128 Vascular Catheter–Related Infections
Catheter-related bloodstream infection (CRBSI) is the third leading device-related infection among U.S. hospitals and ambulatory surgical centers in the United States participating in the National Healthcare Safety Network (NHSN) that report to the Centers for Disease Control and Prevention (CDC).1 In the 2009 report, 14,332 primary bloodstream infections over 7.4 million catheter days (1.93 infections per 1000 catheter days) were identified. This infection rate ranks third in magnitude behind catheter-associated urinary tract infections and ventilator-associated pneumonias.1 CRBSI ranks second worldwide only to ventilator-associated pneumonia.2 The estimates in both of these reports are limited to only central venous catheter (CVC) infections. However, peripheral venous catheters, more permanent cuffed and tunneled catheters, arterial catheters, and peripherally inserted central catheters (PICC) also have associated bloodstream infection rates.3–4
The cost of CRBSI in terms of morbidity is significant to both the patient and the healthcare provider. The impact on resource utilization was summarized by Dimick et al., who conducted a prospective cohort study among surgical ICU patients at a large tertiary care center. A single CRBSI increased hospital costs by $56,167 and hospital length of stay by 22 days.5 The increased mortality of CRBSI was estimated in a meta-analysis by Siempos et al. He analyzed eight different studies that included 2540 ICU patients and determined the relative risk of mortality to be 1.57.6 In a mixed cohort of 2201 medical and surgical patients hospitalized in 15 French ICUs, CRBSI was associated with an estimated excess mortality of 11.5% to 20%.7
Because of the burden of mortality to patient populations and the increased costs to payers, CRBSI was included in the list of eight hospital-acquired conditions, the so-called “never events.” By inclusion as a “never event,” the Centers for Medicare and Medicaid Services (CMS) are prohibited by Congress from reimbursing hospitals for charges associated with these conditions after October 1, 2008.8 Thus, prevention of CRBSI has attracted substantial attention from multiple stakeholders in the healthcare industry.
 Definitions
Definitions
Clinicians and researchers historically have used different definitions for vascular catheter–related infections. Infections can be linked to peripheral, central, venous, and arterial catheters. These catheters can further be designated as permanent, short-term, or long-term. The clinical presentation of a catheter-related infection can be designated as either local (site inflammation, purulent drainage, tenderness) or systemic (bacteremia with or without systemic sepsis). Although it is certain that inanimate objects do not become “infected,” there is strong evidence to suggest that bacteria may be able to live and multiply on catheter surfaces, possibly deriving nutrients from catheter polymers, the deposited glycocalyx of certain bacterial species, and other nonviable bacteria.9,10 Earlier clinical investigations used erroneous descriptions and definitions for catheter contamination, colonization, and infection. These different definitions have led to confusion and incorrect interpretations by previous investigators.11 This is further complicated by confusion regarding subtle differences between surveillance definitions by the NHSN1 and clinical definitions. The commonly accepted clinical definitions have been previously published11,12:
It is important to understand that both microbiological and clinical exit site infections, tunnel infections, and pocket infections, when accompanied by a positive blood culture, will be classified as a CRBSI for hospital surveillance purposes.1,11,12
Culture of drainage around a catheter insertion site may in some situations be helpful in that a positive bacterial culture result assists in confirming the presence of an exit site infection. It is important to also understand that values of 15 CFUs or less for semiquantitative and 103 CFUs or less for quantitative cultures may be regarded as a negative culture, a contaminant, or an insignificant infection that does not require treatment in the absence of a confirmatory blood culture. Insertion site manifestations of inflammation are neither sensitive nor specific for diagnosing CRBSI or catheter colonization. Immunosuppressed patients may manifest local signs of inflammation, and other patient groups may develop intense local insertion site inflammation without associated CRBSI.14
 Pathogenesis
Pathogenesis
Microbial colonization and biofilm formation on intravascular catheters are universal, occurring soon after catheter insertion.9,10,15 The final determinate of whether colonization progresses to clinical infection is multifactorial. A variety of host factors, catheter composition, and the interaction between microorganisms and the catheter surface may all contribute to the ultimate development of CRBSI.
