Chapter 13 Vascular Anomalies of the Brain
INTRODUCTION TO ANGIOGRAPHIC MODALITIES
Catheter Angiography
Cerebral angiography was one of the first diagnostic modalities for brain diseases, and it has remained crucially important and routinely used since its description by Egaz Moniz in 1927.1 However, there has been significant evolution from the direct carotid cut downs and cut film technique of the 1920s to digital subtraction angiography with three-dimensional (3D) reconstruction.
With the transition to non-ionic contrast, smaller 4- and 5-French, and more flexible catheters and guidewires with hydrophilic coating, the risk for permanent neurologic injury was reduced from 1% to 2.4%2,3 to less than 0.1% to 0.3%.4–6 However, given risk of local pain, bruising, and hematoma in up to 25% of cases, noninvasive alternatives should be considered whenever they will provide similar diagnostic information.
Cerebral angiography remains the gold standard in the diagnosis of dural arteriovenous fistulas (dAVFs), as well as small aneurysms and arteriovenous malformations (AVMs). In addition, super-selective angiography can provide functional and physiological data important for clinical decision analysis, although pressure measurements are not commonly performed. It is still strongly recommended that a magnetic resonance imaging (MRI) study and a four-vessel angiogram be obtained to delineate the anatomy of an AVM.7 Computed tomography (CT) angiography can outline enlarged abnormal cortical veins but may miss subtle dural venous drainage or small arteriovenous malformations.8 MRI may show important indirect signs such as edema and abnormal cortical enhancement. In our practice, all patients with unexplained subarachnoid, subdural, or atypical parenchymal hemorrhage undergo digital subtraction angiography (DSA).
Three-Dimensional Digital Subtraction Angiography
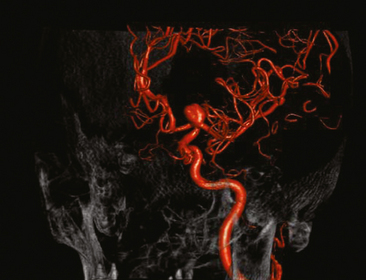
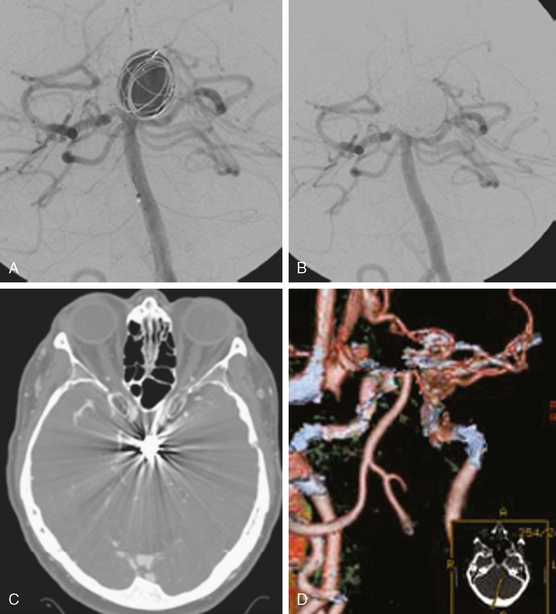
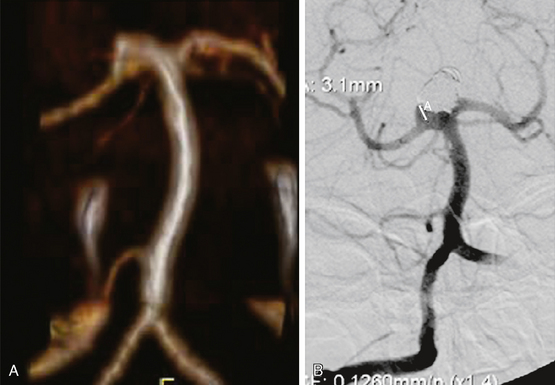
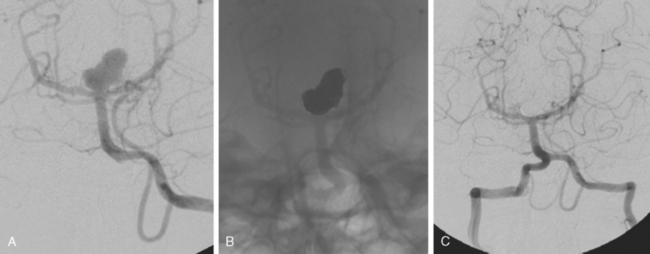
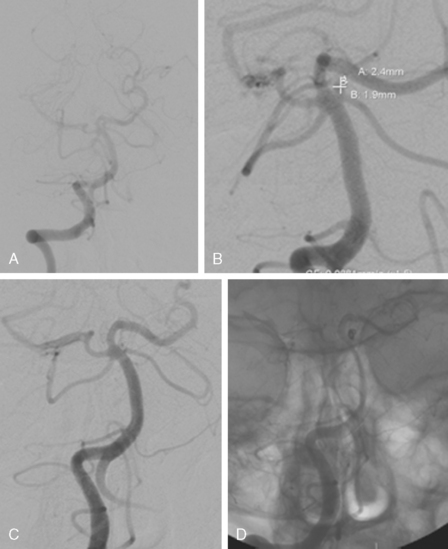
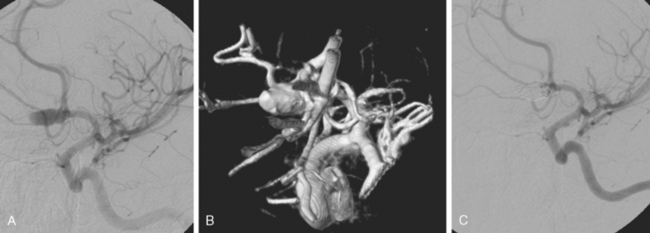
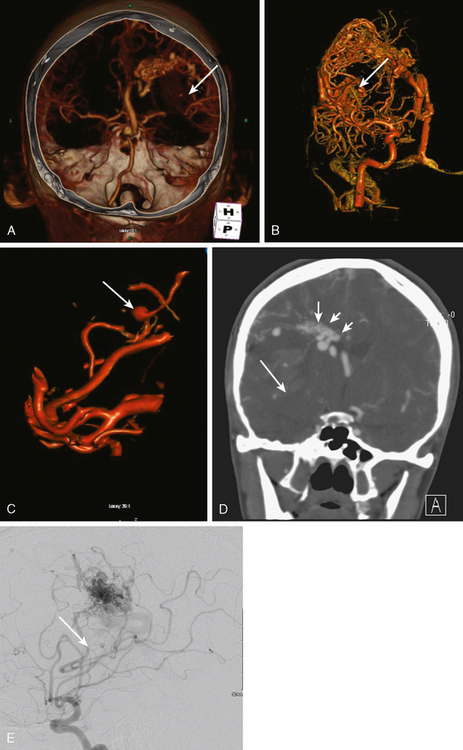
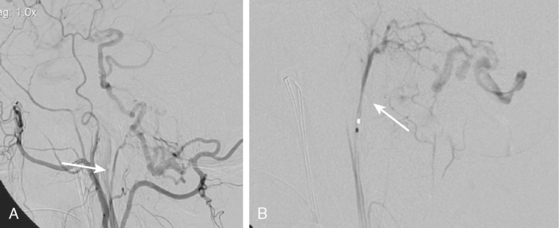
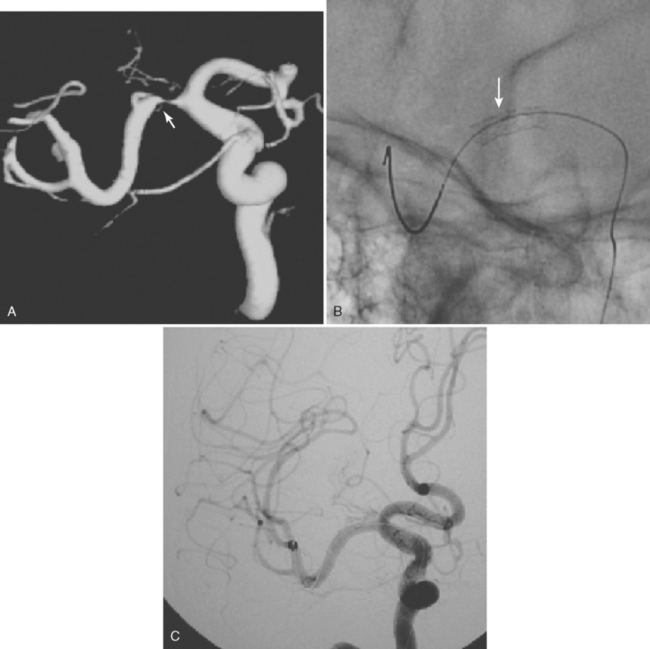
Excellent spatial resolution and lack of artifact has made 3D DSA an attractive tool for treatment planning and follow-up (Figure 13-1). It further increases the sensitivity for detection of aneurysms smaller than 3 mm9 and provides invaluable conformational information for endovascular treatment planning (Figure 13-2).
