Chapter 66 Vascular Considerations in Neurotologic Surgery
VASCULAR ANATOMY OF THE PETROUS BONE AND CEREBELLOPONTINE ANGLE
Internal Carotid Artery
The petrous segment of the internal carotid artery (ICA) begins as the vessel enters the periosteal-lined carotid canal, whereas the cavernous segment originates as it exits the apex of the petrous portion of the temporal bone and traverses the posterior part of the cavernous sinus. The petrous portion of the ICA, which lies immediately posterior to the bony eustachian tube but anterior to the jugular foramen, can be divided into vertical and horizontal segments that connect at the genu. The ICA is lined by cervical sympathetic ganglia throughout its course in the petrous canal, and aneurysms or lesions of the vessel that compress the nerves can cause a classic Horner’s syndrome.1 The ICA is also lined by a venous plexus in the distal part of the canal. The greater superficial petrosal nerve, which originates from the geniculate ganglion of the facial nerve, usually courses above and parallel to the horizontal portion of the petrous ICA. Special caution should be used when exposing the middle fossa floor and the petrous ICA because excessive traction of this nerve can cause postoperative facial nerve palsy.2 Similarly, the trigeminal ganglion lies over the medial portion of the petrous ICA, but it is usually separated from the vessel by dura or a variable layer of bone.
The petrous ICA has four main branches of importance to the neurotologic surgeon: (1) caroticotympanic artery, (2) stapedial artery, (3) artery of the pterygoid canal (vidian), and (4) periosteal artery.1,3 The caroticotympanic artery is often said to be a remnant of the embryonic hyoid artery, and arises from the proximal vertical part of the petrous ICA laterally and supplies the tympanic cavity. It anastomoses most commonly with the inferior tympanic artery. The stapedial artery is the source of the middle meningeal artery during development, but it rarely persists into adulthood. If present, a persistent stapedial artery passes through the floor of the middle ear and runs superiorly in a bony canal, in the obturator foramen of the stapes, and finally through the fallopian canal into the middle cranial fossa.3 Finally, the artery of the pterygoid canal (vidian) and the periosteal artery are often thought of as small collateral routes most likely branching off from the horizontal segment of the petrous portion or from the internal maxillary artery, and may have relevance in interventional embolization procedures as described later on.1
The intracavernous segment of the ICA begins at the level of the petrolingual ligament near the foramen lacerum, and most consistently gives off two branches known as the dorsal (meningohypophyseal trunk) and lateral (artery of the inferior cavernous sinus) main stem arteries.4 The intracavernous ICA is commonly divided into five segments for anatomic orientation, including a posterior vertical, posterior bend, horizontal, anterior bend, and anterior vertical segments.1
The dorsal main stem artery generally branches from the central one third of the convex outer margin of the posterior bend of the ICA. There is great variability in the origin of the branches of the dorsal main stem, but most investigators agree that three vessels—the tentorial artery (artery of Bernasconi-Cassinari), dorsal meningeal artery, and inferior hypophyseal artery—exist in some form.3 The tentorial arteries travel along the tentorium and may contribute blood supply for tentorial or proximal falcine meningiomas and tentorial dural arteriovenous fistulas, and blood supply to portions of CN III and IV.3,5 The inferior hypophyseal artery supplies the periphery of the anterior pituitary gland and forms a “circulus arteriosus” with the dorsal meningeal arteries around the root of the dorsum sella. The distal branches of the dorsal meningeal arteries may enter the internal auditory meatus as described in a few patients, and may represent a remnant of the fetal trigeminal artery.3
Although the dorsal and lateral main stem vessels are the most common branches given off by the intracavernous segment of the ICA, other arteries have been described. McConnell’s capsular artery, when present in about 8% of the population, originates from the horizontal segment of the intracavernous ICA, and supplies any combination of the inferior and peripheral aspect of the anterior lobe of the pituitary gland, the diaphragma sella, and the floor of the sella turcica.6 A persistent fetal trigeminal artery, although rare with an incidence of 0.06% to 0.6%, usually arises from the posterolateral or posteromedial aspect of the intracavernous ICA.3 It is most notable in the literature for producing unique clinical scenarios, including trigeminal neuralgia, oculomotor palsies, hyperprolactinemia, and an increased incidence of intracranial aneurysms.3 Finally, the ophthalmic artery and a superior hypophyseal artery have been shown to originate from the cavernous ICA in 1% to 7.5% and 16% of individuals, respectively.3,7
External Carotid Artery
Proximal to its terminal bifurcation, the external carotid artery gives rise to an anterior and posterior group of vessels, the latter of which consists of three arteries that relate to the petrous portion of the temporal bone. The ascending pharyngeal artery usually originates at or near the main bifurcation of the ICA and external carotid artery, and supplies the meninges around the jugular foramen as it passes through the foramen lacerum. As it travels upward with the carotid arteries, it gives off the inferior tympanic artery, which travels through the tympanic cavity with Jacobson’s nerve (inferior tympanic) via the tympanic canaliculus.2 The occipital artery arises from the posterior surface of the external carotid artery and traverses upward between the posterior belly of the digastric muscle and the internal jugular vein, and then medial to the mastoid process. After passing the longissimus capitis muscle, the vessel courses deep to the splenius capitis muscle, finally terminating at the fascia between the attachment of the sternocleidomastoid and the trapezius muscles at the superior nuchal line.2 Its branches supply several muscular and meningeal branches and often anastomose with branches of the external carotid and vertebral arteries.2 Its most notable branch is the mastoid artery, which supplies the posterior portion of the mastoid bone and runs through the mastoid foramen. Finally, the posterior auricular artery arises above the posterior belly of the digastric and travels between the parotid gland and the styloid process. The artery divides into posterior auricular and occipital branches, and develops a stylomastoid branch, which supplies the structures of the stylomastoid foramen and the facial nerve.2
Although not technically a direct branch of the external carotid artery, but rather a branch of the internal maxillary artery, the middle meningeal artery has clinical relevance in the various neurotologic approaches. As this vessel courses through the foramen spinosum of the sphenoid bone between the two roots of the auriculotemporal nerve, it enters the middle cranial fossa, dividing into two or three main branches. When performing extradural approaches to the floor of the middle fossa, the foramen spinosum is located anteromedial to the geniculate ganglion and anterolateral to the carotid canal. These branches—the anterior branch (frontalis), posterior branch (parietal), and middle branch—supply most of the supratentorial dura and calvaria. On entering the cranium, it also gives off the superficial petrosal artery, which enters the hiatus of the facial canal and supplies the facial nerve. Manipulation of this branch during transpetrosal approaches can result in facial nerve injury.2 Finally, the middle meningeal artery also gives rise to the superior tympanic artery just superior to the foramen spinosum, which runs with the superficial petrosal nerve through the superior tympanic canaliculus and supplies the canal of the tensor tympani muscle.
