Chapter 6 Vascular Supply of the Brain and Spinal Cord
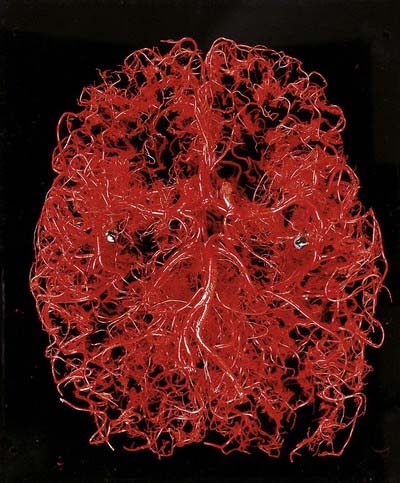
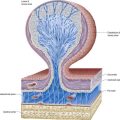
The brain is a highly vascular organ and its profuse blood supply is characterized by a densely branching arterial network (Fig. 6.1). It has high metabolic activity due in part to the energy requirements of constant neural activity. It demands about 15% of the cardiac output and uses 25% of the total oxygen consumed by the body. The brain is supplied by two internal carotid arteries and two vertebral arteries that form a complex anastomosis (circulus arteriosus, or the circle of Willis) on the base of the brain. Vessels diverge from this anastomosis to supply the various cerebral regions. In general, the internal carotid arteries and the vessels arising from them supply the forebrain, with the exception of the occipital lobe of the cerebral hemisphere; the vertebral arteries and their branches supply the occipital lobe, the brain stem and the cerebellum. Venous blood from the brain drains into sinuses within the dura mater. Acute interruption of the blood supply to the brain for more than a few minutes causes permanent neurological damage. Such ischaemic strokes along with intracranial haemorrhages are major sources of morbidity and mortality.
Arterial Supply of the Brain
Internal Carotid Artery
The internal carotid artery (Figs 6.2, 6.3) arises from the bifurcation of the common carotid artery, ascends in the neck and enters the carotid canal of the temporal bone. Its subsequent course is said to have petrous, cavernous and cranial parts.
Cerebral Part
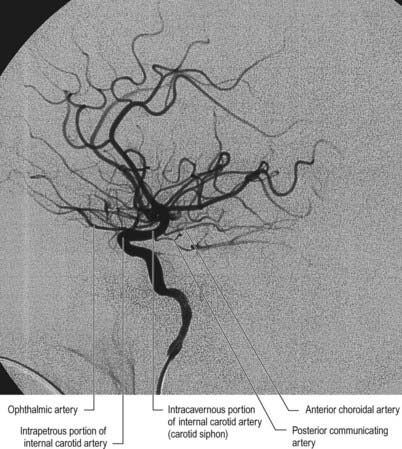
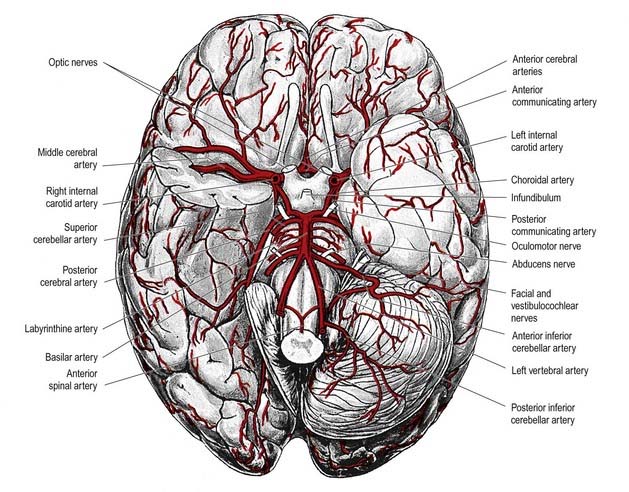
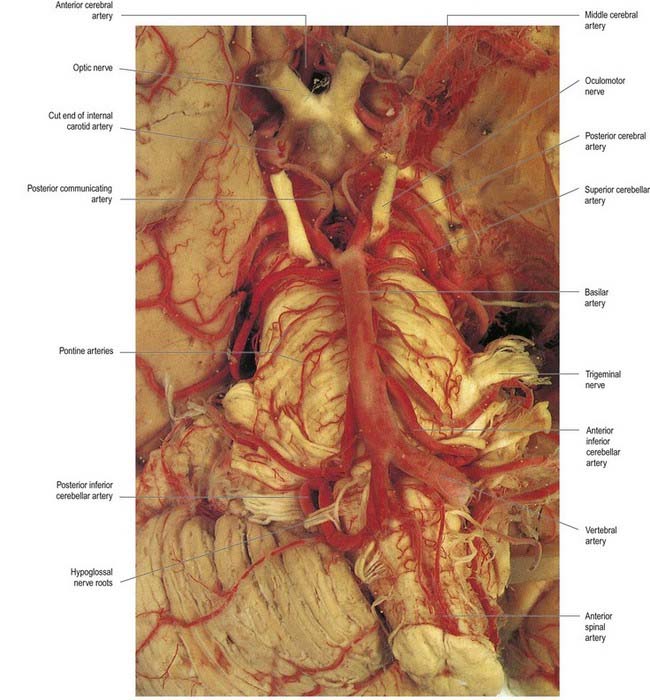
Several preterminal vessels leave the cerebral portion of the internal carotid. The ophthalmic artery arises from the internal carotid as it leaves the cavernous sinus, often at the point of piercing the dura, and enters the orbit through the optic canal. The posterior communicating artery (Figs 6.4–6.6) runs back from the internal carotid above the oculomotor nerve and anastomoses with the posterior cerebral artery (which is a terminal branch of the basilar artery), thereby contributing to the circulus arteriosus around the interpeduncular fossa. The posterior communicating artery is usually very small. Sometimes, however, it is so large that the posterior cerebral artery is supplied via the posterior communicating artery rather than the basilar artery (‘fetal posterior communicating artery’). It is often larger on one side. Small branches from its posterior half pierce the posterior perforated substance, together with branches from the posterior cerebral artery. Collectively, they supply the medial thalamic surface and walls of the third ventricle. The anterior choroidal artery leaves the internal carotid near its posterior communicating branch and passes back above the medial part of the uncus. It crosses the optic tract to reach and supply the crus cerebri of the midbrain; it then turns laterally, recrosses the optic tract and gains the lateral side of the lateral geniculate body, which it supplies with several branches. It finally enters the inferior horn of the lateral ventricle via the choroid fissure and ends in the choroid plexus. This small but important vessel also contributes to the blood supply of the globus pallidus, caudate nucleus, amygdala, hypothalamus, tuber cinereum, red nucleus, substantia nigra, posterior limb of the internal capsule, optic radiation, optic tract, hippocampus and fimbria of the fornix.