There are four established routes for catheter contamination leading to CRBSI:
Following insertion, the intravascular portion of the catheter is quickly coated with a thrombin layer covering both the external and internal surfaces. Thrombin contains a number of proteins including fibronectin, thrombospondin, and laminin which create an adhesive surface on the catheter that promotes adherence of microbial pathogens. Multiple species of Staphylococcus epidermidis, Staphylococcus aureus, Candida albicans, and various gram-negative organisms are all capable of adhering to catheter surfaces.19 A mature biofilm can shield organisms from antibiotics at 10 to 1000 times the concentration required to kill planktonic bacteria.20
This helps explain why the commonly reported pathogens for hospital-acquired bloodstream infections remain coagulase-negative staphylococci (Staphylococcus epidermidis), Staphylococcus aureus, enterococci, and Candida species.20 Gram-negative bacilli account for approximately 20% of CRBSIs reported.20,21
 Risk Factors
Risk Factors
A number of factors potentiate the risk for CRBSI. These are generally similar to the same factors that increase the risk for any hospital-acquired infection. Extremes of age (i.e., pediatric, elderly), immunodeficiency, chronic disease states, remote infection sites, and heavy colonization of the skin with bacteria or fungi may all increase the risk. Alterations in skin integrity (psoriasis, burns) also increase risk. Whereas patient-related factors cannot be significantly modified during an acute illness, they must be considered when developing catheter maintenance protocols. Penel et al. identified age younger than 10 years, difficulties with catheter insertion, and the need for total parenteral nutrition as significant risk factors for intravascular device–related infections.22
In contradistinction to patient-related risk factors, many hospital-related risk factors can be significantly modified, and prevention protocols are designed to focus on these risks.22,23 A number of interventions have been proposed by the CDC to assist in the prevention of CRBSI.24,25 Implementation of educational programs for hospital personnel regarding proper insertion and maintenance of intravascular catheters and appropriate preventive control measures should reduce infection rates. A number of other interventions and measures are also recommended collectively as the “central line bundle.” These recommended procedures and interventions are: hand washing, using full sterile-barrier precautions during insertion of central venous catheters, preparing the insertion skin site with chlorhexidine, avoiding the femoral site if possible, and removing central venous catheters as soon as possible when no longer needed.23 In a large multicenter trial involving 108 intensive care units (ICUs), a central line bundle was initiated to determine its effect on reduction of catheter-related bloodstream infections. Implementing these strategies reduced the mean rate of CRBSI from 7.7 to 1.4 per 1000 catheter-days at 16 to 18 months follow-up (P < 0.002).26 This large multicenter study provided evidence that the guidelines recommended by the CDC24,25 are indeed beneficial in reducing CRBSI rates. Others have suggested that the act of prospective surveillance alone without any specific intervention to reduce CRBSI will also have a beneficial result in decreasing infection rates.27
Although the number of catheter manipulations and the experience of the individual performing the catheter insertion may be risk factors, these often cannot be changed or controlled for the individual patient at risk. The need for total parenteral nutrition, the area within the hospital where the insertion is performed, and the number of catheter lumens have all been associated with increased risk for catheter-related infection.28 Cutdowns should be avoided whenever possible because of the historically high incidence of catheter-related complications.29 The most common risk factors for catheter colonization and CRBSI that can be successfully altered are separately discussed.
Anatomic Insertion Site
An early study by Mermel showed that the use of the internal jugular site, particularly for pulmonary artery catheters, is a significant risk factor for catheter-related infection.30 This may be related to the closer proximity of oropharyngeal secretions, greater catheter motion from neck movement, and greater difficulty in maintaining a sterile occlusive dressing.30 However, it is still recommended that the internal jugular or the femoral vein site be used over the subclavian vein site for short-term hemodialysis catheters to reduce the risk of subsequent subclavian vein stenosis.24,25
The femoral site is also more likely to become heavily colonized and thus is also at higher risk for CRBSI. Merrer et al. in a randomized controlled clinical trial involving eight different ICUs identified a threefold higher incidence of clinical sepsis (with or without bloodstream infection) and a tenfold increase in thrombotic complications when femoral catheters were compared to subclavian catheters.31
Data collected in our own center suggest that colonization rates for femoral sites, even with the use of chlorhexidine and silver sulfadiazine-bonded catheters, are significantly higher than for subclavian or internal jugular catheter sites.32
Duration of Catheter Use
Bacterial colonization of catheter surfaces begins shortly after insertion and is directly proportional to the length of time a catheter remains in place. The risk of CRBSI increases over time. Nonetheless, the optimal timing of catheter removal remains uncertain. The risk of an individual catheter causing CRBSI is low if inserted under optimal sterile conditions and removed within 4 to 7 days. However, critically ill patients typically require venous access for prolonged periods, and the timing of catheter removal must be weighed against clinical necessity. Central venous catheters and pulmonary artery catheters do not have predetermined lifespans.33
Recommendations and guidelines for catheter exchange may be used to minimize CRBSI and to prolong site use on the basis of existing published data. However, it is important to realize that CRBSI risk factors are multifactorial and that global recommendations for catheter maintenance or removal may not be applicable to the individual patient. Generally, catheters should be removed (1) when they are no longer needed, or (2) if CRBSI is suspected clinically and appropriate cultures confirm clinical suspicions (see Diagnostic Techniques). Individual hospitals, individual ICUs, and in certain situations individual practitioners should study their catheter infection rates to develop specific guidelines appropriate to their practice patterns and environment. Rates of CRBSI per 1000 catheters-days can be calculated and compared with published standards.1,2,24,25