Vertebrobasilar Arteries
The AICA typically originates from the lower part of the basilar artery and divides into rostral and caudal trunks as it traverses the central part of the CPA. This main bifurcation often occurs proximal to the facial and vestibulocochlear nerves, and the two trunks formed are usually nerve-related.8 These branches can be divided into a premeatal segment, meatal segment, and subarcuate loop. In one study, a laterally convex curve or “loop” from the meatal segment was found to be medial of the internal acoustic meatus lying in the CPA in 33%, at the entrance of the meatus in 27%, and entering the internal auditory canal (IAC) in 40% of patients.8 A second laterally convex curve, known as a subarcuate loop, can also be seen in the subarcuate fossa and often gives the appearance of an M configuration.8 Although rare, in a few cases the lateral convex loop of the AICA can be embedded in the dura or bone covering the subarcuate fossa, or both.9 In such cases, freeing the artery from the dura and, if necessary, drilling the bone around the margin may aid in freeing this segment in anticipation for exposure of the posterior wall of the internal acoustic meatus.9
In the area of the CPA, the various branches of the AICA give rise to several nerve-related arterial branches from which originate the (1) labyrinthine (IAC), (2) recurrent perforating, (3) subarcuate, and, less commonly, (4) cerebellosubarcuate arteries. The labyrinthine arteries follow the vestibulocochlear nerve into the IAC, supplying the nerves, dura, and bone of the canal, and eventually terminate by giving rise to the anterior vestibular and common cochlear arteries, which supply the inner ear. Although the labyrinthine arteries most commonly arise from the premeatal segment of the AICA, some studies have reported anomalous cases where it originated directly from the basilar artery or from the PICA, or recurrent perforating, subarcuate, and cerebellosubarcuate arteries; however, this difference may be partially explained by the exact anatomic definition of the arteries in the different studies.8 The recurrent perforating arteries often course near the meatus and along the facial and vestibulocochlear nerves before supplying the brainstem. Finally, the subarcuate artery usually travels medial to the meatus, traversing the subarcuate fossa and petromastoid canal, sending branches to the petrous apex, the bone of the semicircular canals, and the posterosuperior portion of the vestibule.
The SCA originates most commonly from the basilar artery below, but adjacent to the origin of the posterior cerebral artery. The SCA generally bifurcates into rostral and caudal trunks, and branches from either make up the cortical arteries, which supply the upper two thirds of the petrosal surface, including both lips of the petrosal fissure.8 The first branch of the cortical arteries, when present in about half of the population, is referred to as a marginal branch, and its surface area supplied is inversely related to the supply of the AICA. Several anastomoses exist between the marginal artery and the AICA.
Dural Venous Sinuses
One must have an intimate knowledge of venous anatomy in neurotologic surgery to decrease the risk of complications from venous congestion. The petrosal vein (Dandy’s vein) parallels the trigeminal nerve just beneath the tentorium and drains into the superior petrosal sinus. The superior petrosal sinus, which lies at the attachment of the tentorium cerebelli to the superior margin of the petrous ridge, connects the cavernous sinus to the lateral extent of the sigmoid sinus. In addition to draining the petrosal veins that drain the cerebellum and brainstem, it also receives vessels from the inferior surface of the temporal lobe and the cavernous sinus. The transverse sinus begins as a confluence of sinuses at the level of the internal occipital protuberance between the tentorium cerebelli and occipitalis bone and runs laterally to join the sigmoid sinus. The anastomotic vein of Labbé, which drains the temporoparietal region, bridges the inferior surface of the temporal lobe to the transverse sinus. This vein is of special importance in petrosal approaches to the skull base because its injury can lead to speech disturbance and contralateral hemiparesis or hemiplegia.2 This vein is particularly at risk during subtemporal exposures. Excessive retraction of the temporal lobe can result in avulsion of the vein from its insertion into the relatively nonmobile transverse-sigmoid junction.
At its entrance to the jugular foramen, the jugular vein is separated from the inferior petrosal sinus by CN IX, X, and XI. This foramen is located at the lower end of the petro-occipital fissure, and is divided into a larger lateral opening that drains the sigmoid sinus and into a small medial petrosal part that receives the inferior petrosal sinus.2 The jugular bulb is inferior to the posterior floor of the middle ear cavity and generally lies inferior to the ampulla of the posterior semicircular canal; however, its location within the tympanic cavity is highly variable. Often, a high-riding jugular bulb can extend superiorly as high as the lateral semicircular canal and can interfere with visualization in Trautmann’s triangle in transpetrosal approaches as discussed in the next section.2 Finally, the internal jugular vein runs inferiorly from the bulb and is located posterolaterally to the ICA.
VASCULAR CONSIDERATIONS IN NEUROTOLOGIC APPROACHES
Transpetrosal Approaches
The transpetrosal approach, also known as the transtemporal approach, consists of three craniotomy techniques that open the posterior fossa dura anterior to the sigmoid sinus, through the posterior aspect of the petrous pyramid (Fig. 66-1). This set of approaches requires removal of 1 to 2 cm of retrosigmoid bone to allow for posterior displacement of the sigmoid sinus. A detailed explanation of the surgical technicalities and indications and exposure to the various approaches is provided elsewhere.
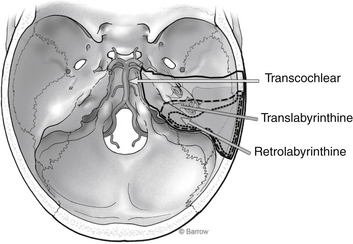
FIGURE 66-1 Surgical corridor afforded by the various transpetrosal approaches.
