Chapter 62 Vascular Compression Syndromes
Thoracic Outlet Syndrome
Thoracic outlet syndrome (TOS) describes a broad spectrum of symptoms and signs all related to compression or injury of the key anatomical structures that traverse this narrow aperture on their way to the upper extremity. This syndrome manifests in three main forms on the basis of the tissues involved: neurogenic, venous, and arterial. Neurogenic thoracic outlet is by far the most common, accounting for 98% of cases.1 At a distant second, venous thoracic outlet occurs in 1.5%, followed by arterial at 0.5%. Considerable controversy surrounds the diagnosis of TOS, especially the neurogenic form. However, decades of research have served to better establish pathophysiology, diagnosis, and treatment of this syndrome.
In 1956, Peet et al. caused a major shift in the modern conception of TOS when they coined the term thoracic outlet syndrome and described a therapeutic exercise program, essentially the first physical therapy program for TOS.2 This coincided with a shift of therapeutic focus to surgical intervention. In 1962, Clagett described high thoracoplasty for first rib resection, an operation requiring division of the trapezius and rhomboid muscles.3 In 1966, Roos described what has become for many the modern treatment of choice for TOS, the transaxillary first rib resection.4 This operation was fashioned after the transaxillary sympathectomy. First rib resection by this route offered reasonable exposure and minimal morbidity, especially when compared with previously employed techniques.
Anatomy of the Thoracic Outlet
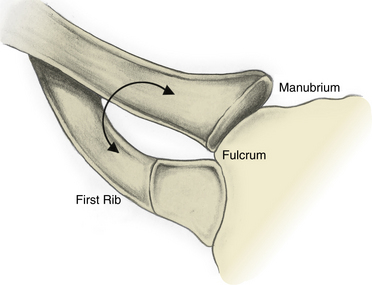
Definitions may vary from author to author, but it is generally accepted that the thoracic outlet is the area from the edge of the first rib extending medially to the upper mediastinum and superiorly to the fifth cervical nerve. The clavicle and subclavius muscles can be pictured as forming a roof, and the superior surface of the first rib forms the floor. Machleder’s description of the thoracic outlet as a triangle with its apex pointed toward the manubrium is helpful in visualizing the three-dimensional (3D) orientation of the structures, as well as the dynamic changes that can lead to injury.5 In this model, the clavicle and its underlying subclavius muscle and tendon form the superior limb, and the base is the first thoracic rib. The point at which these two structures “overlap” medially can be pictured as the fulcrum of a pair of scissors that opens and closes as the arm moves, potentially causing compression of the thoracic outlet contents (Fig. 62-1).
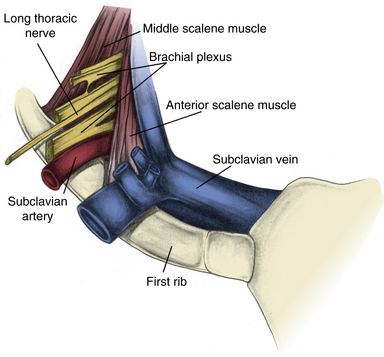
Although most TOS symptoms are related to nerve compression, almost any structure that travels through the thoracic outlet can be involved. Moving from anterior to posterior, one first encounters the exiting subclavian vein, usually positioned adjacent to the region where the first rib and clavicular head fuse to form a fibrocartilaginous joint with the manubrium. Immediately posterior to the vein is the anterior scalene muscle, which inserts onto a prominence on the first rib. Next encountered is the subclavian artery, with the anterior scalene muscle lying between the artery and vein. The brachial plexus is the next structure encountered, oriented superior, posterior, and lateral to the artery. The C4-C6 roots are superiorly oriented, and the C7-T1 roots inferior. Posterior and lateral to the plexus, there is a generally rather broad attachment of the middle scalene muscle to the first rib (Fig. 62-2).
Some authors further classify the thoracic outlet on the basis of three anatomical apertures within this broader space: the interscalene triangle, costoclavicular space, and subpectoral space.6 The most medial aperture that can result in neurovascular compression is within the interscalene triangle. The artery and brachial plexus together pass through this space formed by the anterior scalene anteriorly, middle scalene posteriorly, and first rib inferiorly. Abnormalities of the anterior or middle scalene, presence of a scalenus minimus muscle (seen in <50% of patients, originating between the T1 nerve root and the anterior and inserting onto the pleura and first rib), presence of a cervical rib (0.5% incidence), and presence of fibrous bands (scarring or congenital) can lead to neurovascular compression in this space.6
Lateral and anterior, one can describe the costoclavicular space, bound by the clavicle with its subclavius muscle and tendon anteromedially, the first rib, anterior and middle scalene muscles posteromedially, and the scapula posterolaterally. Bony abnormalities, either congenital or acquired, can narrow this space and result in neurovascular compression. The subclavian vein is especially susceptible to compression in this region as it passes through the narrow space created by the confluence of the clavicle, subclavius muscle and tendon, and the first rib.6
Just deep to the insertion of the pectoralis minor muscle on the coracoid process is the subpectoral aperture. Rarely, neurovascular compression can occur in this space, usually as a result of hyperabduction of the arm, compressing the structures against the chest wall.6
Pathophysiology of Thoracic Outlet Syndrome
The congenital soft-tissue anomalies believed to predispose one to TOS have been well described in the literature. Cadaver studies done by Juvonen, Raymond, and others shed light on the incidence of specific anatomical anomalies in the general population.7 This work is based on the initial observations of Roos, who extensively classified the muscular and fibrous band anomalies seen in patients presenting with TOS.8
Many acquired soft-tissue injuries leading to TOS are the direct result of physical trauma. Studies suggest that soft-tissue injury following motor vehicle trauma (e.g., whiplash) may be the most common underlying etiology.9,10 Other injury patterns include falling onto one’s head and shoulder, causing a lateral stretch injury. In a review of operative TOS patients by Sanders and Hammond,11 86% had a history of trauma. This prevalence of trauma is considerably higher than in many other reports, but stresses the role trauma can play in the disorder.11
Repetitive stress and poor postural habits are also leading causes of TOS.10 Vocations associated with TOS include typists, violinists, or assembly line workers. Other activities include weight lifting or strenuous sports, which lead to hypertrophied scalene musculature. Anatomical studies have documented compression of the subclavian vein into the costoclavicular notch by this muscle.12 This has clear implications for axillosubclavian vein thrombosis. Additionally, some surgeons see a link between Paget-Schroetter’s syndrome and subclavius tendon hypertrophy, particularly in the presence of an enlarged insertion tubercle. Others have implicated a role for the pectoralis minor muscle.13 Rarely, connective tissue disorders have been implicated as a direct causative agent, namely localized scleroderma.14
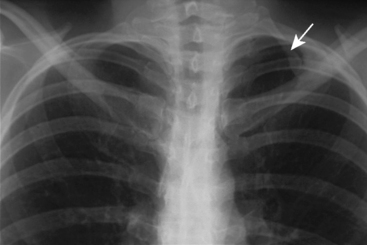
A number of bony abnormalities are found in association with TOS, with the presence of a cervical rib being most common. Autopsy studies indicate that approximately 0.5% of the general population has this structure15 (Fig. 62-3). Further, routine chest radiography demonstrates a cervical rib in roughly 0.7% of individuals.16 Historically, series from the United States report cervical ribs in 10% to 65% of TOS patients, while the European literature reports 25%.15,17 The reason for this discrepancy is not known. It may be at least in part due to variable recognition of neurogenic thoracic outlet in the general population, thus adding bias to these figures. Modern data suggest the percentage of TOS patients with cervical ribs is much lower.
Cervical ribs can be completely formed or rudimentary. In the latter case, there is almost always a compressive band of tissue extending to the first thoracic rib. As they project from transverse processes, cervical ribs displace the involved structures forward. The subclavian artery is particularly vulnerable to damage in this configuration, and some surgeons feel that arterial changes secondary to TOS rarely occur in the absence of a cervical rib. Another common bony anomaly is the presence of an elongated C7 transverse process, which can similarly impinge on the neurovascular structures. Fibrous bands may also be present from an elongated C7 transverse process to the first rib, further worsening the problem.9 Posttraumatic changes following clavicular or first rib fractures are commonly reported, with callous formation at the clavicle and pseudoarthroses of the first rib.18 These changes can frequently be appreciated radiographically.
Presentation of Thoracic Outlet Syndrome
Neurogenic THORACIC OUTLET SYNDROME
Patients can present with the symptoms of neurogenic TOS at any age, although it most commonly occurs in otherwise healthy young to middle-aged individuals. Women are affected three times more frequently than men.1 Neurogenic symptoms can range in severity from nuisance to severely debilitating pain. The most common symptom is pain in an arm, shoulder, and/or the neck. Paresthesias are also commonly present to varying degrees. Although less common, perceived weakness or loss of dexterity can be seen. This occasionally manifests as a decrease in grip strength.1 However, gross motor dysfunction and wasting of the upper extremity is unusual. Gilliat and colleagues description of classic neurogenic TOS with muscle wasting in the hand is seldom seen.19
Pain involving the arm can be focused to a particular nerve distribution or generalized. When localized, ulnar symptoms tend to be the most common, leading to pain and/or paresthesias involving the ring and small fingers. These so-called lower plexus (C8-T1) symptoms are commonly seen. However, upper plexus (C5-C7) manifestations can also be seen, manifesting as pain in the lateral arm and forearm, lateral neck, and deltopectoral region.1
Venous thoracic outlet syndrome (paget-schroetter’s syndrome)
Occasionally, aching pain due to tightness of the skin may also be present, but is absent in the majority of patients.20 However, the typical spectrum of symptoms seen in patients with neurogenic TOS are not usually associated with Paget-Schroetter’s syndrome. Although uncommon, ipsilateral sympathetic hyperactivity can be seen with this condition.
