CHAPTER 17 Vascular supply and drainage of the brain
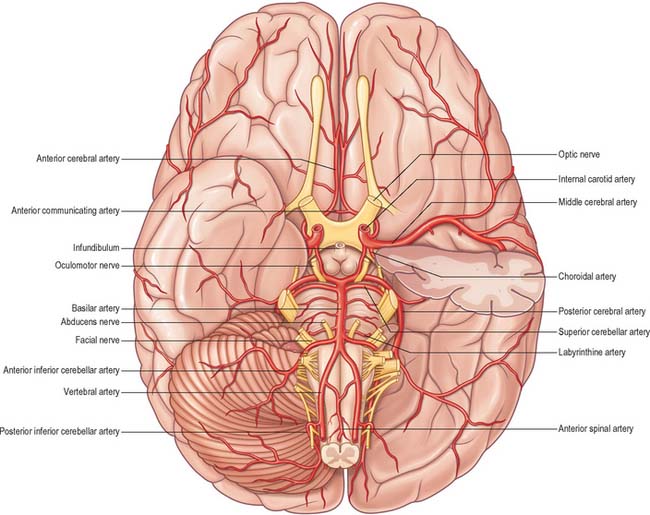
The brain is a highly vascular organ, its profuse blood supply characterized by a densely branching arterial network. It has a high metabolic rate that reflects the energy requirements of constant neural activity. It receives about 15% of the cardiac output and utilizes 25% of the total oxygen consumption of the body. The brain is supplied by two internal carotid arteries and two vertebral arteries that form a complex anastomosis (circulus arteriosus, circle of Willis) on the base of the brain. Vessels diverge from this anastomosis to supply the various cerebral regions. In general, the internal carotid arteries and the vessels arising from them supply the forebrain, with the exception of the occipital lobe of the cerebral hemisphere, and the vertebral arteries and their branches supply the occipital lobe, the brain stem and the cerebellum. Venous blood from the brain drains into sinuses within the dura mater (see Ch. 27). Acute interruption of the blood supply to the brain for more than a few minutes causes permanent neurological damage. Such ischaemic strokes along with intracranial haemorrhage are major contemporary sources of morbidity and mortality.
ARTERIAL SUPPLY OF THE BRAIN
INTERNAL CAROTID ARTERY
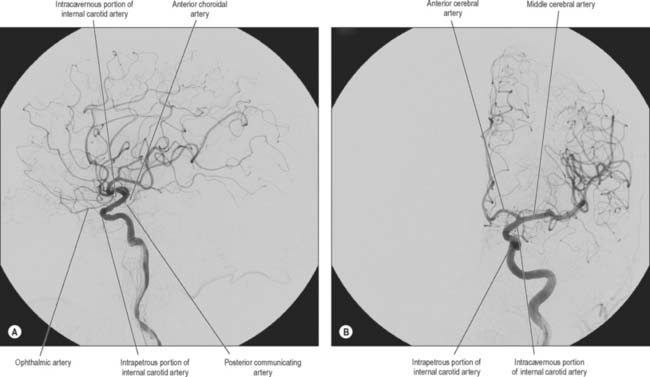
The internal carotid arteries (Fig. 17.1) and their major branches (the internal carotid system or ‘anterior’ circulation) supply blood to the majority of the forebrain. Some parts of the occipital and temporal lobes are supplied by branches of the vertebrobasilar system (see Fig. 17.5).
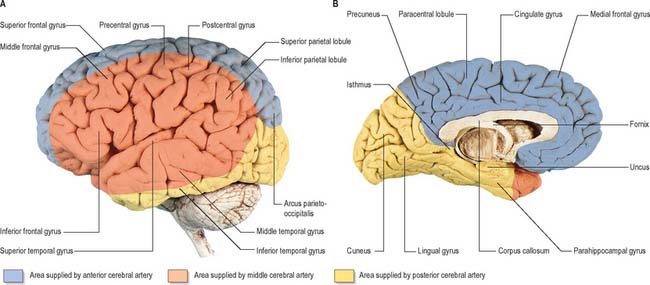
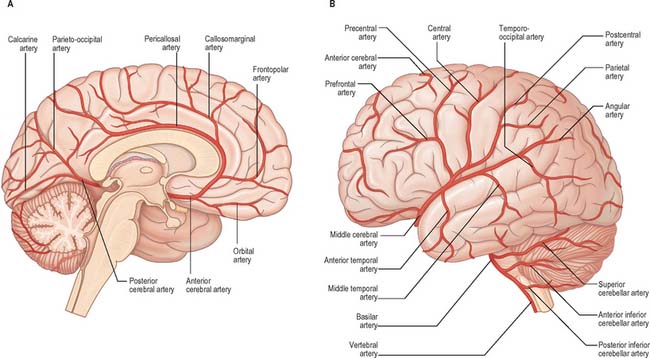
Fig. 17.5 The arteries supplying the left cerebral hemispheres. A, Lateral surface. B, Medial surface.
