CHAPTER 117 Vascular Diseases of the Upper Extremities
LARGE VESSEL DISEASE OF THE UPPER EXTREMITY
Patients with atherosclerotic occlusive disease typically present with upper extremity claudication or steal phenomenon. The most common locations for upper extremity large vessel involvement include the brachiocephalic and subclavian arteries. However, atherosclerosis can also cause small vessel obstruction by atheromatous embolization or thromboembolism. A particularly helpful clinical clue in establishing the correct cause is the age of the patient. Atherosclerotic disease of the upper extremity tends to affect older adult patients, whereas nonatherosclerotic upper extremity arterial occlusive disease affects younger patients.1
The upper extremity is a frequent site for autoimmune vasculitis. Vasculitis is usually defined as a sterile inflammation of the vessel wall. In contrast to patients suffering from atherosclerosis, patients with vasculitis are often younger and have elevated erythrocyte sedimentation rates, antineutrophilic cytoplasmic antibodies (ANCA), and antiendothelial cell antibodies.2 In addition to the local symptoms caused by vessel narrowing, many patients also suffer from concomitant constitutional symptoms such as fever, malaise, myalgia, and arthralgia. The upper extremity is most often affected by Takayasu disease and giant cell arteritis (GCA). These are histologically similar diseases but Takayasu disease tends to affect any of the aortic arch vessels as well as the arch itself, and sometimes the pulmonary and coronary arteries, whereas GCA tends to affect the subclavian arteries in a typical symmetrical pattern.3 A specific clinical constellation associated with GCA is polymyalgia rheumatica. In polymyalgia rheumatica, the vasculitis is located more distally in the cervicocranial arteries. Up to 50% of patients suffer from intermittent claudication of the jaw, tongue, or throat, and up to 33% of patients present with visual complaints such as amaurosis fugax or even blindness.4
The group of neurovascular disorders caused by extrinsic compression of the subclavian and axillary arteries and veins as well as the brachial plexus is called cervicoaxillary compression syndrome. The more popular name for this disorder is the thoracic outlet syndrome (TOS). Symptoms are caused by compression at the interscalene triangle, costoclavicular space, or retropectoralis minor space.5 Compression can be caused by a variety of causes such as a fibrous band, supernumerary cervical ribs, or excessive callus formation subsequent to a clavicular fracture. A large majority of cases (70% to 90%) is caused by involvement of the brachial plexus. In less than one third of cases, arteries and veins are involved.
Prevalence and Epidemiology
The estimated incidence of Takayasu arteritis is 2.6 cases per million in the United States, with higher incidences reported in young women and patients of Asian origin. Women in their second and third decades of life account for the vast majority of patients because they tend to be affected about eight to nine times more often than men. Conversely, GCA tends to affect older patients, peaking in the age group between 70 and 80 years. The estimated incidence of GCA is 2 to 20/million, with women being affected about two to four times more often than men.6
The incidence of thoracic outlet syndrome (TOS) is not well established. Although it is generally accepted that TOS is caused by compression of brachial plexus elements or subclavian vessels in their passage from the cervical area toward the axilla and proximal upper arm, there is much disagreement among clinicians regarding its diagnostic criteria and optimal treatment. Because there is no objective confirmatory test for TOS, the true prevalence of the disease remains elusive. Reported prevalence ranges from 3 to 80 cases/1000 population.7 TOS is mostly considered a diagnosis of exclusion.
It is estimated that vascular injuries to the upper extremity represent approximately 30% to 50% of all peripheral vascular injuries. Usually, the brachial artery is involved and most injuries are caused by penetrating trauma.8 Blunt injuries such as motor vehicle accidents account for 6% to 10% of upper extremity vascular trauma and are often associated with musculoskeletal injuries and neural injuries.9 The functional impact of the trauma is often related to concomitant injury to peripheral nerves. The extent of the vascular compromise following radiation therapy is directly proportional to the amount of radiation given, but symptoms may present only decades after therapy.
Cause and Pathophysiology
Atherosclerosis is considered to be a chronic inflammatory disease of the large arteries.10 The disease starts at an early age and remains clinically silent for decades. For a detailed discussion of the pathophysiology of atherosclerosis, see Chapters 51 and 88. It is important to note that imaging of the vascular lumen alone may underestimate the burden of disease related to atherosclerosis.
Takayasu arteritis is a classic vasculitis of unknown cause involving the upper extremity arteries. Infection, in particular tuberculosis, has been implicated in the pathogenesis of Takayasu arteritis, but a definitive link between the two diseases remains elusive at present.3,11 The disease can be divided into two stages, with an acute period of large vessel vasculitis, followed by fibrosis and scarring. In the acute stage, the adventitial vessels of the arterial walls become inflamed as a result of unknown causes. This leads to a generalized, smooth, circumferential thickening of the affected segment, including the media. In the chronic stage, elastic tissue is replaced by fibrosis, with thickening of all three layers of the vessel wall, leading to irreversible segmental smooth luminal narrowing. Other vasculitides that affect the upper extremities are thought to be of similar pathogenesis, although the clinical manifestations may vary (see earlier).