Arterial thoracic outlet syndrome
Arterial TOS is the most rare and varied form of TOS. Chronic compression of the artery leads to insidiously progressive inflammatory changes and scarring of the vessel. These patients rarely present early in the course of this process. Even after they seek medical care, many will have a history that supports the diagnosis, including symptoms such as episodic pallor, cyanosis, and cold intolerance. Patients may present with an array of complaints and physical findings, ranging from intermittent arm claudication to signs of distal embolization or frank ischemia. Rarely, patients may present with aneurysm rupture from long-standing poststenotic dilatation.16
Patients not infrequently are misdiagnosed with collagen vascular disease, various forms of arteritis, or Raynaud’s disease. However, when symptoms are unilateral and isolated to circulation distal to the subclavian artery, one should be alerted to the possibility of arterial TOS. Because there is overlap between the clinical presentation of arterial insufficiency and neurogenic disorders, patients may be misdiagnosed with carpal tunnel syndrome, cervical disc disease, or even neurogenic TOS. It is important to maintain a high index of suspicion for this entity because its clinical presentation is not straightforward.16
Diagnosis of Thoracic Outlet Syndrome
History and physical examination
Because the overwhelming majority of patients who present with a suspected diagnosis of TOS will be of the neurogenic type, this section mainly focuses on this type of TOS. Venous and arterial forms will be discussed later. As with any initial evaluation, an extensive history should be taken, including any injuries and the patient’s occupation. Exacerbating and ameliorating factors should be identified if present. In conjunction with a good history, most cases of TOS can be diagnosed on the basis of physical examination. A thorough examination should focus not only on the site of complaints but also on other areas commonly involved in neurological conditions. This includes the patient’s general appearance and other signs of symptom impact. Deep tendon reflexes, grip strength, and pulses should also routinely be assessed. Palmar hyperhidrosis should be noted if present. Machleder points out that even in neurogenic TOS, changes in pulses can occasionally be detected.21
After this general neurological assessment, tests more specific for the presence of TOS can be undertaken. The most useful test to aid in the diagnosis of TOS is the elevated arm stress test (EAST), which was originally described by Roos in 1966 as a means of eliciting upper-extremity claudication and neurological symptoms.22 In the test, the patient’s arms are placed in 90 degrees of abduction and external rotation, with 90 degrees of flexion at the elbow (“hold-up position”). The patient is then asked to repeatedly clench and unclench the hands. This positioning is designed to constrict the space within the thoracic outlet in an effort to reproduce the patient’s symptoms. When positive in patients with TOS, this test should bring on weakness and paresthesias in the ulnar and median nerve distributions within 3 minutes. Inability to complete testing owing to symptoms is also considered a positive result. Attention should also be made to the color of the hands during the EAST because one may become pale and ischemic if arterial compromise is involved. Proponents of EAST argue that it is specific for TOS and that the length of time to onset of symptoms correlates with severity of TOS. However, some question the anatomical basis for the test, particularly how clenching and unclenching the hand can lead to stress on the brachial plexus.23 This test is not without its detractors. Although anecdotally reported to have excellent specificity, a study from 1985 found a positive test in more than 80% of patients with carpal tunnel syndrome. Other studies conducted using healthy subjects have also reported a high rate of false positives.24,25
Closely related to the EAST is the abduction and external rotation (AER) test. The arm is abducted and rotated and held in that position. This test works by a similar mechanism and likewise produces the weakness and numbness seen with EAST in a similar distribution, namely the C8 to T1 fibers supplying the median and ulnar nerves. In addition, one can sometimes detect a bruit below the lateral portion of the clavicle that is attributable to partial compression of the axillary artery. Both of these tests appear particularly suited to work-related and repetitive motion–associated TOS.26
Although many clinicians routinely assess for pulse changes with these provocative maneuvers, this adds little useful information to such testing. The original Adson test (chin elevation and head rotation toward affected side) consisted of assessment of the radial pulse; Adson sign is subsequent loss of the radial pulse. Although it has historically been used to screen for TOS, Adson sign is also frequently seen in patients without TOS and is unreliably present in those with confirmed TOS. Therefore, it should not be used to establish the diagnosis.1,27 Additionally, Wright described the hyperabduction position back in 1945, which is also of little clinical utility, given its positive result in most healthy individuals. Furthermore, as many as 60% of healthy subjects who undergo EAST will have diminution of the radial pulse.25
Objective testing
Use of objective neurodiagnostic tests for TOS has met with some success, although it continues to be an area of considerable controversy. This modality came to the forefront in the early 1960s, but the anatomical constraints of attempting to measure changes across the brachial plexus have always made its application in this position difficult. Specific techniques vary, but principally these studies evaluate nerve conduction velocity as well as amplitude. The most common and basic electrophysiological studies involve direct motor and sensory nerve testing at the root, cord, trunk, and/or nerve level. Tests are considered abnormal when the velocities and amplitudes deviate from accepted norms. Perhaps the main criticism of these tests is that they only tend to be positive in patients with advanced disease, in whom history and physical examination should be sufficient. Roos suggests they offer “little definitive diagnostic information” and that one “still must rely on careful history and physical.”24 All of this reflects the fact that most electrophysiological tests evaluate larger myelinated nerve fibers, not the smaller fibers whose injury mediates the pain associated with TOS. A study by Franklin et al. found that of 158 TOS patients, only 7.6% had abnormalities in their electrodiagnostic tests.28 Furthermore, this diagnostic modality is subject to significant interobserver bias. No standardized easily reproducible technique has been adopted, nor has any been extensively studied and proven useful in suspected TOS patients. Beyond the limited role of establishing the diagnosis of TOS, these studies can result in significant discomfort for the patient who is already suffering from disabling pain. They should therefore be used only in special instances. These techniques can be useful, however, to exclude the diagnosis of TOS in cases where another neurological process is suspected (e.g., carpal tunnel syndrome).
Let us look closer at some specific techniques that have been used in patients with neurological disorders of the upper extremity. First, several authors have described their experience using ulnar conduction velocity from Erb’s point (above the clavicle and lateral to the insertion of the sternocleidomastoid muscle) to the elbow to assess for TOS, but this technique has been criticized.21 However, if there is severe disease with concomitant axonal damage, changes in ulnar action potentials can be demonstrated. A reasonable approach to conduction studies includes sensory testing of the median and ulnar nerves at the wrist to screen for carpel tunnel syndrome and TOS, respectively.
Electromyography is capable of providing objective data supporting the diagnosis of TOS in a setting of advanced disease. This study demonstrates spontaneous firing of acutely denervated muscle fibers (positive sharp waves, fibrillation potentials), but this is not the usual clinical situation for TOS. Rather, after reinnervation, prolonged and irregular potentials are seen. Because this is a reflection of previous denervation injury, many of these patients have atrophy of the involved muscle groups, and the electromyogram (EMG) can confirm that the lower trunk of the brachial plexus was injured. However, in patients without evidence of atrophy, this test is not likely to reveal these findings. This fact is supported by studies showing that standard EMG tests are negative in 62% of TOS patients.29 However, these tests can be used to examine the paraspinal muscles, which can be important in ruling out radiculopathy as the cause of the patient’s symptoms.
Somatosensory evoked potentials (SSEPs) can play a role in the workup of TOS. Currently, assessment of the ulnar and median nerves can be used for evidence of their compression at the thoracic outlet. When abnormal, these studies tend to show lower plexus injury (ulnar) with normal median function. This is seen primarily as a blunting of Erb’s point peak. Machleder et al.30 showed that 74% of their patients carrying a clinical diagnosis of TOS had abnormal evoked responses. Furthermore, when these patients were studied following operative decompression of their thoracic outlets, more than 90% had correlation between improved symptoms and normalization of their SSEPs.30 Increases of the sensitivity of these tests can be achieved with provocative maneuvers such as arm positioning, although these maneuvers can also cause SSEP changes in patients with no clinical evidence of TOS. Although neurodiagnostic testing remains useful as exclusionary testing, its role in establishing the diagnosis of neurogenic TOS remains limited.
In a study by Jordan and Machleder, 122 patients underwent EMG-guided scalene blockade for a suspected diagnosis of TOS. Reduction in pain was then assessed with provocative maneuvers (generally the EAST); 38 patients went on to have surgical decompression, 32 of whom had a positive result with muscle blockade. The 94% of patients who had a positive response to blockade had a positive long-term result with surgery, as opposed to only 50% of patients with a negative block.31
Beyond its utility as a predictor of good outcome following surgery, lidocaine blockade does offer patients some transient relief while awaiting surgery. However, this effect rarely lasts beyond 1 month. Some groups advocate botulinum toxin injection over lidocaine because of its longer duration of action. This is particularly useful in patients who may have a delay between scalene block and surgery. Jordan et al. also demonstrated in a cohort of 22 patients that botulinum injection results in a 50% reduction of symptoms for a least 1 month in 64% of patients (mean duration of 88 days). This is in contrast to only 12% of patients with continued symptomatic relief beyond 1 month when receiving lidocaine alone.32
Finally, some centers advocate the use of duplex ultrasonography to assess for the presence of TOS. To perform this test, provocative maneuvers in conjunction with color Doppler are used to assess blood flow velocities in the subclavian artery and vein. In theory, patients without TOS will have minimal velocity changes within the subclavian artery during varying degrees of abduction of the arm. If the patient’s symptoms are related to TOS, one would expect to see velocity changes. First, as the artery becomes partially compressed, velocity in the artery increases. Further abduction will worsen compression to the point where flow is diminished, and one should see a resultant decrease in velocities. Finally, with hyperabduction the compression may be so severe that the vessel becomes occluded, with cessation of flow. Recent data have given support to this modality. In a small cohort of patients suspected of having TOS on the basis of symptoms and physical examination, all patients demonstrated the anticipated hemodynamic changes described. With 120 degrees of abduction, mean peak systolic velocities increased greater than 50%. At 180 degrees of abduction, velocities were reduced below baseline values, and hyperabduction revealed absent flow in all patients for whom data were available. As anticipated, venous duplex revealed changes suggestive of venous compression, namely loss of atrial and respiratory dynamics, increased velocities, and increased turbulence.33 Although this study was underpowered to definitively establish duplex ultrasonography as a proven diagnostic modality in TOS, it does offer promising data. Validating studies should be conducted to further our understanding of the role of ultrasound in TOS.
Diagnosis of venous thoracic outlet syndrome
In the TOS patient who presents with upper-extremity swelling, the diagnosis of axillosubclavian vein thrombosis is suggested by the history and physical examination described earlier. Assessment of the venous system is usually initiated with noninvasive duplex ultrasonography and dynamic phlebography (also see Chapter 12). Provocative positioning, such as external rotation and abduction, can increase the sensitivity of these tests. Other authors describe a two-position technique, with the arms fully adducted and then 90 degrees abducted. It is not clear at this time how useful magnetic resonance venography (MRV) is for axillosubclavian evaluation. It appears to have anatomical limitations similar to duplex ultrasonography, with poor quality of images in the retroclavicular space.
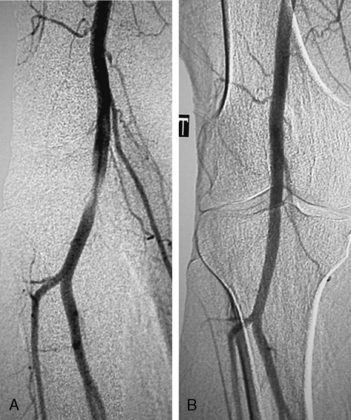
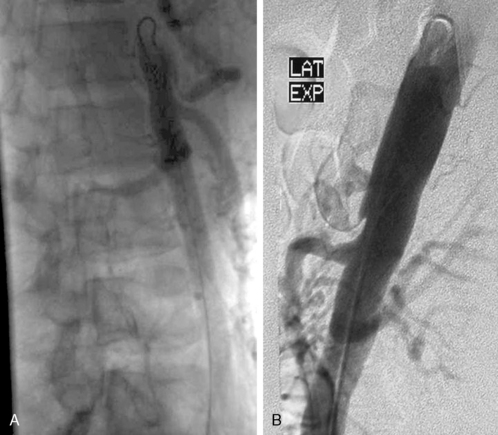
Many patients undergo a diagnostic venogram, which is the gold standard for thrombosis in this situation (Fig. 62-4). Occlusion of the axillosubclavian vein is readily identified with this diagnostic modality. Typically, patients will also have numerous venous collaterals not seen in normal individuals without thrombosis. Although this test confirms the diagnosis of venous thrombosis, the occasional patient needs further clarification as to its cause. Neurogenic symptoms are usually not present, and one must often rely on exclusion of causes of venous thrombosis other than TOS in this situation.
Diagnosis of arterial thoracic outlet syndrome
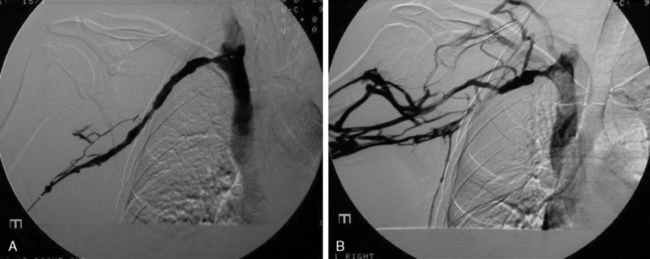
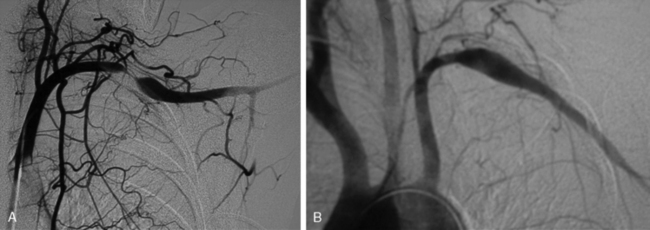
Frequently, arterial compression can be better visualized if the arm is abducted 90 degrees, and most studies are obtained with the arms in two positions (Fig. 62-5). When distal embolization is suggested, the angiography should encompass the target sites, often requiring studies of the hand on the affected side. Provocative positioning (abduction) can be used in conjunction with angiography to demonstrate arterial compression.
