CHAPTER 91 Vascular Malformations of the Spinal Cord
Vascular lesions of the spinal cord are a rare cause of neurologic dysfunction, representing less than 5% of all intraspinal pathology.1 This heterogeneous class of entities encompasses a wide range of etiologic, anatomic, pathophysiologic, and clinical features. They occur throughout the spine and may affect any age group, although the vast majority present between the third and fifth decades of life.1 Symptoms and signs result from ischemia, venous congestion, hemorrhage, or mechanical compression of the spinal cord and roots. Most spinal vascular lesions are characterized by an abnormal arteriovenous shunt, which may be located within the dura, on the spinal cord surface, within the substance of the spinal cord, or rarely extradurally.2–5 The shunt may take the form of a simple direct arteriovenous fistula (AVF) or of a more complex nidus of dysmorphic arteries and veins without an intervening capillary bed. Whereas the latter lesions are typically congenital, the more common fistulous lesions are often acquired.6
Classification
Various nomenclature and classification systems have been employed in the description of spinal vascular lesions.4,7–9 As a first approximation, these lesions can be broadly divided by the presence or absence of an arteriovenous shunt. Those lesions without a shunt include cavernous malformation (a lesion of capillary structure10) and, possibly, hemangioblastoma.4 Spinal cord arteriovenous shunts, on the other hand, have traditionally been separated into four types on the basis of the location and angioarchitecture of the abnormal arteriovenous connection (Box 91–1). Types I and IV represent direct AVFs that occur within the dural root sleeve (type I) or on the spinal cord surface (type IV). Type II arteriovenous malformations (AVMs) are true congenital malformations, similar to their intracranial pial counterparts. Type III AVMs are also congenital but demonstrate extensive contiguous involvement of intramedullary, intradural-extramedullary, and extradural/paraspinal tissues.
Type I
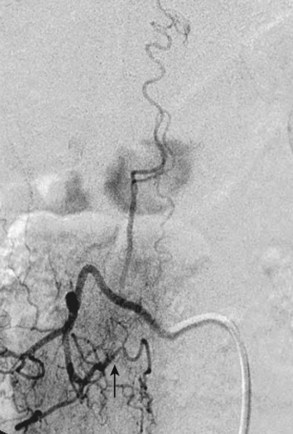
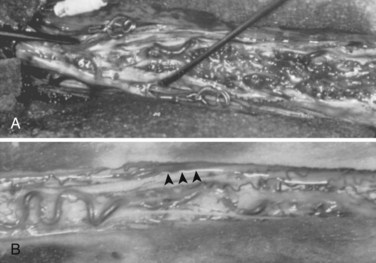
Type I AVFs, the most commonly occurring type of spinal vascular malformation, have also been termed long dorsal AVMs; single coiled vessel AVMs; angioma venosum racemosum; dural AVF; micro-AVF; and, more recently, intradural dorsal AVF.4,7,8,11–17 A more sophisticated understanding of the anatomy and pathophysiology of these fistulas has developed since the seminal description by Wyburn-Mason,17 who characterized these as purely venous lesions. The advent of selective spinal angiography in the 1960s reclassified them as slow-flow AVFs. Early surgical treatment was directed at stripping the long dorsal vein off the spinal cord surface. It was assumed that tiny feeding vessels, too small to be seen angiographically, supplied this dilated vein throughout its length. This treatment approach did stabilize or improve symptoms in some patients, but postoperative neurologic deterioration was seen in many others. Kendall and Logue,13 in 1977, correctly recognized these lesions as simple AVFs between a radicular or radiculomedullary artery and a medullary vein, with the fistulous connection located in the dural root sleeve (Fig. 91–1). The entire intradural portion of the malformation, therefore, represents the enlarged spinal cord venous system that has been pathologically engorged from retrograde flow from the fistula into the spinal cord veins (Fig. 91–2). These malformations are likely often acquired and arise predominantly at thoracic and thoracolumbar levels. As mentioned earlier, these shunts and their venous drainage are almost universally dorsally located with ventral communications being exceedingly rare.18
Type II
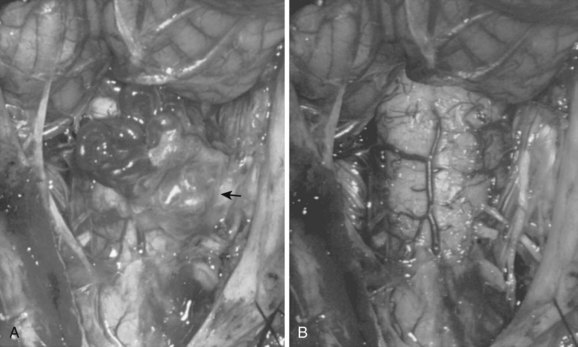
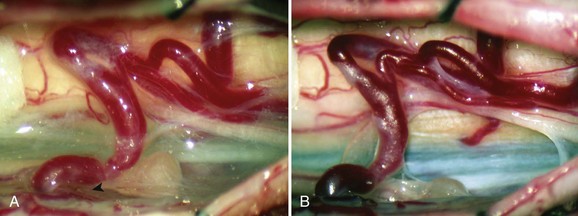
These malformations are known as glomus, nidus, or simply intramedullary type AVMs.4,7 They are angiographically and operatively well-defined lesions consisting of a distinct conglomeration of dysmorphic arteries and veins in direct communication without an intervening capillary bed (Figs. 91-3 and 91-4).19,20 The location of the nidus may be completely or partially intramedullary and only rarely is confined to the epipial tissue of the cord. These latter lesions are sometimes referred to as perimedullary type II AVMs and most commonly occur dorsally at the cervicomedullary junction (Fig. 91–5).