 Diagnostic Techniques
Diagnostic Techniques
The clinical diagnosis of CRBSI is often inaccurate, leading to premature catheter removal. Assuming that appropriate sterile technique during insertion and appropriate site care have been followed, the presence of entry-site inflammation is neither sensitive nor specific for CRBSI.14 Qualitative broth cultures collected through the CVC are generally discouraged for determining CRBSI for short-term, non-tunneled catheters. The positive predictive value of blood cultures obtained through the catheter is significantly less than from a peripheral venipuncture,34,35 and additional cultures are usually necessary to make the definitive diagnosis. However, a negative culture from either a peripheral venipuncture or a CVC has excellent negative predictive value, and cultures obtained through the catheter are frequently performed to rule out CRBSI.35
Diagnostic Techniques Requiring Removal of the Central Venous Catheter
Quantitative Catheter Cultures
This type of culture involves flushing, sonicating, or vortexing the catheter segment with broth. This is designed to retrieve organisms from both the internal and external catheter surface. This technique is particularly useful for catheters in situ for more than 7 days.11 In this situation, intraluminal spread from the hub is the most likely mechanism for catheter colonization. Therefore, obtaining a culture from both the internal and external surface should be more sensitive and specific. A culture yielding over 103 CFU is diagnostic for CRBSI if accompanied by the appropriate clinical diagnosis, a positive peripheral blood culture with the same organism, and no other likely source for the infection. A meta-analysis conducted by Safdar in 2005 showed that the pooled sensitivity and specificity for this culture technique was 83% and 87% respectively.36
Semiquantitative Catheter Culture
The semiquantitative (roll-plate) technique developed by Maki and colleagues remains the most common diagnostic technique for determining catheter-related infection.37 A 5-cm segment (either catheter tip or intracutaneous segment) is rolled across a blood-agar plate in a reproducible, defined manner. In the original study, a positive result was defined as more than 15 CFUs per plate, although most of the culture-positive catheters in the original study yielded confluent growth.37 A positive catheter segment culture result (>15 CFUs) resulted in a 16% risk of CRBSI. This technique is probably most accurate for catheters that are removed within the first 7 days.36 It may become less sensitive for more long-term catheters, because this technique does not culture the internal lumen. A recent meta-analysis of 19 studies using the semiquantitative catheter culture technique identified an overall sensitivity of 85% and specificity of 82%.36
Central Venous Catheter–Sparing Diagnostic Techniques
A number of techniques have been developed as an alternative for diagnosing CRBSI in patients for whom catheter removal is undesirable because of limited vascular access.11
Paired Device–Collected Quantitative Blood Cultures
This technique involves obtaining quantitative cultures of paired blood samples—one obtained through the central venous catheter hub and the other from a peripheral venipuncture site. The samples should be obtained less than 10 minutes apart using the same blood volume for each culture. Central venous catheter cultures yielding a colony count at least fivefold greater than the colony count obtained from the peripheral venipuncture sample is considered predictive of CRBSI.11,38 This technique is used more frequently for long-term tunneled catheters. A comparative meta-analysis of various diagnostic methods reviewed 7 studies utilizing the differential quantitative blood culture method. The overall pooled sensitivity was 75% to 93%, and the specificity was 97% to 100%.36
Differential Time to Positivity for Central Venous Catheter versus Peripheral Blood Cultures
This method makes use of continuous blood culture monitoring for positivity.11 Radiometric methods are utilized comparing the differential time to positivity for qualitative cultures of blood samples drawn from the catheter and from a peripheral vein. This test is based on the hypothesis that the time to positivity of a culture is closely related to the inoculum size of the microorganisms. The difference between the time required for culture positivity in simultaneously drawn samples of catheter blood and peripheral blood are measured. Raad determined that the cutoff time for positivity was 120 minutes.39 A subsequent meta-analysis revealed an overall sensitivity of 85% and a specificity of 81%.36
 Catheter and Site Maintenance
Catheter and Site Maintenance
Adjuncts to Catheter and Site Maintenance
Several trials have compared various antiseptic solutions’ efficacy in preventing CRBSI. Parienti et al. randomized 223 catheters to either a 10% aqueous povidone-iodine solution or a 5% povidone-iodine solution in 70% ethanol.40 They observed that the ethanol-based solution was associated with a lower catheter colonization rate and a longer time to catheter colonization compared to the aqueous solution. However, the rates of catheter-related bacteremia were similar in both groups.40 Mimoz et al. compared 5% povidone-iodine in 70% ethanol to a solution of 0.25% chlorhexidine gluconate, 0.025% benzalkonium chloride, and 4% benzylic alcohol.41 A total of 538 catheters were randomized, with 481 of these providing evaluable culture results. The solutions were used for skin preparation and then as a single application during subsequent dressing changes. There was a 50% decrease in the incidence of catheter colonization and a trend toward lower rates of CRBSI in the chlorhexidine group.41 Other studies have focused on trials of chlorhexidine-impregnated dressing materials as a strategy to decrease CRBSI. A meta-analysis of eight studies was conducted by Ho et al.42 The chlorhexidine-impregnated dressing demonstrated an odds ratio (OR) for catheter or exit site bacterial colonization of 0.47, P <0.001. Like other investigators, they observed a trend towards reduction in CRBSI. Interestingly, they estimated that the dressings would have to be used on 142 catheters, with a total cost of $532.50, to prevent one episode of CRBSI.42 It is noteworthy that although the studies cited here achieved impressive reductions in colonization, none demonstrated significant reduction in CRBSI. Thus, dressing materials alone are not sufficient to realize decreases in CRBSI rates.
