, POISONING, AND CERTAIN OTHER CONSEQUENCES OF EXTERNAL CAUSES
ADVERSE EVENTS
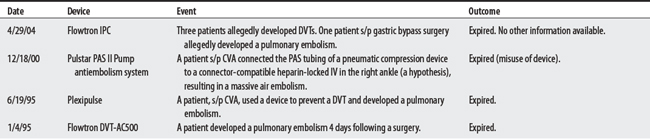
FOOD AND DRUG ADMINISTRATION REPORTS3
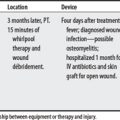
IPCD-Related Injuries
| Date | Summary Outcomes |
|---|---|
| FDA, 11/12/97 to 6/29/04 |
Note: FDA reports do not necessarily establish cause-effect relationships between equipment and injury. Incidences may be due to equipment or user error. Also, some reports are alleged by attorneys.
1 Cameron MH. Physical agents in rehabilitation: from research to practice. St. Louis: Saunders, 2003.
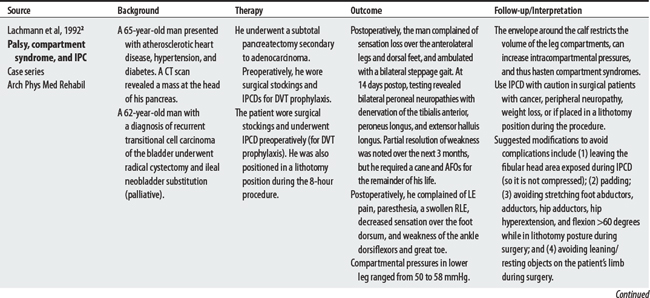
2 Lachmann EA, Rook JL, Tunkel R, et al. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehabil. 1992;73(5):482-485.
3 U.S. Food and Drug Administration. Center for Device and Radiological Health [web page]. Available at: http://www.fda.gov/cdrh/mdr/. Accessed: November 7, 2005
4 Hecox B, Jacobs LF. External compression. In: Hecox B, Mehreteab TA, Weisberg J, editors. Integrating physical agents in rehabilitation. Upper Saddle River (NJ): Pearson Prentice Hall, 2006.
5 Hooker DN. Intermittent compression devices. In Prentice WE, editor: Therapeutic modalities for physical therapists, ed 2, New York: McGraw-Hill, 2002.
6 Grieveson S. Intermittent pneumatic compression pump settings for the optimum reduction of oedema. J Tissue Viability. 2003;13(3):98-110.
7 McGrory BJ, Burke DW. Peroneal nerve palsy following intermittent sequential pneumatic compression. Orthopaedics. 2000;23(10):1103-1105.