Anterior Cerebral Artery
The anterior cerebral artery is the smaller of the two terminal branches of the internal carotid artery (see Figs 6.5, 6.6).
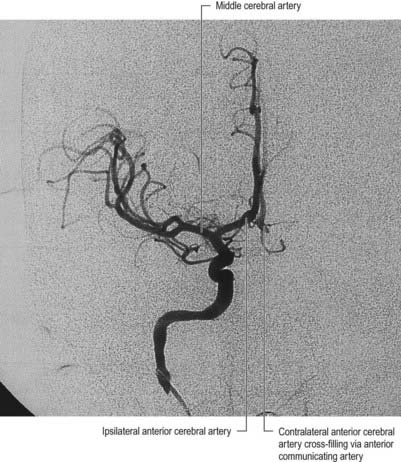
The two anterior cerebral arteries travel together in the great longitudinal fissure. They pass around the curve of the genu of the corpus callosum (Fig. 6.7) and then along its upper surface to its posterior end, where they anastomose with posterior cerebral arteries. They give off cortical and central branches.
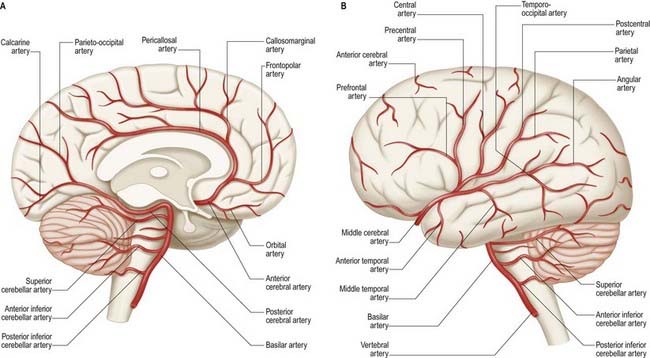
The cortical branches of the anterior cerebral artery are named by distribution. Two or three orbital branches ramify on the orbital surface of the frontal lobe and supply the olfactory cortex, gyrus rectus and medial orbital gyrus. Frontal branches supply the corpus callosum, cingulate gyrus, medial frontal gyrus and paracentral lobule. Parietal branches supply the precuneus, and the frontal and parietal branches both send twigs over the superomedial border of the hemisphere to supply a strip of territory on the superolateral surface (Fig. 6.8). Cortical branches of the anterior cerebral artery, therefore, supply the areas of the motor and somatosensory cortices that represent the lower limb.
Central branches of the anterior cerebral artery arise from its proximal portion and enter the anterior perforated substance (see Fig. 6.6) and lamina terminalis. Collectively, they supply the rostrum of the corpus callosum, the septum pellucidum, the anterior part of the putamen, the head of the caudate nucleus and adjacent parts of the internal capsule. Immediately proximal or distal to its junction with the anterior communicating artery, the anterior cerebral artery gives rise to the medial striate artery, which supplies the anterior part of the head of the caudate nucleus and adjacent regions of the putamen and internal capsule.
Middle Cerebral Artery
The middle cerebral artery is the larger terminal branch of the internal carotid.
The middle cerebral artery runs first in the lateral cerebral fissure, then posterosuperiorly on the insula; it divides into branches distributed to this and the adjacent lateral cerebral surface (see Figs 6.5, 6.6, 6.8). Like the anterior cerebral artery, it has cortical and central branches.
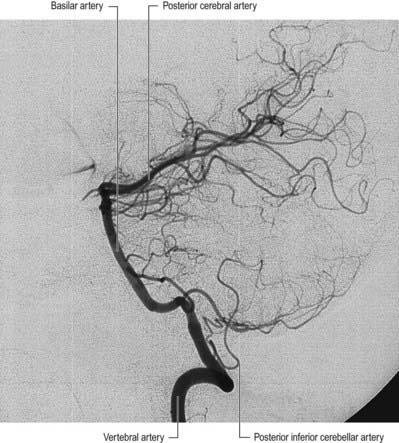
Vertebral Artery
The vertebral arteries and their major branches (sometimes referred to as the vertebrobasilar system) essentially supply blood to the upper spinal cord, brain stem, cerebellum and occipital lobe of the cerebrum (Figs 6.9, 6.10). In addition, other branches have a wider distribution.
The vertebral arteries are derived from the subclavian arteries. They ascend through the neck in the foramina transversaria of the upper six cervical vertebrae and enter the cranial cavity through the foramen magnum, close to the anterolateral aspect of the medulla (see Fig. 6.5). They converge medially as they ascend the medulla and unite to form the midline basilar artery at approximately the level of the junction between the medulla and the pons.
Basilar Artery
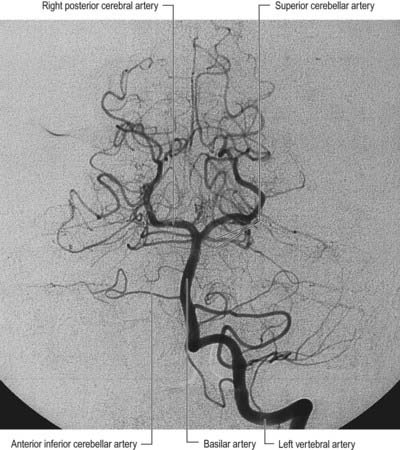
This large median vessel is formed by the union of the vertebral arteries at the mid-medullary level and extends to the upper border of the pons (see Figs 6.5, 6.6). It lies in the pontine cistern and follows a shallow median groove on the ventral pontine surface. The basilar artery terminates by dividing into two posterior cerebral arteries at a variable level, but most frequently in the interpeduncular cistern, behind the dorsum sellae.
