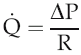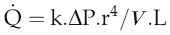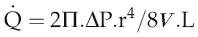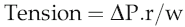Chapter 5 Vascular system
The volume flow of blood around the circulation per unit time (
) can be expressed in terms of the pressure gradient (ΔP) and the absolute resistance to flow of the vasculature (R):
Intravascular pressure at the downstream end of the vascular circuit where it drains into the heart is virtually the same as atmospheric pressure and can be regarded as 0 mmHg. The value of ΔP, therefore, depends primarily on the value of arterial blood pressure. In the last chapter we looked at the aspects of cardiac function that affect this and reviewed briefly the involvement of the peripheral vasculature. We need now to examine in more detail the properties of blood vessels and how these contribute to resistance to flow.
PHYSICAL FACTORS AFFECTING PERIPHERAL RESISTANCE
Vessel radius
The dependence of resistance on absolute vessel size also indicates that the major component of total peripheral resistance must be localized to the smaller precapillary vessels (the microcirculation) rather than the large distributing arteries. Not only are these microcirculatory vessels smaller in radius but they also branch repeatedly every mm or so. Although total cross-sectional area increases with each branching, total surface area increases even more, so that resistance rises rapidly along quite a short distance.
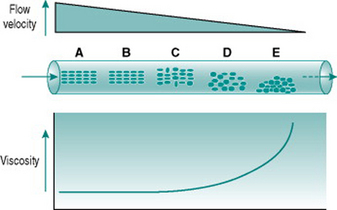
Blood viscosity
Figure 5.1 illustrates the processes that underlie anomalous viscosity in the bloodstream. When the blood is flowing relatively fast (A–B), the cellular components travel as a core in the centre of the vessel, surrounded by a cell-free layer of plasma. The cells are oriented so that they travel edge-on, producing minimal friction between the cell layers and between cells and plasma. The viscosity of the blood in this situation is around 50% greater than that of plasma alone. If the flow velocity falls sufficiently then the orientation of the suspension becomes less organized, with some cells starting to rotate and collide with adjacent cells. This process absorbs some of the energy creating the pressure gradient and so viscosity rises (C–D). If flow rate falls even further, the cellular constituents fall out of suspension and form an aggregate on the gravitationally lowest surface of the vessel. Because of the mass of the aggregate, a substantial amount of energy is required to lift the cells back into suspension again, reflected in a very high viscosity (E).
The dependence of blood viscosity on the cellular components has several implications. First, alteration of the haematocrit will change the size of the cell-rich core relative to surrounding plasma. Therefore, increased haematocrit will proportionately elevate viscosity. This can impose a substantial extra cardiac workload particularly during exercise. Thus, ‘blood doping’ with erythropoietin, although it is likely to improve maximal exercise performance by enhancing oxygen delivery to muscles, also carries a significant risk of damage to the heart. A second consequence of anomalous viscosity is that very low rates of blood flow can result in cells falling out of suspension. If the cell aggregates remain unsuspended for more than a few minutes, they begin to stick together and, in small vessels, may completely obstruct the lumen. This situation is most likely to occur in the postcapillary venules and will be discussed in Chapter 10 in relation to hypotensive states associated with prolonged exercise.
Blood flow through systemic capillaries is rather different to that through either precapillary or postcapillary vessels. All these other vessels have diameters greater than that of blood cells, whereas the typical capillary diameter of around 6 μm is marginally less than that of an erythrocytes (8 μm). In consequence, blood cells have to be partially folded in order to pass along the capillary. This process is important in that it ensures the closest contact possible between erythrocyte and endothelial membranes and so minimizes the distance for gas diffusion between blood and tissue. However, in theory it should also produce very high frictional forces between blood cells and the capillary wall, greatly increasing local viscosity and impeding the efficiency of capillary perfusion. To avoid such a disadvantageous situation, the endothelium of capillaries secretes a lubricant mucopolysaccharide that virtually eliminates frictional interaction with the blood cells and results in local viscosity that is almost as low as that of cell-free plasma.