Typical type II AVMs may arise anywhere within the spinal cord but predominantly are found at the cervical and lumbar enlargements or the conus medullaris. Type II AVMs of the cervical spinal cord frequently have multiple feeding vessels of anterior spinal, posterior spinal, and radiculomedullary arteries, whereas type II AVMs of the thoracic spinal cord or conus are often supplied via a single enlarged branch of the anterior spinal artery. Latent anastomotic channels invariably exist, however, which may emerge after proximal ligation or endovascular occlusion of the primary feeding vessel (Fig. 91–6).
Type III
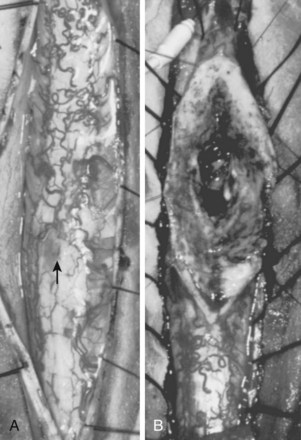
Also known as juvenile or metameric AVMs, type III malformations are fortunately rare. These lesions do not possess a discrete nidus but rather consist of diffuse arteriovenous shunts with variable degrees of involvement of the spinal cord, vertebral, and paraspinal tissues (Fig. 91–7). Other metameric anomalies of associated organs and the skin are commonly associated with these lesions.
Type IV
Type IV malformations are completely intradural fistulas, variably referred to as perimedullary AVF, macro-AVF, or intradural ventral AVF.4,7,8,12,21,22 Most occur in the thoracolumbar region as a fistula between the anterior spinal artery (ASA) and vein (ASV) on the ventral spinal cord surface. Gueguen and colleagues23 as well as Anson and Spetzler12 subdivided these arteriovenous shunts according to the complexity and size of the lesion. A type IV-A malformation is a small, simple direct fistula with a single ASA feeder. Type IV-B lesions are medium-sized fistulas and have additional, smaller feeding vessels arising from either the ASA or posterior spinal artery (PSA). Type IV-C fistulas are giant sized and demonstrate several enlarged ASA and PSA feeding branches with dilated venous outflow. The subtypes may represent progressive changes resulting from venous congestion, thrombosis, collateral vessel recruitment, or ischemia. The sporadic nature of these malformations suggests an acquired rather than congenital nature, but their rarity makes their etiology difficult to determine.
Pathophysiology and Symptomatology
Type I
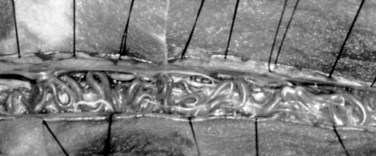
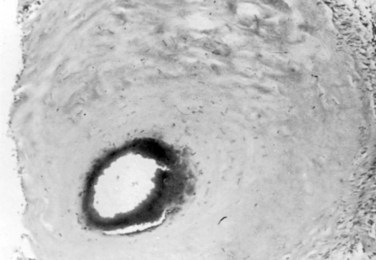
Type I AVFs produce progressive spinal cord ischemia from venous hypertension.7,11,15,16,24–28 Despite slow flow through the shunt, intradural venous pressures become markedly elevated and may approach systemic mean arterial pressure. Because spinal cord perfusion pressure is equal to mean systemic arterial pressure minus venous pressure, progressive spinal cord ischemia may ensue. The elevation of intraspinal venous pressures during activity or exercise accounts for the reversible ischemic symptoms often seen in these patients. Venous hypertension may be further exacerbated by structural changes such as reductions in venous diameter and compliance secondary to intimal thickening and hyalinization seen with chronic exposure to high intravenous pressures (Fig. 91–8). Episodic acute neurologic deterioration in these patients may occur as a result of venous thrombosis. In rare cases, patients may present with subarachnoid hemorrhage mimicking that of intracranial aneurysm rupture.29,30
It follows then that type I AVFs produce symptoms usually in middle and advanced adult years with a mean age at symptom onset around 50 years. Men are four to five times more commonly affected than women. The majority occur in the thoracic or thoracolumbar region. Symptoms typically arise insidiously with back and leg pain and mild sensorimotor dysfunction. In early stages, symptoms may mimic those of neurogenic claudication secondary to spinal stenosis. Neurologic examination, however, often reveals mixed upper and lower motor neuron disease and patchy sensory loss, clearly differentiating the clinical picture from degenerative lumbar stenosis. The natural history of type I AVFs produces an inexorable progression of symptoms occasionally punctuated by episodes of acute worsening, as in the classic Foix-Alajouanine syndrome. If untreated, these lesions lead to significant disability and wheelchair dependence in most patients within 6 months to 3 years after symptom onset.15
Numerous investigators have demonstrated that preoperative neurologic status is the most important predictor of post-treatment outcomes.28,31–33 Median time from symptom onset to diagnosis in modern series ranges from 15 to 23 months.32–36 If type I AVFs are treated early in their course, symptoms are often reversible. However, in more chronic cases, progressive ischemia ultimately leads to irreversible neuronal loss and infarction. Therefore definitive treatment should be accomplished as early in the disease as possible because function is unlikely to improve in the presence of severe incapacity.