(Courtesy of Barrow Neurological Institute.)
Retrolabyrinthine
After the initial incision is made behind the postauricular sulcus, and the mastoid air system is thoroughly exenterated, the posterior limit for drilling is the sigmoid sinus. Using a large diamond burr, the sigmoid sinus must be skeletonized carefully. Some authors have suggested that a thin bone plate be left intact over the anterior aspect of the sinus to protect the vessel from the rotating shaft of the drill as it goes deeper into the temporal bone.2 If this bone plate begins to restrict sigmoid retraction, however, one can always fragment it to facilitate posterior mobilization. Other surgeons believe there is no need for preservation of this bony island, and they remove it and guard the vessel with a retractor. Either way, further exposure must be attained by retracting the sinus superiorly from the jugular bulb. In selected patients, the sigmoid sinus can be ligated to improve exposure; however, this can occur only when angiography shows a patent communication between the two transverse sinuses. Mastoid emissary veins are usually encountered and can be controlled with bipolar cautery; similarly, any lacerations to the sinus can readily be controlled with extraluminal tamponade with hemostatic gauze. Intraluminal packing of the sigmoid sinus is not recommended because there is always a risk for embolization of the packing material.
Translabyrinthine
As the dural flap is reflected posteriorly to expose the meatal structures and the CPA, the subarcuate artery or AICA may be encountered. As noted earlier, the subarcuate artery usually stems from the AICA and passes through the dura on the upper posterior wall of the meatus. This vascular anatomy tends to be variable, however, and in some patients the subarcuate artery, along with its origin from the AICA, may be incorporated into the dura on the posterior face of the temporal bone.9
VASCULAR LESIONS OF THE PETROUS BONE AND CEREBELLOPONTINE ANGLE
Cerebral Aneurysms
Petrous Internal Carotid Artery Aneurysms
Presentation and Pathophysiology
Aneurysms of the petrous ICA are rare, although their true incidence is unknown. They are often discovered incidentally on computed tomography (CT) scans for unrelated purposes. They are usually asymptomatic; however, patients can present to an otolaryngologist with a wide variety of signs and symptoms, including cranial nerve palsies, Horner syndrome, epistaxis, vertigo, pulsatile tinnitus, and otorrhagia.1 The triad of otorrhagia, epistaxis, and neurologic deficit is almost pathognomonic for a petrous ICA aneurysm.10 On otoscopic examination, patients with aural symptoms can present with a vascular retrotympanic mass, which is often mistaken for a glomus tympanicum tumor. Biopsy of such a lesion can lead to severe hemorrhage and can be catastrophic. Spontaneous rupture can cause dramatic hemorrhage into the eustachian tube or middle ear or both, and manifest as massive epistaxis or otorrhagia or both with the risk of death from exsanguination.
These lesions can be classified further as true aneurysms or pseudoaneurysms, which can alter treatment. True aneurysms have walls that are continuous with the unaffected portion of the parent vessel and can develop from a congenitally weakened wall, whereas pseudoaneurysms solely involve the adventitial layer of the vessel and develop when a thrombus and fibrous tissue capsule form in response to a traumatic injury. The pathophysiology of petrous ICA aneurysms is unclear, but traumatic, mycotic (by direct seeding from the middle ear and eustachian tube), and congenital causes have been implicated.11 Trauma is a significant cause of petrous ICA pseudoaneurysms in particular because of the predisposing anatomic arrangement, with a stationary ICA segment connected to a distal mobile cervical segment, predisposing the vessel to stretch forces from forceful trauma. Iatrogenic trauma, especially from temporal bone surgery and myringotomy, has been a well-documented source of petrous ICA aneurysms.1
Management and Treatment
Various interventional options exist for patients with petrous ICA aneurysms, including endovascular and surgical therapies. Endovascular techniques consisting of coil embolization or stent coiling should be considered the first line of therapy in symptomatic patients or in patients with ruptured aneurysms. For aneurysms that are not amenable to reconstructive procedures, parent vessel sacrifice (deconstructive procedures) should be considered. A balloon occlusion test with hypotensive challenge is usually performed to evaluate for adequate collateral flow and to assess for any neurologic symptoms or signs of insufficient blood supply. This test produces a high false-negative rate, however, and 2% to 22% of patients with negative findings have been found to have early or delayed ipsilateral cerebral ischemia after permanent occlusion.1 Also, a false-positive result may be obtained if the procedure is complicated by thromboemboli.
If the transient occlusion produces no new neurologic deficits, and if adequate collateral flow exists, occlusion of the ICA can be considered. Detachable balloons were frequently used in the past. More recently, coil occlusion has become the predominant modality for parent vessel sacrifice. Although coils have the advantage of providing a rapid and permanent arterial occlusion, they also have an increased risk of clot formation and distal embolization compared with immediate vessel occlusion with balloon embolization.12 Some reports have shown formation or growth of cerebral aneurysms after balloon or coil embolization.1
Numerous case reports have documented the successful use of stents to treat ruptured petrous ICA aneurysms. Cheng and colleagues12 and Auyeung and associates13 described the successful use of covered stents in three hemodynamically unstable patients who had massive epistaxis as a result of rupture of petrous ICA pseudoaneurysms that were radiation induced. A covered stent may be placed in the petrous ICA because of its lack of major arterial branches, and can be considered for pseudoaneurysms because of their lack of surrounding support. Cheng and colleagues12 concluded that porous stents can alter blood flow pattern, which readily results in stasis and thrombosis of the aneurysm; they cited numerous reports of the successful use of secondary coiling in case of stent failure. Drawbacks to stents include stent-induced intimal hyperplasia, which can cause a hemodynamically significant stenosis and thromboembolic events.14 Long-term studies are still ongoing to determine its overall efficacy and patency rates; however, initial studies show a promising role for this technology.
If a patient develops neurologic sequelae after a balloon occlusion test, revascularization should be considered through an extracranial-intracranial bypass. Similarly, a patient with no neurologic deficits on temporary occlusion who exhibits marked asymmetric decrease in hemispheric blood flow (<30 mL/100 g/min) should be a candidate for a revascularization procedure because of the high false-negative rate of the screening test.15 Some groups advocate universal revascularization in patients who undergo carotid artery sacrifice. The rationale for universal revascularization includes the high incidence of false-negative results from balloon test occlusion, the risk of delayed ischemic complications, and the risk for delayed intracranial aneurysm formation because of changes in intracranial flow and increased shear stress at points of bifurcation.
