CHAPTER 87 Vascular Surgery
CAROTID ENDARTERECTOMY
Indications
Vascular surgeons are particularly stringent about the indications for carotid endarterectomy because this is one of the few prophylactic operations. Indications for carotid endarterectomy are based on two large, multicenter, randomized trials comparing best medical therapy (antiplatelet) and surgical therapy for carotid stenosis. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) examined patients with a previous history of stroke, transient ischemic attack, or amaurosis fugax within 3 months of enrollment.1 During 2 years, the risk of stroke in patients with a 70% to 99% stenosis was reduced from 26% to 9% with carotid endarterectomy. The risk of stroke in patients with a 50% to 69% stenosis was reduced from 22% to 16% during 5 years. Given the less substantial benefit seen with the moderate-grade lesions, most vascular surgeons would recommend carotid endarterectomy to symptomatic patients with 50% to 69% stenosis only if they have a substantial life expectancy and low risk of complications related to the surgical intervention. Finally, subgroup analysis revealed an increased risk of stroke in medically treated patients with contralateral carotid occlusions and ulcerated plaques.
The Asymptomatic Carotid Atherosclerosis Study (ACAS) randomized asymptomatic patients to medical and surgical therapy.2 Carotid endarterectomy reduced the 5-year risk of stroke from 11% to 5% in patients with 60% to 99% stenosis compared with medical therapy. Given an absolute stroke risk reduction of 6% during 5 years, further studies have shown that surgical therapy is most beneficial in patients with 80% to 99% stenosis and in men compared with women. This equates to changing the outcome of approximately 1 in 20 to 30 patients, making the safety and risk reduction in the conduct of intervention for asymptomatic patients of paramount importance. This fact significantly affects the decision-making process regarding carotid imaging for either carotid endarterectomy or carotid stenting. In the ACAS trial, there was a 1.5% risk of stroke from diagnostic angiography alone.
Contraindications
Further studies examined carotid endarterectomy and found that it can be safely performed in patients deemed at high risk, including those 80 years or older and others with significant comorbid conditions, with combined stroke and mortality rates comparable to those found in the NASCET and ACAS studies. Contralateral occlusion was the only predictor for moderately increased perioperative risk of stroke and reduced long-term survival.3
Outcomes and Complications
One rare complication is hyperperfusion syndrome, which is characterized by a severe, unilateral headache occurring 3 to 7 days after endarterectomy. This has been attributed to the sudden restoration of normal arterial pressures in a vascular bed that has not seen pulsatility and normal pressures for a long period, thus leading to atrophy of the arteriolar restrictive mechanisms and impaired ability of the cerebrovascular circulation to autoregulate after the reestablishment of cranial blood flow. MRI may show reversible vasogenic edema similar to that observed in the posterior leukoencephalopathy syndrome.4 Treatment includes control of the hypertension that is frequently associated with this phenomenon to prevent cerebral hemorrhage until cerebral autoregulation is reestablished, typically for a few days to 1 week.
Imaging Findings
Preoperative Assessment
Duplex ultrasonography is the most common technique used to assess patients suspected of having extracranial cerebrovascular disease. B-mode ultrasonography is used to define location of the stenotic lesion, and Doppler examination is used to measure velocities across the stenosis (Fig. 87-1). B-mode ultrasonography not only delineates the location of a stenosis but also describes the characteristics of the plaque itself. Plaque ulceration as well as plaque calcification or hemorrhage may be seen. B-mode ultrasonography can also evaluate plaque echogenicity and the presence of thrombus. Soft, friable plaques on ultrasound examination are typically less stable than echoic plaques are. Mobile thrombus on ultrasound examination has been associated with an increased risk of stroke.
Velocity measurements across a stenosis are the most important aspect of the preoperative assessment for the vascular surgeon because these measurements are directly correlated with degree of stenosis. Strandness5 first reported a sensitivity of 99% and a specificity of 84% with duplex ultrasound criteria for the evaluation of carotid disease compared with conventional angiography (Table 87-1). More recently, it has been observed that diagnostic criteria must be altered in the setting of a contralateral severe stenosis or complete occlusion of the internal carotid artery because of a compensatory increase in carotid blood flow. By use of the criteria of AbuRahma,6 the accuracy of duplex ultrasound measurements can be increased to 96%. No matter which criteria are used, they must be validated by individual vascular laboratories as this technique may be operator and instrument dependent.
TABLE 87-1 Duplex Ultrasound Criteria for Diagnosis of Internal Carotid Artery Stenosis
| Stenosis | Criteria | |
|---|---|---|
| Strandness | AbuRahma | |
| Normal | PSV < 125 cm/sec | PSV < 125 cm/sec |
| No SB | No SB | |
| Flow reversal in bulb | ||
| 1%-15% | PSV < 125 cm/sec | PSV < 125 cm/sec |
| No or minimal SB | Minimal SB | |
| Flow reversal in bulb absent | ||
| 16%-49% | PSV > 125 cm/sec | PSV < 140 cm/sec |
| Marked SB | EDV < 140 cm/sec | |
| 50%-79% | PSV > 125 cm/sec | PSV ≥ 140 cm/sec |
| EDV < 140 cm/sec | EDV < 140 cm/sec | |
| 80%-99% | PSV < 125 cm/sec | PSV > 140 cm/sec |
| EDV > 140 cm/sec | EDV < 140 cm/sec | |
| Occlusion | No flow | No flow |
EDV, end-diastolic velocity; PSV, peak systolic velocity; SB, spectral broadening.