Cavernous part
The cavernous part of the internal carotid artery ascends to the posterior clinoid process. It turns anteriorly to the side of the sphenoid within the cavernous sinus and then curves up medial to the anterior clinoid process, to emerge through the dural roof of the sinus. The oculomotor, trochlear, ophthalmic and abducens nerves are lateral to it within the cavernous sinus (see Fig. 27.9). Occasionally, the two clinoid processes form a bony ring round the artery.
This part of the artery gives off a number of small vessels. Cavernous branches supply the trigeminal ganglion, the walls of the cavernous and inferior petrosal sinuses and the nerves contained therein. A minute meningeal branch passes over the lesser wing of the sphenoid to supply the dura mater and bone in the anterior cranial fossa and also anastomoses with a meningeal branch of the posterior ethmoidal artery. Numerous small hypophysial branches supply the neurohypophysis, and are of particular importance because they form the pituitary portal system (Fig. 21.11).
Intracranial part
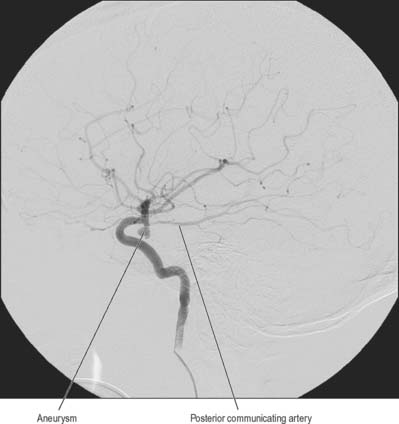
Several preterminal vessels leave the cerebral portion of the internal carotid. The ophthalmic artery arises from the anterior part of the internal carotid as it leaves the cavernous sinus, often at the point of piercing the dura, and enters the orbit through the optic canal. The posterior communicating artery (Fig. 17.2, Fig. 17.3) runs back from the internal carotid above the oculomotor nerve, and anastomoses with the posterior cerebral artery (a terminal branch of the basilar artery), thereby contributing to the circulus arteriosus around the interpeduncular fossa. The posterior communicating artery is usually very small. However, sometimes it is so large that the posterior cerebral artery is supplied via the posterior communicating artery rather than from the basilar artery (‘fetal posterior communicating artery’); it is often larger on one side only. Small branches from its posterior half pierce the posterior perforated substance together with branches from the posterior cerebral artery. Collectively they supply the medial thalamic surface and the walls of the third ventricle. The anterior choroidal artery leaves the internal carotid near its posterior communicating branch and passes back above the medial part of the uncus. It crosses the optic tract to reach and supply the crus cerebri of the midbrain, then turns laterally, recrosses the optic tract, and gains the lateral side of the lateral geniculate body, which it supplies with several branches. It finally enters the inferior horn of the lateral ventricle via the choroidal fissure and ends in the choroid plexus. This small, but important, vessel also contributes to the blood supply of the globus pallidus, caudate nucleus, amygdala, hypothalamus, tuber cinereum, red nucleus, substantia nigra, posterior limb of the internal capsule, optic radiation, optic tract, hippocampus and the fimbria of the fornix.
ANTERIOR CEREBRAL ARTERY
The anterior cerebral artery is the smaller of the two terminal branches of the internal carotid (Fig. 17.3).
The two anterior cerebral arteries travel together in the great longitudinal fissure. They pass around the curve of the genu of the corpus callosum and then along its upper surface to its posterior end, where they anastomose with posterior cerebral arteries (Fig. 17.4). They give off cortical and central branches.