Type II
Type II AVMs present in childhood or adult years. An acute presentation from subarachnoid hemorrhage or intramedullary hemorrhage is most common.16 As mentioned earlier, the presence of venous aneurysms seems to increase the risk of a hemorrhagic event. The acute onset of severe neck or back pain (“coup de poignard”37) approximates the level of the malformation and is typically the first symptom of AVM hemorrhage. The occurrence and progression of neurologic deficit are variable and dependent on the location and severity of the hemorrhage. For example, typical headache and nuchal rigidity may be the only symptoms of a spinal subarachnoid hemorrhage and usually lead to the standard evaluation for an intracranial ruptured aneurysm.38 On the other hand, objective deficits are typically seen with intramedullary hemorrhage. These deficits evolve over minutes to hours, and some degree of recovery is usually seen after incomplete lesions.
Types III and IV
Similar to type II AVMs, these high-flow lesions produce symptoms due to vascular steal/ischemia, hemorrhage, and mass effect. Type III AVMs have been associated with genetic syndromes such as Klippel-Trenaunay-Weber.39 Type IV lesions have an equal incidence in males and females. Symptoms may arise at any age, although most patients present in childhood and early to middle adult years. Associations with hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) and Kartagener syndrome have been noted in younger patients.7,8,40,41
Radiology
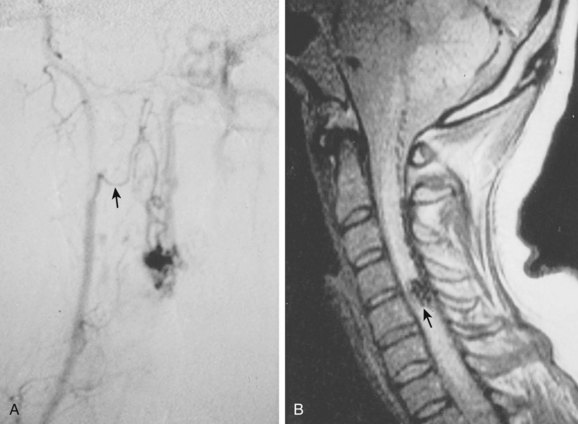
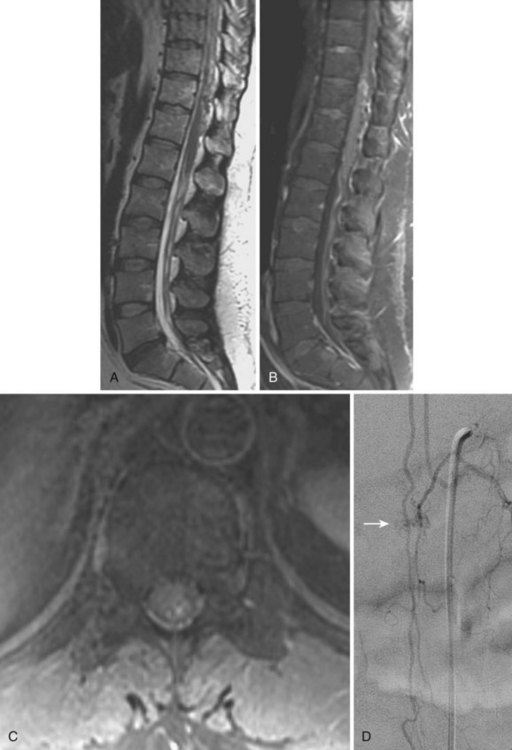
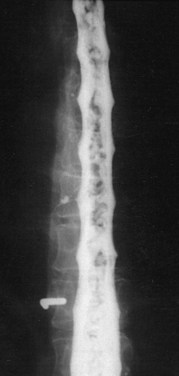
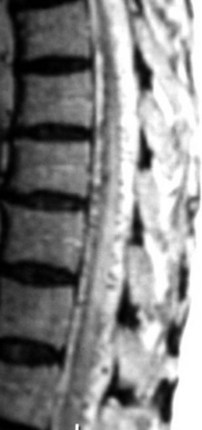
Selective spinal angiography remains the gold standard for the definitive diagnosis and characterization of spinal vascular malformations. This imaging study identifies the locations and flow characteristics of vascular shunts, as well as the sites of critical radiculomedullary arteries (e.g., artery of Adamkiewicz).13,20–22,42–46 Its inherently invasive nature, however, has led to the development and use of alternative modalities as screening procedures. These currently include magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), computed tomographic (CT) angiography (CTA), and CT myelography. Myelography has a long history in the diagnosis of spinal AVMs, particularly for type I AVFs in which the characteristic serpentine filling defect on the dorsal spinal cord surface is often apparent (Fig. 91–9). These enlarged vessels are commonly identified on screening magnetic resonance scans. (Fig. 91–10). If the filling defect extends toward a neural foramen, the level and side of the fistula may further be suggested. The dilated venous filling defect should be differentiated from the venous dilatation seen rostral to high-grade extradural stenotic blocks.