Manifestations of Disease
Clinical Presentation
The most common presentations of chronic large vessel upper extremity occlusive disease are arm claudication, or steal phenomena. Clinical clues are helpful to elucidate the underlying disease process and a thorough medical, surgical, occupational and sports history should be obtained in every patient. Symptoms of upper extremity occlusive disease that suggest nonatherosclerotic causes are young age, long-standing fatigue and malaise, high erythrocyte sedimentation rate, and vigorous occupational or sports activities involving repetitive strain to the shoulder and hand, such as frequent baseball pitching, mountain biking, and using the hand to pound structures (e.g., carpenters). Patients with vasculitis typically report having had vague complaints for months or even years prior to consulting a physician. A possible explanation for the relative rarity of arm claudication symptoms is the reduced muscle mass, less vigorous use, and abundance of numerous and well-developed collateral pathways compared with the lower extremity.12
Patients with steal syndromes present with upper extremity weakness, dizziness, and sometimes angina. The most well-known steal syndrome is subclavian steal, or reversal of antegrade flow in the vertebral artery caused by the presence of ipsilateral significant subclavian artery stenosis or occlusion, proximal to the origin of the vertebral artery. Another well-known steal phenomenon can be seen in the coronary artery circulation after coronary artery bypass grafting using the internal thoracic artery. In the presence of a subclavian artery stenosis, flow may reverse in the internal thoracic artery to supply the upper extremity arterial bed instead of augmenting flow in the coronary arteries. It is important to realize that an angiographic steal phenomenon does not necessarily imply symptoms. In fact, only about one third of all patients with angiographically proven steal syndromes suffer from characteristic complaints.13–15
MEDIUM AND SMALL VESSEL DISEASE OF THE UPPER EXTREMITY
The prototypical disease affecting the medium-sized arteries (and veins) of the upper limb is thromboangiitis obliterans, or Buerger disease (or Winiwarter-Buerger’s disease). Although the lower extremity is involved far more often, Buerger disease may affect the radial and ulnar arteries and palmar arch. There are numerous other diseases that affect the distal forearm, hand, and digital arteries of the upper extremity. A full review of all conditions associated with distal upper extremity arterial disease is beyond the scope of this chapter but can be found in the excellent review by Greenfield and colleagues.16 In many patients, arterial disease of the distal forearm and hand is not isolated but is associated with underlying systemic connective tissue disease such as scleroderma and CREST syndrome (calcinosis cutis, Raynaud syndrome, esophageal motility disorder, sclerodactyly, and telangiectasia) or rheumatologic disorders such as rheumatoid arthritis, mixed connective tissue disease, systemic lupus erythematosus, antiphospholipid syndrome, polymyositis, and dermatomyositis.17–19
Raynaud phenomenon is a common ancillary finding in patients suffering from upper extremity vascular disease and may be the presenting symptom. It is defined as a reversible spasm of the small and medium-sized arteries, resulting in a characteristic triphasic white-blue-red color response. First, cessation of digital artery flow produces well-demarcated finger pallor. This is followed by vasorelaxation and return of arterial flow and subsequent postcapillary venule constriction, resulting in desaturated blood and producing cyanosis. Finally, postischemic hyperemia replaces cyanosis with rubor.16,20,21 A distinction is made between primary Raynaud phenomenon (formerly Raynaud disease), if there is no underlying illness, and secondary Raynaud phenomenon (formerly Raynaud syndrome) if there is an associated disorder detected on assessment. In approximately one third of cases, Raynaud phenomenon is an isolated and benign condition not associated with underlying disease. In cases of inflammatory arteritis, Raynaud phenomenon is common.
Prevalence and Epidemiology
The incidence of Buerger disease is estimated at 8 to 12.6/100,000.22 Patients with Buerger disease are almost always young (younger than 45 years), use large amounts of tobacco, and characteristically show segmental occlusions in the radial, ulnar, palmar, and digital arteries, with typical bridging corkscrew collaterals. The disease is increasingly seen in women, commensurate with the increase in the proportion of female smokers.
Hypothenar hammer syndrome (HHS) is an infrequent condition and its true prevalence is not known. Marie and associates23 have found HHS to be the cause of symptoms in 47 of 4148 patients (1.1%) referred for evaluation of Raynaud phenomenon. Serious complications, defined as requiring surgical intervention, caused by iatrogenic injury of the upper extremity arteries are also relatively rare. Myers and coworkers24 have reported 11 patients over 4 years, whereas Deguara and colleagues25 have reported 6 patients over 20 years. The incidence of serious iatrogenic upper extremity injury depends largely on the case mix of patients seen in the hospital and on the types of procedures performed.
Raynaud’s complex of symptoms is very common. Various studies that have been conducted in the general population in several different countries found the prevalence to range from 3% to 6% up to 30%. In these studies, between 70% and 90% of all reported patients were women. In a large United States registry of 1137 patients presenting with Raynaud, 356 (31.3%) suffered from pure vasospasm with no associated disease, 391 patients (34.4%) had associated connective tissue disease, and 389 patients (34.3%) suffered from other underlying diseases.26
Etiology and Pathophysiology
For a detailed discussion of the pathophysiology of atherosclerosis, see Chapters 51 and 88.
Involvement of the small arteries of the upper extremity is frequently encountered in rheumatic diseases. Szekanecz and Koch27 have recently reviewed the vascular biology underlying this process. Vascular injury is caused primarily by activated neutrophils and inflammatory mediators released by these cells. Rheumatic diseases are associated with accelerated atherosclerosis and increased cardiovascular morbidity and mortality.