Treatment of Thoracic Outlet Syndrome
For many TOS surgeons, referral patterns are such that patients have already undertaken unsuccessful conservative therapy before seeking further consultation. It is important for surgeons to be aware of this selection bias. It is also important to have an algorithm for conservative treatment so that the correct patients are selected for operation. A minimum of 6 weeks of physical therapy is required before its effects can be evaluated. It is also important that the correct program be used because it has been recognized that inappropriate physical therapy can worsen TOS symptoms. In general, these programs are designed to relax muscle groups that tighten the thoracic outlet while conditioning those that open it. Aligne and Barral described a program in which the trapezius, levator scapulae, and sternocleidomastoid muscles are strengthened and the middle scalene, subclavius, and pectoralis muscles relaxed.34
Surgical treatment
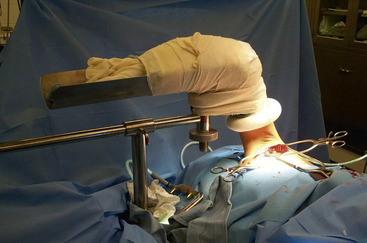
When a symptomatic patient who has sought treatment fails to improve with physical therapy, surgical intervention is warranted. Resection of the first rib and anterior scalenectomy can be performed via either the transaxillary or supraclavicular approach. In our practice, we preferentially use the transaxillary approach owing to its excellent exposure of the first rib, minimal morbidity, and better cosmetic appearance. The patient is placed in the lateral decubitus position, with the head neutral. No paralytic agents are used beyond short-acting agents for induction. Various devices are available for elevation of the arm on the operative side, all of which should permit easy lowering of the extremity intermittently throughout the procedure to allow periods of increased arterial inflow and decreased tension on stretched nerves (Fig. 62-6). The incision is placed between the pectoralis major and the latissimus dorsi in the lower aspect of the axilla. Dissection is carried down to the chest wall, with care taken to identify intercostal brachial cutaneous nerves. These structures should be avoided and preserved when possible, but it is preferable to sacrifice them rather than leave them injured, thereby subjecting the patient to possible causalgic pain.
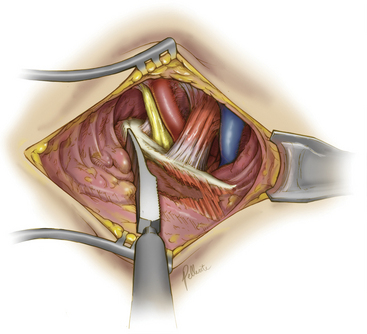
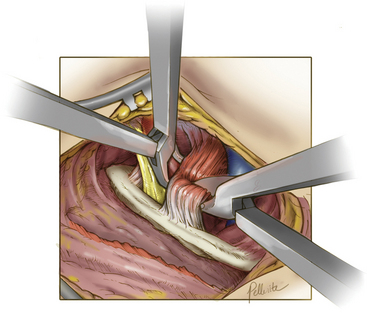
Deep dissection begins by bluntly exposing the external surface of the first rib. Using a periosteal elevator, intercostal muscle and soft-tissue attachments to the first rib are cleared (Fig. 62-7). The parietal pleura is then bluntly dissected away from the internal surface of the first rib. The anterior surface of the first rib is cleared of soft tissue and middle scalene fibers, again using the periosteal elevator. Care is taken to not cut the fibers of the middle scalene. The long thoracic nerve often traverses the muscle in this region, and injury to the long thoracic nerve will result in a winged scapula and is associated with significant long-term disability. Tissue is then cleared bluntly overlying the nerve, artery, and vein. The anterior scalene muscle is now carefully separated from the subclavian vessels, and its attachment to the first rib is divided (Fig. 62-8). The subclavius tendon is also divided.
The supraclavicular approach for scalenectomy (with or without first rib resection) is considered in three situations. The first is when the patient’s symptoms are particularly suggestive of upper brachial plexus involvement (as opposed to the more common lower plexus). As described by Roos,35 it is reasonable to use an approach in which these nerves can be more directly decompressed. The second situation is in patients who have undergone transaxillary operation but now have upper plexus symptoms. The third situation is a matter of preference when a surgeon feels the supraclavicular approach is as effective as and safer than the transaxillary operation. The first rib can also be resected as a component of this procedure, although some argue that it cannot be done with the same margins as the transaxillary approach.
A brief mention should be made here of some alternative surgical approaches for patients with TOS. Some advocate for a thoracoscopic approach to rib resection and scalenectomy. Although offered at several centers, this procedure has not gained widespread acceptance. Many question the stated benefits over the transaxillary approach.36 Also reported in the literature is endoscopic-assisted transaxillary rib resection and scalenectomy. Again, this has not been demonstrated in any large series to be superior to the traditional approaches.
Treatment of venous thrombosis
Venous occlusion at the thoracic outlet should be treated expeditiously. Although this disease was historically treated with a conservative approach of anticoagulation and arm elevation, most therapeutic protocols now emphasize thrombolysis, anticoagulation, and surgical decompression as the key components of treatment. When patients present acutely, as is often the case, they should undergo catheter-directed fibrinolysis of the clot expeditiously. Currently, fibrinolytics such as alteplase and reteplase are used and have largely replaced streptokinase and urokinase, owing to improved safety profiles. Patients tend to respond better to thrombolytic therapy instituted within days of the onset of symptoms, but many may still benefit as far out as 4 to 6 weeks.37 Following thrombolytic therapy, patients should receive systemic anticoagulation.
Traditionally, clinicians would advocate for a 1- to 3-month period of anticoagulation following thrombolysis, prior to surgical intervention. Previous work by Machleder and Kunkle demonstrated that this protracted time frame allowed for intimal healing of the damaged vein and reduction in the inflammatory response, thus facilitating a successful surgical outcome.38 In the modern era, most clinicians advocate for earlier surgical intervention in attempts to decrease the period of disability.39,40 Some authors even promote first rib resection during the initial hospitalization. Moreover, this approach has been demonstrated to be safe. Angle et al.41 recently reported a series of patients treated in this fashion and noted no increased morbidity compared with patients with delayed operation. In particular, none of the theoretical concerns for bleeding following the use of thrombolytics were realized, nor were there particular technical problems secondary to the thrombosis-mediated inflammatory response seen in these patients. In 2007, Molina et al. reported a series of 97 patients undergoing immediate surgical decompression following thrombolysis, with only one bleeding complication.42 Conversely, many suggest that early surgical intervention minimizes the incidence of residual symptoms, postthrombotic syndrome, and rethrombosis.37,43
Although controversial, recent data published by our group suggest that thrombolysis may not even be necessary. In a series of 110 first rib resection and scalenectomies, the number of patent vessels at 1 year was equal at 91% in patients undergoing preoperative thrombolysis and those who only had anticoagulation.44 This needs to be validated by additional studies, but may further evolve the management approach to patients presenting with this disease in the future.
Beyond details outlined earlier for surgical treatment of TOS, it is important to emphasize a few points specific to venous TOS. Because venous compression commonly occurs within the costoclavicular aperture, it is imperative to adequately decompress this space. Complete lysis of the subclavius tendon is integral in this process. Furthermore, the first rib should be cut as far anterior as possible, well into the costal cartilage. Many advocate lysis of any fibrotic tissue surrounding the vein, although this may increase the incidence of a vein injury. Some authors even remove a portion of the medial clavicle, although most clinicians do not advocate this aggressive approach.40 In some cases, the vein is so severely damaged that the only operative repair possible is a jugular-subclavian bypass, although this is rarely undertaken.45
Following surgical intervention, most clinicians use routine venography to both diagnose and treat residual disease.39,46 Angioplasty for residual strictures appears to work quite well after surgical decompression.41 Although enticing from a theoretical perspective, intravascular stenting for residual stenosis following angioplasty has been shown to be detrimental. Multiple authors report higher rethrombosis rates with stent placement compared to patients undergoing angioplasty alone.47–49
Outcomes for Thoracic Outlet Syndrome
In a recent series published by our group, quality-of-life (QOL) measures were prospectively assessed in both neurogenic and venous TOS patients using the short form (SF)-12 and DASH (Disabilities of the Arm, Shoulder, and Hand) instruments.50 Patients completed QOL surveys preoperatively and then at 3, 6, 12, 18, and 24 months. Of an eligible 105 patients, 2-year follow-up data were available for 70 patients, with 44 subjects having neurogenic TOS. These patients were followed out to 2 years postoperatively, with promising results. At baseline, the physical component score of the SF-12 was the most notably decreased score, with a median of 33.8 points. Postoperatively, this value increased on average 0.24 points per month over the 2-year follow-up period, with return to normal QOL for the physical component by 23 months on average. Notably, the return to normal quality for the mental component score was on average 12 months.50
For the venous TOS patients in the study, mean recovery period was notably shorter. Recovery to normal QOL occurred at a mean of 11 months for the physical component score and 8 months for the mental component score. Return to full activity or work in both groups occurred earlier than complete functional recovery (50% at 4 months and 77% at 5 months). Notably, fewer patients in the venous subset had return to full activity at 2 years (77%), since only 8% of patients were disabled or unemployed preoperatively. Reasons for this are unclear.50
In line with this study, Cordobes-Gual et al. similarly reported prospective results, also using the DASH instrument. A full 78% of neurogenic patients reported significant improvement in functional status and reduction in symptoms over the study period. There was a significant reduction in mean DASH scores from 54 preoperatively to 18 postoperatively at a mean follow-up interval of 40 months.51
Scali et al. also recently reported promising retrospective results on a small cohort of neurogenic patients. They noted a 91% reduction in the use of therapeutic adjuncts (e.g., narcotics, physical therapy, scalene blocks) following surgical decompression. Furthermore, patients’ self-assessment of functional status was rated as good or excellent in 68% of patients postoperatively, compared to only 14% reporting a status of good or fair preoperatively.52
Despite these encouraging results, others have reported poorer outcomes. In a series of 254 procedures performed for neurogenic TOS, only 46% had a successful outcome after the first operation, defined as greater than 50% reduction in symptoms. Furthermore, 80 patients underwent a repeat procedure for “failure,” with an overall secondary success rate of 66%. Of the failures, 89% occurred within 12 months of surgery.53 The reasons for variable success rates are unclear, but likely related to variability in patient selection, operative intervention, and lack of standardized outcome measures.
A study by Sharp et al. focused on patients’ ability to return to work following TOS surgery, noting that 80% of the patients in their study were able to do so, and that 85% of the patients subjectively described their outcomes as good to excellent.54 Employment and disability issues complicate outcomes in TOS. In the Washington State workers’ compensation study done by Franklin et al., 40% of postoperative TOS patients were still not working 2 years after their operations; this percentage was actually worse at 5 years (44%).28 Interestingly, the conservatively treated patients in this study did better in this regard, although this was a retrospective study where the cohorts were not particularly well matched. Numerous other studies have shown worse outcomes when the patients have work-related or legal issues related to their TOS.55
Other factors predicting outcome following TOS surgery have been studied. Trauma as the event precipitating TOS is associated with poor outcomes in several series, but not in others.56,57 Preoperative depression has been linked to worse outcome as well. In a report by Axelrod et al., those patients with preoperative depression were more likely to have continued functional and vocational disability following operation.58 This study combined preoperative and postoperative interviews, psychological evaluations, and patient examinations; overall, 67% subjectively reported good or average outcome. At an average of 47 months’ follow-up, 64% of patients were satisfied with their outcomes, and 69% reported they would undergo operation again if faced with the same symptoms. Eighteen percent of the patients considered themselves disabled.