Timsit et al. performed a prospective randomized multicenter study in 2009 comparing standard catheter dressings and site care to a chlorhexidine gluconate–impregnated sponge dressing to determine the effect on catheter colonization and the incidence of major catheter-related infection (defined as either catheter-related clinical sepsis without bloodstream infection or catheter-related bloodstream infection).43 This study also randomized patients to receive dressing changes at either 3 or 7 days. The novel chlorhexidine dressing reduced catheter colonization from 15.8/1000 catheter-days to 6.3/1000 catheter-days (hazard ratio 0.36, 0.28–0.46, P < .001). Similar hazard risk reduction was identified for both major catheter-related infection (1.4/1000 catheter-days versus 0.6/1000 catheter-days) and CRBSI (1.3/1000 catheter-days versus 0.4/1000 catheter-days). It should be mentioned that almost 50% of the catheters studied were arterial catheters. Also, the majority of the catheter sites required more frequent dressing changes before the 3- or 7-day time periods expired. The authors concluded that 117 catheters would require management with the chlorhexidine gluconate–impregnated sponges to prevent one major catheter-related infection.43 Use of these dressings with central venous catheters and arterial catheters in the ICU reduced the risk of infection even when background infection rates were low. Reducing the frequency of changing unsoiled adherent dressings from every 3 days to every 7 days modestly decreased the total number of dressing changes and appeared to be safe.43
Investigation to find other effective adjuncts to CRBSI prevention has extended into use of antimicrobials as flush (or lock) solutions. Safdar and Maki published a meta-analysis of seven prospective, randomized trials comparing vancomycin-heparin to heparin alone as lock solutions for prevention of CRBSI. The study cohorts included patients with cancer, those requiring parenteral nutrition, and critically ill neonates. The vancomycin-heparin lock solution was associated with an odds reduction of 0.49 for CRBSI compared to heparin alone.44 When vancomycin was used as a true lock solution, it conferred a greater benefit, with an OR of 0.34. The authors concluded that this strategy warranted consideration for high-risk patients requiring central access.44 Other antibiotic-based solutions have been tested in various populations, with similarly impressive reductions in CRBSI rates.45
In addition to evaluating topical application of antimicrobial solutions and lock solutions, investigators have tested various strategies of catheter replacement as a means to reduce CRBSI by decreasing prolonged exposure to any individual catheter. Both new-site replacement and guidewire exchange protocols have been examined. Cook et al. systematically reviewed the literature consisting of 12 relevant trials of catheter replacement over a guidewire versus new-site placement.46 They observed that new-site placement presented a higher risk of mechanical complications compared to guidewire exchange. However, guidewire exchange, regardless of whether the patient was suspected of having an infection, was associated with trends toward higher rates of catheter site infection and CRBSI. Additionally, exchanging catheters routinely every 3 days, either by new-site placement or by guidewire exchange, was not effective in reducing CRBSI compared to exchange on an as-needed basis. They concluded that if guidewire exchange is necessary, meticulous sterile technique is required.46
Suggested Method for Guidewire Exchange
The following procedure of guidewire exchange is recommended:
A chest radiograph is generally not required after guidewire exchange.
Whereas strategies aimed at reducing CRBSI are traditionally focused on isolated technical interventions, there is accumulating evidence that systems-based interventions are also very effective in improving patient outcomes. Common themes in the various systems-based strategies are education of nursing and physician staff in evidence-based practices of hand hygiene and catheter site preparation. Additionally, these interventions should employ ongoing compliance and CRBSI surveillance and feedback to the teams with observed compliance and CRBSI event rates. By utilizing evidence-based practices, monitoring compliance and CRBSI rates, and updating the care teams concerning their progress, an environment of conscientious quality improvement and patient safety is created. Several investigators have studied this type of intervention and realized 50% to greater than 70% reductions in CRBSI rates across various critical care settings.47–49 It is clear that individual technical innovations offer the means to decrease CRBSI rates. However, initiating a multimodal approach that incorporates evidence-based practices, team education, results tracking, and feedback may offer the most robust and sustainable improvements in patient outcomes.