The anterior inferior cerebellar artery (see Fig. 6.6) is given off from the lower part of the basilar artery and runs posterolaterally, usually ventral to the abducens, facial and vestibulocochlear nerves. It commonly exhibits a loop into the internal acoustic meatus below the nerves, and when this occurs, the labyrinthine artery may arise from the loop. The anterior inferior cerebellar artery supplies the inferior cerebellar surface anterolaterally and anastomoses with the posterior inferior cerebellar branch of the vertebral artery. A few branches supply the inferolateral parts of the pons and occasionally also supply the upper medulla oblongata.
The superior cerebellar artery (see Fig. 6.6) arises near the distal portion of the basilar artery, immediately before the formation of the posterior cerebral arteries. It passes laterally below the oculomotor nerve, which separates it from the posterior cerebral artery, and curves around the cerebral peduncle below the trochlear nerve to gain the superior cerebellar surface. There it divides into branches that ramify in the pia mater and supply this aspect of the cerebellum and also anastomose with branches of the inferior cerebellar arteries. The superior cerebellar artery supplies the pons, pineal body, superior medullary velum and tela choroidea of the third ventricle.
Posterior Cerebral Artery
The posterior cerebral artery (see Figs 6.5, 6.6) is a terminal branch of the basilar artery.
The surgical nomenclature identifies three segments: P1, from the basilar bifurcation to the junction with the posterior communicating artery; P2, from the junction with the posterior communicating artery to the portion in the perimesencephalic cistern; and P3, the portion running in the calcarine fissure.
The central branches supply subcortical structures. Several small posteromedial central branches arise from the beginning of the posterior cerebral artery (see Fig. 6.6) and, together with similar branches from the posterior communicating artery, pierce the posterior perforated substance and supply the anterior thalamus, subthalamus, lateral wall of the third ventricle and globus pallidus. One or more posterior choroidal branches pass over the lateral geniculate body and supply it before entering the posterior part of the inferior horn of the lateral ventricle via the lower part of the choroid fissure. Branches also curl around the posterior end of the thalamus and pass through the transverse fissure, go to the choroid plexus of the third ventricle or traverse the upper choroid fissure. Collectively, these supply the choroid plexuses of the third and lateral ventricles and the fornix. Small posterolateral central branches arise from the posterior cerebral artery beyond the cerebral peduncle and supply the peduncle and the posterior thalamus, superior and inferior colliculi, pineal gland and medial geniculate body.
Circulus Arteriosus
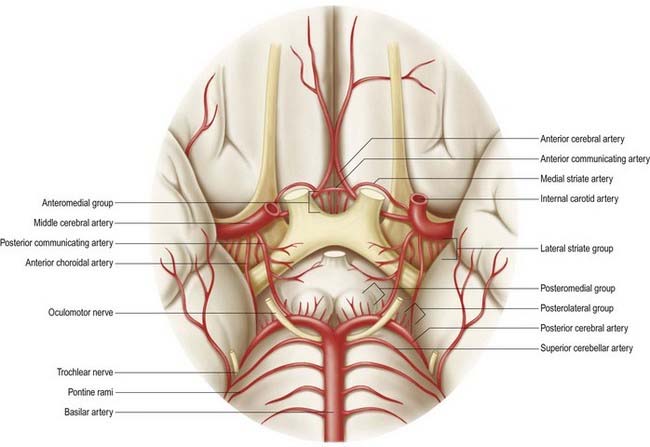
The circulus arteriosus (circle of Willis) is a large arterial anastomosis that unites the internal carotid and vertebrobasilar systems (see Figs 6.5, 6.6). It lies in the subarachnoid space within the deep interpeduncular cistern and surrounds the optic chiasma, the infundibulum and other structures of the interpeduncular fossa. Anteriorly, the anterior cerebral arteries, which are derived from the internal carotid arteries, are joined by the small anterior communicating artery. Posteriorly, the two posterior cerebral arteries, which are formed by the division of the basilar artery, are joined to the ipsilateral internal carotid artery by a posterior communicating artery. In the majority of cases, the posterior communicating arteries are very small; however, a limited flow is possible between the anterior and posterior circulations. This is important because the primary purpose of the vascular circle is to provide anastomotic channels if one vessel is occluded. The normal-sized posterior communicating artery usually cannot fulfill this role.
There are considerable individual variations in the pattern and calibre of vessels that make up the circulus arteriosus. Although a complete circular channel almost always exists, one vessel is usually sufficiently narrowed to reduce its role as a collateral route. Cerebral and communicating arteries may be absent, variably hypoplastic, double or even triple. The circle is rarely functionally complete.
CASE 1 Subarachnoid Haemorrhage
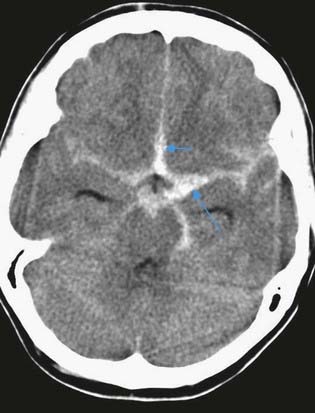
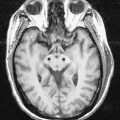
A 42-year-old woman develops neck pain followed by a severe headache after lifting a laundry basket. She has a history of poorly controlled hypertension and smokes a pack of cigarettes daily. She is initially awake and alert, but within an hour she becomes somnolent. On examination, she can be aroused briefly, can answer simple questions and can follow one-step commands. She has no weakness. A head computed tomography (CT) scan shows diffuse subarachnoid blood with hydrocephalus (Fig. 6.11). Emergent angiography demonstrates a left middle cerebral artery aneurysm, which is treated by endovascular coiling.