The cortical branches of the anterior cerebral artery are named according to their distribution. Two or three orbital branches ramify on the orbital surface of the frontal lobe and supply the olfactory cortex, gyrus rectus and medial orbital gyrus. Frontal branches supply the corpus callosum, cingulate gyrus, medial frontal gyrus and paracentral lobule. Parietal branches supply the precuneus, while the frontal and parietal branches both send twigs over the superomedial border of the hemisphere to supply a strip of territory on the superolateral surface (Fig. 17.5). Cortical branches of the anterior cerebral artery therefore supply the areas of the motor and somatosensory cortices that represent the lower limb.
Central branches of the anterior cerebral artery arise from its proximal portion and enter the anterior perforated substance (Fig. 17.3) and lamina terminalis. Collectively, they supply the rostrum of the corpus callosum, the septum pellucidum, the anterior part of the putamen, the head of the caudate nucleus and adjacent parts of the internal capsule. Immediately proximal or distal to its junction with the anterior communicating artery, the anterior cerebral artery gives rise to the medial striate artery which supplies the anterior part of the head of the caudate nucleus and adjacent regions of the putamen and internal capsule.
MIDDLE CEREBRAL ARTERY
The middle cerebral artery is the larger terminal branch of the internal carotid.
The middle cerebral artery runs at first in the lateral fissure, then posterosuperiorly on the insula, and divides into branches distributed to the insula and the adjacent lateral cerebral surface (Fig. 17.3, Fig. 17.4, Fig. 17.5). Like the anterior cerebral artery, it has cortical and central branches.
VERTEBRAL ARTERY
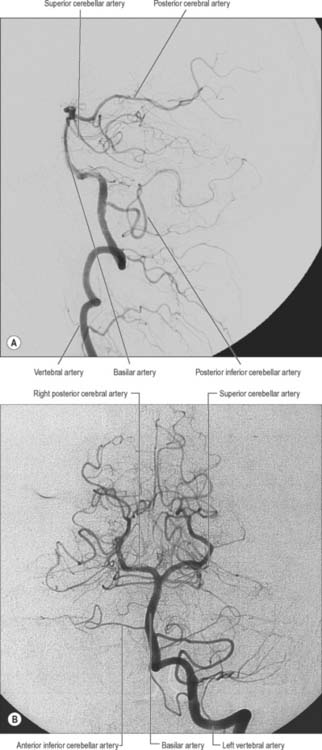
The vertebral arteries and their major branches (sometimes referred to as the ‘vertebrobasilar system’) essentially supply blood to the upper spinal cord, the brain stem and cerebellum and a significant but variable part of the posterior cerebral hemispheres (Fig. 17.6).
The vertebral arteries are derived from the subclavian arteries (see Ch. 30). They ascend through the neck in the foramina transversaria of the upper six cervical vertebrae and enter the cranial cavity through the foramen magnum, close to the anterolateral aspect of the medulla (Fig. 17.2). They converge medially as they ascend the medulla and unite to form the midline basilar artery at approximately the level of the junction between the medulla and pons.
The largest branch of the vertebral artery is the posterior inferior cerebellar artery (Fig. 17.6A). It arises near the lower end of the olive and then ascends behind the roots of the glossopharyngeal and vagus nerves to reach the inferior border of the pons. Here it curves and descends along the inferolateral border of the fourth ventricle before it turns laterally into the cerebellar vallecula between the hemispheres, and divides into medial and lateral branches. The medial branch runs back between the cerebellar hemisphere and inferior vermis, and supplies both. The lateral branch supplies the inferior cerebellar surface as far as its lateral border and anastomoses with the anterior inferior and superior cerebellar arteries (from the basilar artery). The trunk of the posterior inferior cerebellar artery supplies the medulla oblongata dorsal to the olivary nucleus and lateral to the hypoglossal nucleus and its emerging nerve roots. It also supplies the choroid plexus of the fourth ventricle and sends a branch lateral to the cerebellar tonsil to supply the dentate nucleus. The posterior inferior cerebellar artery is sometimes absent.
BASILAR ARTERY
The basilar artery is a large median vessel formed by the union of the vertebral arteries at the mid-medullary level (Figs 17.2, 17.3, 17.6). It lies in the pontine cistern, and follows a shallow median groove on the ventral pontine surface, extending to the upper border of the pons. It ends by dividing into two posterior cerebral arteries at a variable level behind the dorsum sellae, usually in the interpeduncular cistern.