Modified from Strandness DE Jr. Extracranial arterial disease. In Strandness DE Jr (ed). Duplex Scanning in Vascular Disorders, 2nd ed. New York, Raven Press, 1993, pp 113-158; and AbuRahma AF, Richmond BK, Robinson PA, et al. Effect of contralateral severe stenosis or carotid occlusion on duplex criteria of ipsilateral stenoses: comparative study of various duplex parameters. J Vasc Surg 1995; 22:751-761.
CTA has been used to delineate extracranial cerebrovascular and carotid arch anatomy and has the advantages of minimal discomfort for the patient, relatively low radiation doses, and demarcation of calcific plaques in both the carotid arteries and the aortic arch. Recent three-dimensional reconstructions provide the surgeon with a greater ability in preoperative planning (Fig. 87-2).
MRA is another tool of the vascular surgeon and can be particularly useful in patients with an allergy to intravenous contrast material. Time-of-flight MRA has a tendency to overestimate the degree of stenosis because of local blood flow turbulence. Overestimation of time-of-flight MRA can be reconciled with the performance of gadolinium-enhanced three-dimensional MRA.7 Both techniques also demonstrate intracranial vessel anatomy along with patency of the communicating arteries.
Catheter angiography (Fig. 87-3), previously considered the gold standard for the assessment of extracranial cerebrovascular disease, has been used less frequently in uncomplicated patients because of the approximately 1.5% risk of stroke. Catheter angiography is now typically reserved for evaluation of patients in whom results from duplex ultrasonography and either CTA or MRA are discordant.
CAROTID-SUBCLAVIAN BYPASS
Description
Carotid-subclavian bypass may be performed through a remote cervical incision just lateral to the sternocleidomastoid muscle. The jugular vein is reflected medially to expose the common carotid artery. The anterior scalene muscle is then divided to expose the subclavian artery. Once proximal and distal control of each artery is obtained, bypass is typically performed with a prosthetic graft as patency is superior to autogenous conduit in this position.8 On occasion, concomitant disease of the ipsilateral common carotid artery precludes its use as an inflow vessel. In these cases, the contralateral common carotid may be used. The prosthetic graft would then be tunneled across the midline through the retropharyngeal space; this is a more direct path and avoids erosion of the overlying skin or interference with possible subsequent sternotomy or tracheostomy.
Outcomes and Complications
Outcomes from carotid-subclavian bypass are excellent, with 10-year patency rates of 84%.9 Complications range from a 0% to 1% stroke rate to a 0% mortality rate.10,11
Imaging Findings
Preoperative Assessment
Duplex ultrasonography may be used to screen for subclavian stenosis but is limited by the bony structures of the mediastinum. Its usefulness lies in its ability to assess for reversal of blood flow in the vertebral artery with upper extremity exercise (Fig. 87-4) and the adequacy of the common carotid artery for inflow.
Preoperative Planning
Once a subclavian stenosis has been identified, preoperative planning may be accomplished with CTA or MRA (Fig. 87-5). Both evaluate the exact location of the lesion as well as assess possible inflow and outflow vessels. Full appraisal of the aortic arch, its branches, and neck extracranial cerebrovascular vessels is necessary to rule out other pathologic processes. Resolution of the aortic arch and these large-caliber vessels with CTA and MRA is excellent, and they may replace standard angiography.
Digital subtraction angiography may be a useful adjunct for preoperative planning before carotid subclavian bypass. Angiography can identify major inflow and outflow vessels as well as important collaterals (Fig. 87-6).
THORACOABDOMINAL AORTIC ANEURYSM REPAIR
Description
Thoracoabdominal aortic aneurysm (TAAA) repair is performed through a retroperitoneal approach combined with thoracotomy. Intraoperative management of patients with thoracoabdominal aneurysms is dependent on the extent of the aneurysmal degeneration of the aorta. Patients with Crawford extent I and II TAAAs generally require either continuous distal perfusion or left-sided heart bypass; these types of aneurysms are associated with the greatest risk of paraplegia (Fig. 87-7). In the thorax, the recurrent laryngeal and vagus nerves are gently retracted off from the aorta. After cross-clamping of the aorta, the proximal anastomosis is sewn in an endoaneurysmal fashion (end-to-end within the aneurysm sac). All large intercostal arteries from T7 to L2 are reimplanted into the graft, followed by the visceral vessels. If stenoses at the origin of these arteries are encountered, an endarterectomy may be performed. Finally, the distal anastomosis is performed in endoaneurysmal fashion to an uninvolved portion of the distal aorta.
Contraindications
TAAA repair represents one of the most morbid vascular surgical procedures not only because of the risk of paraplegia (up to 13% electively) but also because of a mortality rate of 8%.12 Patients with prohibitive cardiopulmonary risk are not candidates for this procedure. These patients are typically identified on preoperative cardiac stress testing as well as on preoperative pulmonary function testing. Relative contraindications include decreased life expectancy related to other medical issues.
Outcomes and Complications
The 30-day survival after TAAA repair is approximately 95%. Late survival rates are 55% at 5 years, 29% at 10 years, and 21% at 15 years.12 Complications related to the surgery are typically pulmonary (32%), cardiac (8%), renal (6%), and spinal (4% to 13%) in nature. Spinal cord ischemia is significantly increased in Crawford extent I and II aneurysms.