Surgical options should also be considered if endovascular techniques fail, and include cerebral revascularization procedures with high-flow or low-flow bypass. Umenzu and coworkers16 described a 21-year-old man who had angiographic evidence of a petrous ICA. He failed balloon occlusion twice and eventually was successfully treated via a superficial temporal artery–to–middle cerebral artery bypass.16 Five patients with petrous ICA aneurysms were treated successfully by saphenous vein interposition graft through an extradural cervical carotid artery–to–petrous ICA bypass at another institution.17 Surgical techniques described include a modified infratemporal fossa approach and a combined subtemporal and preauricular infratemporal fossa approach.
Liu and colleagues1 describe a less invasive approach in which a submandibular cervical carotid artery–to–supraclinoid ICA interpositional saphenous vein graft precluded the need for direct exposure of the aneurysm or extensive drilling of the temporal bone. From our experience of eight patients with traumatic ICA dissections, we have had great success with saphenous vein ICA bypasses (cervical ICA–to–petrous ICA) with only one patient experiencing early postoperative graft occlusion that was amenable to immediate thrombectomy.18 Relative contraindications to direct surgical repair or bypass include poorly controlled active hemorrhage and lesion extending distal to the carotid canal, which would impede exposure.
High Cervical Internal Carotid Artery Aneurysms
Aneurysms of the high cervical ICA are rare and usually occur as a result of trauma, dissection, and atherosclerosis, and iatrogenically from carotid artery surgery.19 Most often, these vascular lesions reach clinical attention after causing symptomatic distal embolization, thrombosis, mass effect, or hemorrhage. Management of an aneurysm in this location is similar to management of aneurysms in the petrous ICA; a balloon occlusion test should be performed to assess for collateral circulation. If the test shows insufficient supply, a revascularization procedure is necessary. Several techniques have been proposed, including a sacrifice of the ICA followed by a standard bypass graft to the middle cerebral artery, exclusion of the aneurysmal ICA by an extra-anatomic cervicopetrous bypass, and a cervicosupraclinoid bypass.19 If the aneurysm is small, and if the ICA tortuosity permits, treatment with a stent (bare or covered) can be considered. Small cervical ICA aneurysms typically can be managed conservatively with the patient placed on aspirin therapy to prevent thromboembolic complications.
Vertebrobasilar Aneurysms
Numerous approaches have been described for vertebrobasilar aneurysms, and most often have depended on the precise location of the lesion in the vessel. Aneurysms of the upper third of the basilar trunk are considered more accessible via an orbitozygomatic or subtemporal approach, whereas aneurysms arising from the vertebral artery usually can be accessed via a retrocondylar far-lateral approach.20,21 Surgical access to aneurysms of the lower basilar trunk and vertebrobasilar junction have been controversial, however, and various approaches have been attempted, including the subtemporal with drilling of Kawase’s triangle, suboccipital-retromastoid, far-lateral transcondylar, and transoral-transclival.20 Exposure is often limited because these vascular lesions are deep within the surgical field and surrounded by vital structures, including brainstem and cranial nerves.
More recently, lateral approaches that traverse the petrous bone have gained favor as the approach of choice for such aneurysms. In a series of nine patients with aneurysms of the lower basilar trunk and vertebrobasilar junction, direct clipping via a transpetrosal approach was successfully performed in eight.20 The operative technique consisted of a combined supratentorial/infratentorial craniotomy via burr holes bordering the transverse sinus that were connected using a high-speed drill. After craniotomy, a posterior petrosectomy is performed with careful attention to preserve the labyrinthine structures (particularly the posterior semicircular canal). The temporal dura is excised parallel to the transverse sinus, followed by removal of the presigmoid dura in Trautmann’s triangle to the level of the superior petrosal sinus. This sinus is ligated, the tentorium is transected, and the structures are retracted to expose the basilar artery to the level of the vertebrobasilar junction and to the vertebral artery. The origin of the aneurysm is dissected and clipped. A major advantage over this approach and other skull base approaches compared with standard craniotomies is minimal retraction of brain tissue.
Dural Arteriovenous Malformations
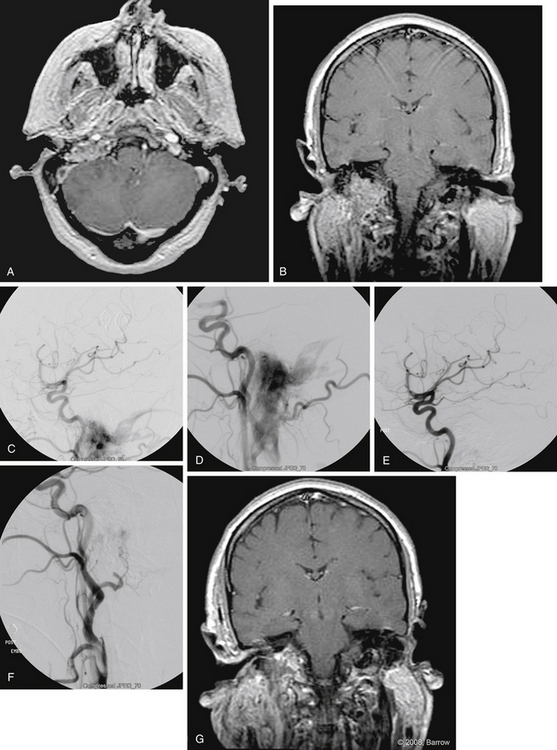
Dural arteriovenous malformations are extremely rare, constituting 10% to 15% of all intracranial arteriovenous malformations.5 This vascular anomaly is believed to derive from venous thrombosis of a dural sinus with consequent collateral revascularization that can lead to abnormal fistulous connection between arteries and veins. Dural arteriovenous malformations can be high-risk or low-risk lesions; this differentiation is based primarily on the presence of venous drainage into the cortical veins, which confers high risk for future hemorrhage. Most patients with high-risk cases present with intracranial hemorrhage or progressive neurologic deficit attributed to venous hypertension. Low-risk fistulas are frequently discovered incidentally or manifest with pulse-synchronous bruit.