Repetitive occupational or recreational trauma may damage the ulnar artery when it enters the hand through Guyon’s canal. The type of arterial abnormality often depends on the nature of the damage to the vessel. Intimal damage favors thrombotic occlusion, whereas injury to the media favors palmar aneurysms.28,29 Thrombosis and aneurysm are common features seen angiographically with HHS.30
In primary Raynaud phenomenon, patients have an abnormally strong vasospastic response to cold or emotional stimuli, with anatomically normal arteries. Primary Raynaud typically occurs in young women, is bilateral and not associated with ischemic ulcerations, has a benign course, and requires only symptomatic treatment. Secondary Raynaud is suggested by the following findings: an age of onset older than 30 years; episodes that are intense, painful, asymmetrical, or associated with skin lesions; clinical features suggestive of a connective tissue disease (e.g. arthritis and abnormal lung function); specific autoantibodies and evidence of microvascular disease on microscopy of nail fold capillaries.21,31,32
Manifestations of Disease
Imaging Techniques and Findings
Radiography
When there is clinical suspicion of proximal upper extremity arterial disease, conventional radiographs of the thoracic outlet should be ordered. This will allow identification of supernumerary ribs, elongated transverse processes, or excessive callus formation in close proximity to vascular structures (Fig. 117-1). In patients with upper extremity trauma, conventional radiographs enable identification of fracture sites and the subsequent apposition of bone fragments and presence of radiopaque foreign material.
Ultrasound
Ultrasonography is a powerful first-line screening technique for evaluation of extrathoracic upper extremity arteries and veins. In contrast to the central large arteries and veins close to the heart or underneath the clavicle, the arteries of the upper extremity are easily accessible for ultrasonographic evaluation. There are enough imaging windows available so that the transducer can be placed over the artery of interest, without the presence of overlying bone. High-frequency transducers (more than 5 MHz) can normally be used because arteries and veins lie close to the skin, usually at a depth of several centimeters at most. Gray-scale imaging is useful for evaluating vascular diameter and the presence of atherosclerotic plaque or thrombotic material. Color Doppler flow imaging enables characterization of blood flow patterns and vascular patency. Because it is a noninvasive technique, ultrasonography is well suited for serial examinations. Arteries and veins can be evaluated reliably with ultrasonography from the axillary artery down to the distal radial and ulnar arteries, as well as the palmar arch and smaller digital branches. The normal flow pattern is triphasic. Expert ultrasonographers may be able to evaluate the subclavian artery for the presence of wall thickening associated with GCA or the presence of aneurysm formation and mural thrombus in patients with suspected TOS (Fig. 117-2).
A particularly powerful application of color Doppler ultrasonography is the evaluation of forearm arteries with regard to suitability for coronary artery bypass grafting as well as suspected pseudoaneurysms. Blood flow signals within a mass contiguous to an artery suggest the diagnosis of pseudoaneurysm, which is a complication that sometimes develops following arterial catheterization or penetrating trauma (Fig. 117-3). With an iatrogenic pseudoaneurysm of a native artery (e.g., after catheterization, unsuccessful arterial puncture, or placement or removal of indwelling arterial catheters), there is usually a small-diameter channel communicating with a larger contained collection of (partially thrombosed) blood. Color Doppler imaging shows blood flow signals and the pseudoaneurysm cavity. A jet and/or swirling motion or color yin yang sign is typically seen within the collection itself.33 Color DUS is also well suited for the emergency evaluation of the upper extremity arteries in cases of suspected acute arterial embolus—for example, in patients with atrial fibrillation.
Computed Tomography
Properly executed, CTA allows recognition of arterial stenoses (Fig. 117-4) and aneurysm formation (Fig. 117-5) as seen in atherosclerosis, and the typical circumferential wall thickening as seen in vasculitis. An added benefit of CT over MRI is the improved ability to recognize and display bony abnormalities. However, in most cases, these have already been detected with conventional radiography, which remains the first-line imaging study in patients with upper extremity complaints. With the current generation of 64-detector row CT scanners, care should be taken not to outrun the arrival of the contrast bolus—because incomplete vascular filling may erroneously suggest embolic occlusion of the vessel—as a result of the extremely fast acquisition speeds of these machines. Submillimeter collimation, a low pitch, and slower gantry speed are options to avoid this problem. Arterial embolism is usually characterized by an acute filling defect in the affected vessel, and is sometimes accompanied by increased density of contrast medium proximal to the occlusion, which often shows a typical meniscus sign.
When imaging the distal upper extremity, best image quality is achieved with the patient in the supine position, with the forearm and hand raised above the head. In patients with Buerger disease, arterial (and sometimes venous) occlusion can be seen, with typical bridging corkscrew collaterals that reconstitute distal flow. Key findings in small vessel disease are generally diminished enhancement in the radial, ulnar, and interosseous arteries and poor enhancement of the palmar and digital arteries in the hand (Fig. 117-6). Sometimes, narrowing and tapering of digital vessels can be seen.
In cases of suspected trauma, it is important to review the scout images for the presence of foreign material, such as bullets or shrapnel (Fig. 117-7).
Magnetic Resonance Imaging
There is a paucity of literature on the efficacy and diagnostic accuracy of nonenhanced techniques, so this section will focus on contrast-enhanced techniques. The limited maximum field of view (FOV) of 40 to 50 cm in commercially available MR scanners necessitates dedicated protocols for the central vessels in the chest and forearm and hand vessels, analogous to the dedicated protocols as described earlier for CT. An important pitfall of MRI is susceptibility-induced pseudostenosis in the subclavian artery ipsilateral to the site where the gadolinium (Gd) chelate contrast medium is injected. Because of the high concentration of Gd in the subclavian vein during the initial intravenous injection, it is possible to encounter signal drop-off (also known as T2* susceptibility artifact), which obscures visualization of the subclavian vein but also of the adjacent subclavian artery (Fig. 117-8). To avoid this artifact, it is necessary to acquire early and late arterial phase images; the signal drop-off will disappear on the later phase images because of the natural reduction in the concentration of Gd over time, with hemodilution of the contrast agent.