THORACIC AORTIC ANEURYSM HYBRID PROCEDURES
Description
Thoracic aortic aneurysm (TAA) repair is a morbid procedure because of the necessity for a median sternotomy or posterolateral thoracotomy. Whereas endovascular TAA repair avoids the need for these approaches, anatomic factors such as small access vessels may preclude this. In these cases, hybrid TAA repair may be performed by creating open vascular surgical access to the aorta either distally or proximally. In the case of distal open access, an aortofemoral bypass may be performed either alone or in conjunction with concomitant debranching or repair of an aortic aneurysm (Fig. 87-8). The common iliac artery is oversewn, allowing retrograde blood flow through the aortofemoral limb to the internal iliac artery through the external iliac artery. In the case of proximal access, the arch vessels are debranched from the aorta and reanastomosed to a four-branched prosthetic graft that is anastomosed directly to the ascending aorta (Fig. 87-9). The sheath is then advanced through one of the limbs of the graft, and the TAA is repaired in standard endovascular fashion. At the conclusion of the case, the access limb of the graft is oversewn.
Contraindications
Contraindications to hybrid TAA repair include cardiopulmonary risk factors as well as anatomic factors of the thoracic aneurysm. Currently, only three devices have been approved by the Food and Drug Administration for endovascular TAA repair (Cook TX, Gore TAG, and Medtronic Talent devices). Anatomic requirements include 20 mm or more of proximal and distal neck length and distal neck diameters of 20 to 42 mm. In cases in which the left subclavian artery needs to be covered with the endovascular stent graft for proximal fixation, contraindications to covering the left subclavian artery with the endograft include a patent left internal mammary artery to left anterior descending coronary artery bypass, incomplete circle of Willis, and dominant left vertebral artery. These factors increase the risk of myocardial infarction and stroke.13 Otherwise, most patients have enough collateral circulation to prevent left upper extremity ischemia, with claudication developing in approximately 16% and rest pain in 5%.14 In patients with upper extremity rest pain or tissue loss, a left carotid–subclavian bypass may be performed.
Outcomes and Complications
Outcomes of hybrid TAA repairs are similar to those of standard endovascular TAA repair. In the perioperative period, 10% of patients require reintervention; freedom from reintervention approaches 81% by 48 months.15 Spinal cord ischemic complications, which may occasionally be transient, are experienced by 7% of patients. The perioperative mortality rate is approximately 10% and is half that of an open repair.
Imaging Findings
ABDOMINAL AORTIC ANEURYSM REPAIR
Indications
The size at which asymptomatic AAA repair is indicated is determined by a composite of the morphology and anatomy of the aneurysm, the medical comorbidities of the patient, and the morbidity and mortality demonstrated by the surgeon. Broadly speaking, 5.5 cm is the size at which the risk of death from the surgical procedure (1% to 5%) is outweighed by the annual risk of rupture (5% to 10%). Other patient groups who may be repaired before this include women with 5-cm AAAs, who have a higher risk of rupture with smaller aneurysm size, and patients with a rapidly expanding AAA (>1 cm per year). According to the Aneurysm Detection and Management (ADAM) study16 and the U.K. Small Aneurysm Trial,17 AAAs between 4.5 and 5.5 cm can be safely observed in compliant patients as the risk of open surgery outweighs the risk of rupture. Symptomatic AAAs presenting with abdominal or back pain are repaired immediately as discussed previously for thoracoabdominal aneurysms.
Outcomes and Complications
Late complications of AAA repair occur in approximately 7% of patients within 5 years. Aortic graft infection is a dreaded complication. Patients frequently present with fever, bacteremia, or failure to thrive. CT findings include air or fluid around the graft and inflammatory changes in normal tissue planes (Fig. 87-10). Patients undergoing AAA repair may normally have perigraft fluid up to 6 months postoperatively. Differentiation between a graft infection and normal postoperative fluid collections can be accomplished with CT-guided aspiration or indium In 111–tagged white blood cell nuclear scintigraphy.
Anastomotic pseudoaneurysms occur in 0.2% of aortic anastomoses, 1.2% of iliac anastomoses, and 3% of femoral anastomoses.18 This incidence increases over time, which stresses the importance of follow-up in young patients undergoing AAA repair.
Imaging Findings
Preoperative Assessment
Ultrasonography is the primary modality for the identification of abdominal aortic aneurysmal disease because of its low cost and ease of detection. Studies have shown that it is helpful not only in patients with pulsatile abdominal masses but also in generalized screening of men older than 60 years19 or those with a family history of AAA. CT provides more accurate assessment of the diameter as well as extent of aneurysmal degeneration of the aorta and is particularly helpful in aneurysms approaching 4.5 cm.
Preoperative Planning
Similar to preoperative TAAA planning, CTA is often the preferred modality for preoperative planning before AAA repair. Not only does it provide a more accurate diameter measurement than ultrasonography, but it assesses the entire aorta for pathologic changes (Fig. 87-11A). CTA can identify heavy calcification, accessory renal vessels, and retroaortic left renal veins, all of which have implications for intraoperative clamp placement. The recent improvements in three-dimensional image postprocessing and reconstruction of CTA data have enhanced the ability of the vascular surgeon to assess aortic disease and to plan open repair by longitudinally distinguishing intraluminal thrombus from calcification as well as noncircumferential dilation of the aortic wall that might not be as easily seen on standard axial CT images.
Postoperative Surveillance
Postoperative assessment of AAA repair is accomplished almost exclusively with CTA (Fig. 87-11B). Open AAA repairs are typically scanned once at 6 to 12 months after surgery, if there are no anatomic concerns. Further scanning is indicated for the development of late symptoms. CTA is helpful not only in assessing the durability of the repair but also for identifying aneurysmal degeneration of the remaining abdominal aorta and its branches, especially the iliac arteries (Fig. 87-12). This becomes clinically relevant beyond 3 mm of dilation and is seen in approximately 13% of patients.20
ABDOMINAL AORTIC ANEURYSM HYBRID PROCEDURES
Description
In patients with AAA and bilateral common iliac artery aneurysms, hybrid repair may be performed by a femorofemoral bypass, followed by an aorto–uni-iliac endograft and then either a contralateral external to internal iliac covered stent or ligation of the contralateral common iliac artery (Fig. 87-13).