Inadequate access to the arterial or draining venous system or multiple sites of fistulization may necessitate surgical management. Endovascular and surgical techniques should complement each other in the management of this group of vascular malformations. For patients with high-risk dural arteriovenous fistulas, treatment with single-mode or multimodal therapy results in a high likelihood of achieving excellent long-term outcomes: 95% have attained Glasgow Outcome Scale scores of 4 or 5 in certain studies.5 The following sections discuss the surgical approaches to dural arteriovenous fistulas traversing the two most common locations for this lesion to develop: the transverse-sigmoid junction and the tentorium.
Cavernous Malformations and Cavernous Hemangiomas
Cavernous malformations and hemangiomas of the petrous bone are rare and often manifest with deficits of CN VII and VIII. Other, more life-threatening presentations are possible, however, including epidural hemorrhage as described by Kessler and associates in 1956.22 There are two subtypes of cavernous malformations, and they are histologically identical. Extra-axial cavernous hemangiomas typically affect the petrous bone, and intra-axial cavernous malformations within the posterior fossa are typically located within the brainstem or cerebellum. Cavernous hemangiomas are most commonly located at the fundus of the IAC or in the region of the geniculate ganglion, and can often spread to the CPA.23 These lesions generally produce symptoms by compressing the surrounding cranial nerves causing resultant palsies. They have the capability of perineural invasion, however, which would necessitate resection of the nerve with a cable graft placement.23
These vascular malformations can often resemble an acoustic neuroma in presentation clinically and radiographically, but they can be distinguished from a meningioma based on negative angiography.24 Diagnosis is best made with thin-section, gadolinium-enhanced magnetic resonance imaging (MRI). They are often sharply defined and enhanced compared with cholesteatomas and schwannomas.23 The presence of calcification within the lesion strongly suggests a hemangioma.
Sundaresan and colleagues24 described three patients with vascular lesions in the petrous bone, two of which were cavernous hemangiomas, and concluded that these lesions can most likely be distinguished from neuromas by their presentation. The authors observed that small IAC or CPA hemangiomas tend to have early-onset hearing loss associated with marked facial weakness or fasciculation or both. In contrast, neuromas in the same locations, which also manifest with hearing loss, seldom involve such a severe motor palsy of CN VII.24
Madden and Sirimanna25 described a case of a cavernous hemangioma located in the IAC, with the same presentation. Because the patient had permanent sensorineural hearing loss, the group decided on a translabyrinthine approach with a facial nerve graft. The patient did not regain function after 1 year, however.25 Rocco and coworkers26 described a similar presentation of hearing loss and severe facial weakness (House-Brackmann grade IV) except that final pathology revealed a cavernous malformation in the IAC with hematoma formation into the CPA.26
Posterior fossa cavernous malformations are rare and account for only a few cavernous malformations affecting the craniospinal axis. Most of these lesions are approached via standard posterior fossa craniotomies. Cavernous malformations with a ventral and deep location may require a transpetrosal approach, however, to minimize brain retraction during exposure. An anterior transpetrosal approach (Kawase’s approach) was used successfully for a symptomatic cavernous hemangioma that was located in the ventrolateral surface of the pons as described by Saito and colleagues.27 MacDonald and associates28 used an anterior, subtemporal, medial transpetrosal approach for a pontine cavernous hemangioma, which caused intraparenchymal hemorrhage resulting in a moderate right hemiparesis. Most of these lesions can be accessed by a retrosigmoid craniotomy with a generous unroofing of the sigmoid sinus and a few millimeters of the presigmoid bone to allow for retraction of the sinus (see Fig. 66-2).
VASCULAR TUMORS OF THE PETROUS BONE AND CEREBELLOPONTINE ANGLE
Role of Preoperative Embolization
Surgical excision of hypervascular central nervous system tumors in the temporal bone and CPA can be dangerous, with the potential for staggering blood loss. The need to mitigate intraoperative tumoral hemorrhage has fostered the development and refinement of various neuroendovascular techniques for preoperative embolization of tumors. This concept of interrupting blood supply to a tumor in anticipation for surgical excision has gained popularity for hypervascular tumors such as meningiomas, hemangiopericytomas, hemangioblastomas, paragangliomas, and juvenile nasopharyngeal angiofibromas.29 In the following sections, the principles of embolization, choice of embolic agents, and potential complications associated specifically with glomus tumors, hemangiomas, and hemangioblastomas of the petrous bone and CPA are discussed.
Glomus Tumors (Paragangliomas)
Paragangliomas consist of a slow-growing group of benign neoplasms known as glomus tumors that arise from remnants of neural crest paraganglion cells. These tumors are of special interest to neurotologic and head and neck surgeons as they occur in the temporal bone (glomus tympanicum and glomus jugulare), the carotid bifurcation (glomus caroticum), and the upper parapharyngeal space (glomus vagale). Four percent of paragangliomas have documented catecholamine secretion, which can cause a pheochromocytoma-type syndrome.29 If catecholamine secretion is suspected and documented, the patient must be pretreated with α and β blockade before embolization and surgical resection. Similarly, preoperative testing should include angiography to define intracranial and extracranial arterial supply to the tumor and involvement of any dural venous sinus. In such cases, the patency of both transverse-sigmoid systems must be evaluated to determine if the involved sinus can be sacrificed without causing intracranial venous congestion.
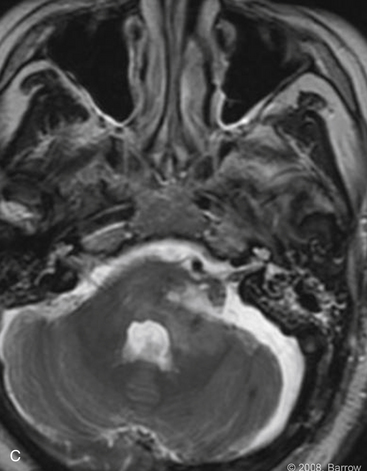
Temporal glomus tumors often obtain their blood supply from the petrous branches of the ICA, the most common of which is the vidian artery, and from the cavernous-carotid branches, the most common of which is the clival branch of the meningohypophyseal trunk. The most frequent neoplasm arising in the middle ear—glomus tympanicum—seldom requires preoperative embolization, and can usually be resected with conventional tympanoplasty techniques (Fig. 66-3).