Discussion: Bleeding into the subarachnoid space is frequently caused by a ruptured cerebral aneurysm (Fig. 6.12). The circle of Willis and cerebral arteries lie within the subarachnoid space. It also contains CSF. Blood in the subarachnoid space disrupts the circulation of CSF and frequently leads to hydrocephalus.
Central or Perforating Arteries
Numerous small central (perforating or ganglionic) arteries arise from the circulus arteriosus or from vessels near it (see Fig. 6.6). Many of these enter the brain through the anterior and posterior perforated substances (see Fig. 15.9). Central branches supply nearby structures on or near the base of the brain, along with the interior of the cerebral hemisphere, including the internal capsule, basal ganglia and thalamus. They form four principal groups. The anteromedial group arises from the anterior cerebral and anterior communicating arteries and passes through the medial part of the anterior perforated substance. These arteries supply the optic chiasma; lamina terminalis; anterior, preoptic and supraoptic areas of the hypothalamus; septum pellucidum; paraolfactory areas; anterior columns of the fornix; cingulate gyrus; rostrum of the corpus callosum; anterior part of the putamen and head of the caudate nucleus. The posteromedial group comes from the entire length of the posterior communicating artery and from the proximal portion of the posterior cerebral artery. Anteriorly, these arteries supply the hypothalamus and pituitary and the anterior and medial parts of the thalamus via thalamo-perforating arteries. Caudally, branches of the posteromedial group supply the mammillary bodies, subthalamus, lateral wall of the third ventricle, including the medial thalamus, and globus pallidus. The anterolateral group comprises mostly branches from the proximal part of the middle cerebral artery; they are also known as striate, lateral striate or lenticulostriate arteries. They enter the brain through the anterior perforated substance and supply the posterior striatum, lateral globus pallidus and anterior limb, genu and posterior limb of the internal capsule. The medial striate artery, which is derived from the middle or anterior cerebral arteries, supplies the rostral part of the caudate nucleus and putamen and the anterior limb and genu of the internal capsule. The posterolateral group is derived from the posterior cerebral artery distal to its junction with the posterior communicating artery and supplies the cerebral peduncle, colliculi, pineal gland and, via thalamogeniculate branches, posterior thalamus and medial geniculate body.
Regional Arterial Supply of the Brain
Brain Stem
The pons is supplied by the basilar artery and the anterior inferior and superior cerebellar arteries. Direct branches from the basilar artery enter the pons along the ventral medial groove (basilar sulcus). Other vessels enter along the trigeminal, abducens, facial and vestibulocochlear nerves and from the pial plexus.
Basal Ganglia
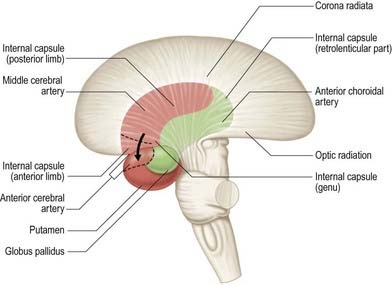
The majority of the arterial supply to the basal ganglia comes from the striate arteries, which are branches from the roots of the anterior and middle cerebral arteries (Fig. 6.13). They enter the brain through the anterior perforated substance and also supply the internal capsule. The caudate nucleus receives blood additionally from the anterior and posterior choroidal arteries. The posteroinferior part of the lentiform complex is supplied by the thalamostriate branches of the posterior cerebral artery. The anterior choroidal artery, a preterminal branch of the internal carotid artery, contributes to the blood supply of both segments of the globus pallidus and the caudate nucleus. In a well known case, ligation of this vessel during a neurosurgical procedure on a patient suffering from Parkinson’s disease led to the alleviation of Parkinsonian symptoms, presumably as a consequence of infarction of the globus pallidus. This chance observation led to the initiation of pallidal surgery (pallidotomy) for this condition.
Internal Capsule
The internal capsule is supplied by central, or perforating, arteries that arise from the circulus arteriosus and its associated vessels (see Fig. 6.13). These include the lateral and medial striate arteries, which come from the middle and anterior cerebral arteries and also supply the basal ganglia. The lateral striate arteries supply the anterior limb, genu and much of the posterior limb of the internal capsule and are commonly involved in ischaemic and haemorrhagic strokes. One of the larger striate branches of the middle cerebral artery is known as Charcot’s artery of cerebral haemorrhage.
Cerebral Cortex
The lateral surface of the hemisphere is supplied mainly by the middle cerebral artery (see Fig. 6.8). This includes the territories of the motor and somatosensory cortices, which represent the whole body except for the lower limb and also the auditory cortex and language areas. The anterior cerebral artery supplies a strip next to the superomedial border of the hemisphere, as far back as the parieto-occipital sulcus. The occipital lobe and most of the inferior temporal gyrus (excluding the temporal pole) are supplied by the posterior cerebral artery.
CASE 2 Cerebrovascular Disease
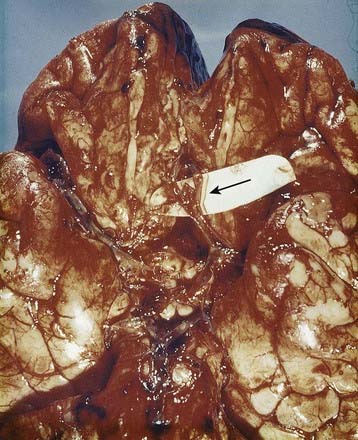
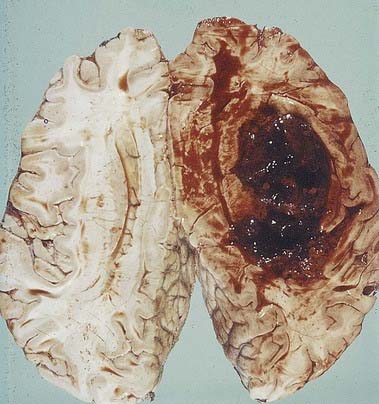
Haemorrhagic stroke occurs when a cerebral blood vessel ruptures. Depending on the location of the vessel, bleeding may be epidural, subdural, intraparenchymal, intraventricular or subarachnoid (Fig. 6.14). The most common cause of intracerebral haemorrhage is hypertension; other causes include cerebral aneurysms, vascular malformations and amyloid angiopathy. Treatment options for intracerebral haemorrhage are limited and are largely directed toward preventing secondary damage from the haemorrhage mass.