Atherosclerotic lesions present as stenoses or occlusions in the aortic arch and brachial vessels over a relative short length and can be recognized by their typical serrated or jagged appearance (Fig. 117-9). This is opposed to the smooth, longer segmented stenoses with concomitant circumferential arterial wall thickening, which may be seen with vasculitis (i.e., arteritis). In cases of stenoses in one of the arch vessels, it is useful to supplement the MRI examination with phase contrast imaging of the cervical vessels to detect the presence of retrograde flow, or steal, in the vertebral artery.
Bilateral symmetrical smooth stenoses in the subclavian arteries are highly suggestive of GCA (Fig. 117-10). In patients with clinically suspected temporal arteritis, it is also useful to evaluate the superficial temporal artery with a dedicated high spatial resolution imaging protocol. The degree of arterial wall enhancement in this vessel has been found to correlate well with the amount of inflammatory activity at histology. Conversely, a return to lower degrees of enhancement and normal wall thickness detected by MRI after the institution of therapy reliably signifies a reduction of clinical disease activity.34,35
Aneurysmal widening of the subclavian artery can be seen in vasculitis but is more often encountered in patients with TOS. It is important to identify the presence of luminal thrombus in the artery, because this may pose a risk for distal embolization. It is often useful to image patients in the neutral anatomic position and with the arm(s) elevated above the head to assess the effect of postural changes on vascular patency (Fig. 117-11). Sagittal T1- and T2-weighted images in the neutral and elevated positions may also be of use to identify nonosseous compression of vascular structures such as fibrous bands. In patients with Buerger disease, arterial (and sometimes venous) occlusion can be seen, with typical bridging corkscrew collaterals (see later, “Angiography”).
As is the case with CT, the best image quality is achieved with the patient in the supine position, with the forearm and hand raised above the head when imaging the distal upper extremity. Key findings in small vessel disease are slow and generally diminished enhancement of the forearm arteries and poor enhancement of the palmar and digital arteries in the hand (Fig. 117-12). The ability to perform time-resolved MR imaging is particularly helpful in this regard because it can readily demonstrate differential filling between both upper extremities or globally slowed arterial opacification. The introduction of the blood pool agent gadofosveset trisodium (Lantheus Medical Imaging; North Billerica, Mass) has facilitated ultrahigh spatial resolution equilibrium phase imaging of the vascular system in the hand and fingers, with voxel sizes as small as 64 µm. Narrowing and tapering of digital vessels can easily be appreciated on these images (Fig. 117-13).
Nuclear Medicine and Positron Emission Tomography
The introduction of scanners capable of combined 18fluorodeoxyglucose (18FDG ) positron emission tomography (PET) and CT imaging is potentially attractive for imaging inflammatory large vessel disease. Although no large-scale studies have been performed to date, there is anecdotal evidence that the combination of CT with PET (PET-CT) is of use for the evaluation of patients with large vessel vasculitis. A recent study by Henes and associates36 in 13 patients who were newly diagnosed or re-evaluated for large vessel vasculitis has found that PET-CT could provide additional information over conventional serologic markers, such as erythrocyte sedimentation rate and C-reactive protein levels in blood. Stenotic lesions at CT were found in only 8 of 13 patients, whereas all patients with clinically active disease (N = 12) demonstrated uptake of 18FDG in the aortic arch and branch vessels, indicating that PET imaging may be more sensitive in the early phases of the disease, when angiographic evaluation does not yet show luminal abnormalities (Fig. 117-14). Whether nuclear medicine techniques will still be used in the future remains to be determined because of the very high radiation doses involved (10 to 20 mSv/examination). No large comparative studies between MRI and PET-CT have been published so it is unclear to what extent these imaging modalities are complementary. Because of the high radiation burden and because many patients with vasculitis are young women, PET-CT is not a very attractive technique for repetitive imaging, as is often needed in the follow-up of vasculitis. In the foreseeable future, MRI remains the modality of first choice for these patients.
Angiography
Although CTA and MRA have largely overtaken DSA for the evaluation of the central thoracic arteries and veins, IA-DSA with the administration of vasodilators remains the standard for evaluation of digital arteries in most centers because of its unparalleled spatial and temporal resolution (Fig. 117-15). The major drawback, however, is the invasiveness and discomfort of the procedure, especially when vasodilators are used. Typical findings with regard to atherosclerotic or aneurysmal arterial disease, as well as findings suggestive of vasculitis or Buerger disease, are identical to those described earlier for CTA and MRA (Fig. 117-16). A limitation of IA-DSA is its inability to image the vessel wall. In clinical practice, IA-DSA of the central vessels is primarily performed in the context of endovascular treatment.
REPORTING: INFORMATION FOR THE REFERRING PHYSICIAN
KEY POINTS
 Symptoms of upper extremity vascular disease are the result of a heterogeneous set of conditions that affect the arteries and/or veins primarily, secondarily (associated with underlying systemic conditions), or by extrinsic compression or other trauma.