Indications
Hybrid procedures for AAA repair may be performed for technical issues when the femoral or iliac access vessels are smaller than the EVAR device diameter, heavily calcified, or tortuous. Hybrid AAA repair may also be performed in patients with concomitant bilateral common iliac artery aneurysms and inadequate distal landing zones (Fig. 87-13A and B). In these patients, standard EVAR covering both internal iliac arteries with the endograft limbs would lead to a high likelihood of pelvic ischemia. Hybrid repair is useful in these situations to avoid the morbidity of an open repair.
Hybrid repairs may also be necessary when there is abnormally low takeoff of a renal vessel or a dominant accessory renal artery (Fig. 87-14). In these situations, an aortorenal or iliorenal bypass may be necessary before the placement of the endograft.
AORTOBIFEMORAL BYPASS
Outcomes and Complications
Outcomes of aortobifemoral bypass are excellent, with 5-year patency rates ranging from 85% to 95%. Complications occur infrequently and are typically cardiopulmonary and renal in nature. One unique complication related to this procedure is the development of thigh and buttock claudication in up to 30% of patients undergoing aortobifemoral bypass.21 This occurs because of a lack of blood flow in the hypogastric system and may be increased in patients with an end-to-end proximal anastomosis. Operative mortality rates range from 2% to 3%.
Imaging Findings
Preoperative Planning
Angiography is the gold standard for preoperative planning because it can show the precise pattern of disease and may assess the patency of the internal iliac arteries. In cases of complete aortic or bilateral iliac artery occlusion, brachial access may be necessary to evaluate the aorta proximal to the occlusion (Fig. 87-15).
Postoperative Surveillance
Postoperative surveillance may be performed with noninvasive vascular laboratory testing. Duplex ultrasonography can be used to assess graft patency, although this may be limited by bowel gas. More detailed information about graft patency is typically obtained with CTA or MRA. In patients without an iodinated contrast agent allergy, CTA has the advantage of being performed typically in a timelier manner. Compromise of graft patency requires angiographic assessment for anastomotic or intraluminal narrowing amenable to open or endovascular therapies. Finally, more distal peripheral vascular disease may lead to increased resistance within a graft limb and subsequent thrombosis (Fig. 87-16). Evaluation of lower extremity blood flow may be prudent in patients at risk for graft failure.
LOWER EXTREMITY BYPASS
Outcomes and Complications
Outcomes of lower extremity bypass are summarized in Table 87-2. The patency rates of this meta-analysis show that vein bypasses are the conduit of choice.
TABLE 87-2 Summary of Patency Rates in Patients Undergoing Lower Extremity Bypass
| Type of Bypass | Primary 5-Year Patency (%) |
|---|---|
| Above-knee vein bypass | 80 |
| Above-knee prosthetic bypass | 66 |
| Below-knee vein bypass | 72 |
| Below-knee prosthetic bypass | 38 |
Complications of lower extremity bypass can be divided into graft-related and non–graft-related categories. Graft thrombosis is the most common complication and a major reason for postoperative graft surveillance (Fig. 87-17). Early graft thrombosis (0 to 30 days) is most likely due to technical error in either performance of the bypass or determination of adequacy of the runoff. Mid graft thrombosis (30 days to 2 years) is typically due to intimal hyperplasia. Late graft thrombosis is usually due to progression of atherosclerosis. Graft infection is another graft-related complication. Infections of autogenous grafts may be treated with antibiotic therapy. Attempts at salvage of infected prosthetic grafts can be made, although these frequently need to be excised, especially with involvement of an anastomosis. Fluid collections surrounding a graft on duplex ultrasonography or CTA may be suggestive of graft infection (Fig. 87-18). Non–graft-related complications are typically cardiopulmonary or renal in nature.
Imaging Findings
Preoperative Assessment
Doppler ankle pressure measurements are the most commonly used noninvasive vascular laboratory tests to assess for peripheral vascular disease. The ankle-brachial index is determined by dividing the systolic blood pressure in the brachial artery by the systolic blood pressure at the ankle (the higher of the two values for the dorsalis pedis artery and the posterior tibial artery). The ankle-brachial index has been correlated to degree of symptoms (Table 87-3). The ankle-brachial index may be falsely elevated in heavily calcified arteries, as is typically seen in diabetic patients. Finally, in patients with claudication, the ankle-brachial index may be normal at rest but decreased with exercise. An abnormal exercise response is defined as a 20% decrease from baseline or more than 3 minutes to recover to baseline.
TABLE 87-3 Ankle-Brachial Indices and Correlation to Symptoms
| Symptom | Ankle-Brachial Index |
|---|---|
| Normal | 0.80-1.0 |
| Claudication | 0.50-0.60 |
| Critical ischemia | <0.30 |
Measurement of pulse volume recordings in the lower extremity is a useful technique to identify significant stenosis in the lower extremity (Fig. 87-19). Blunting of the normal cardiac cycle waveform is typically seen distal to a hemodynamically significant stenosis.