Cerebral Bloodflow
Cerebral bloodflow in the human brain is approximately 50 ml/g/min. Global cerebral bloodflow is autoregulated—that is, it remains constant in normal individuals despite variations in mean arterial blood pressure over a range of approximately 8.7 to 18.7 kPa (65 to 140 mm Hg). If the blood pressure falls below this range, cerebral bloodflow decreases. Alternatively, if the pressure rises above this range, cerebral bloodflow may increase. Arterial and arteriolar intraluminal pressures directly control the contraction of intramural muscle, so an increase in arterial pressure, for example, causes arterial constriction, and bloodflow remains constant.
Not all substances circulating in arterial blood have access to the brain parenchyma. Particulate matter, such as bacteria, is excluded. In general, lipophilic molecules and small molecules, such as oxygen and carbon dioxide, can cross the blood–brain barrier, but hydrophilic ones (excluding glucose) cannot. The cellular basis for the blood–brain barrier is discussed in Chapter 2.
Venous Drainage of the Brain
Veins of the Brain Stem
The veins of the brain stem form a superficial venous plexus deep to the arteries.
Veins of the Cerebral Hemisphere
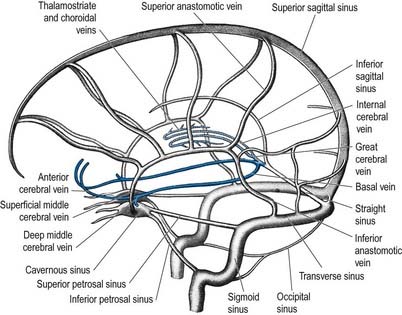
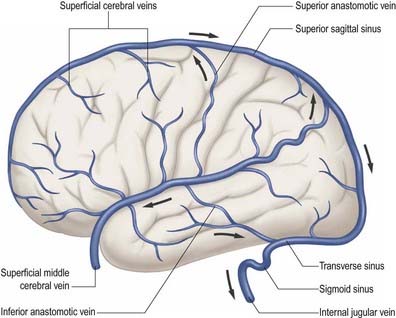
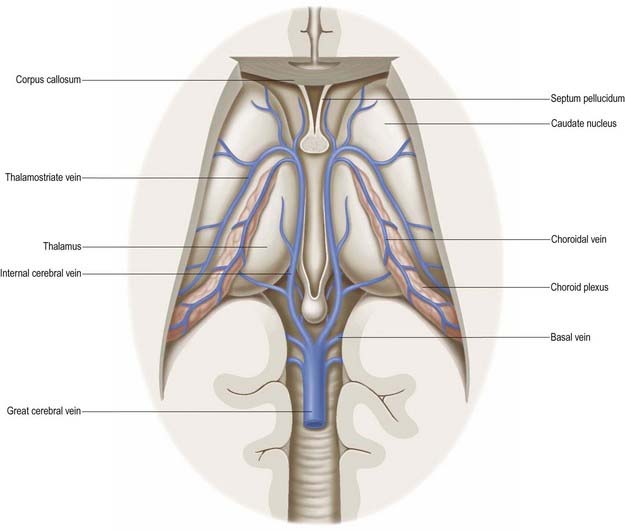
External and internal cerebral veins (Figs 6.15–6.19) drain the surfaces and the interior of the cerebral hemisphere. External cerebral veins may be divided into three groups: superior, middle and inferior.
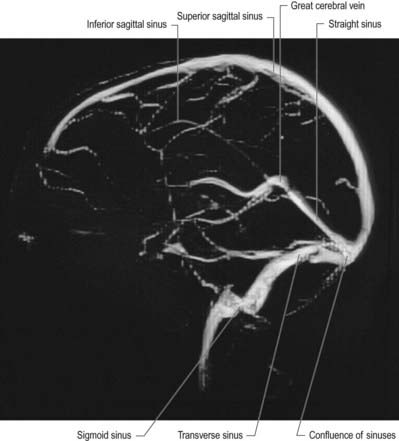
Fig. 6.18 Lateral projection from a magnetic resonance venogram using time-of-flight methods.
(Courtesy of Professor P. D. Griffiths, Academic Unit of Radiology, University of Sheffield.)
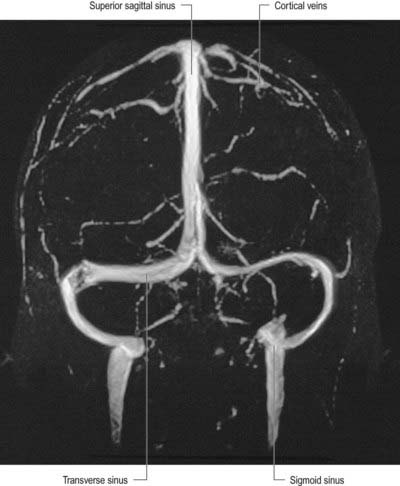
Fig. 6.19 Frontal projection from a magnetic resonance venogram using time-of-flight methods.
(Courtesy of Professor P. D. Griffiths, Academic Unit of Radiology, University of Sheffield.)
Eight to 12 superior cerebral veins drain the superolateral and medial surfaces of each hemisphere. They mainly follow the sulci, although some do pass across gyri. They ascend to the superomedial border of the hemisphere, where they receive small veins from the medial surface, and then open into the superior sagittal sinus. Superior cerebral veins in the anterior part of the hemisphere join the sinus almost at right angles. The larger posterior veins are directed obliquely forward, against the direction of flow in the sinus, an arrangement that may resist their collapse when intracranial pressure is raised.