MRI and MRA are useful for the initial screening of AVFs and are critical in the evaluation of intramedullary malformations, particularly with regard to operative planning and understanding the relationship of the malformation to normal spinal cord. As with CT myelography, both MRA and CTA may permit more targeted selective angiography.47,48
Three-dimensional angiography is also emerging as a potential aid in angiographic delineation of the structural anatomy of spinal vascular malformations and their relationship to the surrounding spine and normal vessels.49
Treatment
Successful treatment of spinal vascular malformations requires the total obliteration or excision of the abnormal shunt.* Procedures that only partially reduce the shunt or address only proximal feeders may provide temporary benefit but all too often lead to delayed recurrences.
Surgical Therapy: General Considerations
The surgical approach to spinal cord vascular malformations depends on the level and anatomic position of the lesion. Nevertheless, the majority are dorsal or dorsolateral and therefore can be approached via a standard posterior laminectomy of appropriate number of levels. In general, routine perioperative antibiotics and corticosteroids are administered at the time of surgery. Neurophysiologic monitoring including somatosensory evoked potential (SSEP) and motor evoked potential (MEP) are also routinely used. Whereas authors such as Malis53 have described the use of the sitting or oblique positions for the posterior removal of malformations, we prefer the prone position for all such laminectomies. Although the sitting position decreases venous pressure and respiratory excursions, it also precludes the effective use of an assistant during the operation. With the patient in the prone position, the surgeon and assistant work together across the operating table.
For complex cases, intraoperative confirmation of shunt elimination may be obtained. The standard method employed is intraoperative angiography.54,55 This, however, has its limitations with regard to both patient positioning and image resolution. For types I and IV fistulas, some authors have described the use of intraoperative micro-Doppler ultrasound or infrared imaging of spinal vascular blood flow to demonstrate successful obliteration of the shunt.56–58
Endovascular Therapy: General Considerations
The role of endovascular techniques in the treatment of spinal vascular malformations continues to evolve. Advances in catheter technology, image resolution, and embolization materials have allowed increasing success and safety in the elimination of certain vascular lesions. The wide variety of embolic materials used in the endovascular treatment of spinal vascular malformations includes N-butyl cyanoacrylate (NBCA), Onyx, polyvinyl alcohol, coils, cellulose acetate polymer, and trisacryl gelatin microspheres.33,59–62
The more widespread use of liquid adhesive embolic agents such as N-butyl cyanoacrylate (NBCA) and ethylene vinyl alcohol copolymer (Onyx Micro Therapeutics Inc., Irvine, Calif.) has generally been associated with more durable results and less recanalization as compared with particulate embolic materials (e.g., polyvinyl alcohol). Both neurophysiologic monitoring and pharmacologic provocative testing have emerged as important adjuncts in the endovascular treatment of spinal vascular malformations. SSEPs and MEPs and testing with intra-arterial injections of agents such as amobarbital (Amytal) and lidocaine have been shown to allow safer embolization of spinal AVMs.62–64
Whether endovascular techniques occupy a primary or adjunctive role depends not only on the type of vascular lesions but also on institutional experience. Whereas embolization may clearly be the procedure of choice for extradural fistulas and type III vascular malformations,2 surgery remains the “gold standard” for type II lesions. For types I and IV lesions, some authors advocate initial treatment attempts with embolization, reserving surgery for failures, but others prefer surgery as first-line treatment.