Transcutaneous oxygen tension (TcPo2) measurements are particularly helpful in determining the ability of a wound to heal (Table 87-4). TcPo2 quantifies oxygen molecules transferred to the skin after it is heated with a transducer above 40°C. TcPo2 can be in the form of absolute oxygen tension or regional index, the TcPo2 of the leg divided by the TcPo2 measured at a reference point (chest). This technique has also been shown to be useful in diabetic patients, in whom the ankle-brachial index is unreliable.22
TABLE 87-4 Transcutaneous Oxygen Measurements and Correlation to Symptoms
| Symptom | Transcutaneous Oxygen Tension (absolute) | Transcutaneous Oxygen Tension (regional index) |
|---|---|---|
| Likely to heal | 35-40 mm Hg | >0.6 |
| Borderline or delayed healing | 25-35 mm Hg | 0.4-0.6 |
| Unlikely to heal | <20-25 mm Hg | <0.4 |
Preoperative Planning
Preoperative planning for a lower extremity bypass is typically performed by use of catheter angiography, but CTA and MRA have emerged as suitable preoperative planning examinations at some sites. Preoperative planning requires the vascular surgeon to identify the exact location of stenoses but also to assess the inflow (site of proximal anastomosis), outflow (site of distal anastomosis), and runoff vessels (arterial tree beyond the distal anastomosis) (Fig. 87-20). Runoff scores have been shown to be one of the strongest predictors for bypass patency and are a key consideration during angiography.23
Preoperative vein mapping with duplex ultrasonography can provide detailed information about the quality of a potential vein graft. It can detect constriction of veins secondary to sclerosis, previous manipulation, or thrombophlebitis. It can also assess length and diameter of the vein. These measurements can be particularly important in performing a tibial bypass for which a long segment may be necessary. Diameters of more than 3 mm for reverse24 and 2 mm for in situ25 saphenous vein grafts have been recommended to obtain adequate long-term patency.
Postoperative Surveillance
Long-term follow-up of lower extremity bypasses is aimed at early identification of a failing graft and is critical to the long-term success of all lower extremity bypasses as restenosis is frequent. Duplex ultrasonography is frequently used to interrogate a bypass and to identify areas of stenosis with increased velocities (Fig. 87-21). Duplex ultrasonography may also show significantly decreased velocities throughout the graft that may be related to a distal anastomotic stenosis or worsening of the runoff arterial bed. Once a failing graft is identified, angiography must be performed to more accurately delineate the problem. Revision may be performed with both open and endovascular techniques.
AORTOILIAC AND LOWER EXTREMITY OCCLUSIVE DISEASE HYBRID PROCEDURES
Imaging Findings
Preoperative Planning
Duplex ultrasonography or arteriography of the femoral arteries typically shows heavy calcification (Fig. 87-22).
Postoperative Surveillance
KEY POINTS
 Different imaging modalities are relevant at different points during the course of vascular surgical disease management. An understanding of their utility at each point is key to the care of the vascular surgical patient.
Different imaging modalities are relevant at different points during the course of vascular surgical disease management. An understanding of their utility at each point is key to the care of the vascular surgical patient. Duplex ultrasonography is useful in the preoperative and postoperative care of patients with carotid artery disease as well as in the follow-up of lower extremity bypass.
Duplex ultrasonography is useful in the preoperative and postoperative care of patients with carotid artery disease as well as in the follow-up of lower extremity bypass. Three-dimensional CT angiography is becoming the preferred modality for preoperative planning of aortic aneurysmal disease because of its ability to provide accurate diameter measurements as well as to assess for other aortic disease.
Three-dimensional CT angiography is becoming the preferred modality for preoperative planning of aortic aneurysmal disease because of its ability to provide accurate diameter measurements as well as to assess for other aortic disease.Ascher E, Marks N. Preprocedural imaging: new options to reduce need for contrast angiography. Semin Vasc Surg. 2007;20:15-28.
Beebe HG, Kritpracha B. Imaging of abdominal aortic aneurysm: current status. Ann Vasc Surg. 2003;17:111-118.
Chiles C, Carr JJ. Vascular diseases of the thorax: evaluation with multidetector CT. Radiol Clin North Am. 2005;43:543-569.
Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334:1257.
Fillinger MF. New imaging techniques in endovascular surgery. Surg Clin North Am. 1999;79:451-475.
Hiatt MD, Fleischmann D, Hellinger JC, Rubin GD. Angiographic imaging of the lower extremities with multidetector CT. Radiol Clin North Am. 2005;43:1119-1127.
Ho VB, Corse WR. MR angiography of the abdominal aorta and peripheral vessels. Radiol Clin North Am. 2003;41:115-144.
Martin ML, Tay KH, Flak B, et al. Multidetector CT angiography of the aortoiliac system and lower extremities: a prospective comparison with digital subtraction angiography. AJR Am J Roentgenol. 2003;180:1085-1091.
Nederkoorn PJ, van der Graaf Y, Hunink MG. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review. Stroke. 2003;34:1324-1332.
Seifert B, Struwe A, Heilmaier C, et al. Assessment of aortoiliac and renal arteries: MR angiography with parallel acquisition versus conventional MR angiography and digital subtraction angiography. Radiology. 2007;245:276-284.
1 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
2 Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421-1428.
3 Reed AB, Gaccione P, Belkin M, et al. Preoperative risk factors for carotid endarterectomy: defining the patient at high risk. J Vasc Surg. 2003;37:1191-1199.
4 Karapanayiotides T, Meuli R, Devuyst G, et al. Postcarotid endarterectomy hyperperfusion or reperfusion syndrome. Stroke. 2005;36:21-26.
5 Strandness DEJr. Extracranial arterial disease. In: Strandness DEJr, editor. Duplex Scanning in Vascular Disorders. 2nd ed. New York: Raven Press; 1993:113-158.
6 AbuRahma AF, Richmond BK, Robinson PA, et al. Effect of contralateral severe stenosis or carotid occlusion on duplex criteria of ipsilateral stenoses: comparative study of various duplex parameters. J Vasc Surg. 1995;22:751-761.