Although complications are rare, they do occur. Inadvertent embolization of the vasa vasorum can lead to a palsy of the lower cranial nerves (CN IX-XII). Marangos and Schumacher30 and Herdman and colleagues31 documented two cases of facial palsy after embolization of a glomus jugulare tumor. One patient had recovered completely 1 year after treatment, and the other had improved significantly.30,31 Pandya and coworkers32 described a herniation syndrome that followed direct puncture and absorbable gelatin sponge (Gelfoam) embolization of a glomus jugulare tumor. They theorized that significant tumor infarction exacerbated posterior fossa edema.32
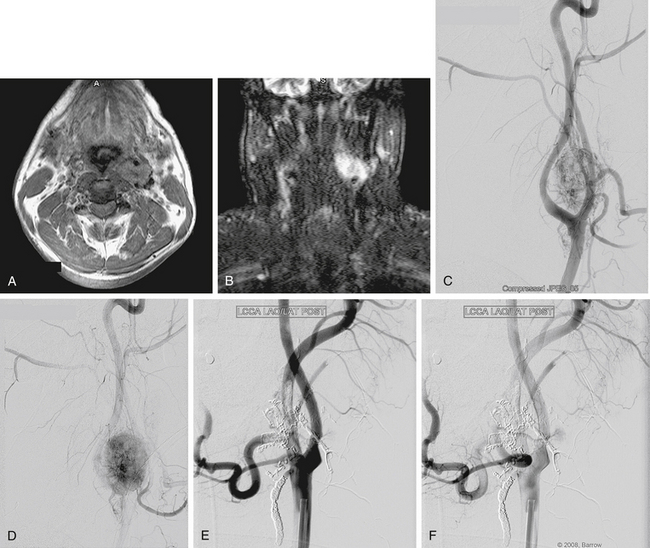
Carotid body tumors usually occur sporadically, whereas bilateral tumors occur in 5% to 10% of cases, with 2% to 6% of lesions showing malignant characteristics such as distant metastatic spread.29 Surgical excision of these tumors is particularly challenging because of their hypervascular nature and propensity to involve cranial nerves (Fig. 66-4). Relevant literature on the use of preoperative embolization techniques for carotid body tumors is scarce, and mostly relegated to a few small single-institution reports.
LaMuraglia and associates33 preoperatively embolized 11 patients with carotid body tumors supplied by the ascending pharyngeal artery, ascending cervical branches of the thyrocervical trunk, and vertebral arteries. They found that the technique significantly decreased intraoperative bleeding compared with nonembolized lesions. One patient had transient aphasia that resolved in 24 hours. The authors concluded that preoperative embolization should be performed for tumors larger than 3 cm, and should be followed by surgical resection within a few days. They also advocated for temporary balloon dilation occlusion with hypotensive challenge if the carotid artery is extensively involved because embolization might be difficult owing to the tumor’s location at the carotid bifurcation.33 Feeders to carotid body tumors tend to have short pedicles, which makes stable microcatheter positioning for embolization challenging, and increases the risk for nontarget embolization.
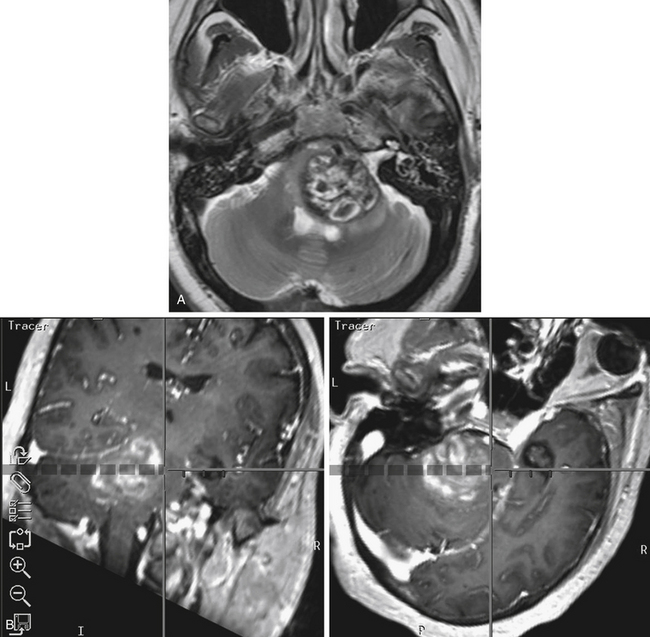
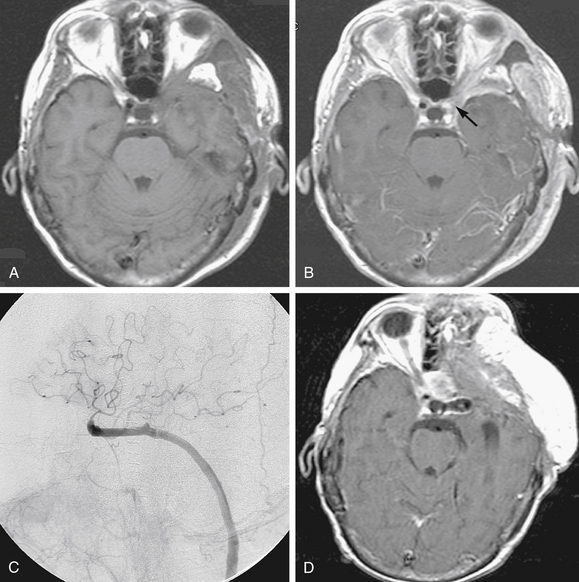
Hemangioblastomas
Hemangioblastomas constitute 1.1% to 2% of craniospinal tumors, and most frequently occur within the cerebellar hemispheres and less commonly at the CPA, vermis, or brainstem.29 These benign, highly vascularized tumors most commonly arise sporadically. Twenty percent are associated, however, with the autosomal dominant genetic disorder, von Hippel–Lindau disease. Because these neoplasms are hypervascular, they are exceedingly difficult to resect, particularly in the intricate areas from where they tend to develop. Severe intraoperative hemorrhage is a significant contributor to the morbidity and mortality rates associated with the resection of hemangioblastomas.