Type I
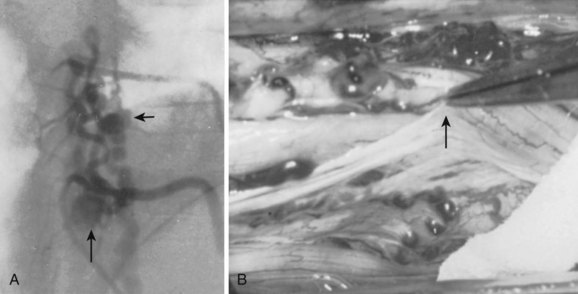
Operatively, type I shunts may be treated by excision of the dural fistula or interruption of the intradural draining vein (Fig. 91–11).11,24,25 This can easily be accomplished via a two-level hemilaminectomy and partial medial facetectomy, exposing the dural root sleeve and foramen. A paramedian longitudinal dural incision allows exposure of the intradural nerve root and initial segment of the associated draining vein of the shunt. Simple interruption of the draining vein is the generally preferred technique, particularly in circumstances in which the radicular artery that supplies the fistula also supplies a spinal cord medullary artery. This, fortunately, occurs in less than 10% of cases. In cases of more complex or recurrent type I AVFs, fistula excision definitively prevents re-establishment of retrograde intradural venous drainage through collateral longitudinal extradural venous channels at adjacent radicular levels. Several millimeters of the feeding radicular artery and intradural draining vein may be cauterized, divided, and contiguously excised along with a small window of dura on the root sleeve. This dural defect can be primarily repaired with sutures. The wound is then closed in layers in a typical fashion.
Outcomes following the treatment of type I AVFs are generally good, with most reports demonstrating neurologic improvement or stabilization in 70% to 99% of patients.31,32,36,65–68 Interestingly, motor and gait disturbances seem to improve to a greater degree than sensory or sacral deficits.31,65 Surgery produces a 98% fistula obliteration rate, and endovascular embolization produces a 25% to 66% obliteration rate.32,35,36,66,68–70 In general then, for most type I lesions we recommend surgery as a primary therapy to avoid the progressive myelopathy associated with a delay in definitive treatment that can be seen with endovascular treatment failures.
Type II
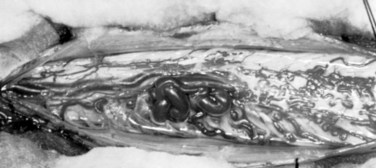
Malformations that are largely intramedullary are similarly treated incorporating techniques used for intramedullary spinal cord tumors. The locus of the lesion is visualized, and a myelotomy is made over the dorsal surface of the malformation identifying both rostral and caudal poles of the malformation. Typically, irrigating bipolar cautery is used during the obliteration of the malformation. Only the largest vessels are clipped; and from a practical point of view, this means clipping few or no arteries because the application of metallic clips to intramedullary lesions often proves difficult or dangerous. As expected, the AVM is approached from the arterial side first, serially coagulating and dividing arterial contributions to the AVM as it is gently peeled away from the cord substance on its venous pedicle, which is ligated just prior to final removal. As mentioned earlier, early interruption of the venous drainage should be avoided. Aneurysmal venous dilatations are prone to rupture even with minor surgical manipulation. Bipolar cautery can be used to shrink these venous aneurysms, but care must be taken not to violate the thin vessel wall. In some cases, the venous aneurysm may be gently teased out of the cord (Fig. 91–12).
Type III
These lesions are the most difficult to treat. They penetrate the spinal cord, and histologic examination indicates violation of presumably functional cord tissue interspersed with vascular channels of the malformation. These AVMs do not have well-defined margins and comprehensively involve the intramedullary, intradural-extramedullary, and extradural compartments over many spinal segments. Although these are generally unresectable lesions, partial treatment through endovascular embolization, surgical decompression, and limited arterial ligation may produce some clinical benefit (Fig. 91–13).4
Type IV
Treatment of type IV AVFs depends particularly on the size and complexity of the lesion.21,22 For small type IV-A shunts, surgical ligation of the fistula is definitive. Adequate exposure of these ventral intradural shunts often requires more aggressive posterior or posterolateral bone removal including facetectomy, as well as spinal cord rotation with suture retraction of the dentate ligament. Alternatively, anterior or anterolateral approaches to such ventral lesions may be used, with special attention paid to the need for spinal reconstruction and the attendant risk of cerebrospinal fluid fistulas.71,72 Intraoperative intravenous administration of near-infrared indocyanine can be useful in assessing the completeness of the resection of difficult spinal cord vascular malformations.73
For more complex, higher-flow lesions (types IV-B and C), endovascular obliteration of the shunt may be the preferred primary treatment or at least a preoperative adjunct.22,40,74
Cavernous Malformations
The surgical technique for cavernous malformation resection depends on the relationship of the lesion to the spinal cord. For pial-based lesions, a circumscribing pial incision allows detachment and delivery of the cavernoma from the superficial substance of the spinal cord. Deeper intramedullary lesions are exposed by a midline myelotomy. Although unencapsulated, these malformations are generally well circumscribed and present a clear dissection plane.10 Uncommonly, a more diffuse lesion permeates the spinal cord tissue and precludes surgical removal. Epidural cavernous malformations are exceedingly rare entities, but treatment follows that of other well-demarcated extradural pathologic processes.75
Summary
The primary aim in the treatment of spinal cord vascular malformations is obliteration or excision of the abnormal arteriovenous shunt. This is routinely achieved with the current techniques available in microsurgery and endovascular therapy for most malformations of types I, II, and IV. And, as with the excision of most cavernous malformations, successful treatment can be accomplished with preservation of neurologic function. Type III AVMs remain a clinical challenge awaiting novel approaches for treatment. The role of new therapeutic modalities such as stereotactic radiosurgery (e.g., CyberKnife) in the management of these lesions has only begun to be explored.76
Pearls
Pitfalls
Key Points
1 Cenzato M, Versari P, Righi C, et al. Spinal dural arteriovenous fistulae: Analysis of outcome in relation to pretreatment indicators. Neurosurgery. 2004;55:815-822. discussion 822-823
2 Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg Spine. 2002;96:145-156.