7 Remonda L, Senn P, Barth A, et al. Contrast-enhanced 3D MR angiography of the carotid artery: comparison with conventional digital subtraction angiography. AJNR Am J Neuroradiol. 2002;23:213-219.
8 Ziomek S, Quiñones-Baldrich WJ, Busuttil RW, et al. The superiority of synthetic arterial grafts over autologous veins in carotid-subclavian bypass. J Vasc Surg. 1986;3:140-145.
9 Kline RA, Kazmers A, Friedland MS. Cervical reconstruction of the supra-aortic trunks: a 16-year experience. J Vasc Surg. 1999;29:239-246.
10 AbuRahma AF, Bates MC, Stone PA, et al. Angioplasty and stenting versus carotid-subclavian bypass for the treatment of isolated subclavian artery disease. J Endovasc Ther. 2007;14:698-704.
11 AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32:411-418.
12 Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg. 2007;83:S856-S861.
13 Feezor RJ, Martin TD, Hess PJ, et al. Risk factors for perioperative stroke during thoracic endovascular aortic repairs (TEVAR). J Endovasc Ther. 2007;14:568-573.
14 Riesenman PJ, Farber MA, Mendes RR, et al. Coverage of the left subclavian artery during thoracic endovascular aortic repair. J Vasc Surg. 2007;45:90-94.
15 Stone DH, Brewster DC, Kwolek CJ, et al. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006;44:1188-1197.
16 Aneurysm Detection and Management Veterans Affairs Cooperative Study Group. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444.