 Infection Risks of Specific Catheters
Infection Risks of Specific Catheters
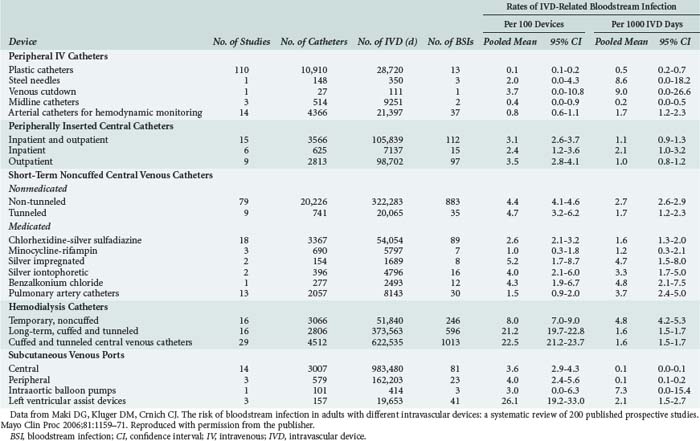
Previously we have discussed CRBSI as a uniform phenomenon without distinguishing the specific burden of risk associated with specific catheters. Each type of catheter carries an associated degree of risk for CRBSI. Many investigators have focused on individual catheter types when reporting these risks. Maki et al. conducted a meta-analysis of 200 published prospective studies encompassing 65,105 intravascular catheters ranging from peripheral IV catheters to left ventricular assist devices. The pooled mean CRBSI rates vary from 0.1/1000 catheter-days observed in subcutaneous venous ports to 9.0/1000 catheter-days reported for venous cutdowns.4 In the following sections, we will discuss the most commonly used catheters and their associated CRBSI infection risks.
Multiple-Lumen Central Venous Catheters
Zürcher et al. conducted a meta-analysis of five published reports from randomized controlled trials to test whether the number of catheter lumens influenced catheter colonization and CRBSI. The authors observed a statistically significant difference in the CRBSI rate between single and multiple-lumen catheters. The multiple-lumen catheters were associated with an 8.4% rate of CRBSI, while single-lumen catheter rates were 3.1%.50 The report is limited because the number of infections per 1000 catheter-days is not reported. Lorente et al. conducted a prospective study of all patients admitted to a 24-bed ICU in Spain. They observed an overall CRBSI rate of 2.79/1000 catheter-days. Data were analyzed by anatomic site. Femoral, jugular, and subclavian sites were analyzed, with the CRBSI risk decreasing in that order.51 Maki et al. observed a range of CRBSI for short-term, noncuffed central venous catheters from 1.2 to 4.8/1000 catheter-days.4
Arterial Catheters
Arterial catheterization for hemodynamic monitoring is a common procedure in the ICU. The anatomic sites used for arterial access include the radial artery (the site most commonly used), brachial, dorsalis pedis, axillary, and femoral arteries. Lorente et al. prospectively observed 2018 ICU patients over 3 years to analyze the incidence of CRBSI according to different access sites. The overall incidence of CRBSI for arterial catheters in their study was 0.59/1000 catheter-days. They observed no infections in the brachial and dorsalis pedis sites, although these sites combined accounted for less than 10% of the total number of catheters included in the study. The incidence of CRBSI was 0.25/1000 catheter-days for the radial site and 1.92/1000 catheter-days for the femoral site. They concluded that using the femoral site increases the risk of arterial catheter-related infection.3 The incidence rates for arterial CRBSI noted by Lorente was lower than the pooled mean of 1.7/1000 catheter-days published in the meta-analysis by Maki et al.4 Of note, the study by Lorente was published in 2006, the same year as the review by Maki, and was not included in Maki’s report.