The anterior inferior cerebellar artery (Fig. 17.3) is given off from the lower part of the basilar artery and runs posterolaterally, usually ventral to the abducens, facial and vestibulocochlear nerves. It commonly exhibits a loop into the internal acoustic meatus below the nerves, and when this occurs, the labyrinthine artery may arise from the loop. The anterior inferior cerebellar artery supplies the inferior cerebellar surface anterolaterally and anastomoses with the posterior inferior cerebellar branch of the vertebral artery. A few branches supply the inferolateral parts of the pons and occasionally also supply the upper medulla oblongata.
The superior cerebellar artery (Figs 17.3, 17.6) arises near the distal portion of the basilar artery, immediately before the formation of the posterior cerebral arteries. It passes laterally below the oculomotor nerve, which separates it from the posterior cerebral artery, and curves round the cerebral peduncle below the trochlear nerve to gain the superior cerebellar surface. Here it divides into branches which ramify in the pia mater and supply this aspect of the cerebellum, and also anastomose with branches of the inferior cerebellar arteries. The superior cerebellar artery supplies the pons, pineal body, superior medullary velum and tela choroidea of the third ventricle.
POSTERIOR CEREBRAL ARTERY
The posterior cerebral artery (Figs 17.2, 17.3, 17.4) is a terminal branch of the basilar artery.
The central branches supply subcortical structures. Several small posteromedial central branches arise from the beginning of the posterior cerebral artery (Fig. 17.3) and, together with similar branches from the posterior communicating artery, pierce the posterior perforated substance to supply the anterior thalamus, subthalamus, lateral wall of the third ventricle and globus pallidus. One or more posterior choroidal branches pass over the lateral geniculate body and supply it before entering the posterior part of the inferior horn of the lateral ventricle via the lower part of the choroidal fissure. Branches also curl round the posterior end of the thalamus and pass through the transverse fissure, or go to the choroid plexus of the third ventricle, or traverse the upper choroidal fissure. Collectively these branches supply the choroid plexuses of the third and lateral ventricles and the fornix. Small posterolateral central branches arise from the posterior cerebral artery beyond the cerebral peduncle and supply the peduncle and the posterior thalamus, superior and inferior colliculi, pineal gland and medial geniculate body.
CIRCULUS ARTERIOSUS
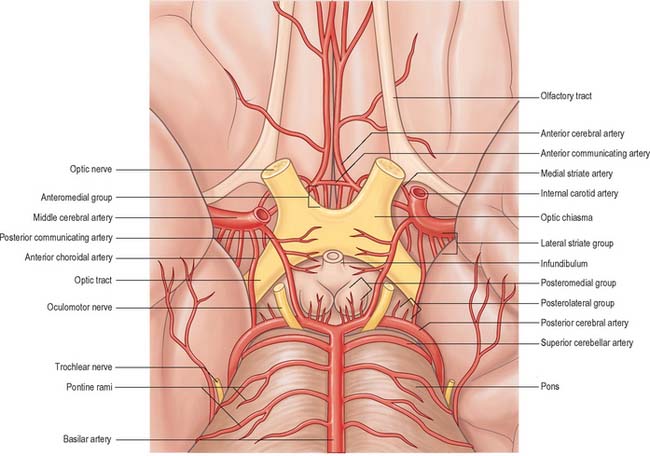
The circulus arteriosus (circle of Willis) is a large arterial anastomosis which unites the internal carotid and vertebrobasilar systems (Figs 17.3, 17.6B). It lies in the subarachnoid space within the interpeduncular cistern, and surrounds the optic chiasma and infundibulum. Anteriorly, the anterior cerebral arteries, derived from the internal carotid arteries, are linked by the small anterior communicating artery. Posteriorly, the two posterior cerebral arteries, formed by the division of the basilar artery, are joined to the ipsilateral internal carotid artery by a posterior communicating artery.
Aneurysms are balloon-like swellings which occur on arteries as a result of defects in the vessel wall. They are most commonly found on the vessels of the circulus arteriosus, particularly at or near the junctions of vessels. Aneurysms on the internal carotid artery near its termination may compress the lateral aspect of the optic chiasma, and compromise axons derived from the temporal side of the ipsilateral retina, which causes a defect in the nasal visual field. Aneurysms in the vicinity of the oculomotor nerve, e.g. on the posterior communicating artery, superior cerebellar artery, or the tip of the basilar artery, can cause third nerve palsy by compression (Fig. 17.7).