Poiseuille’s law
The equation relating flow, pressure and resistance (see p. 45) can be rewritten by substitution of vessel radius (r) and length (L) and blood viscosity (V) for resistance, and adding a factor for numerical accuracy (k), thus:
The practical importance of Poiseuille’s law is that it describes the main factors affecting the relationship between flow and pressure. It does not of course take account of homeostatic feedback so does not necessarily predict the end result of manipulating these factors in the whole body. For example, if blood pressure were elevated by a sudden increase in blood volume, there would normally be baroreflex compensation that would lower peripheral resistance and return the pressure to its initial level (see Chapter 7, p. 81). A second difference from the events in the intact circulation is that Poiseuille’s law assumes that the vasculature is a system of rigid tubes. As soon as we deal with tubes that might distend in response to internal pressure, then obviously the association between pressure and flow becomes different.
RESISTANCE TO FLOW OF DISTENSIBLE VESSELS
Roles of connective tissue and muscle in determining distensibility
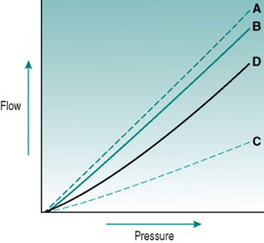
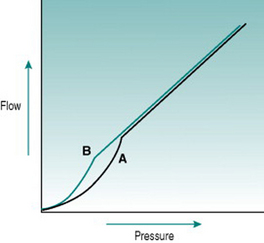
In rigid tubes, there is a linear relationship between the pressure gradient and the resulting volume flow, presuming that resistance remains constant (Fig 5.2A). In distensible tubes, by contrast, increased internal pressure will cause some increase in tube volume, so that the rate of volume flow rise with pressure will be lower. In blood vessels, distensibility is conferred by the presence of elastin in the vessel wall, while the presence of collagen confers rigidity (Fig. 5.2B–D). The property of distensibility is usually referred to as compliance.
For any given mix of elastin and collagen, absolute vessel compliance depends also on the amount of smooth muscle and whether the muscle cells are relaxed or contracted. Although arterioles have elastin in their walls, they behave as virtually rigid tubes because of the thickness of the muscle layer. The walls of large veins contain large amounts of elastin and relatively small amounts of muscle, so they are normally highly distensible. However, if the muscle contracts fully then this has the effect of stiffening the vein dramatically, so that even high internal pressure does not cause distension. This transition becomes practically important when one is inserting an intravenous needle for blood sampling. Unsuccessful insertion frequently causes local muscle spasm in the vein wall, with the result that the vessel shrinks so much that it is impossible to cannulate.
Measurement of arterial distensibility
The compliance of the arterial system determines the magnitude of arterial pulse pressure, the pulsatility of peripheral blood flow and the velocity of blood flow through the larger arteries. This will in turn affect shear stress on the endothelium and the release of endothelial factors that alter peripheral resistance (see Chapter 6, p. 64). The arteries become progressively stiffer with age due to loss of elastin: at any given age higher stiffness is predictive of cardiovascular morbidity, while reduction of blood pressure associated with, for example, chronic exercise programmes is linked to reduced stiffness. Measuring arterial stiffness is, therefore, potentially useful not only for evaluating the basis of circulatory parameters such as blood pressure, but also for assessing cardiovascular health and monitoring the beneficial effects of interventions like exercise.
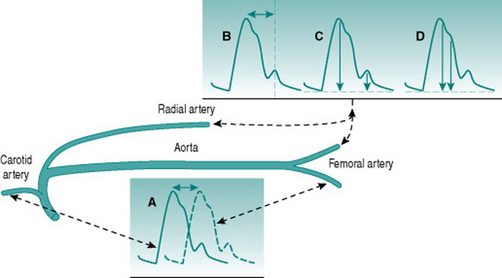
A number of techniques exist for evaluating arterial stiffness, all of which utilize non-invasive detection of the phasic blood pressure waveform and varying degrees of technology. Conceptually simplest, and the original standard, is measurement of pulse wave velocity by timing arrival of the peak pressure wave at two sites different distances away from the heart, traditionally in the carotid and the femoral arteries (Fig. 5.3A). Other methods can utilize pressure waves recorded from one peripheral arterial site (usually the femoral or radial) and involve measurement of the time interval between the systolic pressure peak and the reflected wave that follows it (Fig. 5.3B), comparison of the amplitudes of these two events (Fig. 5.3C) or assessment of the shape of the primary systolic pressure waveform (Fig. 5.3D).