Long-Term Central Venous Catheters
Although seldom used in the acute critical care setting, catheters for long-term central venous access in both the inpatient and outpatient setting are frequently employed for total parenteral nutrition and chemotherapy. In cancer patients, the catheters most frequently used have been long-dwelling tunneled devices (Hickman, Broviac, Groshong).52 These catheters allow for long-term IV therapy without the need for frequent catheter exchanges. Darouiche et al. conducted a randomized controlled trial comparing antimicrobial-impregnated, non-tunneled, long-term central venous catheters to nonimpregnated tunneled catheters in terms of rates of catheter colonization and CRBSI. Their study included 312 catheters. They observed no significant difference in CRBSI rates between the two types of catheters.53 The tunneled catheters were associated with 1.43/1000 catheter-days, whereas the impregnated catheters had a rate of 0.36/1000 catheter-days, P = 0.13.53 In the meta-analysis by Maki et al. the CRBSI rate for long-term cuffed and tunneled central venous and hemodialysis catheters was 1.6/1000 catheter-days. The rate for subcutaneous ports was 0.1/1000 catheter-days.4
The rates of colonization per 1000 catheter-days observed in Darouiche’s study were 7.9 for antimicrobial-impregnated catheters and 6.3 for tunneled catheters. These rates were not significantly different, P=0.46.53
Peripherally Inserted Central Venous Catheters
Peripherally inserted central venous catheters (PICCs) have become a standard approach to securing long-term IV access for patients in both the inpatient and outpatient settings. PICCs are regarded as durable and associated with easier insertion and removal compared to long-term central venous catheters. Despite the ease of placement and removal, PICCs are no less vulnerable to CRBSI than other forms of vascular access. Safdar and Maki prospectively studied patients from two randomized trials assessing the efficacy of chlorhexidine-impregnated sponge dressings and chlorhexidine for cutaneous antisepsis. In total, 115 patients had 251 PICCs placed for a mean duration of catheterization of 11.3 days. A CRBSI rate of 3.5/1000 catheter-days was observed in this cohort. It is important to note that the CRBSI rate was calculated from the pooled control groups of both trials.54 A lower rate of CRBSI was observed by Walshe et al., who prospectively followed 351 patients with PICC lines over a 1-year period. A CRBSI rate of 2.46/1000 catheter-days including 19 primary and 7 secondary bloodstream infections were found in this cohort.55
Table 128-1 from Maki et al.4 lists rates of intravascular device–related bloodstream infection caused by various types of devices used for vascular access.
 Adjuncts To Prevent CRBSI
Adjuncts To Prevent CRBSI
Antiseptic-Impregnated and Antibiotic-Impregnated Catheters
Central venous catheters impregnated with various antiseptic and antibiotic agents are now commonly used to reduce the frequency of CRBSI. There are conflicting studies in the literature concerning whether or not such catheters are cost-effective.56–59 Among the most commonly used antiseptic impregnated catheters is one in which both the inner and outer lumens are bonded with silver sulfadiazine and chlorhexidine antiseptics. Both silver sulfadiazine and chlorhexidine possess broad-spectrum antimicrobial properties, and the two agents exhibit a synergistic activity, reducing the risk of the emergence of resistant strains of bacteria.56,60 Reports of hypersensitivity to chlorhexidine have emerged as its use has become more commonplace.61
In the late 1990s, polyurethane CVCs impregnated with minocycline and rifampin on both the internal and external surfaces were developed.62 Initial concerns that widespread use of surface antibiotics for preventing CRBSI may contribute to the emergence of antibiotic-resistant organisms have not been identified.63,64
 Recommendations
Recommendations
The following recommendations are based on the studies reviewed in this chapter and published CDC guidelines.24,25 These guidelines are currently in revision, and the reader is encouraged to refer to the CDC website for any updates.
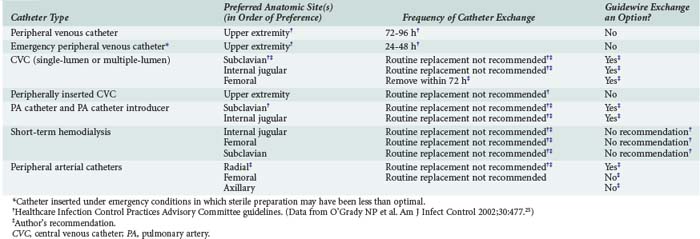
Recommendations for short-term catheter placement are outlined in Table 128-2. Any catheter (peripheral or central) that is placed under less than ideal conditions should be treated as a potential source of infection. Generally, such a catheter should be removed and a new catheter inserted at a different site if catheterization is needed for longer than 48 hours. Ideal conditions for catheter insertion include:
The indication for removal of a non-tunneled central venous catheter is the presence of an unexplained bacteremia. In the critical care setting, fever is an unreliable indicator of CRBSI. The authors think that guidewire exchange using the strict protocol described in this chapter is an acceptable alternative to placing a catheter at a different site, particularly in patients with difficult or compromised venous access. The most recent CDC guidelines discourage this practice24,25 because 20% to 25% of catheters removed for suspected infection yield positive semiquantitative culture results. Despite these culture results, less than 10% of catheters removed are associated with CRBSI.
Key Points
Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783-805.
Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159-1171.
Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167-193.
Mermel LA, Farr BM, Sheretz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249-1272.
Safdar N, Maki DG. The pathogenesis of catheter-related bloodstream infection with non-cuffed short-term central venous catheter. Intensive Care Med. 2004;30:62-67.