Computed Tomography Angiography
Multidetector CT angiography (MDCTA) has higher spatial resolution and decreased, but not eliminated, propensity to motion artifacts (Figure 13-33) than MRA.10,11 For these reasons, MDCTA has largely replaced MR angiography in our practice.
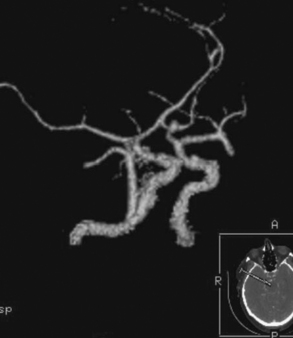
Figure 13-33 Computed tomography (CT) angiography with motion artifact. The CT angiogram suggested moderate perianeurysmal vasospasm, which was not confirmed by digital substraction angiography (see Figure 13-34 A and B,).
The sensitivity for detection of even small aneurysms has substantially increased. One study found MDCTA to be actually more sensitive than DSA for the detection of small aneurysms.12 Yet DSA remains the gold standard for aneurysm detection. Colen et al.13 found that during screening of patients with nontraumatic subarachnoid hemorrhage, MDCTA missed the primary aneurysm in 11 of 211 cases. Regions with overprojecting bone (such as the cavernous segment or supraclinoid carotid artery) may not be optimally visualized,14,15 and MDCTA has lower sensitivity in detecting branches incorporated into the aneurysmal sac.16
The bony structures of the sella are often manually excluded on the reconstruction and thus an aneurysm can easily be “removed” in the cavernous and supraclinoid region (as seen in Figure 13-31, F). Careful analysis of the source images is crucial to preserve high sensitivity. Inadequate bolus timing with poor arterial and dense venous filling can render interpretation of CT angiography much more challenging. Still, CT angiography can be superior to DSA for aneurysms with competing flow, such as some basilar tip, basilar trunk, or anterior communicating aneurysms because it avoids the problem of contrast “washout” (see Figure 13-18).
CT angiography is valuable for follow-up of aneurysms treated with titanium alloy clips, but it is not useful when cobalt alloy clips have been used.17 Image quality is also degraded by orientation of the clip perpendicular to the imaging axis or presence of multiple clips. Streak artifacts caused by platinum coils make it an unsuitable method to assess for recanalization of embolized aneurysms. (See Figures 13-3 and 13-4.)
In our institution, patients with subarachnoid or atypical intracranial hemorrhage are generally screened with CT angiography. An aneurysmal source of bleeding can often be identified in the emergency room, allowing triage to endovascular versus surgical treatment.
If the CT angiography is nondiagnostic, DSA is always performed to evaluate for a missed aneurysm or nonaneurysmal sources, such as a dural arteriovenous (AV) fistula. See Figure 13-5.
However, protocols relying directly on DSA are also very acceptable and may actually reduce cost.