In one of the largest series of TOS patients, Roos reported that in 1844 transaxillary first rib resections, 90% of patients were able to return to performing tasks they had been unable to perform preoperatively. In addition, 97% said that they would recommend rib resection to other patients with symptoms from TOS. There was a 5% recurrence, which was better than the 19% seen with scalenectomy (although this was without rib resection).35 Green et al. also reported higher recurrence following anterior approach, although other authors have reported that the two approaches yielded equivalent recurrence rates.56,59
The prognosis for untreated axillosubclavian venous thrombosis had been one of progressive disability. Recurrence rates of 58% to 75% have been reported for patients receiving only anticoagulation.60,61 Following modern treatment protocols, most patients can expect an excellent functional recovery and can resume normal activities in a relatively short period of time. Urschel and Patel reported 96% of 506 patients experiencing a good to excellent response to the current treatment paradigm of early thrombolysis with prompt rib resection and scalenectomy.61 These promising results are of particular importance to this group of predominantly young and otherwise healthy patients. The most important factor is the time to treatment following thrombosis, with most treatment failures occurring if treatment is delayed. Feugier et al. reported that all treated patients were asymptomatic after an average follow-up of 45 months.62 Long-term sequelae are not common if patients are treated expeditiously and if complete recanalization of the vein is achieved and the compressive rib is removed. If this cannot be accomplished, repeated venous problems occur, and these can lead to debilitating outcomes.
May-Thurner’s Syndrome
Presentation
May-Thurner’s syndrome is the clinical condition caused by extrinsic compression of the left common iliac vein (CIV) by the right common iliac artery (CIA). Typically the presentation of this syndrome involves left lower-extremity swelling, pain, or other signs of venous hypertension as a manifestation of deep venous thrombosis (DVT).63,64 This condition usually manifests in early adulthood, and there is a female predominance. Children can develop May-Thurner’s syndrome, although this is infrequent.65,66 Interestingly, patients with a patent foramen ovale have experienced stroke as the first symptom of the syndrome, with occult pelvic deep venous thrombus crossing the foramen and traveling to the brain.67,68 Pregnancy can also precipitate DVT in patients with this mechanical finding, likely due to the added compression on the pelvic veins caused by a gravid uterus. Rupture of the left iliac vein has also been reported as an initial presentation.69
Pathophysiology
The right CIA crosses anterior to the left CIV at the confluence with the aorta and vena cava. Mechanical compression of the left CIV can occur, with resultant left lower-extremity venous hypertension and thrombosis. However, mechanical compression of the left CIV is frequently identified in patients who are asymptomatic, thus calling into question the contribution of mechanical compression in the syndrome.70,71 Prothrombotic states have been implicated in the occurrence of this syndrome,72–74 and it is not known whether mechanical compression is the sole instigator of symptoms or if other factors are involved for most patients.
Diagnosis
Diagnosis can be delayed until symptoms persist or multiple thrombotic events occur, because computed tomography (CT) venography,75 MR venography,76 or conventional venography of the pelvis are needed to confirm the presence of May-Thurner’s syndrome. Lower-extremity ultrasound frequently fails to confirm the diagnosis, especially in the setting of extensive iliofemoral DVT.77 Although quite rare, right-sided and vena cava compression have also been reported.67,78–80
Treatment
Endovenous stents for May-Thurner’s syndrome have gained increasing popularity to avoid the laparotomy and arterial transection required for the open procedure.81–85 Stents appear to have the radial force necessary to overcome the compression, and stent fractures from repeated trauma have not been reported. Stents are subject to migration and restenosis that may require reintervention.86 Stents are frequently deployed in the left CIV and extended into the vena cava. This placement can lead to thrombosis of the right iliac venous system. Endovascular treatment may be preferable when patients presenting with acute complications due to May-Thurner’s syndrome are also pregnant.87,88 No randomized trials have been performed to compare endovenous stenting to open surgery, and treatment options are typically individualized for each patient.
Outcomes
Endovascular outcomes have been excellent, with reported initial technical success greater than 90% and an equally high rate of symptom resolution.82,83,89,90 Complications included mainly bleeding, usually related to administration of thrombolytic agents. Over an average of 2 years of follow-up, stent thrombosis occurs in approximately 10% of patients. Most of these stents are reopened using endovascular techniques.
Reports of open surgical procedures for May-Thurner’s syndrome are scarce. Frequently the results are reported for a mix of clinical presentations including May-Thurner’s syndrome. The Mayo Clinic has published two reports in the past 10 years.91,92 These show secondary patency rates of 70% at 5-year follow-up. Most of these patients received surgical bypass or a hybrid procedure and had received other interventions before the surgical bypass was performed.
Nutcracker Syndrome
Presentation
Nutcracker syndrome is a clinical condition that results from venous hypertension of the left renal vein and its branches. When found radiographically in its asymptomatic form, it is referred to as nutcracker phenomenon. Both males and females are affected, with a slight female preponderance. Age of presentation is quite variable, but most commonly is young adulthood.93–95 There are many reports documenting childhood presentation.96,97 Symptoms can include hematuria (micro- and macroscopic), orthostatic proteinuria, left flank pain, abdominal pain, left lower-extremity varicose veins, left varicocele (males), and pelvic congestion syndrome (females). Pelvic congestion syndrome symptoms include dyspareunia, dysuria, dysmenorrhea, vulvar varicosities, and vaginal tenderness. Children and adolescents with nutcracker syndrome frequently have associated medical problems that include urolithiasis,98 Henoch-Schönlein purpura,99 immunoglobulin (Ig)A nephropathy,100 hypercalciuria,101 and familial Mediterranean fever.102
Pathophysiology
The course of the left renal vein passes between the SMA and the aorta before emptying into the inferior vena cava (IVC). The gap between the SMA and the aorta is variable, and mechanical compression of the left renal vein can develop when the angle between the two arteries is too sharp. Acute weight loss, which diminishes the amount of mesenteric and retroperitoneal soft tissue, can lead to shrinking of the angle between the aorta and SMA. However, acute weight loss is a variable of unpredictable importance; many patients with this problem do not experience antecedent weight loss. Hypertension of the renal vein can cause hematuria and hypertension of the veins that empty into the left renal vein, which include the left gonadal vein. Retroaortic location of the left renal vein is also associated with nutcracker syndrome, with the left renal vein being entrapped between the aorta and the adjacent vertebral body. This is known as posterior nutcracker syndrome.103 A left-sided vena cava can also lead to nutcracker syndrome.104
Diagnosis
Cross-sectional imaging that demonstrates left abdominal and pelvic venous hypertension can confirm the diagnosis of nutcracker syndrome. Ultrasound, CT, MRI, and traditional venography with pressure measurements have all been used for this purpose. Typically, demonstration of increased pressures distal to the area of compression is sought before invasive treatment is recommended. Ultrasound findings suggestive of nutcracker syndrome include elevated peak systolic velocities in the aortomesenteric portions of the left renal vein as compared to the hilar portion, typically with a ratio of >5.105
Left renal vein acute angle (beak sign) and a diameter ratio of greater than 4.9 between the hilum and the aortomesenteric segment are CT findings that demonstrate the highest correlation with venographic pressure gradients.106 Computed tomography findings correlate with proteinuria in children.107 Computed tomography has also demonstrated usefulness when patients have a presentation consistent with nutcracker syndrome where compression of the left renal vein is caused by unusual structures such as the splenic vein, pancreas, duodenum, and the right diaphragmatic cruz.108 Magnetic resonance fast-spin echo (FSE) T2-weighted imaging can demonstrate stagnant venous flow in the left renal vein and associated varicosities, potentially obviating the need for venography.109
Treatment
Conservative management has proven effective in selected cases.110,111 In particular, pediatric patients with minimal hematuria may improve with further growth. Weight gain may also enlarge the aorto-SMA angle. For patients with concomitant medical problems that may induce acute weight loss, supportive treatment and attainment of weight gain may trigger spontaneous recovery. Use of angiotensin-converting enzyme (ACE) inhibitors has demonstrated efficacy in pediatric patients for relief of proteinuria.112,113
For patients whose symptoms are too severe for conservative management, or for those who do not improve with conservative measures, a wide variety of percutaneous minimally invasive and open surgical techniques are available to address symptoms of nutcracker syndrome. A large number of surgical procedures have been employed for relief of left renal vein compression. These include left renal vein transposition (to an area lower on the vena cava),111,114 autotransplantation of the kidney,115,116 laparoscopic placement of external left renal vein stent,117 laparoscopic spleno–renal vein bypass,118 laparoscopic left renal vein transposition,119 and laparoscopic transposition120 and ligation121 of the left ovarian vein. Synthetic bypass of the left renal vein has also been performed.122 Rarely is nephrectomy indicated for this condition.
Endovascular stenting has also been performed and may be gaining in popularity.123–126 This treatment is controversial because many treated patients are very young, and the implanted stent must last for many years.
Interventions performed should be chosen to address the patient’s specific symptoms. Varicocele and pelvic congestion symptoms can be addressed by gonadal vein embolization or open surgical and laparoscopic resection of the affected engorged venous structures. Treatment of the left renal vein in isolation frequently does not improve pelvic symptoms and secondary procedures are needed to address these symptoms directly.111
Outcomes
The rarity of nutcracker syndrome, fairly recent recognition of this entity, and the success of conservative management for some patients means that outcomes data from large series are lacking. A few small reports regarding long-term outcomes following interventions for nutcracker syndrome do exist.94 Two series and two reviews for open surgical techniques document that the most frequently performed procedure is left renal vein transposition.93,111,127,128 Outcomes reported are excellent, with no mortality and minimal morbidity. Symptom resolution was seen in more than 80% of treated patients. Follow-up has been inconsistent but ranges over the course of many years. In certain instances, secondary procedures were needed for additional treatment of pelvic symptoms or recanalization of left renal veins that occluded after initial treatment. This rethrombosis occurred exclusively in patients with left renal vein occlusion at the time of initial presentation.111
Outcomes for stenting also show a very high rate of symptom resolution and similarly low rates of complications.127,129,130 No procedure-related deaths have been reported. However, two stents have embolized and required retrieval.127,130 Stent thrombosis and stenosis have also been reported.93 As with other endovascular modalities, left renal vein stenting offers a significantly shorter recovery time. However, more data are needed to determine the optimal treatment for nutcracker syndrome.
Popliteal Entrapment Syndrome
Presentation
Popliteal entrapment is a rare syndrome caused by congenital anomalous pathology of the popliteal artery and presents with claudication-like symptoms. Patients most commonly present in early adulthood, although the syndrome has been diagnosed in children.131,132 The most frequent symptom is unilateral lower-extremity pain with exertion. This pain resolves within minutes of activity cessation. The physical examination is typically normal at rest. Provocative maneuvers, which include forced plantar and passive dorsiflexion, will cause diminution or loss of pedal pulses. The presentation may also be bilateral.133 If the syndrome has been long-standing, patients may present with total occlusion or aneurysmal degeneration of the popliteal artery. Occlusion of the popliteal artery aneurysm may cause significant ischemia.134,135 The popliteal vein is involved rarely in the entrapment, so below-knee DVT may be a presenting symptom as well.136,137 Functional entrapment of the popliteal artery has also been reported and is seen in asymptomatic patients as well.138–140
Pathophysiology
The congenital anomalies seen in popliteal entrapment syndrome disturb the normal relationship between the popliteal artery and the muscular structures within the popliteal fossa.141 There are six distinct anatomical presentations of this syndrome (Box 62-1). The most common type (type I) causes the popliteal artery to traverse the popliteal fossa medial to the medial head of the normally configured gastrocnemius muscle. This configuration results in compression of the artery against the head of the tibia when the muscle contracts (typically during ambulation). One variant also causes entrapment of the popliteal vein either in combination with the artery or in isolation.