Symptoms of upper extremity vascular disease are the result of a heterogeneous set of conditions that affect the arteries and/or veins primarily, secondarily (associated with underlying systemic conditions), or by extrinsic compression or other trauma. A thorough medical, surgical, occupational, and sports history, including any habitual drug and/or alcohol use, is crucial to elucidate the source of the complaints; this often yields important information to complement imaging findings.
A thorough medical, surgical, occupational, and sports history, including any habitual drug and/or alcohol use, is crucial to elucidate the source of the complaints; this often yields important information to complement imaging findings. Large artery disease of the upper extremity most often presents as intermittent claudication of the upper extremity, neck, tongue, or jaw. Alternatively, steal phenomena may be in the foreground.
Large artery disease of the upper extremity most often presents as intermittent claudication of the upper extremity, neck, tongue, or jaw. Alternatively, steal phenomena may be in the foreground. Suspected arterial occlusion of the forearm and hand arteries can be caused by distal embolization of thrombus fragments from the heart or from mural thrombus present in a proximal aneurysm.
Suspected arterial occlusion of the forearm and hand arteries can be caused by distal embolization of thrombus fragments from the heart or from mural thrombus present in a proximal aneurysm.Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357:2042-2048.
Demondion X, Herbinet P, Van Sint Jan S, et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26:1735-1750.
Greenfield LJ, Rajagopalan S, Olin JW. Upper extremity arterial disease. Cardiol Clin. 2002;20:623-631.
Huang JH, Zager EL. Thoracic outlet syndrome. Neurosurgery. 2004;55:897-902.
Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512-1523.
Mills JLSr. Buerger’s disease in the 21st century: diagnosis, clinical features, and therapy. Semin Vasc Surg. 2003;16:179-189.
Nastri MV, Baptista LP, Baroni RH, et al. Gadolinium-enhanced three-dimensional MR angiography of Takayasu arteritis. Radiographics. 2004;24:773-786.
Tann OR, Tulloh RM, Hamilton MC. Takayasu’s disease: a review. Cardiol Young. 2008;18:250-259.
1 Azakie A, McElhinney DB, Higashima R, et al. Innominate artery reconstruction: over 3 decades of experience. Ann Surg. 1998;228:402-410.
2 Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512-1523.
3 Nastri MV, Baptista LP, Baroni RH, et al. Gadolinium-enhanced three-dimensional MR angiography of Takayasu arteritis. Radiographics. 2004;24:773-786.
4 Michet CJ, Matteson EL. Polymyalgia rheumatica. BMJ. 2008;336:765-769.
5 Demondion X, Herbinet P, Van Sint Jan S, et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26:1735-1750.
6 Caselli RJ, Hunder GG. Giant cell (temporal) arteritis. Neurol Clin. 1997;15:893-902.
7 Huang JH, Zager EL. Thoracic outlet syndrome. Neurosurgery. 2004;55:897-902.
8 Andreev A, Kavrakov T, Karakolev J, Penkov P. Management of acute arterial trauma of the upper extremity. Eur J Vasc Surg. 1992;6:593-598.
9 Fitridge RA, Raptis S, Miller JH, Faris I. Upper extremity arterial injuries: experience at the Royal Adelaide Hospital, 1969 to 1991. J Vasc Surg. 1994;20:941-946.
10 Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91:3A-6A.
11 Tann OR, Tulloh RM, Hamilton MC. Takayasu’s disease: a review. Cardiol Young. 2008;18:250-259.
12 Kaufman JA. Upper-extremity arteries. In: Kaufman JA, Lee MJ, editors. Vascular and Interventional Radiology: The Requisites. Philadelphia: Mosby; 2004:142-162.
13 Cherry KJJr, McCullough JL, Hallett JWJr, et al. Technical principles of direct innominate artery revascularization: a comparison of endarterectomy and bypass grafts. J Vasc Surg. 1989;9:718-723.
14 Kieffer E, Sabatier J, Koskas F, Bahnini A. Atherosclerotic innominate artery occlusive disease: early and long-term results of surgical reconstruction. J Vasc Surg. 1995;21:326-336.
15 Reul GJ, Jacobs MJ, Gregoric ID, et al. Innominate artery occlusive disease: surgical approach and long-term results. J Vasc Surg. 1991;14:405-412.
16 Greenfield LJ, Rajagopalan S, Olin JW. Upper extremity arterial disease. Cardiol Clin. 2002;20:623-631.
17 McLafferty RB, Edwards JM, Taylor LMJr, Porter JM. Diagnosis and long-term clinical outcome in patients diagnosed with hand ischemia. J Vasc Surg. 1995;22:361-367.
18 Mills JL, Friedman EI, Taylor LMJr, Porter JM. Upper extremity ischemia caused by small artery disease. Ann Surg. 1987;206:521-528.
19 Seibold JR. Critical tissue ischaemia in scleroderma: a note of caution. Ann Rheum Dis. 1994;53:289-290.
20 Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357:2042-2048.
21 Wigley FM. Clinical practice. Raynaud’s Phenomenon. N Engl J Med. 2002;347:1001-1008.
22 Mills JLSr. Buerger’s disease in the 21st century: diagnosis, clinical features, and therapy. Semin Vasc Surg. 2003;16:179-189.
23 Marie I, Herve F, Primard E, et al. Long-term follow-up of hypothenar hammer syndrome: a series of 47 patients. Medicine (Baltimore). 2007;86:334-343.