3 Steinmetz MP, Chow MM, Krishnaney AA, et al. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single-institution series and meta-analysis. Neurosurgery. 2004;55:77-87. discussion 88
4 Wyburn-Mason R. The Vascular Abnormalities and Tumours of the Spinal Cord and Its Membranes. London: Henry Klimpton; 1943.
1 Youmans JR, editor. Neurological Surgery, 2nd ed, Philadelphia: WB Saunders, 1982.
2 Rodesch G, Lasjaunias P. Spinal cord arteriovenous shunts: From imaging to management. Eur J Radiol. 2003;46:221-232.
3 Chuang NA, Shroff MM, Willinsky RA, et al. Slow-flow spinal epidural AVF with venous ectasias: Two pediatric case reports. AJNR Am J Neuroradiol. 2003;24:1901-1905.
4 Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg Spine. 2002;96:145-156.
5 Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: Arteriovenous fistula and arteriovenous malformations. Neurosurgery. 2006;59:S195-S201.
6 Vankan Y, Demaerel P, Heye S, et al. Dural arteriovenous fistula as a late complication of upper cervical spine fracture: Case report. J Neurosurg Spine. 2004;100:382-384.
7 Rodesch G, Hurth M, Alvarez H, et al. Angio-architecture of spinal cord arteriovenous shunts at presentation: Clinical correlations in adults and children. The Bicetre experience on 155 consecutive patients seen between 1981-1999. Acta Neurochir (Wien). 2004;146:217-226. discussion 226-227
8 Rodesch G, Hurth M, Alvarez H, et al. Classification of spinal cord arteriovenous shunts: Proposal for a reappraisal-the Bicetre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery. 2002;51:374-379. discussion 379-380
9 Marsh WR. Vascular lesions of the spinal cord: History and classification. Neurosurg Clin North Am. 1999;10:1-8.
10 McCormick PC, Michelsen WJ, Post KD, et al. Cavernous malformations of the spinal cord. Neurosurgery. 1988;23:459-463.
11 Afshar JK, Doppman JL, Oldfield EH. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg. 1995;82:196-200.
12 Anson JA, Spetzler RF. Classification of spinal arteriovenous malformations and implications for treatment. Barrow Neurol Inst Q. 1992;8:2-8.
13 Kendall BE, Logue V. Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology. 1977;13:181-189.
14 McCormick PC, Stein BM. Management of spinal vascular malformations. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke: Pathophysiology, Diagnosis, and Management. 2nd ed. New York: Churchill Livingstone; 1992:1135-1143.
15 Oldfield EH, Di Chiro G, Quindlen EA, et al. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg. 1983;59:1019-1030.
16 Rosenblum B, Oldfield EH, Doppman JL, Di Chiro G. Spinal arteriovenous malformations: A comparison of dural arteriovenous fistulas and intradural AVM’s in 81 patients. J Neurosurg. 1987;67:795-802.
17 Wyburn-Mason R. The Vascular Abnormalities and Tumours of the Spinal Cord and Its Membranes. London: Henry Klimpton; 1943.
18 Niimi Y, Setton A, Berenstein A. Spinal dural arteriovenous fistulae draining to the anterior spinal vein: Angiographic diagnosis. Neurosurgery. 1999;44:999-1003. discussion 1004
19 Doppman JL. The nidus concept of spinal cord arteriovenous malformations: A surgical recommendation based upon angiographic observations. Br J Radiol. 1971;44:758-763.
20 Houdart R, Djindjian R, Hurth M. Vascular malformations of the spinal cord: The anatomic and therapeutic significance of arteriography. J Neurosurg. 1966;24:583-594.
21 Barrow DL, Colohan AR, Dawson R. Intradural perimedullary arteriovenous fistulas (type IV spinal cord arteriovenous malformations). J Neurosurg. 1994;81:221-229.