![]() Box 62-1 Types of Popliteal Entrapment
Box 62-1 Types of Popliteal Entrapment
| Type I | Popliteal artery deviates medial to medial head of normally configured gastrocnemius |
| Type II | Abnormally lateral insertion of medial head of gastrocnemius displacing popliteal artery medially |
| Type III | Normal popliteal artery is compressed by accessory “slips” of gastrocnemius muscle |
| Type IV | Compression of popliteal artery by popliteus muscle, nerve tissue, or fibrous bands |
| Type V | Any of the above also involving compression of the popliteal vein |
| Type VI | Functional entrapment |
Diagnosis
MRI/MRA is the study of choice for popliteal entrapment syndrome owing to its excellent visualization of both the soft tissue and arterial anatomy in this region. It is not only diagnostic but also provides enough anatomical information to facilitate operative planning.142–144 Catheter-based angiography may also be performed, but forced plantar or dorsiflexion may be required to demonstrate the abnormality, since flow may be normal in a neutral position145 (Fig 62-9). A medial course of the popliteal artery during angiography also suggests the diagnosis. In cases of suspected popliteal artery occlusion, angiography may be necessary for operative planning of a distal bypass. Computed tomography angiography has also been used and studied for its utility in diagnosing popliteal entrapment syndrome.146,147 Like MRA, Computed tomography angiography demonstrates both the vascular structures and soft-tissue relationships, ideal for confirming the diagnosis and planning treatment.148
Treatment
Open surgical repair is typically required to relieve the mechanical compression. Prone positioning is commonly used, and an S-shaped incision is made over the popliteal fossa. Ligation of the offending muscular segments is usually tolerated with no subsequent loss of function. In instances where the entire medial head of the gastrocnemius muscle must be divided, it should be relocated and reattached to the tibial condyle. If indicated, arterial reconstruction can easily be performed through this approach as well. For patients with short-segment disease, repair can be performed with resection of the affected segment, mobilization of the vessels distally and proximally, and construction of an end-to-end anastomosis. If the affected segment is too long, thus precluding primary repair, reconstruction using an interposition saphenous vein graft should be performed.149
Endovascular treatment can include thrombolysis when thrombus is present and the clinical presentation is not advanced irreversible ischemia.150 Decompression of the mechanical compression should be performed during the same hospitalization. Stenting the arterial abnormality is contraindicated, especially if decompression of the compression has not been performed.151,152 In this circumstance, the stent is subjected to the same mechanical trauma that produced the arterial injury, thus leading to stent fracture and the potential for further arterial injury. This approach may lead to a more substantial open repair (e.g., distal bypass) when decompression alone, with or without primary repair, may have sufficed.
Outcomes
Outcomes following intervention for popliteal artery entrapment syndrome depend significantly on the amount of vascular damage that has been incurred prior to diagnosis. The rarity of the syndrome and the low index of suspicion for arterial insufficiency in this atypical patient population frequently delay diagnosis until significant arterial damage has occurred and signs of advanced ischemia are present. In these situations, complex repair may be required,153 and eventual limb loss may be unavoidable. When the syndrome is diagnosed in early stages, with symptoms of claudication and no arterial occlusion, simple decompression of the offending musculotendinous structures should be curative.149
Cystic Adventitial Disease
Presentation
Cystic adventitial disease is a rare entity characterized by accumulation of mucin within the adventitia, resulting in vascular compression. In its most common form, the cyst compresses the popliteal artery. However, many case reports demonstrate the occurrence of these cysts in other locations,154,155 sometimes resulting in compression of veins156–159 instead of arteries. Presenting symptoms are typically those of unilateral claudication. Cases with bilateral cysts have been reported, which can result in bilateral symptoms.160,161 Occasionally, acute occlusion of the popliteal artery may be the event causing someone to initially seek medical care.162 The age of presentation is younger than patients with lower-extremity atherosclerosis, but more varied than patients with popliteal entrapment.163,164 There appears to be a male predominance in patients with this disorder.165 The cyst is benign but has a tendency to recur. The quality of substance within the cyst is gelatinous.
Pathophysiology
The cause of the cyst remains unknown, but there are suggestions that they occur as mucin-producing cells relocate from an adjacent joint.164,166 There is also the possibility that the cyst communicates directly with the synovium of the joint.167
Diagnosis
Cross-sectional imaging including ultrasound,168 CT,169 and MRI170,171 are all useful in establishing the diagnosis of cystic adventitial disease by demonstrating the cyst in close proximity to the vascular structures being compressed. Catheter angiography can confirm arterial compression. Signs typical of cystic adventitial disease include a scalloped appearance of the artery wall.
Treatment
Treatment is directed at full resection or enucleation of the cyst, which also involves the artery wall. Revascularization is typically performed with a saphenous vein interposition graft where appropriate. Despite the goal of treatment being full resection of the cyst, they are known to recur. Alternatives to surgical resection and revascularization include aspiration of the cyst172 and aspiration combined with introduction of a sclerosing agent into the cyst.173 Endovascular stenting in isolation has not been successful in treating cystic adventitial disease,174,175 although treatment of recurrence with angioplasty alone has been reported.176
Outcomes
Determining outcomes of treatment for cystic adventitial disease is difficult owing to the rarity of this disease process. Most available literature consists of case reports. Long-term resolution has been reported with image-guided cyst aspiration.172 Small series (three patients) document good outcomes with surgical resection and revascularization.163,164
Median Arcuate Ligament Syndrome
Presentation
Median arcuate ligament syndrome (MALS) is a rare syndrome characterized by celiac artery compression by the diaphragmatic crura and median arcuate ligament. This syndrome is essentially a diagnosis of exclusion, so many patients have experienced symptoms for many years before a diagnosis of MALS is established. It presents at variable ages (adolescence to late adulthood), with a female preponderance.177–179 The most common symptoms are abdominal pain and weight loss. Vomiting is frequent, and an epigastric bruit is frequently present on physical examination. The pain can be episodic, postprandial, or constant. Exercise-related abdominal pain has also been reported.180 Recently the suggestion that MALS may be a familial syndrome has been made.177,181
Pathophysiology
The celiac artery is the first branch originating from the aorta in the abdomen. The celiac take-off forms a 90-degree angle with the aorta. In this location, it is susceptible to compression by the diaphragmatic crura during thoracic motion throughout respiration. The median arcuate ligament connects the two crura. This compression can result in stenosis or occlusion of the celiac artery. In most patients, this is asymptomatic and can frequently be found incidentally.182,183 Symptoms may manifest in those patients who do not have adequate collateral flow from the SMA to carry the demand of the foregut structures. Some patients experience chronic pain, likely due to compression of neural structures surrounding the celiac artery, and their symptoms are unrelated to visceral perfusion. Increased flow through the SMA can rarely lead to aneurysm formation or rupture in branches of the SMA.184–186
Diagnosis
Presenting symptoms for MALS are very nonspecific. As mentioned, celiac stenosis can be seen in asymptomatic patients. These two factors make it mandatory that these patients go through a thorough evaluation to eliminate other possible explanations for their symptoms.187–189 There is no standard battery of examinations, but any structural or functional abnormality of the upper abdomen should be evaluated and treated first. Following exclusion of other pathologies, it is important to document the celiac artery stenosis or occlusion. If the artery is not occluded, demonstrating that the stenosis varies with respiration is important to rule out other more typical causes of celiac artery stenosis such as atherosclerosis.189 Some clinicians recommend injection of vasodilators190 during angiography or measurement of gastric exercise tonometry191 as adjunctive diagnostic tools. The anatomy can be imaged using catheter angiography, MRI,192 CT,193–197 and ultrasound.198 Ultrasound has the advantage of also providing information about flow through the celiac artery. When angiography is performed, images should be captured during both inspiration and expiration because the degree of celiac compression is worse during full expiration (Fig. 62-10). However, compression of the celiac axis during expiration can be demonstrated in asymptomatic individuals and further underscores the challenge of establishing this diagnosis.
Treatment
Two primary goals must be addressed for treatment of MALS. The first is to divide the median arcuate ligament and all the dense fibrotic tissue that surrounds the celiac artery. This relieves mechanical compression on the artery. The second goal is to perform revascularization for patients who require this as well. Traditionally, both these steps are performed in one open surgical procedure. Less invasive interventional methods proliferated recently and have probably increased the number of patients with this disease who undergo treatment. Many publications have advocated laparoscopic surgery to treat the mechanical compression by skeletonizing the celiac artery (removing all crural components and other investing tissue).189,199–204 If revascularization is needed, it is performed in a second endovascular or surgical procedure. Robotic surgery205 and retroperitoneal endoscopic release206 have also been utilized to treat MALS.
It is unclear when clinicians should perform revascularizations of the celiac artery. The most straightforward scenario would be an occluded celiac artery with minimal collateral flow and symptoms of postprandial pain and weight loss. However, many patients present with varying degrees of celiac stenosis that may not be flow-limiting, a widely patent SMA, and symptoms that are not typical of intestinal malperfusion. With the increasing use of laparoscopic techniques to treat MALS, there is a corresponding increase in experience in patients where celiac revascularization is not performed. Some authors advocate decompression alone with minimally invasive techniques, whereas others decompress and revascularize all or most patients immediately via an open approach.207,208
Rupture or aneurysm of an SMA branch associated with celiac stenosis or occlusion is extremely rare, and treatment of this is difficult. Patients may present with sudden and severe abdominal pain in hypovolemic shock. Endovascular embolization of this entity is preferable to open surgery184,185 because these aneurysms are very difficult to locate and expose surgically. Gastroduodenal branches are frequently involved, and definitive open surgery may include a pancreatic resection because of the rich collateral network of arteries in this location.
Outcomes
Patient selection is a key component of obtaining good outcomes for invasive treatment of MALS. Improvement in symptoms is unlikely if the original diagnosis is incorrect. Symptoms can recur even after initial improvement.206 Minimally invasive approaches to MALS are technically difficult and prone to bleeding complications, requiring conversion to an open procedure.189,203 However, when successful, laparoscopic approach to MALS reduces length of stay and significantly decreases recovery time. It also prevents future complications from a laparotomy.
Regardless of approach and procedures performed, most series show favorable outcomes. The majority of patients experience valuable symptom relief. There is generally limited morbidity and mortality. One report documented that in a series of six patients with long-term follow-up, all patients would have the surgery again.209 Contemporary management for this disease is still evolving, as is the ability to accurately recognize it. Future outcomes research will hopefully shed light on the ideal intervention for patients with this challenging and elusive disorder.
1 Brantigan C.O., Roos D.B. Diagnosing thoracic outlet syndrome. Hand Clin. 2004;20:27–36.
2 Peet R.M., Henriksen J.D., Anderson T.P., et al. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31:281–287.
3 Clagett O. Research and prosearch. J Thorac Cardiovasc Surg. 1962;44:153–166.
4 Roos D.B. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann Surg. 1966;163:354–358.
5 Machleder H., ed. Vascular disorders of the upper extremity, ed 2, Mt Kisco, NY: Futura Press, 1989.
6 Atasoy E. Thoracic outlet syndrome: anatomy. Hand Clin. 2004;20:7–14. v
7 Juvonen T., Satta J., Laitala P., et al. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170:33–37.
8 Roos D. Congenital anomalies associated with thoracic outlet syndrome: anatomy, symptoms, diagnosis, and treatment. Am J Surg. 1976;132:771–778.