Inferior cerebral veins on the orbital surface of the frontal lobe join the superior cerebral veins and thus drain to the superior sagittal sinus. Those on the temporal lobe anastomose with basal veins and middle cerebral veins and drain to the cavernous, superior petrosal and transverse sinuses.
The basal vein begins at the anterior perforated substance by the union of a small anterior cerebral vein, which accompanies the anterior cerebral artery; a deep middle cerebral vein, which receives tributaries from the insula and neighbouring gyri and runs in the lateral cerebral fissure; and striate veins, which emerge from the anterior perforated substance. The basal vein passes back around the cerebral peduncle to the great cerebral vein (see Fig. 6.15) and receives tributaries from the interpeduncular fossa, inferior horn of the lateral ventricle, parahippocampal gyrus and midbrain.
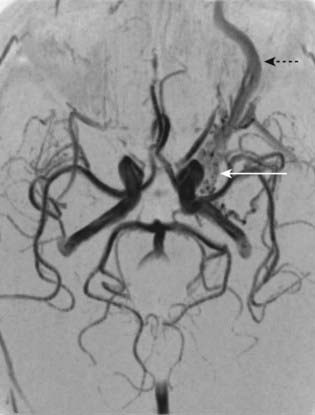
CASE 3 Carotid Cavernous Fistula
Discussion: This man has developed a carotid cavernous fistula secondary to head trauma, with laceration of the carotid artery within the cavernous sinus (Fig. 6.20). In a small proportion of cases the lesion is spontaneous, secondary to rupture of a previously unrecognized carotid aneurysm. Several structures within the cavernous sinus are variously affected, along with the pulsatile exophthalmos, orbital congestion and oedema secondary to involvement of the carotid artery within the sinus itself. The differential diagnosis includes, most importantly, cavernous sinus thrombosis secondary to spread of sepsis from infection in the sinuses or the upper face. Under these circumstances, the patient is generally febrile and acutely ill; physical findings are otherwise much the same as in a traumatic carotid cavernous fistula, as observed in this patient.
Vascular Supply of the Spine (see also Crock 1996)
Arteries
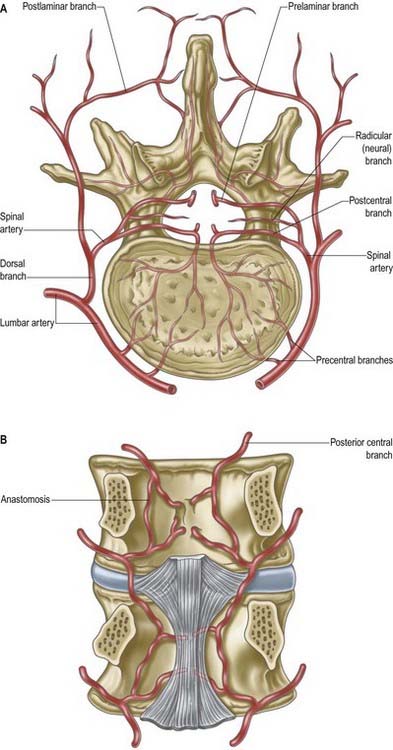
The vertebral column, its contents and its associated soft tissues all receive their arterial supply from derivatives of dorsal branches of the embryonic intersegmental somatic arteries (Fig. 6.21). The relevant named artery depends on the level of the column. These intersegmental vessels persist in the thoracic and lumbar regions as the posterior intercostal and lumbar arteries. In the cervical and sacral regions, longitudinal anastomoses between the intersegmental vessels persist as longitudinal vessels, which themselves give spinal branches to the vertebral column. In the neck, the postcostal anastomosis becomes most of the vertebral artery, whereas the post-transverse anastomosis forms most of the deep cervical artery. The ascending cervical artery and the lateral sacral artery are persistent parts of the precostal anastomosis.
In the thorax and abdomen, the primitive arterial pattern is retained by the paired branches of the descending aorta, which supply the vertebral column (see Fig. 6.21A). On each side, the main trunk of the artery (posterior intercostal or lumbar) passes around the vertebral body, giving off primary periosteal and equatorial branches to the body and then a major dorsal branch. The dorsal branch gives off a spinal branch, which enters the intervertebral foramen before supplying the facet joints, posterior surfaces of the laminae and overlying muscles and skin. There is free anastomosis between these dorsal articular and soft tissue branches, extending over several segments (Crock and Yoshizawa 1976; Boelderl et al 2002). At cervical and sacral levels, the longitudinally running arteries described earlier have direct spinal branches. The spinal branches are the main arteries of supply to all bony elements of the vertebrae and to the dura and epidural tissues; they also contribute to the supply of the spinal cord and nerve roots via radicular branches (see later). As they enter the vertebral canal, the spinal arteries divide into postcentral, prelaminar and radicular branches. The postcentral branches, which are the main nutrient arteries to the vertebral bodies and to the periphery of the intervertebral discs, anastomose beneath the posterior longitudinal ligament with their fellows above and below, as well as across the midline (see Fig. 6.21). This anastomosis also supplies the anterior epidural tissues and dura. The majority of the vertebral arch, the posterior epidural tissues and dura and the ligamentum flavum are supplied by the prelaminar branches and their anastomotic plexus on the posterior wall of the vertebral canal.
Veins
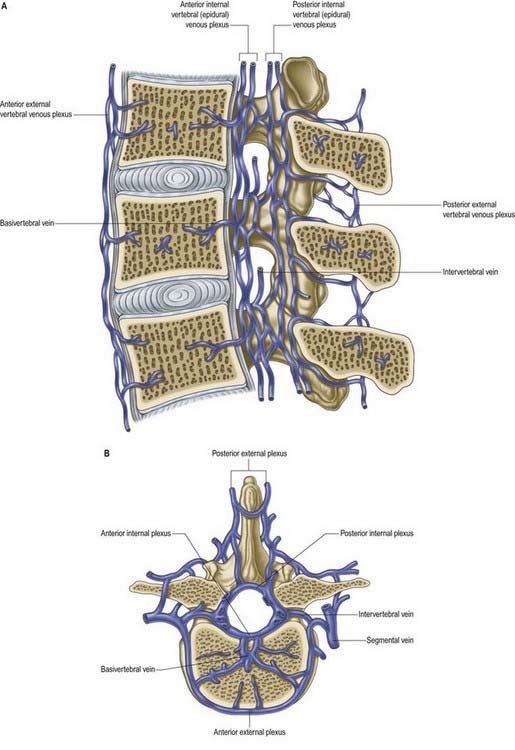
Veins of the vertebral column form intricate plexuses along the entire column, external and internal to the vertebral canal (Fig. 6.22). Both groups are devoid of valves, anastomose freely with each other and join the intervertebral veins. Interconnections are widely established between these plexuses and longitudinal veins early in fetal life. When development is complete, the plexuses drain into the caval and azygos ascending lumbar systems via named veins that accompany the arteries described earlier.