22 Mourier KL, Gobin YP, George B, et al. Intradural perimedullary arteriovenous fistulae: Results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993;32:885-891. discussion 891
23 Gueguen B, Merland JJ, Riche MC, Rey A. Vascular malformations of the spinal cord: Intrathecal perimedullary arteriovenous fistulas fed by medullary arteries. Neurology. 1987;37:969-979.
24 Aminoff MJ, Logue V. Clinical features of spinal vascular malformations. Brain. 1974;97:197-210.
25 Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974;97:211-218.
26 Logue V. Angiomas of the spinal cord: Review of the pathogenesis, clinical features, and results of surgery. J Neurol Neurosurg Psychiatry. 1974;42:1-11.
27 Rodriguez FJ, Crum BA, Krauss WE, et al. Venous congestive myelopathy: A mimic of neoplasia. Mod Pathol. 2004. (Epub, Dec 3)
28 Kataoka H, Miyamoto S, Nagata I, et al. Venous congestion is a major cause of neurological deterioration in spinal arteriovenous malformations. Neurosurgery. 2001;48:1224-1229. discussion 1229-1230
29 Aviv RI, Shad A, Tomlinson G, et al. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: Report of two cases and literature review. AJNR Am J Neuroradiol. 2004;25:854-858.
30 Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage: Case report and review of the literature. J Neurosurg Spine. 2004;100:385-391.
31 Cenzato M, Versari P, Righi C, et al. Spinal dural arteriovenous fistulae: Analysis of outcome in relation to pretreatment indicators. Neurosurgery. 2004;55:815-822. discussion 822-823
32 Atkinson JLD, Miller GM, Krauss WE, et al. Clinical and radiographic features of dural arteriovenous fistula, a treatable cause of myelopathy. Mayo Clin Proc. 2001;76:1120-1130.
33 Ushikoshi S, Hida K, Kikuchi Y, et al. Functional prognosis after treatment of spinal dural arteriovenous fistulas. Neurol Med Chir (Tokyo). 1999;39:206-212. discussion 212-213
34 Jellema K, Canta LR, Tijssen CC, et al. Spinal dural arteriovenous fistulas: Clinical features in 80 patients. J Neurol Neurosurg Psychiatry. 2003;74:1438-1440.
35 Schick U, Hassler W. Treatment and outcome of spinal dural arteriovenous fistulas. Eur Spine J. 2003;12:350-355.
36 Westphal M, Koch C. Management of spinal dural arteriovenous fistulae using an interdisciplinary neuroradiological/neurosurgical approach: Experience with 47 cases. Neurosurgery. 1999;45:451-457. discussion 457-458
37 Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: A literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26:1-49.
38 Kasdon DL, Wolpert SM, Stein BM. Surgical and angiographic localization of spinal arteriovenous malformations. Surg Neurol. 1976;5:279-283.
39 Alexander MJ, Grossi PM, Spetzler RF, McDougall CG. Extradural thoracic arteriovenous malformation in a patient with Klippel-Trenaunay-Weber syndrome: Case report. Neurosurgery. 2002;51:1275-1278. discussion 1278-1279
40 Mont’Alverne F, Musacchio M, Tolentino V, et al. Giant spinal perimedullary fistula in hereditary haemorrhagic telangiectasia: Diagnosis, endovascular treatment and review of the literature. Neuroradiology. 2003;45:830-836.
41 Rosenow J, Rawanduzy A, Weitzner IJr, Couldwell WT. Type IV spinal arteriovenous malformation in association with familial pulmonary vascular malformations: Case report. Neurosurgery. 2000;46:1240-1244. discussion 1244-1245
42 Cogen P, Stein BM. Spinal cord arteriovenous malformations with significant intramedullary components. J Neurosurg. 1983;59:471-478.
43 Djindjian R. Embolization of angiomas of the spinal cord. Surg Neurol. 1975;4:411-420.
44 Houdart R, Djindjian R, Hurth M, Rey A. Treatment of angiomas of the spinal cord. Surg Neurol. 1974;2:186-194.
45 Kunc Z, Bret J. Diagnosis and treatment of vascular malformations of the spinal cord. J Neurosurg. 1969;30:436-445.
46 Stein BM. Arteriovenous malformations of the brain and spinal cord. In: Hoff J, editor. Practice of Surgery. New York: Harper & Row, 1979.
47 Pui MH. Gadolinium-enhanced MR angiography of spinal arteriovenous malformation. Clin Imaging. 2004;28:28-32.
48 Terae S, Kudo K, Asano T, et al. CT angiography with multidetector-row helical CT in spinal arteriovenous malformation. Clin Imaging. 2004;28:23-27.
49 Prestigiacomo CJ, Niimi Y, Setton A, Berenstein A. Three-dimensional rotational spinal angiography in the evaluation and treatment of vascular malformations. AJNR Am J Neuroradiol. 2003;24:1429-1435.