9 Brantigan C.O., Roos D.B. Etiology of neurogenic thoracic outlet syndrome. Hand Clin. 2004;20:17–22.
10 Sanders R.J., Hammond S.L. Etiology and pathology. Hand Clin. 2004;20:23–26.
11 Sanders R.J., Hammond S.L. Management of cervical ribs and anomalous first ribs causing neurogenic thoracic outlet syndrome. J Vasc Surg. 2002;36:51–56.
12 Daskalakis E., Bouhoutsos J. Subclavian and axillary vein compression of musculoskeletal origin. Br J Surg. 1980;67:573–576.
13 Dijkstra P., Westra D. Angiographic features of compression of the axillary artery by the musculus pectoralis minor and the head of the humerus in the thoracic outlet compression syndrome. Case report. Radiol Clin. 1978;47:423–427.
14 Le E.N., Freischlag J.A., Christo P.J., et al. Thoracic outlet syndrome secondary to localized scleroderma treated with botulinum toxin injection. Arthritis Care Res (Hoboken). 2010;62:430–433.
15 Roos D. Historical perspectives and anatomic considerations. Thoracic outlet syndrome. 1996.
16 Patton G.M. Arterial thoracic outlet syndrome. Hand Clin. 2004;20:107–111. viii
17 Firsov G. Cervical ribs and their distinction from under-developed first ribs. Arkh Anat Gistol Embriol. 1974;67:101.
18 Terabayashi N., Ohno T., Nishimoto Y., et al. Nonunion of a first rib fracture causing thoracic outlet syndrome in a basketball player: a case report. J Shoulder Elbow Surg. 2010;19:e20–e23.
19 Gilliatt R., Le Quesne P., Logue V., et al. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psych. 1970;33:615–624.
20 Sanders R.J., Hammond S.L. Venous thoracic outlet syndrome. Hand Clin. 2004;20:113–118. viii
21 Machleder H.I. Thoracic outlet syndromes: new concepts from a century of discovery. Cardiovasc Surg. 1994;2:137–145.
22 Roos D., Owens J. Thoracic outlet syndrome. Arch Surg. 1966;93:71–74.
23 Wilbourn A. The thoracic outlet syndrome is overdiagnosed. Arch Neurol. 1990;47:328–330.
24 Costigan D., Wilbourn A. The elevated arm stress test: specificity in the diagnosis of thoracic outlet syndrome. Neurology. 1985;35:74.
25 Plewa M., Delinger M. The false-positive rate of thoracic outlet syndrome shoulder maneuvers in healthy subjects. Acad Emerg Med. 1998;5:337–342.
26 Toomingas A., Nilsson T., Hagberg M., et al. Predictive aspects of the abduction external rotation test among male industrial and office workers. Am J Ind Med. 1999;35:32–42.
27 Roos D. The place for scalenectomy and first-rib resection in thoracic outlet syndrome. Surgery. 1982;92:1077–1085.
28 Franklin G., Fulton-Kehoe D., Bradley C., et al. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54:1252–1258.
29 Abe M., Ichinohe K., Nishida J. Diagnosis, treatment, and complications of thoracic outlet syndrome. J Orthop Sci. 1999;4:66–69.
30 Machleder H., Moll F., Nuwer M., et al. Somatosensory evoked potentials in the assessment of thoracic outlet compression syndrome. J Vasc Surg. 1987;6:177–184.
31 Jordan S.E., Machleder H.I. Diagnosis of thoracic outlet syndrome using electrophysiologically guided anterior scalene blocks. Ann Vasc Surg. 1998;12:260–264.
32 Jordan S.E., Ahn S.S., Freischlag J.A., et al. Selective botulinum chemodenervation of the scalene muscles for treatment of neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2000;14:365–369.
33 Wadhwani R., Chaubal N., Sukthankar R., et al. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20:795–801.
34 Aligne C., Barral X. Rehabilitation of patients with thoracic outlet syndrome. Ann Vasc Surg. 1992;6:381–389.
35 Cronenwett, ed. Rutherford’s vascular surgery, ed 3, Philadelphia: WB Saunders, 1989. Roos DB, ed
36 Ohtsuka T., Wolf R.K., Dunsker S.B. Port-access first-rib resection. Surg Endosc. 1999;13:940–942.
37 Alla V., Natarajan N., Kaushik M., et al. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11:358–362.
38 Kunkel J.M., Machleder H.I. Treatment of Paget-Schroetter syndrome. A staged, multidisciplinary approach. Arch Surg. 1989;;124:1153–1157. discussion 1157–1158
39 Brooke B., Freischlag J. Contemporary management of thoracic outlet syndrome. Curr Opin Cardiol. 2010;25:535–540.
40 Illig K.A., Doyle A.J. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg. 2010;51:1538–1547.
41 Angle N., Gelabert H.A., Farooq M.M., et al. Safety and efficacy of early surgical decompression of the thoracic outlet for Paget-Schroetter syndrome. Ann Vasc Surg. 2001;15:37–42.
42 Molina J., Hunter D., Dietz C. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45:328–334.
43 Molina J., Hunter D., Dietz C. Protocols for Paget-Schroetter syndrome and late treatment of chronic subclavian vein obstruction. Ann Thorac Surg. 2009;87:416–422.
44 Guzzo J.L., Chang K., Demos J., et al. Preoperative thrombolysis and venoplasty affords no benefit in patency following first rib resection and scalenectomy for subacute and chronic subclavian vein thrombosis. J Vasc Surg. 2010;52:658–662.
45 Thompson R. Venous thoracic outlet syndrome: paraclavicular approach. Op Tech Gen Surg. 2008;10:113–121.
46 de Leon R.A., Chang D.C., Hassoun H.T., et al. Multiple treatment algorithms for successful outcomes in venous thoracic outlet syndrome. Surgery. 2009;145:500–507.
47 Lee J., Karwowski J., Harris E., et al. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43:1236–1243.
48 Meier G.H., Pollak J.S., Rosenblatt M., et al. Initial experience with venous stents in exertional axillary-subclavian vein thrombosis. J Vasc Surg. 1996;;24:974–981. discussion 981–973
49 Kreienberg P., Chang B., Darling R. Long-term results in patients treated with thrombolysis, thoracic inlet decompression, and subclavian vein stenting for Paget-Schroetter syndrome. J Vasc Surg. 2001;33:100–105.
50 Chang D.C., Rotellini-Coltvet L.A., Mukherjee D., et al. Surgical intervention for thoracic outlet syndrome improves patient’s quality of life. J Vasc Surg. 2009;49:630–635.
51 Cordobes-Gual J., Lozano-Vilardell P., Torreguitart-Mirada N., et al. Prospective study of the functional recovery after surgery for thoracic outlet syndrome. Eur J Vasc Endovasc Surg. 2008;35:79–83.
52 Scali S., Stone D., Bjerke A., et al. Long-term functional results for the surgical management of neurogenic thoracic outlet syndrome. Vasc Endovasc Surg. 2010;44:550–555.
53 Altobelli G.G., Kudo T., Haas B.T., et al. Thoracic outlet syndrome: pattern of clinical success after operative decompression. J Vasc Surg. 2005;42:122–128.
54 Sharp W.J., Nowak L.R., Zamani T., et al. Long-term follow-up and patient satisfaction after surgery for thoracic outlet syndrome. Ann Vasc Surg. 2001;15:32–36.
55 Lepäntalo M., Lindgren K. Long-term outcome after resection of the first rib for thoracic outlet syndrome. Br J Surg. 1989;76:1255–1256.
56 Green R., McNamara J., Ouriel K. Long-term follow-up after thoracic outlet decompression: an analysis of factors determining outcome. J Vasc Surg. 1991;14:739–745.
57 Sanders R., Pearce W. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10:626–634.
58 Axelrod D.A., Proctor M.C., Geisser M.E., et al. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33:1220–1225.
59 Sessions R. Recurrent thoracic outlet syndrome: causes and treatment. South Med J. 1982;75:1453–1466.
60 Tilney N., Griffiths H., Edwards E. Natural history of major venous thrombosis of the upper extremity. Arch Surg. 1970;101:792–796.
61 Urschel H., Patel A. Surgery remains the most effective treatment for Paget-Schroetter syndrome: 50 years’ experience. Ann Thorac Surg. 2008;86:254–260.
62 Feugier P., Aleksic I., Salari R., et al. Long-term results of venous revascularization for Paget-Schroetter syndrome in athletes. Ann Vasc Surg. 2001;15:212–218.
63 Dhillon R.K., Stead L.G. Acute deep vein thrombus due to May-Thurner syndrome. Am J Emerg Med. 2010;28(254):e253–e254.
64 Dogan O., Boke E. Three cases with May-Thurner syndrome: a possibly under-reported disorder. Vasa. 2005;34:147–151.
65 Oguzkurt L., Tercan F., Sener M. Successful endovascular treatment of iliac vein compression (May-Thurner) syndrome in a pediatric patient. Cardiovasc Interv Radiol. 2006;29:446–449.
66 Vyas S., Roberti I., McCarthy C. May–Thurner syndrome in a pediatric renal transplant recipient—case report and literature review. Pediatr Transplant. 2008;12:708–710.
67 Im S., Lim S.H., Chun H.J., et al. Leg edema with deep venous thrombosis-like symptoms as an unusual complication of occult bladder distension and right May-Thurner syndrome in a stroke patient: a case report. Arch Phys Med Rehabil. 2009;90:886–890.
68 Kiernan T.J., Yan B.P., Cubeddu R.J., et al. May-Thurner syndrome in patients with cryptogenic stroke and patent foramen ovale: an important clinical association. Stroke. 2009;40:1502–1504.
69 Kim Y., Ko S., Kim H. Spontaneous rupture of the left common iliac vein associated with May Thurner syndrome: successful management with surgery and placement of an endovascular stent. Br J Radiol. 2007;80:e176–e179.
70 Kibbe M.R., Ujiki M., Goodwin A.L., et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39:937–943.
71 Raju S., Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44:136–144.
72 De Bast Y., Dahin L. May-Thurner syndrome will be completed? Thromb Res. 2009;123:498–502.
73 Sharma R., Joshi W. A case of May-Thurner syndrome with antiphospholipid antibody syndrome. Conn Med. 2008;72:527–530.
74 Murphy E.H., Davis C.M., Journeycake J.M., et al. Symptomatic iliofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May-Thurner syndrome. J Vasc Surg. 2009;49:697–703.
75 Jeon U.B., Chung J.W., Jae H.J., et al. May-Thurner syndrome complicated by acute iliofemoral vein thrombosis: helical CT venography for evaluation of long-term stent patency and changes in the iliac vein. Am J Roentgenol. 2010;195:751–757.
76 Gurel K., Gurel S., Karavas E., et al. Direct contrast-enhanced MR venography in the diagnosis of May-Thurner syndrome. Eur J Radiol. 2011;80:533–536.
77 Oguzkurt L., Ozkan U., Tercan F., et al. Ultrasonographic diagnosis of iliac vein compression (May-Thurner) syndrome. Diagn Interv Radiol. 2007;13:152–155.
78 Fretz V., Binkert C. Compression of the inferior vena cava by the right iliac artery: a rare variant of May-Thurner syndrome. Cardiovasc Interv Radiol. 2010;33:1060–1063.
79 Abboud G., Midulla M., Lions C., et al. Right-sided May-Thurner syndrome. Cardiovasc Interv Radiol. 2010;33:1056–1059.
80 Burke R.M., Rayan S.S., Kasirajan K., et al. Unusual case of right-sided May-Thurner syndrome and review of its management. Vascular. 2006;14:47–50.
81 Grunwald M., Goldberg M., Hofmann L. Endovascular management of May-Thurner syndrome. AJR Am J Roentgenol. 2004;183:1523–1524.