All of these methods involve some assumptions about the physics of the circulation and some require significant amounts of computation. For example, the central augmentation index method (Fig. 5.3D) involves a computerized transfer function that estimates the aortic waveform from the shape of a peripheral systolic wave. Comparison of values obtained with a range of methods in healthy subjects and patient groups has indicated that there is relatively poor agreement between different techniques. This is not surprising since some methods use central and some use peripheral arteries: these two types of artery have different wall characteristics and are at very different distances from reflection sites in the peripheral microcirculation. Optimal assessment of arterial wall behaviour should, therefore, employ more than one technique, so as to involve both vessel types. However, it remains to be determined which combination of methods is most useful. For the present, the choice is based primarily on what facilities are available in particular laboratories.
EFFECT OF INTRAVASCULAR PRESSURE ON WALL TENSION
Laplace’s law and large vessel function
In rigid tubes, it is clear that wall tension is independent of intravascular pressure. If the tube is distensible, by contrast, increased intravascular pressure will not only increase r, but also reduce w, so that wall tension rises rapidly. With the magnitudes of pressure and vessel size characteristic of the aorta during systolic ejection, increases in vessel radius by around 50% would be likely to cause wall rupture. The protection against this is the presence of a non-distensible layer of collagen fibres around the aorta. These are arranged so that they allow aortic radius to increase by about 20% during systolic ejection, consistent with its function in facilitating diastolic flow (see Chapter 4, p. 31), but form a rigid outer sleeve to the vessel that prevents further increases in radius (Fig 5.4). Although this protective mechanism is highly effective, it can be disrupted in people with pathologies that cause selective loss of collagen from the body. If these individuals are subject to sudden rises in blood pressure, they may, therefore, experience uncontrolled aortic distension leading to wall rupture (a dissecting aneurysm) and potentially fatal haemorrhage.
Laplace’s law and small vessel function
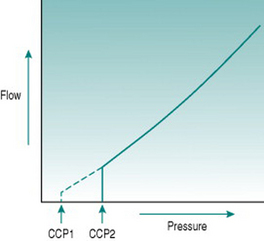
In the precapillary microcirculation, although intravascular pressure is of the same order of magnitude as in the aorta, the luminal size is too small for absolute wall tension to ever reach values that might cause wall damage. In these small arterial vessels, Laplace’s law has functional significance of another sort. The walls of small arteries and arterioles contain little connective tissue and consist primarily of smooth muscle that usually has some degree of active tone – that is, is in a state of partial contraction – due to sympathetic vasoconstrictor nerve activity and circulating vasoconstrictor hormones. Under these circumstances, the vessel luminal size must reflect a balance between the outwardly directed pressure of the blood and the inwardly directed pressure exerted by the muscle. If intravascular pressure falls suddenly, then the vessel will constrict without opposition down to a minimal luminal diameter. The absolute pressure necessary to balance muscle tone is termed the critical closing pressure, and imposes a further modification on the relationship between pressure and flow, since it means that in the microcirculation flow ceases while there is still a measurable pressure gradient (Fig. 5.5). In arterioles with high vasoconstrictor tone, the critical closing pressure may be as great as 60–70 mmHg. If the vessels are fully dilated, then flow would persist with very low pressure gradients of the order of 4–5 mmHg.
The vessel segment most likely to be subject to critical closure is the region of transition between arteriole and capillary, known as the metarteriole, because within the muscular arteriolar tree this last region is the smallest in diameter and has least supporting tissue. The relationship between contractile state and metarteriolar closure is an important determinant of blood flow distribution in tissues, such as skeletal muscle, and will be discussed further in the next chapter (pp. 69–73).
MEASUREMENT OF PERIPHERAL BLOOD FLOW
Temperature
Skin temperature reflects a balance between the rate at which heat arrives from the body core in the cutaneous blood supply and the rate at which this heat is lost into the environment. Provided that the local environmental conditions can be controlled, therefore, skin temperature is often used as a semi-quantitative index of skin blood flow. The main advantage of this technique is that it can provide moment-to-moment indicators of skin perfusion over a wide area of the body surface, making it useful, for example, for evaluating cutaneous vasomotor responses to thermoregulatory stimuli. Aside from the obvious fact that no calibration of flow is possible, other limitations of this technique include the need to prevent interference from sweat-induced evaporative heat loss and the fact that high metabolic rates of underlying tissues, such as muscle, may distort the temperature recorded at the skin surface.