The veins also communicate with cranial dural venous sinuses and with the deep veins of the neck and pelvis. The venous complexes associated with the vertebral column can dilate considerably and can form alternative routes of venous return in patients with major venous obstruction in the neck, chest or abdomen. The absence of valves allows pathways for the wide and sometimes paradoxical spread of malignant disease and sepsis. Pressure changes in the body cavities are transmitted to these venous plexuses and thus to the CSF, although the cord itself may be protected from such congestion by valves in the small veins that drain from the cord into the internal vertebral plexus.
Basivertebral Veins
The basivertebral veins emerge from the posterior foramina of the vertebral bodies. They are large and tortuous channels in bone, like those in the cranial diploë. The basivertebral veins also drain into the anterior external vertebral plexuses through small openings in the vertebral bodies. Posteriorly, they form one or two short trunks that open into the transverse branches and unite the anterior internal vertebral plexuses. They enlarge in advanced age.
Intervertebral Veins
The intervertebral veins accompany the spinal nerves through intervertebral foramina, draining the spinal cord and internal and external vertebral plexuses and ending in the vertebral, posterior intercostal, lumbar and lateral sacral veins. Upper posterior intercostal veins may drain into the caval system via brachiocephalic veins, whereas the lower intercostals drain into the azygos system. Lumbar veins are joined longitudinally in front of the transverse processes by the ascending lumbar veins, in which they may terminate. Alternatively, they may proceed around the vertebral bodies to drain into the inferior vena cava. Whether the basivertebral or intervertebral veins contain effective valves is uncertain, but experimental evidence strongly suggests that their bloodflow can be reversed (Batson 1957). This may explain how pelvic neoplasms (e.g. prostate carcinoma) may metastasize in vertebral bodies: the cells spread into the internal vertebral plexuses via their connections with the pelvic veins when bloodflow is temporarily reversed by raised intra-abdominal pressure or postural alterations.
Vascular Supply of Spinal Cord, Roots and Nerves
Arteries
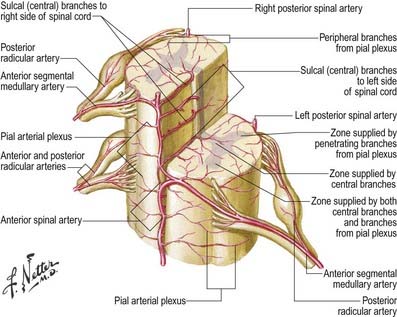
The spinal cord, its roots and nerves are supplied with blood by both longitudinal and segmental vessels. Three major longitudinal vessels—a single anterior and two posterior spinal arteries (each of which is sometimes doubled to pass on either side of the dorsal rootlets)—originate intracranially from the vertebral artery and terminate in a plexus around the conus medullaris (Fig. 6.23). The anterior spinal artery forms from the fused anterior spinal branches of the vertebral artery and descends in the anterior median fissure of the cord. Each posterior spinal artery originates either directly from the ipsilateral vertebral artery or from its posterior inferior cerebellar branch and descends in a posterolateral sulcus of the cord. The segmental arteries are derived in craniocaudal sequence from spinal branches of the vertebral, deep cervical, intercostal and lumbar arteries. These vessels enter the vertebral canal through the intervertebral foramina and anastomose with branches of the longitudinal vessels to form a pial plexus on the surface of the cord. The segmental spinal arteries send anterior and posterior radicular branches to the spinal cord along the ventral and dorsal roots. Most anterior radicular arteries are small and end in the ventral nerve roots or in the pial plexus of the cord. The small posterior radicular arteries also supply the dorsal root ganglia; branches enter at both ganglionic poles to be distributed around ganglion cells and nerve fibres.
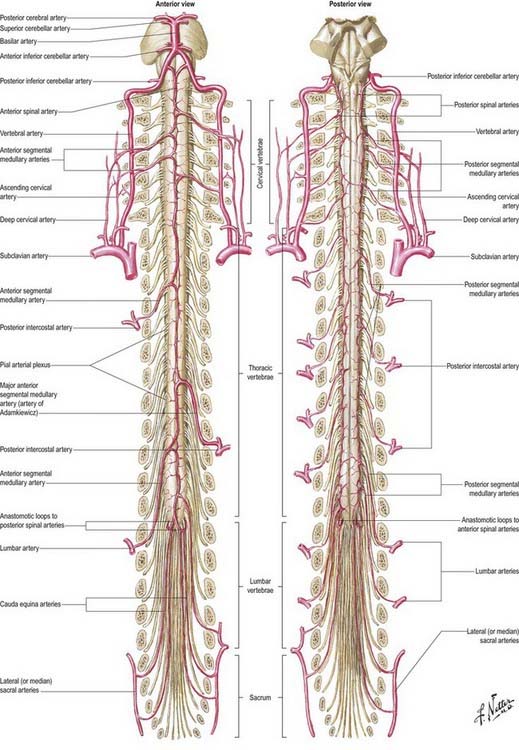
Fig. 6.23 Arteries of the spinal cord.
(Netter illustration from www.netterimages.com. © Elsevier Inc. All rights reserved.)