82 Kwak H.S., Han Y.M., Lee Y.S., et al. Stents in common iliac vein obstruction with acute ipsilateral deep venous thrombosis: early and late results. J Vasc Interv Radiol. 2005;16:815–822.
83 Kim J.Y., Choi D., Guk Ko Y., et al. Percutaneous treatment of deep vein thrombosis in May-Thurner syndrome. Cardiovasc Interv Radiol. 2006;29:571–575.
84 Moudgill N., Hager E., Gonsalves C., et al. May-Thurner syndrome: case report and review of the literature involving modern endovascular therapy. Vascular. 2009;17:330–335.
85 Canales J.F., Krajcer Z. Intravascular ultrasound guidance in treating May-Thurner syndrome. Tex Heart Inst J. 2010;37:496–497.
86 Mullens W., De Keyser J., Van Dorpe A., et al. Migration of two venous stents into the right ventricle in a patient with May-Thurner syndrome. Int J Cardiol. 2006;110:114–115.
87 Hartung O., Barthelemy P., Arnoux D., et al. Management of pregnancy in women with previous left ilio-caval stenting. J Vasc Surg. 2009;50:355–359.
88 Zander K.D., Staat B., Galan H. May-Thurner syndrome resulting in acute iliofemoral deep vein thrombosis in the postpartum period. Obstet Gynecol. 2008;111:565–569.
89 O’Sullivan G.J., Semba C.P., Bittner C.A., et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
90 Titus J., Moise M.A., Bena J., et al. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53:706–712.
91 Jost C.J., Gloviczki P., Cherry K.J. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320–328.
92 Garg N., Gloviczki P., Karimi K.M., et al. Factors affecting outcome of open and hybrid reconstructions for nonmalignant obstruction of iliofemoral veins and inferior vena cava. J Vasc Surg. 2011;53:383–393.
93 Ahmed K., Sampath R., Khan M. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg. 2006;31:410–416.
94 Kurklinsky A.K., Rooke T.W. Nutcracker phenomenon and nutcracker syndrome. Mayo Clinic Proc. 2010;85:552–559.
95 Menard M. Nutcracker syndrome: when should it be treated and how? Perspect Vasc Surg Endovasc Ther. 2009;21:117–124.
96 Park Y.H., Choi J.Y., Chung H.S., et al. Hematuria and proteinuria in a mass school urine screening test. Pediatr Nephrol. 2005;20:1126–1130.
97 Shin J.I., Lee J.S. Unexpected superimposition of nutcracker effect in various conditions: is it an unrecognized confounding factor? Eur J Pediatr. 2007;166:1089–1090.
98 Altugan F.S., Ekim M., Fitöz S., et al. Nutcracker syndrome with urolithiasis. J Pediatr Urol. 2010;6:519–521.
99 Shin J., Park J., Shin Y., et al. Superimposition of nutcracker syndrome in a haematuric child with Henoch Schönlein purpura. Int J Clin Pract. 2005;59:1472–1475.
100 Shin J.I., Park J.M., Shin Y.H., et al. Nutcracker syndrome combined with IgA nephropathy in a child with recurrent hematuria. Pediatr Int. 2006;48:324–326.
101 Shin J.I., Park J.M., Lee J.S., et al. Superimposition of nutcracker syndrome in a hematuric child with idiopathic hypercalciuria and urolithiasis. Int J Urol. 2006;13:814–816.
102 Ozcan A., Gonul I.I., Sakallioglu O., et al. Nutcracker syndrome in a child with familial Mediterranean fever (FMF) disease: renal ultrastructural features. Int Urol Nephrol. 2009;41:1047–1053.
103 Ali-El-Dein B., Osman Y., El-Din A.B.S., et al. Anterior and posterior nutcracker syndrome: a report on 11 cases. Transplant Proc. 2003;35:851–853.
104 Fitoz S., Yalcinkaya F. Compression of left inferior vena cava: a form of nutcracker syndrome. J Clin Ultrasound. 2008;36:101–104.
105 Mohamadi A., Ghasemi-Rad M., Mladkova N., et al. Varicocele and nutcracker syndrome: sonographic findings. J Ultrasound Med. 2010;29:1153–1160.
106 Kim K.W., Cho J.Y., Kim S.H., et al. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. Eur J Radiol. 2011;80:648–654.
107 Cho B.S., Suh J.S., Hahn W.H., et al. Multidetector computed tomography findings and correlations with proteinuria in nutcracker syndrome. Pediatric Nephrol. 2010;25:469–475.
108 Karaosmanoglu D., Karcaaltincaba M., Akata D., et al. Unusual causes of left renal vein compression along its course: MDCT findings in patients with nutcracker and pelvic congestion syndrome. Surg Radiol Anat. 2010;32:323–327.
109 Wong H.L., Chen M.C.Y., Wu C.S., et al. The usefulness of fast-spin-echo T2-weighted MR imaging in nutcracker syndrome: a case report. Korean J Radiol. 2010;11:373–377.
110 Shin J.I., Park J.M., Lee S.M., et al. Factors affecting spontaneous resolution of hematuria in childhood nutcracker syndrome. Pediatr Nephrol. 2005;20:609–613.
111 Reed N.R., Kalra M., Bower T.C., et al. Left renal vein transposition for nutcracker syndrome. J Vasc Surg. 2009;49:386–394.
112 Ha T.S., Lee E.J. ACE inhibition can improve orthostatic proteinuria associated with nutcracker syndrome. Pediatr Nephrol. 2006;21:1765–1768.
113 Shin J.I., Lee J.S. ACE inhibition in nutcracker syndrome with orthostatic proteinuria: how about a hemodynamic effect? Pediatr Nephrol. 2007;22:758.
114 Kim J., Joh J., Choi H., et al. Transposition of the left renal vein in nutcracker syndrome. Eur J Vasc Endovasc Surg. 2006;31:80–82.
115 Salehipour M., Rasekhi A., Shirazi M., et al. The role of renal autotransplantation in treatment of nutcracker syndrome. Saudi J Kidney Dis Transpl. 2010;21:237–241.
116 Xu D., Liu Y., Gao Y., et al. Management of renal nutcracker syndrome by retroperitoneal laparoscopic nephrectomy with ex vivo autograft repair and autotransplantation: a case report and review of the literature. J Med Case Reports. 2009;3:82.
117 Zhang Q., Zhang Y., Lou S., et al. Laparoscopic extravascular renal vein stent placement for nutcracker syndrome. J Endourol. 2010:354–357.
118 Chung B.I., Gill I.S. Laparoscopic splenorenal venous bypass for nutcracker syndrome. J Vasc Surg. 2009;49:1319–1323.
119 Hartung O., Azghari A., Barthelemy P., et al. Laparoscopic transposition of the left renal vein into the inferior vena cava for nutcracker syndrome. J Vasc Surg. 2010;52:738–741.
120 Hartung O., Barthelemy P., Berdah S.V., et al. Laparoscopy-assisted left ovarian vein transposition to treat one case of posterior nutcracker syndrome. Ann Vasc Surg. 2009;23:413.
121 Rogers A., Beech A., Braithwaite B. Transperitoneal laparoscopic left gonadal vein ligation can be the right treatment option for pelvic congestion symptoms secondary to nutcracker syndrome. Vascular. 2007;15:238–240.
122 Shaper K., Jackson J., Williams G. The nutcracker syndrome: an uncommon cause of haematuria. Br J Urol. 1994;74:144–146.
123 Basile A., Tsetis D., Calcara G., et al. Percutaneous nitinol stent implantation in the treatment of nutcracker syndrome in young adults. J Vasc Interv Radiol. 2007;18:1042–1046.
124 Chen W., Chu J., Yang J., et al. Endovascular stent placement for the treatment of nutcracker phenomenon in three pediatric patients. J Vasc Interv Radiol. 2005;16:1529–1533.
125 Cohen F., Amabile P., Varoquaux A., et al. Endovascular treatment of circumaortic nutcracker syndrome. J Vasc Interv Radiol. 2009;20:1255–1257.
126 Gagnon L.O., Ponsot Y., Benko A., et al. Nutcracker syndrome in a 20-year-old patient treated with intravascular stent placement: a case report. Can J Urol. 2009;16:4765–4769.
127 Hartung O., Grisoli D., Boufi M., et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg. 2005;42:275–280.
128 Wang L., Yi L., Yang L., et al. Diagnosis and surgical treatment of nutcracker syndrome: a single-center experience. Urology. 2009;73:871–876.
129 Kim S., Kim C.W., Kim S., et al. Long-term follow-up after endovascular stent placement for treatment of nutcracker syndrome. J Vasc Interv Radiol. 2005;16:428–431.
130 Zhang H., Li M., Jin W., et al. The left renal entrapment syndrome: diagnosis and treatment. Ann Vasc Surg. 2007;21:198–203.
131 Bernheim J.W., Hansen J., Faries P., et al. Acute lower extremity ischemia in a 7-year-old boy: an unusual case of popliteal entrapment syndrome. J Vasc Surg. 2004;39:1340–1343.
132 Haidar S., Thomas K., Miller S. Popliteal artery entrapment syndrome in a young girl. Pediatr Radiol. 2005;35:440–443.
133 Ferreira J., Canedo A., Graça S., et al. Thrombosed popliteal aneurysm-first manifestation of bilateral popliteal entrapment syndrome. Int Angiol. 2010;29:83–86.
134 Tercan F., Oguzkurt L., Kizilkilic O., et al. Popliteal artery entrapment syndrome. Diagn Interv Radiol. 2005;11:222–224.
135 Beuthien W., De Potzolli O., Mellinghoff H.U., et al. Popliteal artery entrapment with peripheral thromboembolism. J Clin Rheumatol. 2004;10:147–149.
136 Misselbeck T., Dangleben D., Celani V. Isolated popliteal vein entrapment by the popliteus muscle: a case report. Vasc Med. 2008;13:37–39.
137 Nottingham J.M., Haynes J.L. Isolated popliteal vein entrapment by a fibrous band. Vasc Endovasc Surg. 2001;35:487–490.
138 Pillai J., Levien L.J., Haagensen M., et al. Assessment of the medial head of the gastrocnemius muscle in functional compression of the popliteal artery. J Vasc Surg. 2008;48:1189–1196.
139 Turnipseed W.D. Functional popliteal artery entrapment syndrome: a poorly understood and often missed diagnosis that is frequently mistreated. J Vasc Surg. 2009;49:1189–1195.
140 Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg. 2008;48:61s–65s.
141 Aktan Ikiz Z.A., Ucerler H., Ozgur Z. Anatomic variations of popliteal artery that may be a reason for entrapment. Surg Radiol Anat. 2009;31:695–700.
142 Holden A., Merrilees S., Mitchell N., et al. Magnetic resonance imaging of popliteal artery pathologies. Eur J Radiol. 2008;67:159–168.
143 Kim H.K., Shin M.J., Kim S.M., et al. Popliteal artery entrapment syndrome: morphological classification utilizing MR imaging. Skeletal Radiol. 2006;35:648–658.
144 Özkan U., Oguzkurt L., Tercan F., et al. MRI and DSA findings in popliteal artery entrapment syndrome. Diagn Interv Radiol. 2008;14:106–110.
145 Kukreja K., Scagnelli T., Narayanan G., et al. Role of angiography in popliteal artery entrapment syndrome. Diagn Interv Radiol. 2009;15:57–60.
146 Anil G., Tay K., Howe T., et al. Dynamic computed tomography angiography: role in the evaluation of popliteal artery entrapment syndrome. Cardiovasc Interv Radiol. 2011;34:259–270.
147 Zhong H., Liu C., Shao G. Computed tomographic angiography and digital subtraction angiography findings in popliteal artery entrapment syndrome. J Comput Assist Tomogr. 2010;34:254–259.