Based on angiography, blood supply is typically via the PICA and, less commonly, via the AICA or branches of the SCA. Dural branches of the vertebral artery, such as the posterior meningeal artery, may supply superficial lesions. The criteria for preoperative embolization of hemangioblastomas include large tumors (often >3 cm), with well-defined arterial feeders that are inaccessible surgically. Embolization using polyvinyl alcohol (PVA) particles or N-butyl cyanoacrylate (NBCA) glue should be performed with a microcatheter tip that is placed beyond the normal branches.34 The risk of embolization is particularly high with hemangioblastomas because the feeding arteries are often pial vessels.
Experience with preoperative embolization of hemangioblastomas in the petrous bone or CPA has been limited mostly to small studies performed at single institutions. Conway and associates35 had mixed success with the use of preoperative embolization in 4 of 40 patients with hemangioblastomas. Although the embolization alone was sufficient to arrest symptom progression in one patient with a sacral hemangioblastoma, another patient had a lateral medullary infarction after attempted embolization of a medullary hemangioblastoma. The authors concluded that embolization should be reserved for tumors with large, surgically inaccessible arterial feeders.35 Tampieri and colleagues36 treated two patients successfully with large hemangioblastomas, one spinal and one involving the posterior fossa, with only minimal blood loss on surgical resection. Eskridge and coworkers37 reported one complication in nine patients with craniospinal hemangioblastomas: A patient developed malignant posterior fossa edema associated with hydrocephalus after treatment with polyvinyl alcohol. The surgeons concluded that embolization facilitated tumor manipulation and surgical resection.37 Finally, from this author’s experience of 35 patients who underwent preoperative embolization with NBCA liquid adhesive, one of the five patients treated for a hemangioblastoma experienced a complication.34 In this case, distal branches of the PICA were inadvertently occluded during glue embolization of a posterior meningeal artery. The patient had initial mild dysmetria; however, the deficit had resolved by the 12 month follow-up examination.
Role of Cerebral Revascularization and Bonnet Bypass
Untreated head and neck cancer that involves the skull base often is associated with a poor prognosis, especially if it involves the ICA. The risk of ICA rupture because of malignant cervical tumor invasion can be 18%, which highlights the need for ICA resection in certain cases.38 ICA sacrifice comes with the inherent risk of stroke, however, and has led many authors to advocate that resection with revascularization should proceed only in patients with unsuccessful results from a balloon test occlusion (radiographically or clinically). The test has significant drawbacks, however, including a high false-negative rate, often leading to a high rate of perioperative stroke despite a successful balloon occlusion test. It is difficult to predict immediate and delayed postoperative ischemic events. Consequently, we advocate universal revascularization in all patients with cranial base tumors that involve the carotid artery. Other groups have suggested that in severe metastatic disease, aggressive surgery including carotid sacrifice should be performed only if there is a chance of complete cure of the tumor. More prospective studies are needed to determine a specific set of guidelines on the controversy (Fig. 66-5).
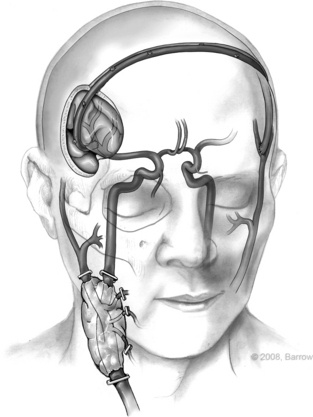
The concept of a contralateral extracranial–to–ipsilateral intracranial bypass was originally introduced in 1978 by the senior author (R.F.S.) using a saphenous vein as an interposition graft, the so-called bonnet bypass (Fig. 66-6).39 Since then, based on our experience, we have found that the radial artery as an interposition graft can be used as a bonnet bypass and works with a high technical success rate with minimal complications. Bonnet bypass should be considered if the ipsilateral carotid artery is unavailable because of previous surgery or radiation, or if a radical neck dissection is planned. The ipsilateral subclavian artery also can be used. In this case, however, the bypass would pass through the surgical field. Experience from this group has shown that the contralateral superficial temporal artery is an excellent donor vessel to perfuse the ipsilateral hemisphere after carotid sacrifice.38 A radial artery interposition graft is positioned between the contralateral superficial temporal artery and the ipsilateral distal middle cerebral artery.
The use of a radial artery and a saphenous vein graft has been described as potential interposition grafts. Revascularization using a radial artery graft is often less taxing because the vessel wall is more resilient than vein grafts, and because the caliber of the artery closely matches that of the superficial temporal artery or middle cerebral artery. The patency rates for radial artery grafts are also higher because the arterial endothelium can support sluggish flow without thrombosis, especially because there is a lack of valves. Although the blood flow in radial artery grafts is high at 40 to 70 mL/min, flow rates with saphenous grafts are even higher at 70 to 140 mL/min.38,40 The lower flow makes radial artery grafts more conducive for anastomosis with smaller distal arteries because less turbulence is created. The grafts can be inadequate, however, for some patients who have very poor or no collateral blood flow. In this situation, the surgeon can divide a radial artery graft into two separate branches of the middle cerebral artery, or use one saphenous vein graft to accommodate the need for higher flow.
1. Liu J.K., Gottfried O.N., Amini A., Couldwell W.T. Aneurysms of the petrous internal carotid artery: Anatomy, origins, and treatment. Neurosurg Focus. 2004;17:1-9.
2. Rhoton A.L. The temporal bone and transtemporal approaches. Neurosurgery. 2000;47:211-265.
3. Tubbs R.S., Hansasuta A., Loukas M., et al. Branches of the petrous and cavernous segments of the internal carotid artery. Clin Anat. 2007;20:596-601.
4. Rhoton A.L. The anterior and middle cranial base. Neurosurgery. 2002;51:273-302.
5. Kakarla U.K., Deshmukh V.R., Zabramski J.M., et al. Surgical treatment of high-risk intracranial dural arteriovenous fistulae: Clinical outcomes and avoidance of complications. Neurosurgery. 2007;61:447-459.