24 Myers SI, Harward TR, Maher DP, et al. Complex upper extremity vascular trauma in an urban population. J Vasc Surg. 1990;12:305-309.
25 Deguara J, Ali T, Modarai B, Burnand KG. Upper limb ischemia: 20 years experience from a single center. Vascular. 2005;13:84-91.
26 Porter JM, Edwards JM. Occlusive and vasospastic diseases involving distal upper extremity arteries—Raynaud’s syndrome. In: Rutherford RB, editor. Vascular Surgery. 5th ed. Philadelphia, WB: Saunders; 2000:1170-1183.
27 Szekanecz Z, Koch AE Vascular involvement in rheumatic diseases: ‘vascular rheumatology ’ Arthritis Res Ther, 10 2008. 224.
28 Kleinert HE, Volianitis GJ. Thrombosis of the palmar arterial arch and its tributaries: etiology and newer concepts in treatment. J Trauma. 1965;83:447-457.
29 Kleinert HE, Burget GC, Morgan JA, et al. Aneurysms of the hand. Arch Surg. 1973;106:554-557.
30 Vayssairat M, Debure C, Cormier JM, et al. Hypothenar hammer syndrome: seventeen cases with long-term follow-up. J Vasc Surg. 1987;5:838-843.
31 Kallenberg CG. Early detection of connective tissue disease in patients with Raynaud’s phenomenon. Rheum Dis Clin North Am. 1990;16:11-30.