This article provides a concise review of the four mechanisms of the pathogenesis of CRBSI.
Raad II, Hanna HA. Intravascular catheter-related infections: new horizons and recent advances. Arch Intern Med. 2002;162:871-878.
O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51:1-29.
1 Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37(10):783-805.
2 Rosenthal VD, Maki DG, Jamulitrat S, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010;38(2):95-104. e2
3 Lorente L, Santacreu R, Martin MM, Jimenez A, Mora ML. Arterial catheter-related infection of 2,949 catheters. Crit Care. 2006;10(3):R83. (doi:10.1186/cc4930)
4 Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159-1171.
5 Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg. 2001;136(2):229-234.
6 Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289.
7 Renaud B, Brun-Buisson C. Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am J Respir Crit Care Med. 2001;163(7):1584-1590.
8 Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ “never events”: an analysis and recommendations to hospitals. Health Care Manag. 2008;27:338-349.
9 Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevent microorganisms. Clin Microbiol Rev. 2002;15(2):167-193.
10 Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881-890.
11 Mermel LA, Farr BM, Sheretz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32(9):1249-1272.
12 Pearson ML. Guideline for prevention of intravascular device-related infections. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1996;17:438-473.
13 Malgrange VB, Escande MC, Theobold S. Validity of earlier positivity of central venous blood cultures in comparison with peripheral blood cultures for diagnosing catheter-related bacteremia in cancer patients. J Clin Microbiol. 2001;39(1):274-278.
14 Safdar N, Maki DG. Inflammation at the insertion site is not predictive of catheter-related bloodstream infection with short-term, noncuffed central venous catheters. Crit Care Med. 2002;30(12):2632-2635.
15 Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277-281.
16 Safdar N, Maki DG. The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheter. Intensive Care Med. 2004;30(1):62-67.
17 Anaissie E, Samonis G, Kontoyiannis D, et al. Role of catheter colonization and infrequent hematogenous seeding in catheter-related infections. Eur J Clin Microbiol Infect Dis. 1995;14(2):134-137.
18 Raad I, Hanna HA, Awad A, et al. Optimal frequency of changing intravenous administration sets: is it safe to prolong use beyond 72 hours? Infect Control Hosp Epidemiol. 2001;22(3):136-139.
19 Raad II, Hanna HA. Intravascular catheter-related infections: new horizons and recent advances. Arch Intern Med. 2002;162:871-878.
20 Mohamed JA, Huang DB. Biofilm formation by enterococci. J Med Microbiol. 2007;56:1581-1588.
21 Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in U.S. hospitals: an analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309-317.
22 Penel N, Neu JC, Clisant S, et al. Risk factors for early catheter-related infections in cancer patients. Cancer. 2007;110(7):586-592.
23 Eggiman P, Harbath S, Constantin M, et al. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868.
24 Center for Disease Control and Prevention. Guidelines for the prevention of intravascular catheter-related infection. MMWR. 2002;51(No. RR-10):1-26.
25 O’Grady NP, Alexander M. Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002;30(8):476-489.
26 Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732.
27 Gastmeier P, Geffers C, Brandt C, et al. Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infection. J Hosp Infect. 2006;64(1):16-22.
28 Chen H, Wang F, Lin M, et al. Risk factors for central venous catheter-related infections in general surgery. J Microbiol Immunol Infect. 2006;39(3):231-236.
29 Moran JM, Atwood RP, Rowe MI. A clinical and bacteriologic study of infections associated with venous cutdowns. N Engl J Med. 1965;272:554-560.
30 Mermel LA, McCormick RD, Springman SR, et al. The pathogenesis and epidemiology of catheter-related infection with pulmonary-artery Swan-Ganz catheters: a prospective study using molecular subtyping. Am J Med. 1991;91(Suppl 3B):197S-205S.
31 Merrer J, Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients. JAMA. 2001;286(6):700-707.
32 Norwood S, Wilkins H, Vallina VL, et al. The safety of prolonging the use of central venous catheters: a prospective analysis of the effects of using antiseptic-bonded catheters with daily site care. Crit Care Med. 2000;28(5):1376-1382.
33 Chen YY, Yen DH, Yang YG, et al. Comparison between replacement at 4 days and 7 days of the infection rate for pulmonary artery catheters in an intensive care unit. Crit Care Med. 2003;31(5):1353-1358.
34 Martinez JA, Desjardin JA, Aronoff M, et al. Clinical utility of blood cultures drawn from central venous or arterial catheters in critically ill surgical patients. Crit Care Med. 2002;30(1):7-13.
35 Desjardin JA, Falagas ME, Ruthazer R, et al. Clinical utility of blood cultures drawn from indwelling central venous catheters in hospitalized patients with cancer. Ann Intern Med. 1999;131(9):641-647.