Bleeding into the subarachnoid compartment, subarachnoid haemorrhage, is the most common pathology that involves the subarachnoid space. There are many causes; in adults the commonest is rupture of an aneurysm of the intracranial vessels that run within the subarachnoid space. A person who has had a subarachnoid haemorrhage usually complains of a very sudden onset of headache that is frequently described as being their ‘worst ever headache’ or ‘like being hit on the head with a hammer’. When this is suspected the first investigation is an X-ray CT examination which has a very high sensitivity for detecting fresh haemorrhage. Blood will be seen in the basal cisterns and entering the depths of the cortical sulci, i.e. delineating the anatomy of the subarachnoid space, a presentation that permits an accurate diagnosis (Fig. 17.8). In marked contrast, the spread of blood in a subdural haemorrhage is limited by the arachnoid mater on its deep surface, and the blood therefore remains on the surface of the brain.
CENTRAL OR PERFORATING ARTERIES
Numerous small central (perforating or ganglionic) arteries arise from the circulus arteriosus, or from vessels near it (Fig. 17.3). Many of these enter the brain through the anterior and posterior perforated substances. Central branches supply nearby structures on or near the base of the brain together with the interior of the cerebral hemisphere including the internal capsule, basal ganglia and thalamus. These branches form four principal groups. The anteromedial group arises from the anterior cerebral and anterior communicating arteries and passes through the medial part of the anterior perforated substance. These arteries supply the optic chiasma, lamina terminalis, anterior, preoptic and supraoptic areas of the hypothalamus, septum pellucidum, paraolfactory areas, anterior columns of the fornix, cingulate gyrus, rostrum of the corpus callosum and the anterior part of the putamen and the head of the caudate nucleus. The posteromedial group comes from the entire length of the posterior communicating artery and from the proximal portion of the posterior cerebral artery. Anteriorly, these arteries supply the hypothalamus and pituitary gland, and the anterior and medial parts of the thalamus via thalamoperforating arteries. Caudally, branches of the posteromedial group supply the mammillary bodies, subthalamus, the lateral wall of the third ventricle, including the medial thalamus, and the globus pallidus. The anterolateral group is mostly comprised of branches from the proximal part of the middle cerebral artery that are also known as striate, lateral striate or lenticulostriate arteries. They enter the brain through the anterior perforated substance and supply the posterior striatum, lateral globus pallidus and the anterior limb, genu and posterior limb of the internal capsule. The medial striate artery, derived from the middle or anterior cerebral arteries, supplies the rostral part of the caudate nucleus and putamen and the anterior limb and genu of the internal capsule. The posterolateral group is derived from the posterior cerebral artery distal to its junction with the posterior communicating artery, and supplies the cerebral peduncle, colliculi, pineal gland and, via thalamogeniculate branches, the posterior thalamus and medial geniculate body.
REGIONAL ARTERIAL SUPPLY OF THE BRAIN
Basal ganglia
The majority of the arterial supply to the basal ganglia comes from the striate arteries, which are branches from the roots of the anterior and middle cerebral arteries. They enter the brain through the anterior perforated substance and also supply the internal capsule. The caudate nucleus receives blood additionally from the anterior and posterior choroidal arteries. The posteroinferior part of the lentiform complex is supplied by the thalamostriate branches of the posterior cerebral artery. The anterior choroidal artery, a preterminal branch of the internal carotid artery, contributes to the blood supply of both segments of the globus pallidus and the caudate nucleus. Famously, the ligation of this vessel during a neurosurgical procedure on a patient suffering from Parkinson’s disease led to alleviation of the Parkinsonian symptoms, presumably as a consequence of infarction of the globus pallidus. This chance observation led to the initiation of pallidal surgery (pallidotomy) for this condition (see also Ch. 22).