50 Krayenbuhl H, Yasargil MG, McClintock HG. Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg. 1969;30:427-435.
51 McCormick PC. Vascular tumors and vascular malformations of the spine. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier, 1996.
52 Yasargil MG, DeLong WB, Guarnaschelli JJ. Complete microsurgical excision of cervical extramedullary and intramedullary vascular malformations. Surg Neurol. 1975;4:211-224.
53 Malis LI. Microsurgery for spinal cord arteriovenous malformations. Clin Neurosurg. 1979;26:543-555.
54 Benes L, Wakat JP, Sure U, et al. Intraoperative spinal digital subtraction angiography: Technique and results. Neurosurgery. 2003;52:603-609. discussion 608-609
55 Schievink WI, Vishteh AG, McDougall CG, Spetzler RF. Intraoperative spinal angiography. J Neurosurg Spine. 1999;90:48-51.
56 Nakagawa A, Hirano T, Uenohara H, et al. Use of intraoperative dynamic infrared imaging with detection wavelength of 7-14 microns in the surgical obliteration of spinal arteriovenous fistula: Case report and technical considerations. Minim Invasive Neurosurg. 2004;47:136-139.
57 Padovani R, Farneti M, Maida G, Ghadirpour R. Spinal dural arteriovenous fistulas: The use of intraoperative microvascular Doppler monitoring. Br J Neurosurg. 2003;17:519-524.
58 Iacopino DG, Conti A, Giusa M, et al. Assistance of intraoperative microvascular Doppler in the surgical obliteration of spinal dural arteriovenous fistula: Cases description and technical considerations. Acta Neurochir (Wien). 2003;145:133-137. discussion 137
59 Warakaulle DR, Aviv RI, Niemann D, et al. Embolisation of spinal dural arteriovenous fistulae with Onyx. Neuroradiology. 2003;45:110-112.
60 Rodiek SO. Successful endovascular treatment of a spinal dural arteriovenous fistula with trisacryl gelatin microspheres. Minim Invasive Neurosurg. 2002;45:173-176.
61 Schaat TJ, Salzman KL, Stevens EA. Sacral origin of a spinal dural arteriovenous fistula: Case report and review. Spine. 2002;27:893-897.
62 Sugiu K, Meguro T, Nakashiama H, Ohmoto T. Successful embolization of a spinal perimedullary arteriovenous fistula with cellulose acetate polymer solution: Technical case report. Neurosurgery. 2001;49:1257-1260. discussion 1260-1261
63 Niimi Y, Sala F, Deletis V, et al. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR Am J Neuroradiol. 2004;25:1131-1138.
64 Sala F, Niimi Y, Berenstein A, Deletis V. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the spinal cord. Ann N Y Acad Sci. 2001;939:126-136.
65 Jellema K, Tijssen CC, van Rooij WJ, et al. Spinal dural arteriovenous fistulas: Long-term follow-up of 44 treated patients. Neurology. 2004;62:1839-1841.
66 Steinmetz MP, Chow MM, Krishnaney AA, et al. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single-institution series and meta-analysis. Neurosurgery. 2004;55:77-87. discussion 78
67 Song JK, Vinuela F, Gobin YP, et al. Surgical and endovascular treatment of spinal dural arteriovenous fistulas: Long-term disability assessment and prognostic factors. J Neurosurg Spine. 2001;94:199-204.
68 Narvid J, Hetts SW, Larsen D, et al. Spinal dural arteriovenous fistulae: Clinical features and long-term results. Neurosurgery. 2008;62:159-167.
69 Eskandar EN, Borges LF, Budzik RFJr, et al. Spinal dural arteriovenous fistulas: Experience with endovascular and surgical therapy. J Neurosurg Spine. 2002;96:162-167.
70 Van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multidisciplinary management of spinal dural arteriovenous fistulas: Clinical presentation and long-term follow-up in 49 patients. Stroke. 2002;33:1578-1583.
71 O’Toole JE, McCormick PC. Midline ventral intradural schwannoma of the cervical spinal cord resected via anterior corpectomy with reconstruction: Technical case report and review of the literature. Neurosurgery. 2003;52:1482-1485. discussion 1485-1486
72 Hida K, Iwasaki Y, Ushikoshi S, et al. Corpectomy: A direct approach to perimedullary arteriovenous fistulas of the anterior cervical spinal cord. J Neurosurg Spine. 2002;96:157-161.
73 Raabe A, Beck J, Gerlach R, et al. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132-139.
74 Hida K, Iwasaki Y, Goto K, et al. Results of the surgical treatment of perimedullary arteriovenous fistulas with special reference to embolization. J Neurosurg Spine. 1999;90:198-205.
75 Nagi S, Megdiche H, Bouzaidi K, et al. Imaging features of spinal epidural cavernous malformations. J Neuroradiol. 2004;31:208-213.
76 Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838-846.