36 Safdar N, Fine JP, Maki DG. Meta-analysis: Methods for diagnosing intravascular device-related bloodstream infection. Ann Intern Med. 2005;142(6):451-466.
37 Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296(23):1305-1309.
38 Fan ST, Teoh-Chan CH, Lau KF. Evaluation of central venous catheter sepsis by differential quantitative blood culture. Eur J Clin Microbiol Infect Dis. 1989;8(2):142-144.
39 Raad I, Hanna HA, Alakech B, et al. Differential time to positivity: A useful method for diagnosing catheter-related bloodstream infection. Ann Intern Med. 2004;140(1):18-25.
40 Parienti JJ, Thirion M, Megarane B, et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA. 2008;299(20):2413-2422.
41 Mimoz O, Villeminey S, Ragot S, et al. Chlorhexidine-based antiseptic solution vs alcohol-based povidone-iodine for central venous catheter care. Arch Intern Med. 2007;167:2066-2072.
42 Ho KM, Litton E. Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemother. 2006;58:281-287.
43 Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults. A randomized controlled trial. JAMA. 2009;301(12):1231-1241.
44 Safdar N, Maki DG. Use of vancomycin-containing lock or flush solutions for prevention of bloodstream infection associated with central venous access devices: a meta-analysis of prospective, randomized trails. Clin Infect Dis. 2006;43:474-484.
45 Bleyer AJ. Use of antimicrobial catheter lock solutions to prevent catheter-related bacteremia. Clin J Am Soc Nephrol. 2007;2:1073-1078.
46 Cook D, Randolph A, Kernerman P, et al. Central venous catheter replacement strategies: a systematic review of the literature. Crit Care Med. 1997;25:1417-1424.
47 Pronovost PJ, Groeschel CA, Colantuoni E, et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010;340:c309. doi: 10.1136/bmj.c309
48 Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37:2167-2173.
49 Collignon PJ, Dreimanis DE, Beckingham WD, Roberts JL, Gardner A. Intravascular catheter bloodstream infections: an effective and sustained hospital-wide prevention program over 8 years. Med J Aust. 2007;187:551-554.
50 Zurcher M, Tramer MR, Walder B. Colonization and bloodstream infection with single- versus multi-lumen central venous catheters: a quantitative systematic review. Anesth Analg. 2004;99:177-182.
51 Lorente L, Henry C, Martin MM, Jimenez A, Mora ML. Central venous catheter-related infection in a prospective and observational study of 2,595 catheters. Crit Care. 2005;9:R631-R635. (doi: 10.1186/cc3824)
52 Farr BM. Vascular catheter related infections in cancer patients. Surg Oncol Clin N Am. 1995;4:493-503.
53 Darouiche RO, Berger DH, Khardori N, et al. Comparison of antimicrobial impregnation with tunneling of long-term central venous catheters: a randomized controlled trial. Ann Surg. 2005;242:193-200.
54 Safdar N, Maki DG. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128:489-495.
55 Walshe LJ, Sharp FM, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol. 2002;20:3276-3281.
56 Veenstra DL, Saint S, Sullivan SD. Cost-effectiveness of antiseptic impregnated central venous catheters for the prevention of catheter-related blood stream infection. JAMA. 1999;282:554-560.
57 Ciresi DL, Albrecht RM, Volkers PA, et al. Failure of antiseptic bonding to prevent central venous catheter-related infection and sepsis. Am Surg. 1996;62:641-646.
58 Pemberton LB, Ross V, Cuddy P, et al. No difference in catheter sepsis between standard and antiseptic central venous catheters. A prospective randomized trial. Arch Surg. 1996;131:986-989.
59 Heard SO, Wagle M, Vijayakumar E, et al. Influence of triple-lumen central venous catheters coated with chlorhexidine and silver sulfadiazine on the incidence of catheter-related bacteremia. Arch Intern Med. 1998;158:81-87.
60 Greenfield JI, Sampath L, Popilskis SJ, et al. Decreased bacterial adherence and biofilm formation on chlorhexidine and silver sulfadiazine-impregnated central venous catheters implanted in swine. Crit Care Med. 1995;23:894-900.
61 Pham NH, Weiner JM, Reisner GS, Baldo BA. Anaphylaxis to chlorhexidine. Case report. Implication of immunoglobulin E antibodies and identification of an allergenic determinant. Clin Exp Allergy. 2000;30:1001-1007.
62 Raad I, Darouiche R, Dupris J, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med. 1997;127:267-274.
63 Chatzinikolaou I, Finkel K, Hanna H, et al. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med. 2003;115:352-357.
64 Hanna H, Benjamin R, Chatzinikolaou I, et al. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients; a prospective randomized clinical trial. J Clin Oncol. 2004;22:3163-3171.