6. McConnell E.M. The arterial blood supply of the human hypophysis cerebri. Anat Rec. 1953;115:175-203.
7. Hitotsumatsu T., Natori Y., Matushima T., et al. Micro-anatomical study of the carotid cave. Acta Neurochir. 1997;139:869-874.
8. Rhoton A.L. The cerebellar arteries. Neurosurgery. 2000;47:29-68.
9. Tanriover N., Rhoton A.L. The anteroinferior cerebellar artery embedded in the subarcuate fossa: A rare anomaly and its clinical significance. Neurosurgery. 2005;57:314-319.
10. Constantino P.D., Russel E., Reisch D., et al. Ruptured petrous carotid aneurysm presenting with otorrhagia and epistaxis. Am J Otol. 1991;12:378-383.
11. McGrail K.M., Heros R.C., Debrun G., Beyerl B.D. Aneurysm of the ICA petrous segment treated by balloon entrapment after EC-IC bypass. J Neurosurg. 1986;65:249-252.
12. Cheng K.M., Chan C.M., Cheung Y.L., et al. Endovascular treatment of radiation-induced petrous internal carotid artery aneurysm presenting with acute haemorrhage: A report of two cases. Acta Neurochir. 2001;143:351-356.
13. Auyeung K.M., Lui W.M., Chow L.C.K., Chan F.L. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: Emergency treatment with covered stent in two cases. AJNR Am J Neuroradiol. 2003;24:1449-1452.
14. Wakhloo A.K., Lanzino G., Lieber B.B., et al. Stents for intracranial aneurysms: The beginning of a new endovascular era? Neurosurgery. 1998;43:377-379.
15. Sekhar L.N., Sen C.N., Jho H.D. Saphenous vein graft bypass of the cavernous internal carotid artery. J Neurosurg. 1990;72:35-41.
16. Umenzu H., Seki Y., Aiba T., Kumakawa K. Aneurysm arising from the petrous portion of the internal carotid artery: Case report. Radiat Med. 1993;11:251-255.
17. Lawton M.T., Hamilton M.G., Morcos J.J., et al. Revascularization and aneurysm surgery: Current techniques, indications, and outcome. Neurosurgery. 1996;38:83-94.
18. Vishteh A.G., Marciano F.F., David C.A., et al. Long-term graft patency rates and clinical outcomes after revascularization for symptomatic traumatic internal carotid artery dissection. Neurosurgery. 1998;43:761-767.
19. Candon E., Canovas F., Kabbaj J., et al. Anatomic basis for the treatment of aneurysms of the upper cervical segment of the internal carotid artery by extra-intracranial cervico-petrous bypass with inverted “in situ” saphenous vein graft. Surg Radiol Anat. 1998;19:1-6.
20. Seifert V., Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85:373-379.
21. Kawase T., Bertalanffy H., Otani M., et al. Surgical approaches for vertebro-basilar trunk aneurysms located in the midline. Acta Neurochir. 1996;138:402-410.
22. Kessler L.A., Lubic L.G., Koskoff Y.D. Epidural hemorrhage secondary to cavernous hemangioma of the petrous portion of the temporal bone. J Neurosurg. 1957;14:329-331.
23. Bottrill I. Imaging quiz case 2: Intraosseous cavernous-type hemangioma of the petrous temporal bone. Arch Otolaryngol Head Neck Surg. 1995;121:348-350.
24. Sundaresan N., Eller T., Ciric I. Hemangiomas of the internal auditory canal. Surg Neurol. 1976;6:119-121.
25. Madden G.J., Sirimanna K.S. Cavernous hemangioma of the internal auditory meatus. J Otolaryngol. 1990;19:288-291.
26. Rocco F.D., Paterno V., Safavi-Abbasi S., et al. Cavernous malformation of the internal auditory canal. Acta Neurochir. 2006;148:695-697.
27. Saito N., Sasaki T., Chikui E., et al. Anterior transpetrosal approach for pontine cavernous angiomas. Neurol Med Chir. 2002;42:272-274.
28. MacDonald J.D., Antonelli P., Day A.L. The anterior subtemporal, medial transpetrosal approach to the upper basilar artery and ponto-mesencephalic junction. Neurosurgery. 1998;43:84-89.
29. Deshmukh V.R., Fiorella D.J., McDougall C.G., et al. Preoperative embolization of central nervous system tumors. Neurosurg Clin N Am. 2005;16:411-432.
30. Marangos N., Schumacher M. Facial palsy after glomus jugulare tumour embolization. J Laryngol Otol. 1999;113:268-270.
31. Herdman R.C., Gillespie J.E., Ramsden R.T. Facial palsy after glomus tumour embolization. J Laryngol Otol. 1993;107:963-966.
32. Pandya S.K., Nagpal R.D., Desai A.P., et al. Death following external carotid artery embolization for a functioning glomus jugulare chemdectoma: Case report. J Neurosurg. 1978;48:1030-1034.
33. LaMuraglia G.M., Fabian R.L., Brewster D.C., et al. The current surgical management of carotid body paragangliomas. J Vasc Surg. 1992;15:1038-1044.
34. Kim L.J., Albuquerque F.C., Aziz-Sultan A., et al. Low morbidity associated with use of n-butyl cyanoacrylate liquid adhesive for preoperative transarterial embolization of central nervous system tumors. Neurosurgery. 2006;59:98-104.
35. Conway J.E., Chou D., Clatterbuck R.E., et al. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001;48:55-62.
36. Tampieri D., Leblanc R., TerBrugge K. Preoperative embolization of brain and spinal hemangioblastomas. Neurosurgery. 1993;33:502-505.
37. Eskridge J.M., McAuliffe W., Harris B., et al. Preoperative endovascular embolization of craniospinal hemangioblastomas. AJNR Am J Neuroradiol. 1996;17:525-531.
38. Deshmukh V.R., Porter R.W., Spetzler R.F. Use of “bonnet” bypass with radial artery interposition graft in a patient with recurrent cranial base carcinoma: Technical report of two cases and review of the literature. Neurosurgery. 2005;56:E202.
39. Spetzler R.F., Roski R.A., Rhodes R.S., Modic M.T. The “bonnet bypass”: Case report. J Neurosurg. 1980;53:707-709.
40. Sekhar L.N., Duff J.M., Kalavakonda C., Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: Techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646-658.