17 United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452.
18 Szilagyi DE, Smith RF, Elliott JP, et al. Anastomotic aneurysms after vascular reconstruction: problems of incidence, etiology, and treatment. Surgery. 1975;78:800-816.
19 Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531-1539.
20 Falkensammer J, Oldenburg WA, Biebl M, et al. Abdominal aortic aneurysm neck remodeling after open aneurysm repair. J Vasc Surg. 2007;45:900-905.
21 Jaquinandi V, Picquet J, Bouyé P, et al. High prevalence of proximal claudication among patients with patent aortobifemoral bypasses. J Vasc Surg. 2007;45:312-318.
22 Williams DT, Price P, Harding KG. The influence of diabetes and lower limb arterial disease on cutaneous foot perfusion. J Vasc Surg. 2006;44:770-775.
23 Seeger JM, Pretus HA, Carlton LC, et al. Potential predictors of outcome in patients with tissue loss who undergo infrainguinal vein bypass grafting. J Vasc Surg. 1999;30:427-435.
24 Wengerter KR, Veith FJ, Gupta SK, et al. Influence of vein size (diameter) on infrapopliteal reversed vein graft patency. J Vasc Surg. 1990;11:525-531.
25 Bergamini TM, Towne JB, Bandyk DF, et al. Experience with in situ saphenous vein bypasses during 1981 to 1989: determinant factors of long-term patency. J Vasc Surg. 1991;13:137-147.

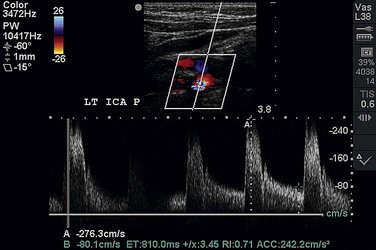
 FIGURE 87-1