148 Hai Z., Guangrui S., Yuan Z., et al. CT angiography and MRI in patients with popliteal artery entrapment syndrome. AJR Am J Roentgenol. 2008;191:1760–1766.
149 Goh B.K.P., Tay K.H., Tan S.G. Diagnosis and surgical management of popliteal artery entrapment syndrome. Aust N Z J Surg. 2005;75:869–873.
150 Shen J., Abu-Hamad G., Makaroun M.S., et al. Bilateral asymmetric popliteal entrapment syndrome treated with successful surgical decompression and adjunctive thrombolysis. Vasc Endovasc Surg. 2009;43:395–398.
151 di Marzo L., Cavallaro A., O’Donnell S.D., et al. Endovascular stenting for popliteal vascular entrapment is not recommended. Ann Vasc Surg. 2010;24:1135.
152 Meier T., Schneider E., Amann-Vesti B. Long-term follow-up of patients with popliteal artery entrapment syndrome treated by endoluminal revascularization. Vasa. 2010;39:189–195.
153 Paraskevas N., Castier Y., Fukui S., et al. Superficial femoral artery autograft reconstruction for complicated popliteal artery entrapment syndrome. Vasc Endovasc Surg. 2009;43:165–169.
154 Gagnon J., Doyle D. Adventitial cystic disease of common femoral artery. Ann Vasc Surg. 2007;21:84–86.
155 Jindal R., Majed A., Hamady M., et al. Cystic adventitial disease of the iliofemoral artery: case reports and a short review. Vascular. 2006;14:169–172.
156 Dix F.P., McDonald M., Obomighie J., Chalmers, et al. Cystic adventitial disease of the femoral vein presenting as deep vein thrombosis: a case report and review of the literature. J Vasc Surg. 2006;44:871–874.
157 Gasparis A.P., Wall P., Ricotta J.J. Adventitial cystic disease of the external iliac vein presenting with deep venous thrombosis. Vasc Endovasc Surg. 2004;38:273–276.
158 Morizumi S., Suematsu Y., Gon S., et al. Adventitial cystic disease of the femoral vein. Ann Vasc Surg. 2010;24:1135e5–1135e7.
159 Seo J.Y., Chung D.J., Kim J.H. Adventitial cystic disease of the femoral vein: a case report with the CT venography. Korean J Radiol. 2009;10:89–92.
160 França M., Pinto J., Machado R., et al. Case 157: Bilateral adventitial cystic disease of the popliteal artery. Radiology. 2010;255:655–660.
161 Ortiz M.W.R., Lopera J.E., Giménez C.R., et al. Bilateral adventitial cystic disease of the popliteal artery: a case report. Cardiovasc Interv Radiol. 2006;29:306–310.
162 Ortmann J., Widmer M., Gretener S., et al. Cystic adventitial degeneration: ectopic ganglia from adjacent joint capsules. Vasa. 2009;38:374–377.
163 Asciutto G., Mumme A., Marpe B., et al. Different approaches in the treatment of cystic adventitial disease of the popliteal artery. Chir Ital. 2007;59:467–473.
164 Setacci F., Sirignano P., de Donato G., et al. Adventitial cystic disease of the popliteal artery: experience of a single vascular and endovascular center. J Cardiovasc Surg. 2008;49:235–239.
165 Chappel P., Gielen J., Salgado R., et al. Cystic adventitial disease with secondary occlusion of the popliteal artery. JBR–BTR. 2007;90:180–181.
166 Tsilimparis N., Hanack U., Yousefi S., et al. Cystic adventitial disease of the popliteal artery: an argument for the developmental theory. J Vasc Surg. 2007;45:1249–1252.
167 Buijsrogge M.P., van der Meij S., Korte J.H., et al. “Intermittent claudication intermittence” as a manifestation of adventitial cystic disease communicating with the knee joint. Ann Vasc Surg. 2006;20:687–689.
168 Taurino M., Rizzo L., Stella N., et al. Doppler ultrasonography and exercise testing in diagnosing a popliteal artery adventitial cyst. Cardiovasc Ultrasound. 2009;7:23.
169 Nano G., Dalainas I., Casana R., et al. Case report of adventitial cystic disease of the popliteal artery presented with the “dog-leg” sign. Int Angiol. 2007;26:75–78.
170 Maged I.M., Turba U.C., Housseini A.M., et al. High spatial resolution magnetic resonance imaging of cystic adventitial disease of the popliteal artery. J Vasc Surg. 2010;51:471–474.
171 Tomasian A., Lai C., Finn J.P., et al. Cystic adventitial disease of the popliteal artery: features on 3T cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:38.
172 Keo H.H., Baumgartner I., Schmidli J., et al. Sustained remission 11 years after percutaneous ultrasound-guided aspiration for cystic adventitial degeneration in the popliteal artery. J Endovasc Ther. 2007;14:264–265.
173 Johnson J.M., Kiankhooy A., Bertges D.J., et al. Percutaneous image-guided aspiration and sclerosis of adventitial cystic disease of the femoral vein. Cardiovasc Interv Radiol. 2009;32:812–816.
174 Khoury M. Failed angioplasty of a popliteal artery stenosis secondary to cystic adventitial disease. Vasc Endovasc Surg. 2004;38:277–280.
175 Rai S., Davies R.S.M., Vohra R.K. Failure of endovascular stenting for popliteal cystic disease. Ann Vasc Surg. 2009;23:410.
176 Maged I.M., Kron I.L., Hagspiel K.D. Recurrent cystic adventitial disease of the popliteal artery: successful treatment with percutaneous transluminal angioplasty. Vasc Endovasc Surg. 2009;43:399–402.
177 Foertsch T., Koch A., Singer H., et al. Celiac trunk compression syndrome requiring surgery in 3 adolescent patients. J Pediatr Surg. 2007;42:709–713.
178 Gander S., Mulder D.J., Jones S., et al. Recurrent abdominal pain and weight loss in an adolescent: celiac artery compression syndrome. Can J Gastroenterol. 2010;24:91–93.
179 Okten R.I.S., Kucukay F., Tola M., et al. Is celiac artery compression syndrome genetically inherited?: a case series from a family and review of the literature. Eur J Radiol. 2011;. epub ahead of print
180 Desmond C., Roberts S. Exercise-related abdominal pain as a manifestation of the median arcuate ligament syndrome. Scand J Gastroenterol. 2004;39:1310–1313.
181 Said S.M., Zarroug A.E., Gloviczki P., et al. Pediatric median arcuate ligament syndrome: first report of familial pattern and transperitoneal laparoscopic release. J Pediatr Surg. 2010;45:e17–e20.
182 Loukas M., Pinyard J., Vaid S., et al. Clinical anatomy of celiac artery compression syndrome: a review. Clin Anat. 2007;20:612–617.
183 Soman S., Sudhakar S.V., Keshava S.N. Celiac axis compression by median arcuate ligament on computed tomography among asymptomatic persons. Ind J Gastroenterol. 2010;29:121–123.
184 Akatsu T., Hayashi S., Yamane T., et al. Emergency embolization of a ruptured pancreaticoduodenal artery aneurysm associated with the median arcuate ligament syndrome. J Gastroenterol Hepatol. 2004;19:482–483.
185 Ogino H., Sato Y., Banno T., et al. Embolization in a patient with ruptured anterior inferior pancreaticoduodenal arterial aneurysm with median arcuate ligament syndrome. Cardiovasc Interv Radiol. 2002;25:318–319.
186 Tsai Y.S., Lee C.H., Pang K.K. Electronic clinical challenges and images in GI. Amb Pediatr. 2009;136:e3–e4.
187 Duncan A.A. Median arcuate ligament syndrome. Curr Treat Options Cardiovasc Med. 2008;10:112–116.
188 Gloviczki P., Duncan A.A. Treatment of celiac artery compression syndrome: does it really exist? Perspect Vasc Surg Endovasc Ther. 2007;19:259–263.
189 Roseborough G.S. Laparoscopic management of celiac artery compression syndrome. J Vasc Surg. 2009;50:124–133.
190 Kalapatapu V.R., Murray B.W., Palm-Cruz K., et al. Definitive test to diagnose median arcuate ligament syndrome: injection of vasodilator during angiography. Vasc Endovasc Surg. 2009;43:46–50.
191 Mensink P.B.F., van Petersen A.S., Kolkman J.J., et al. Gastric exercise tonometry: the key investigation in patients with suspected celiac artery compression syndrome. J Vasc Surg. 2006;44:277–281.
192 Aschenbach R., Basche S., Vogl T.J. Compression of the celiac trunk caused by median arcuate ligament in children and adolescent subjects: evaluation with contrast-enhanced MR angiography and comparison with Doppler US evaluation. J Vasc Interv Radiol. 2011;. epub ahead of print
193 Horton K., Talamini M., Fishman E. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005;25:1177–1182.
194 Ilica A.T., Kocaoglu M., Bilici A., et al. Median arcuate ligament syndrome: multidetector computed tomography findings. J Comput Assist Tomogr. 2007;31:728–731.
195 Karahan Ö, Kahr man G., Y k lmaz A., et al. Celiac artery compression syndrome: diagnosis with multislice CT. Diagn Interv Radiol. 2007;13:90–93.
196 Manghat N., Mitchell G., Hay C., et al. The median arcuate ligament syndrome revisited by CT angiography and the use of ECG gating–a single centre case series and literature review. Br J Radiol. 2008;81:735–742.
197 Özbülbül N.I. CT angiography of the celiac trunk: anatomy, variants and pathologic findings. Diagn Interv Radiol. 2010;. epub ahead of print
198 Wolfman D., Bluth E.I., Sossaman J. Median arcuate ligament syndrome. J Ultrasound Med. 2003;22:1377–1380.
199 Baccari P., Civilini E., Dordoni L., et al. Celiac artery compression syndrome managed by laparoscopy. J Vasc Surg. 2009;50:134–139.
200 Baldassarre E., Torino G., Siani A., et al. The laparoscopic approach in the median arcuate ligament syndrome: report of a case. Swiss Med Wkly. 2007;137:353–354.
201 Mirkia K., Subin B. Minimally invasive approach to median arcuate ligament syndrome in a patient with HIV/AIDS. Int J STD AIDS. 2009;20:209–210.
202 Riess K.P., Serck L., Gundersen S.B., et al. Seconds from disaster: lessons learned from laparoscopic release of the median arcuate ligament. Surg Endosc. 2009;23:1121–1124.
203 Tulloch A.W., Jimenez J.C., Lawrence P.F., et al. Laparoscopic vs. open celiac ganglionectomy in patients with median arcuate ligament syndrome. J Vasc Surg. 2010;52:1283–1289.
204 Vaziri K., Hungness E.S., Pearson E.G., et al. Laparoscopic treatment of celiac artery compression syndrome: case series and review of current treatment modalities. J Gastrointest Surg. 2009;13:293–298.
205 Jaik N., Stawicki S., Weger N., et al. Celiac artery compression syndrome: successful utilization of robotic-assisted laparoscopic approach. J Gastrointest Liver Dis. 2007;16:93–96.
206 van Petersen A.S., Vriens B.H., Huisman A.B., et al. Retroperitoneal endoscopic release in the management of celiac artery compression syndrome. J Vasc Surg. 2009;50:140–147.
207 Grotemeyer D., Duran M., Iskandar F., et al. Median arcuate ligament syndrome: vascular surgical therapy and follow-up of 18 patients. Langenbecks Arch Surg. 2009;394:1085–1092.
208 Wang X., Impeduglia T., Dubin Z., et al. Celiac revascularization as a requisite for treating the median arcuate ligament syndrome. Ann Vasc Surg. 2008;22:571–574.
209 Kohn G.P., Bitar R.S., Farber M.A., et al. Treatment options and outcomes for celiac artery compression syndrome. Surg Innov. 2011;18:338–343.