Ischaemic stroke
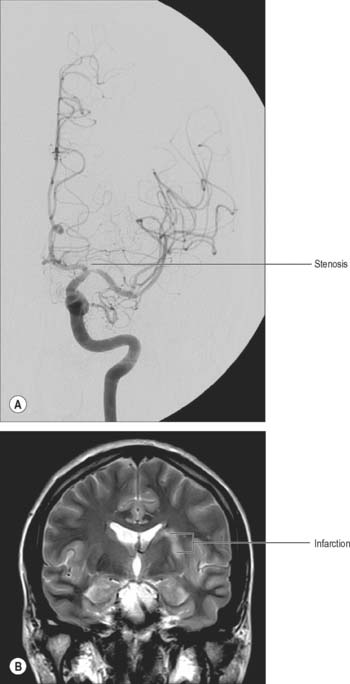
Stroke refers to the clinical syndrome of a rapidly developing focal neurological deficit that is not due to seizure activity. If the cause is lack of, or reduced, blood supply to a portion of the brain then the term ischaemic stroke (see Fig. 17.9) is used, as opposed to haemorrhagic stroke e.g. subarachnoid haemorrhage. The lack of blood flow can be due to pathology in the vessel lumen, such as thrombosis or embolus (common), pathology outside the blood vessel, such as occlusion from mass effect of a tumour or haematoma (rare), or pathology of the vessel wall, such as inflammatory or infective arteritides (rare). The symptoms and signs of ischaemic stroke depend on the location and extent of the arterial infarction. In certain locations, even a small volume stroke can have devastating effects. For example, the internal capsule and most of the adjacent basal ganglia are supplied by small perforating arteries, which are a common site for ischaemic stroke. Corticobulbar and corticospinal motor pathways and third-order thalamocortical sensory fibres all pass through the internal capsule, so that it is effectively the only route connecting the cerebral cortex with other regions of the neuraxis. The neurological deficits that result from stroke in the internal capsule, therefore, may include contralateral spastic hemiparesis, contralateral hemisensory loss and psychological deficits.
Cerebral cortex
The entire blood supply of the cerebral cortex comes from cortical branches of the anterior, middle and posterior cerebral arteries (Fig. 17.4, Fig. 17.5). In general, long branches traverse the cortex and penetrate the subjacent white matter for 3 or 4 cm without communicating. Short branches are confined to the cerebral cortex, and form a compact network in the middle zone of the grey matter, whereas the outer and inner zones are sparingly supplied. Although adjacent vessels anastomose on the surface of the brain, they become end arteries as soon as they enter it. In general, superficial anastomoses only occur between microscopic branches of the cerebral arteries, and there is little evidence that they can provide an effective alternative circulation after the occlusion of larger vessels.
Cerebral blood flow
Not all substances circulating in arterial blood have access to the brain parenchyma. Particulate matter, such as bacteria, is excluded. In general, lipophilic molecules and small molecules, such as oxygen and carbon dioxide, can cross the blood–brain barrier but hydrophilic ones (excluding glucose) cannot. The cellular basis for the blood–brain barrier is discussed in Chapter 3.
VENOUS DRAINAGE OF THE BRAIN
The venous drainage of the brain occurs through a complex system of deep and superficial veins. These veins possess no valves and have thin walls devoid of muscular tissue. They pierce the arachnoid mater and the inner layer of the dura mater to open into the dural venous sinuses (see Chapter 27).
VENOUS DRAINAGE OF THE POSTERIOR FOSSA
The veins of the brain stem form a superficial venous plexus deep to the arteries.
Veins of the medulla oblongata drain into the veins of the spinal cord or the adjacent dural venous sinuses, or into variable radicular veins which accompany the last four cranial nerves to either the inferior petrosal or occipital sinuses, or to the superior bulb of the jugular vein. Anterior and posterior median medullary veins may run along the anterior median fissure and posterior median sulcus, to become continuous with the spinal veins in corresponding positions. Pontine veins, which may include a median vein and a lateral vein on each side, drain into the basal vein, cerebellar veins, the petrosal sinuses, transverse sinus or the venous plexus of the foramen ovale. Veins of the midbrain join the great cerebral vein or basal vein.
VENOUS DRAINAGE OF THE CEREBRAL HEMISPHERE
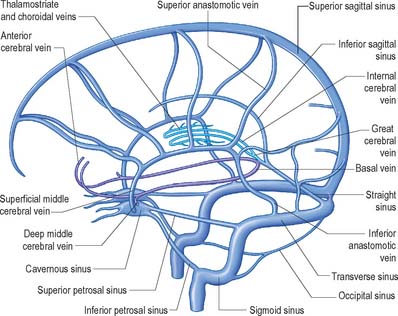
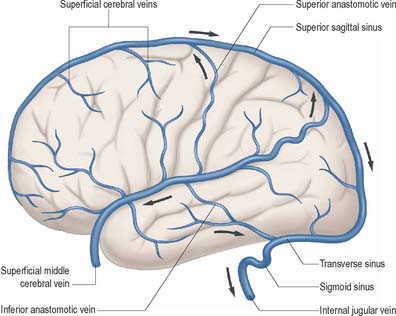
External and internal cerebral veins drain the surfaces and the interior of the cerebral hemisphere (Figs 17.10, 17.11, 17.12, 17.13).