FIGURE 87-1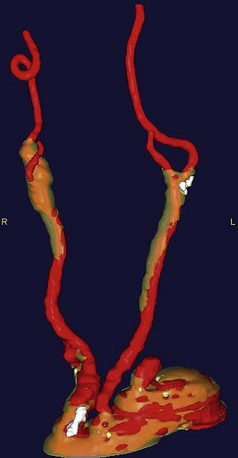
 FIGURE 87-2
FIGURE 87-2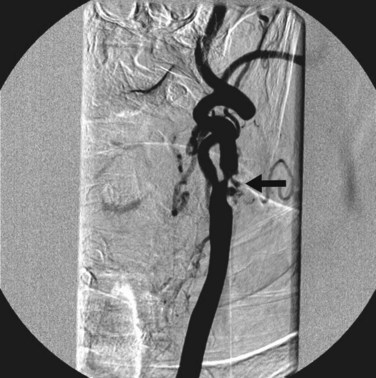
 FIGURE 87-3
FIGURE 87-3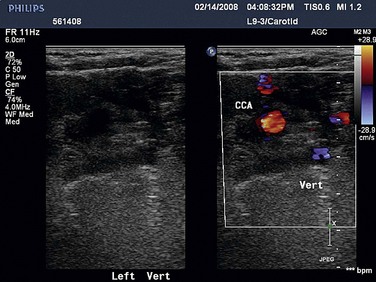
 FIGURE 87-4
FIGURE 87-4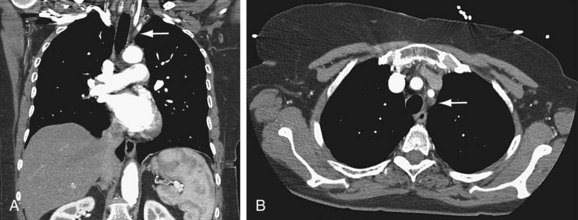
 FIGURE 87-5
FIGURE 87-5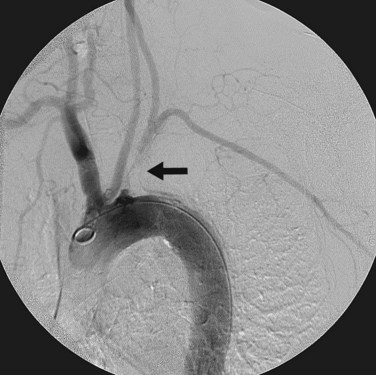
 FIGURE 87-6
FIGURE 87-6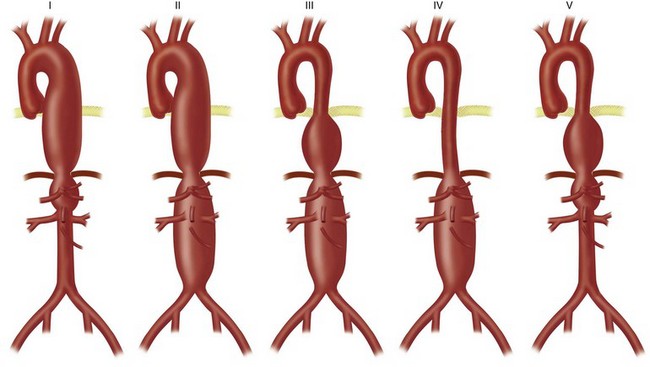
 FIGURE 87-7
FIGURE 87-7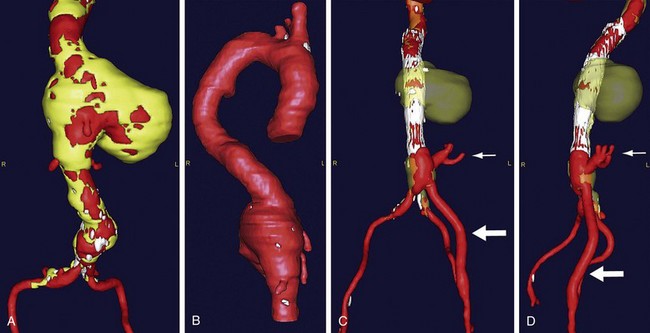
 FIGURE 87-8
FIGURE 87-8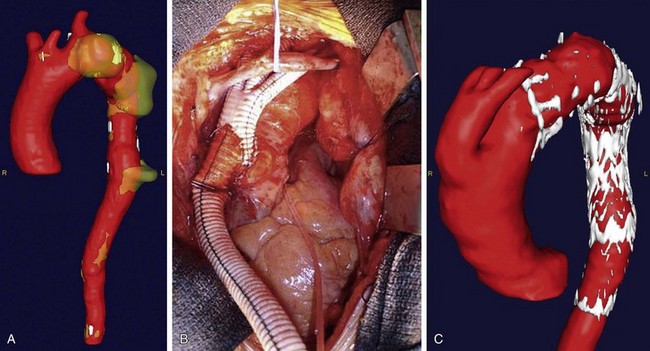
 FIGURE 87-9
FIGURE 87-9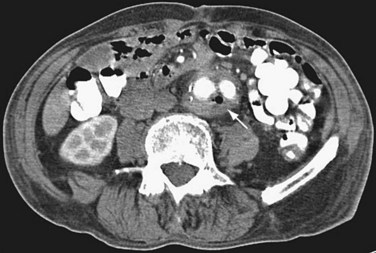
 FIGURE 87-10
FIGURE 87-10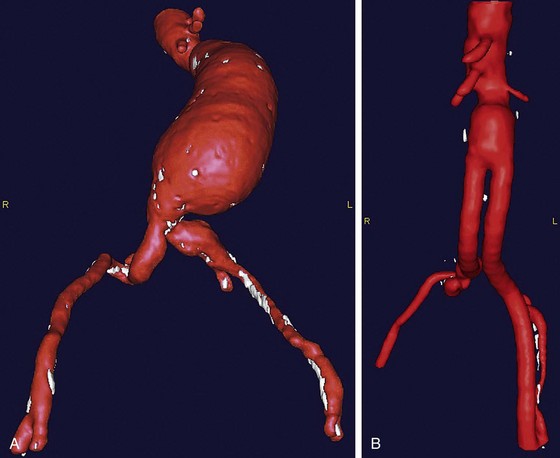
 FIGURE 87-11
FIGURE 87-11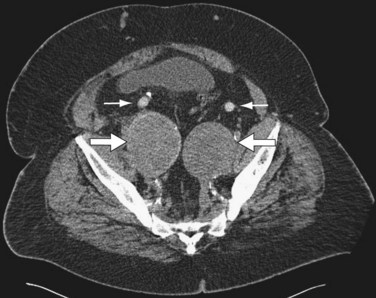
 FIGURE 87-12
FIGURE 87-12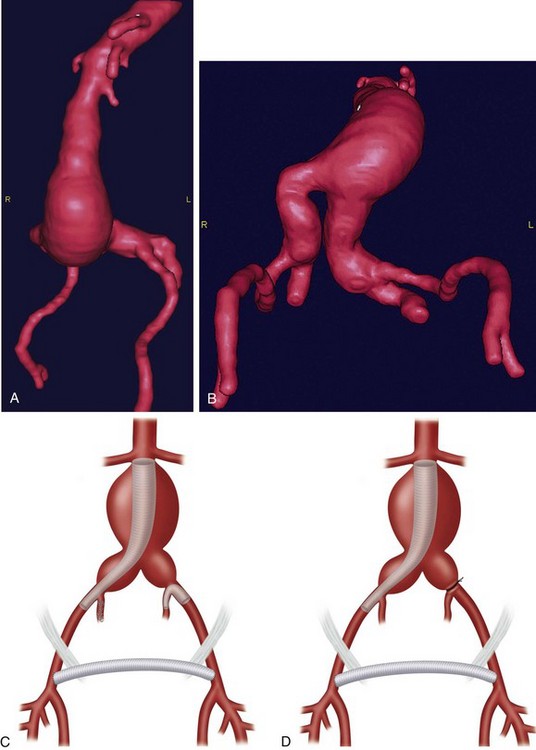
 FIGURE 87-13
FIGURE 87-13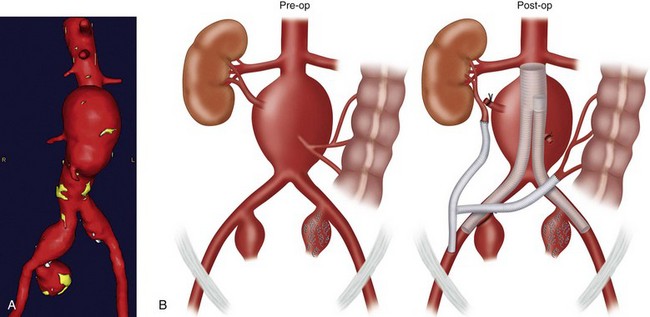
 FIGURE 87-14
FIGURE 87-14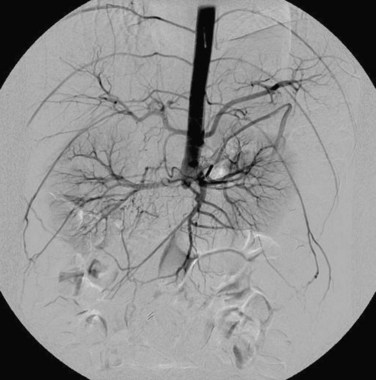
 FIGURE 87-15
FIGURE 87-15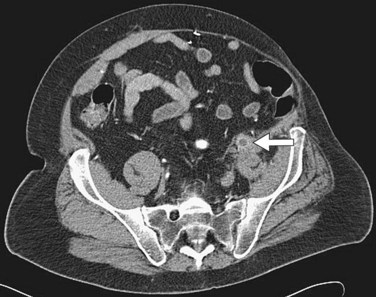
 FIGURE 87-16
FIGURE 87-16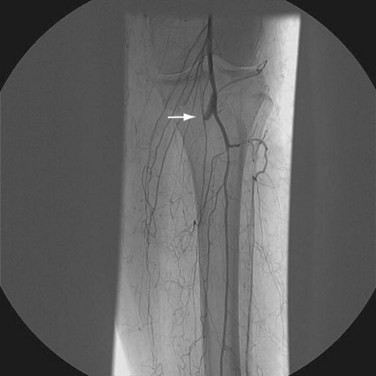
 FIGURE 87-17
FIGURE 87-17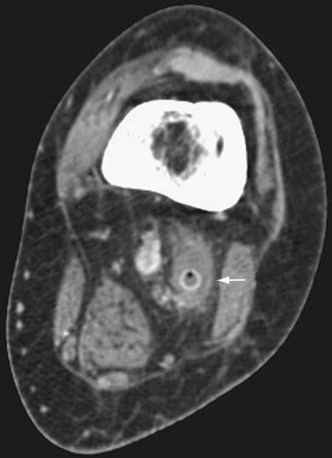
 FIGURE 87-18
FIGURE 87-18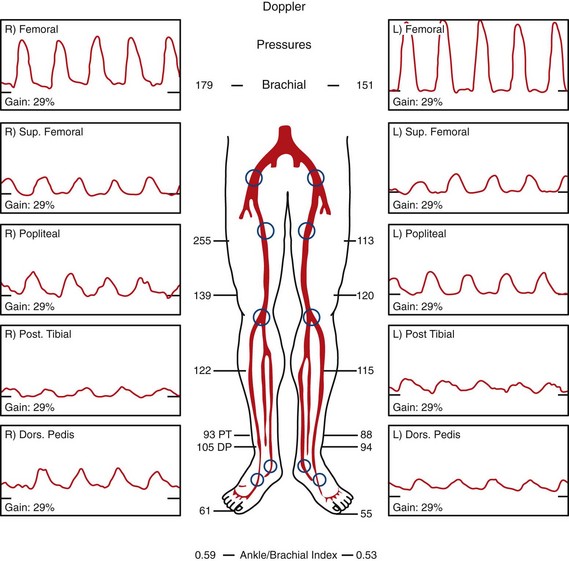
 FIGURE 87-19
FIGURE 87-19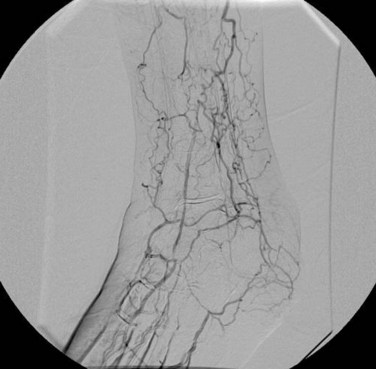
 FIGURE 87-20
FIGURE 87-20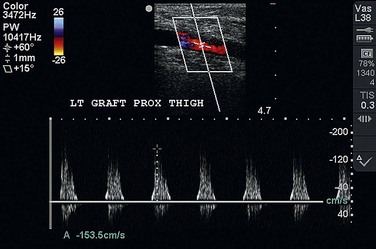
 FIGURE 87-21
FIGURE 87-21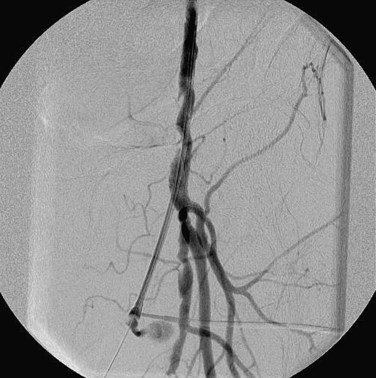
 FIGURE 87-22
FIGURE 87-22