32 Kallenberg CG, Wouda AA, Hoet MH, van Venrooij WJ. Development of connective tissue disease in patients presenting with Raynaud’s phenomenon: a six-year follow-up with emphasis on the predictive value of antinuclear antibodies as detected by immunoblotting. Ann Rheum Dis. 1988;47:634-641.
33 Polak JF, Donaldson MC, Whittemore AD, et al. Pulsatile masses surrounding vascular prostheses: real-time US color flow imaging. Radiology. 1989;170:363-366.
34 Bley TA, Ness T, Warnatz K, et al. Influence of corticosteroid treatment on MRI findings in giant cell arteritis. Clin Rheumatol. 2006.
35 Bley TA, Wieben O, Vaith P, et al. Magnetic resonance imaging depicts mural inflammation of the temporal artery in giant cell arteritis. Arthritis Rheum. 2004;51:1062-1063.
36 Henes JC, Muller M, Krieger J, et al. [18F] FDG-PET/CT as a new and sensitive imaging method for the diagnosis of large vessel vasculitis. Clin Exp Rheumatol. 2008;26:S47-S52.

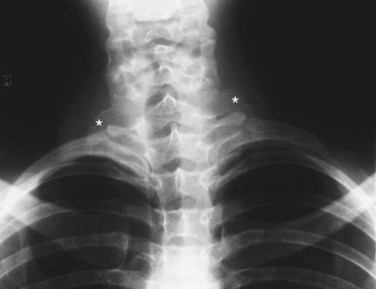
 FIGURE 117-1