External cerebral veins may be divided into three groups, namely superior, middle and inferior.
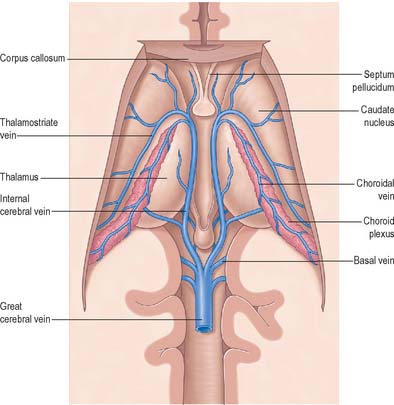
The internal cerebral vein drains the deep parts of the hemisphere and the choroid plexuses of the third and lateral ventricles. It is formed near the interventricular foramen, behind the column of the fornix, primarily by union of the thalamostriate and choroidal veins, although numerous smaller veins from surrounding structures also converge here. The thalamostriate vein runs anteriorly, between the caudate nucleus and thalamus, and receives many tributaries from both areas. The choroidal vein runs a convoluted course along the whole choroid plexus, and receives veins from the hippocampus, fornix, corpus callosum and adjacent structures. After their formation, the two internal cerebral veins travel back parallel to one another beneath the splenium of the corpus callosum, where they unite to form the great cerebral vein. The great cerebral vein is a short median vessel which curves sharply up around the splenium of the corpus callosum and opens into the anterior end of the straight sinus after receiving the right and left basal veins.
Intracranial venosinus thrombosis
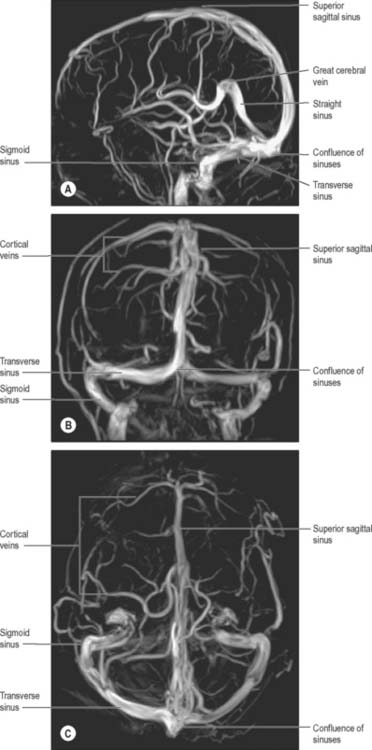
Although they may occur together, clinicians distinguish between thrombosis that affects the ‘superficial’ and ‘deep’ venous systems (Fig. 17.10). Superficial thrombosis usually occurs in the superior sagittal sinus (with or without involvement of the cortical veins), whilst deep intracranial thrombotic disease usually affects the vein of Galen and straight sinus. The parts of the cerebral hemispheres affected are different in the two situations; superficial thrombosis affects the cerebral hemispheres (particularly the white matter) and deep thrombosis affects the thalami and basal ganglia. However, one common feature is the tendency for bilateral involvement, because all of the main draining venous channels are in the midline and accept venous drainage from both hemispheres.
Andeweg J. The anatomy of collateral venous flow from the brain and its value in aetiological interpretation of intracranial pathology. Neurorad. 1996;38:621-628.
Includes a brief historical review..
Bogousslavsky J, Caplan L. Stroke Syndromes, 2nd edn., Cambridge: Cambridge University Press, 2002.
Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519-579.
Duvernoy H, Delon S, Vannson JL. The vascularization of the human cerebellar cortex. Brain Res Bull. 1983;11:419-480.
Kaplan HA, Ford DH. The Brain Vascular System. Amsterdam: Elsevier, 1966.
Plets C, De Reuck J, Vander Eecken H, Van den Bergh R. The vascularization of the human thalamus. Acta Neurol Belg. 1970;70:687-770.
Puchades-Orts A, Nombela-Gomez M, Ortu-o-Pacheco G. Variation in form of the circle of Willis. Some anatomical and embryological considerations. Anat Rec. 1976;185:119-123.
Sengupta RP, McAllister VL. Subarachnoid Haemorrhage. Berlin: Springer-Verlag; 1986:9-31.