Segmental Medullary Feeder Arteries
Some radicular arteries, mainly situated in the lower cervical, lower thoracic and upper lumbar regions, are large enough to reach the anterior median sulcus, where they divide into slender ascending and large descending branches. These are the anterior medullary feeder arteries (Dommisse 1975). They anastomose with the anterior spinal arteries to form a single or partly double longitudinal vessel of uneven calibre along the anterior median sulcus. The largest anterior medullary feeder, the great anterior segmental medullary artery of Adamkiewicz, varies in level, arising from a spinal branch of one of the lower posterior intercostal arteries (T9–11) or subcostal artery (T12) or, less frequently, a spinal branch of the upper lumbar arteries (L1 and L2). It most often arises on the left side (Carmichael and Gloviczki 1999). Reaching the spinal cord, it sends a branch to the anterior spinal artery below and another to anastomose with the ramus of the posterior spinal artery, which lies anterior to the dorsal roots. It may be the main supply to the lower two-thirds of the cord. Central branches of the anterior spinal artery enter the anterior median fissure and then turn right or left to supply the ventral grey column, the base of the dorsal grey column, including the dorsal nucleus, and the adjacent white matter (Fig. 6.24).
Intramedullary Arteries
The central branches of the anterior spinal artery supply about two-thirds of the cross-sectional area of the cord. The rest of the dorsal grey and white columns and peripheral parts of the lateral and ventral white columns are supplied by numerous small radial vessels that branch from posterior spinal arteries and the pial plexus. In a microangiographic study of the human cervical spinal cord, up to six anterior and eight posterior radicular spinal arteries were described, and up to eight central branches arose from each centimetre of the anterior spinal artery (Turnbull, Brieg and Hassler 1966).
Spinal Cord Ischaemia
The spinal cord cannot rely entirely on the longitudinal arteries for either its transverse or its longitudinal blood supply. The anterior longitudinal artery and the intramedullary arteries are functional end-arteries, although overlapping territories of supply have been described. Damage to the anterior longitudinal artery can result in loss of function of the anterior two-thirds of the cord. The longitudinal arteries cannot supply the whole length of the cord, and the input of the segmental medullary feeder vessels is essential. This is especially true of the artery of Adamkiewicz (great anterior segmental medullary artery), which may effectively carry the major supply for the lower cord. The midthoracic cord, distant from the main anterior medullary feeders, is particularly liable to become ischaemic after periods of hypotension.
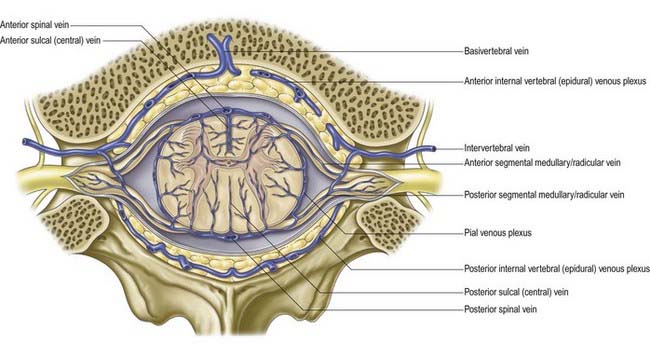
Veins
The venous drainage of the spinal cord (Fig. 6.25) follows a similar pattern to that of its arterial supply (Gillilan 1970). Intramedullary veins within the substance of the cord drain into a plexus of surface veins, the coronal plexus. There are six tortuous longitudinal channels within this plexus—one in each of the anterior and posterior median fissures, and four others that run on either side of the ventral and dorsal nerve roots. Only the anterior median vein, which drains the central grey matter, is consistently complete. These vessels connect freely. They drain superiorly into the cerebellar veins and cranial sinuses and segmentally into mainly the medullary veins. These segmental veins drain into the intervertebral veins and then into the external vertebral venous plexuses, the caval and azygos systems.
Batson, 1957Batson O.V. The vertebral vein system. AJR Am. J. Roentgenol.. 78:1957;195-212.
Boelderl, Daniaux, Kathrein, Maurer, 2002Boelderl A. Daniaux H. Kathrein A. Maurer H. Danger of damaging the medial branches of the posterior rami of spinal nerves during a dorsomedian approach to the spine. Clin. Anat.. 15:2002;77-81.
Carmichael S.W., Gloviczki P. Anatomy of the blood supply to the spinal cord: the artery of Adamkiewicz revisited. Perspect. Vasc. Surg.. 1999;12:113-122.
Crock H.V. Atlas of Vascular Anatomy of the Skeleton and Spinal Cord. London: Martin Dunitz; 1996.
Crock H.V., Yoshizawa H. The blood supply of the lumbar vertebral column. Clin. Orthop.. 1976;115:6-21.
Dommisse G.F. The Arteries and Veins of the Human Spinal Cord from Birth. Edinburgh: Churchill Livingstone; 1975.
Duvernoy H.M., Delon S., Vannson J.L. Cortical blood vessels of the human brain. Brain Res. Bull.. 1981;7:519-579.
Duvernoy H., Delon S., Vannson J.L. The vascularization of the human cerebellar cortex. Brain Res. Bull.. 1983;11:419-480.
Gillilan L.A. Veins of the spinal cord: anatomic details, suggested clinical applications. Neurology. 1970;20:860-868.
Kaplan H.A., Ford D.H. The Brain Vascular System. Amsterdam: Elsevier; 1966.
Plets C., De Reuck J., Vander Eecken H., Van den Bergh R. The vascularization of the human thalamus. Acta. Neurol. Belg.. 1970;70:687-770.
Puchades-Orts A., Nombela-Gomez M., Ortuño-Pacheco G. Variation in form of the circle of Willis: some anatomical and embryological considerations. Anat. Rec.. 1976;185:119-123.
Sengupta, McAllister, 1986Sengupta R.P., McAllister V.L. Subarachnoid Haemorrhage. 1986. Springer-Verlag. Berlin. 9-31.
Includes details on variations of the circle of Willis.
Turnbull I.M., Brieg A., Hassler O. Blood supply of cervical spinal cord in man: a microangiographic cadaver study. J. Neurosurg.. 1966;24:951-965.