Venous occlusion plethysmography
If venous outflow from a tissue is obstructed, the continued arterial inflow will initially produce an equivalent increase in tissue volume. At the same time, intravascular pressure in the tissue capillaries and veins rises progressively towards arterial pressure. When venous pressure reaches the occluding pressure then venous outflow is re-established and no further increase in tissue volume occurs. Provided that the tissue involved is able to swell in response to arterial inflow for at least a few heart beats, which is the case for most tissues, then the volume change gives an accurate measurement of regional blood flow.
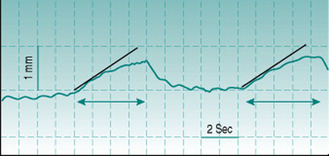
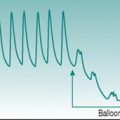
In practice, this technique of measuring volume change (plethysmography) in response to venous occlusion is usually applied to measurement of limb blood flow, and then most commonly to that in forearm. The subject is seated with the forearm in a horizontal position and at or slightly above the level of the heart. An occlusive cuff is applied to the upper arm and inflated repetitively for periods of several seconds to a pressure above that in the arm veins but below diastolic arterial pressure – this pressure is typically set at 40 mmHg and the inflation–deflation cycle is typically of the order of 4 s–6 s. Tissue volume is quantified from arm circumference, measured by a stretchable strain gauge wrapped around the midpoint of the forearm. Without cuff inflation, it is usually possible to observe small pulsatile changes in arm circumference with every pulse wave (Fig. 5.6). Cuff inflation causes a progressive rise in circumference that becomes less pronounced after the first few heart beats as venous pressure rises and will plateau after some seconds when venous pressure exceeds the cuff pressure. Flow is calculated by measuring the gradient of the early, steepest part of the curve and converting this to the rate of volume change of the tissue slice beneath the strain gauge (Fig. 5.6).
Because the distensible tissues of the forearm consist of both muscle and skin, venous occlusion plethysmography measures blood flow through both and interpretation of the vascular bed responsible for experimentally induced flow changes is based largely on the situation. For example, skin perfusion can be held at a low and stable level by maintaining a cool environment and hyperaemia following brief isometric forearm exercise must by definition be primarily localized to muscle. By contrast, increases in flow associated specifically with body warming can be assumed to occur primarily in skin. For accurate assessment of skin blood-flow responses to thermal stimuli it is preferable to measure not only forearm, but also finger blood flow, because the fingers are devoid of muscle. As well, since the finger circulation contains arteriovenous shunts (see Chapter 5, p. 60), simultaneous monitoring of arm and finger flows sometimes provides differential information on the responses of shunt and arteriolar vessels to thermal stimuli.
STRUCTURAL SPECIALIZATION OF BLOOD VESSELS
Two situations exist in which structural specialization of blood vessels provides unique functional properties that are not found in most vascular beds. One of these is the parallel arrangement of arterial and venous vessels that provides an opportunity for recycling of material between arterial and venous blood. The other is a system of wide, low-resistance vessels that allow blood to be shunted from arterial to venous circulations without transiting the capillaries.
Countercurrent exchangers
Countercurrent heat exchange
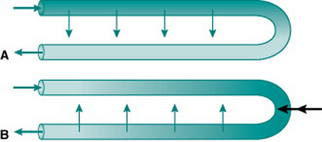
When the body is exposed to a cold environment, by contrast, then even the relatively small volume of blood reaching the extremities under vasoconstricted conditions loses a lot of its internal heat and places an additional metabolic load on the body if core body temperature is to remain stable. For metabolic economy it is, therefore, sensible to minimize heat loss in the extremities. This is achieved by sympathetic changes in venous tone that divert drainage in the limbs to deep veins lying adjacent to the main arteries. In this situation, heat is transferred from arterial to venous blood as it travels down the limbs, and recycled back into the central circulation (Fig. 5.7A).