FIGURE 117-1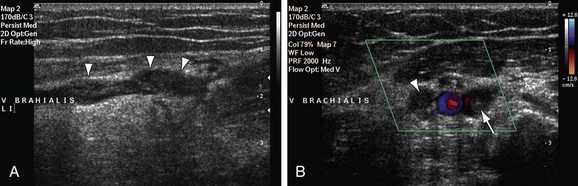
 FIGURE 117-2
FIGURE 117-2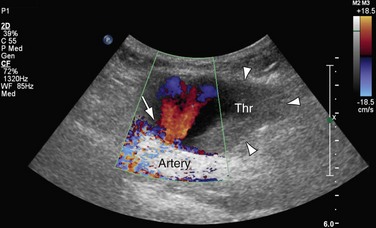
 FIGURE 117-3
FIGURE 117-3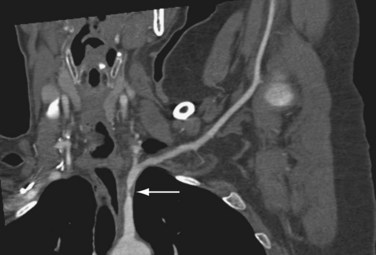
 FIGURE 117-4
FIGURE 117-4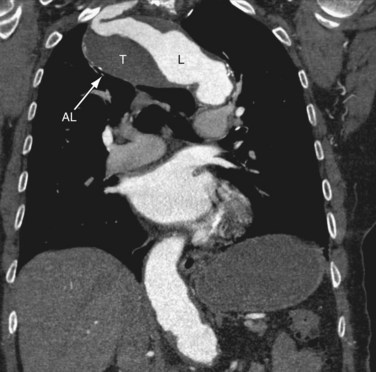
 FIGURE 117-5
FIGURE 117-5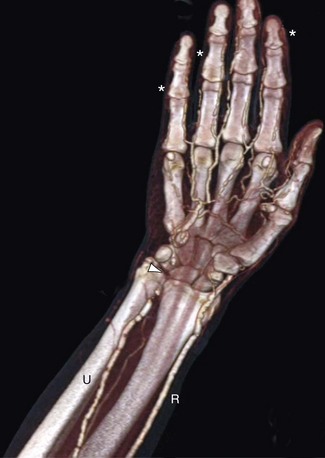
 FIGURE 117-6
FIGURE 117-6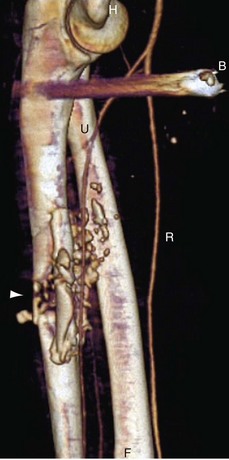
 FIGURE 117-7
FIGURE 117-7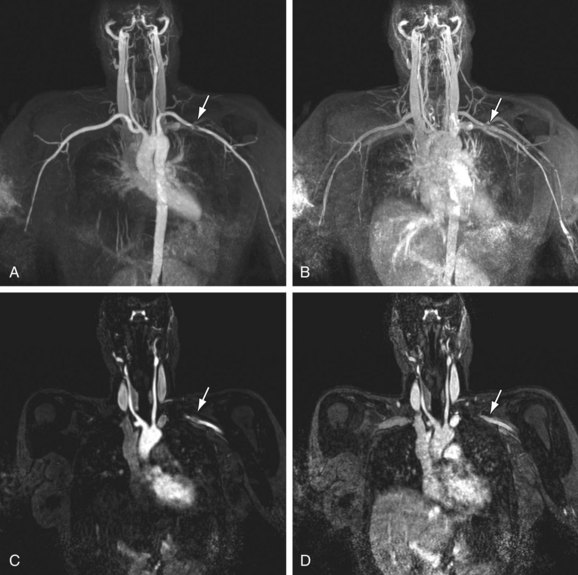
 FIGURE 117-8
FIGURE 117-8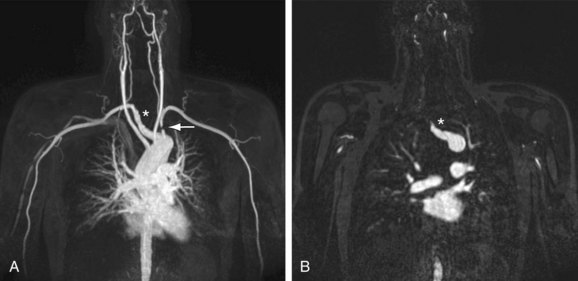
 FIGURE 117-9
FIGURE 117-9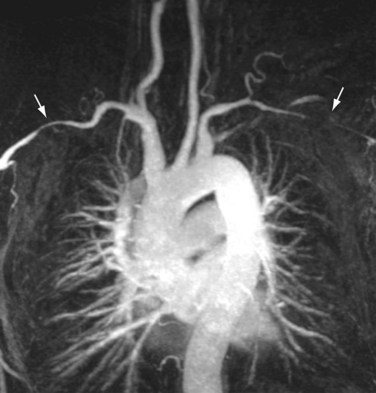
 FIGURE 117-10
FIGURE 117-10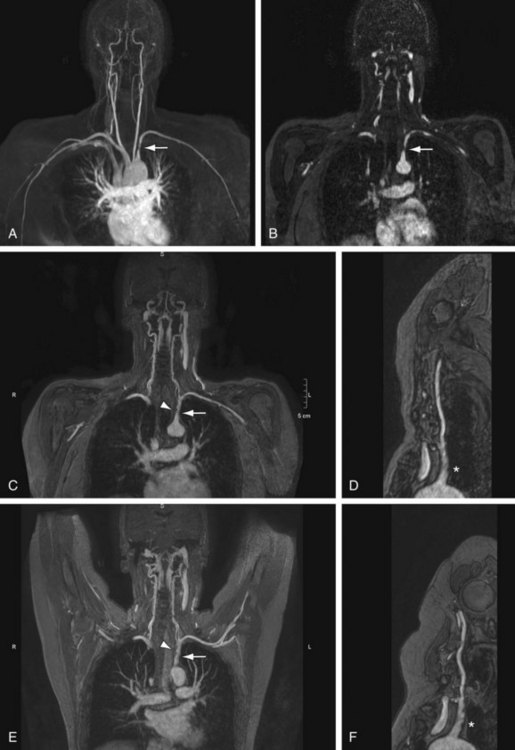
 FIGURE 117-11
FIGURE 117-11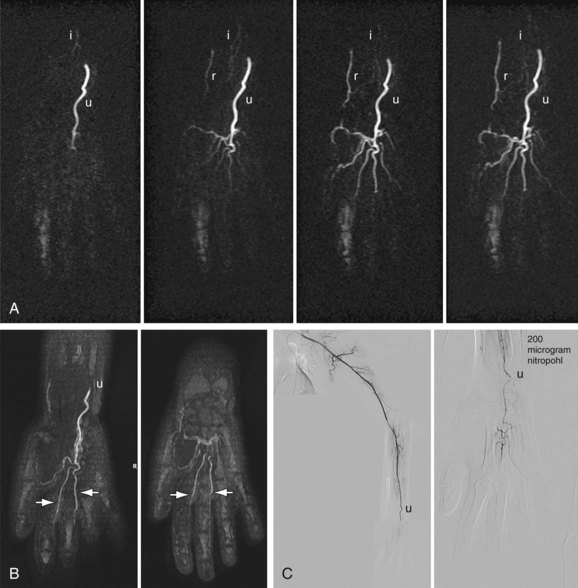
 FIGURE 117-12
FIGURE 117-12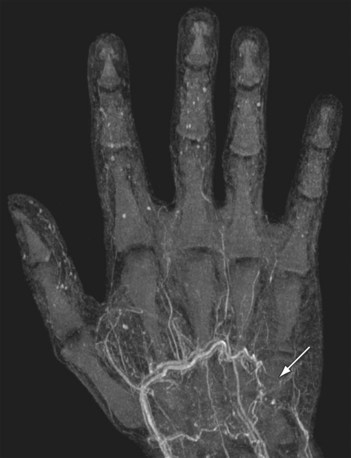
 FIGURE 117-13
FIGURE 117-13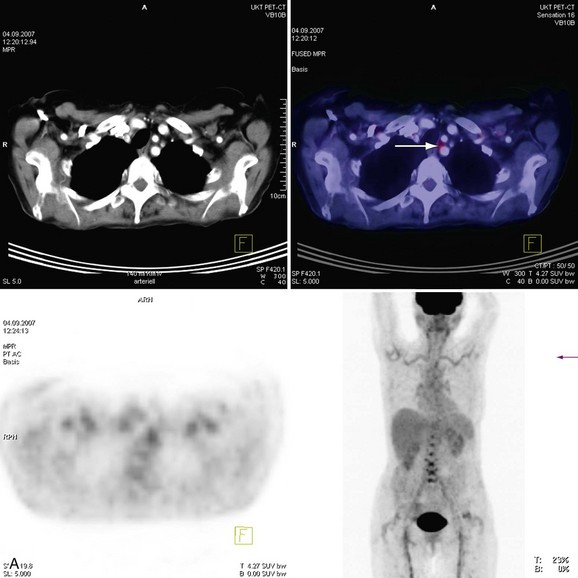
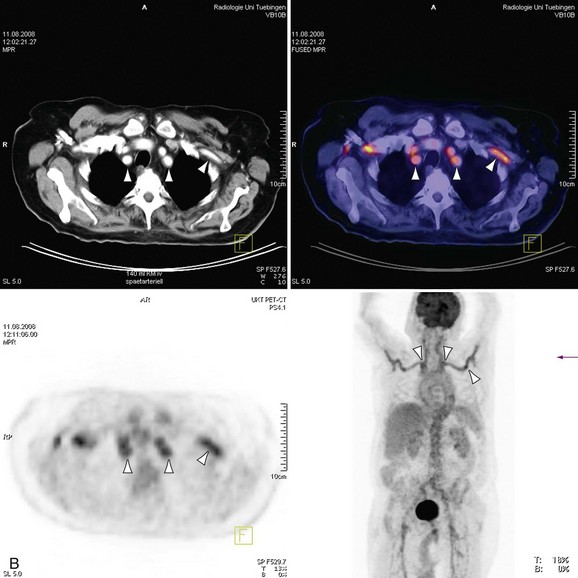
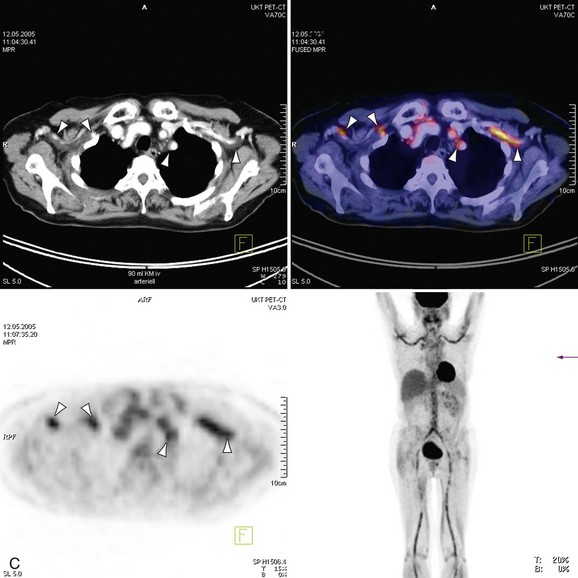
 FIGURE 117-14
FIGURE 117-14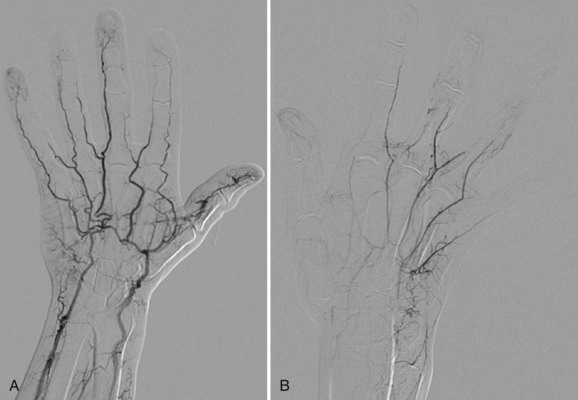
 FIGURE 117-15
FIGURE 117-15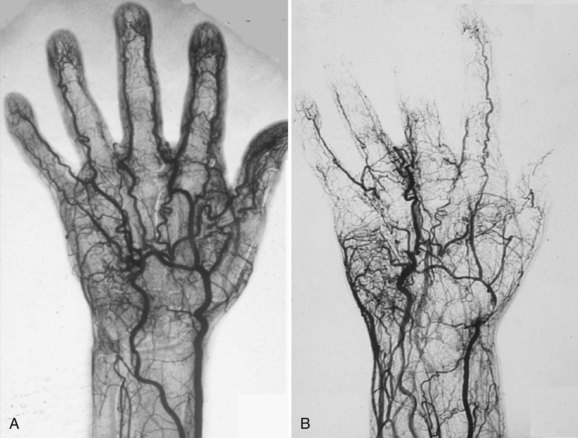
 FIGURE 117-16
FIGURE 117-16