Countercurrent solute exchange
Microcirculatory countercurrent exchange of solutes is functionally important in two regions – the epithelium of the small intestine and the vasa recta of the renal medulla. In each of these situations, the actual vascular organization consists of a quite complex network of vessels but can be envisaged, in simplified form, as a hairpin loop consisting of an arteriole leading to a capillary loop and then a venule that runs parallel and close to the arteriole. By definition, the concentration of oxygen is higher in arterial than in venous plasma, so some incoming arterial oxygen always diffuses across into the parallel stream of venous blood along the length of the loop. Thus, the tissue cells near the loop end are always living in a relatively hypoxic environment even at high total rates of organ blood flow (Fig. 5.7A). These cells are, therefore, unusually susceptible to damage if local blood flow falls. Exchange in the opposite direction will occur with any solutes that are taken up into the capillary loop from the interstitium and so are in higher concentration in the venous than the arterial plasma (Fig. 5.7B). This applies in the intestine to any absorbed nutrients and in the renal medulla to the urea and sodium that have been concentrated by selective movement out of the distal nephron. The result of this countercurrent exchange is to recycle solute from the venous into the arterial plasma and so limit the amount of solute entering the venous return. In the intestine, this ensures that absorbed nutrients do not reach the liver faster than they can be processed and stored. In the kidney, it provides a mechanism for retention of the medullary interstitial hyperosmolarity that is essential for regulation of urinary water loss.
Arteriovenous shunts
In the previous section we saw that the digits are a major site of heat loss from the body because of their large surface area:volume ratios. A similar thermoregulatory function is served by other small superficial tissues with large surface areas, the ears and the nose. In all these tissues, heat exchange is optimized further by the presence of short, large diameter vessels that connect large arterioles directly to large venules, bypassing the resistance of the smaller arterioles. These so-called arteriovenous anastomoses or shunts have thick walls containing circular bands of smooth muscle, which can shut off the lumen of the vessel entirely when contracted. Under those conditions, all flow takes place through capillaries. If the shunt muscle is relaxed, the low resistance of the shunt vessels means that total blood flow to these areas of the skin rises dramatically. Like other vascular smooth muscles, the shunt muscle receives a sympathetic innervation and, therefore, the withdrawal of sympathetic tone to the skin circulation that occurs during during body heating not only increases skin blood flow generally but in addition opens up the shunts.
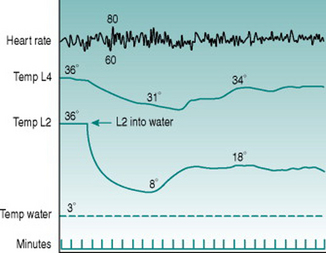
The shunt vessels subserve a second thermoregulatory function in that they open not only during heat stress but also when local skin temperature reaches a value just above freezing point. At these local temperatures, therefore, blood flow through the fingers, toes, ears and nose suddenly rises dramatically from the low values that are characteristic of skin perfusion during sympathetic vasoconstriction (Fig. 5.8). This response, termed cold vasodilatation, is potentially important for preventing freezing of peripheral tissues that cool down particularly rapidly. While this role imposes a significant extra thermal load on the body because it involves loss of additional body heat into the environment, this is minimized for the limbs, where total flow and, therefore, potential heat loss is highest, by the countercurrent recycling of arterial heat into the deep veins (see Countercurrent heat exchange, above).
The mechanism of cold vasodilatation is not well understood. Smooth muscle cells lose their contractile ability when they are cooled below about 10° C, so shunt opening could be due to this cold-induced paralysis overriding the constrictor effect of sympathetic nerve activity. Some data, however, suggest that this is too simplistic an explanation. For one thing, the increased local blood flow that accompanies shunt opening can rewarm the skin to values considerably above those that cause muscle paralysis, but this is not always followed by another bout of shunt closure and recooling. As well, when cooling is restricted to one digit, less exaggerated increases in blood flow occur synchronously in adjacent fingers (Fig. 5.8). Finally, some studies have reported deficits in cold vasodilatation in limbs with damaged innervation and noted that the response normalized as nerve regeneration took place. Together, these findings suggest that the cold vasodilator response may involve activation of dilator nerves rather than being a purely local phenomenon.
Berne RM, Levy MN. Hemodynamics, Cardiovascular Physiology. 8th edn, St Louis: Mosby, 2001
Panerai RB. The critical closing pressure of the cerebral circulation. Medical Engineering and Physics. 2003;25:621-632.
Woodman RJ, Kingwell BA, Beilin LJ, Hamilton SE, Dart AM, Watts G. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. American Journal of Hypertension. 2005;18:249-260.