Magnetic Resonance Angiography
TOF techniques exploit the contrast between the high signal intensity of inflowing, fully magnetized blood and the low signal intensity of saturated stationary tissue. Multiple thin imaging slices (two-dimensional [2D]) or larger slabs (3D) perpendicular to the axis of flow are acquired with a flow-compensated gradient-echo sequence. Three-dimensional TOF angiography allows greater spatial resolution and is more resistant to signal loss from disturbed flow than 2D TOF. However, PC MRA and TOF MRA are sensitive to artifacts caused by turbulent or slow flow (Figure 13-6, B). TOF is also affected by in-plane artifacts.
Contrast-enhanced (TOF) MR angiography has been employed to overcome these problems. It is performed by intravenously injecting a contrast agent to shorten selectively the T1 of the blood to produce the signal, rather than relying on flow. By implementing a T1-weighted imaging sequence during the first pass of the contrast agent, images can be produced showing arteries in striking contrast relative to surrounding stationary tissues and veins. However, synchronizing the acquisition with the arrival of the contrast agent is critical to ensure image quality. For a more detailed discussion on these MR angiography techniques, the reader is referred to the review authored by Aygun.18
Whereas MRA for follow-up of clipped aneurysms is limited by severe “blooming” artifact, it is usually adequate to monitor coiled aneurysms because it has a sensitivity of 75% for detecting a residual neck.19 See Figure 13-7. However, problems related to signal drop-out may occur (Figure 13-8).
No clear information has emerged on whether contrast-enhanced MR angiography is superior to conventional 3D TOF.19,20 Where available, contrast-enhanced 3-Tesla MRA may a superior diagnostic option.21
DSA remains the gold standard for the detection of small AV malformations and dural AV fistulas. Although specialized sequences such as 3T four-dimensional MRA22 and others23 show good correlation for size of feeders and AVM classification, DSA is still more reliable in the diagnosis of small AVMs, which can be missed even by repeated MRA studies.24 Contrast-enhanced MRA can be used to follow dural AV fistulas, but it is less sensitive than DSA (Figure 13-9).25
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
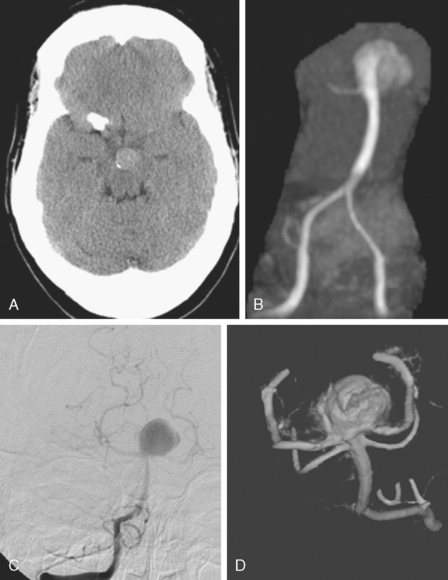
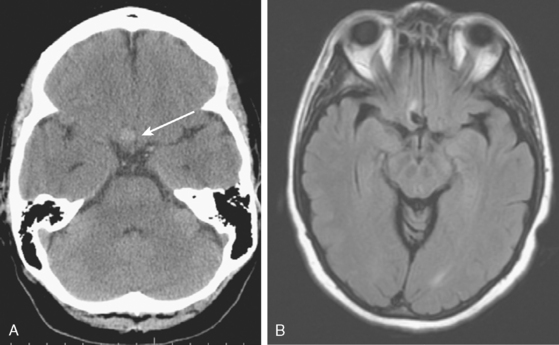
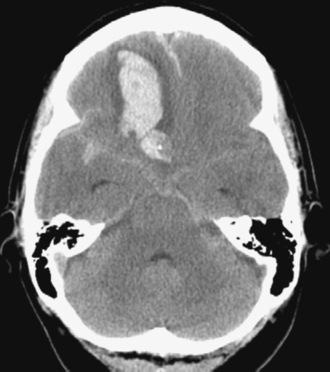
CT without contrast is poorly sensitive for brain aneurysms. However, unruptured aneurysms may present with rim calcification or slight hyperdensity due to the blood contained in the aneurysm. Only relatively large (≥10mm) aneurysms can be seen on plain CT scan, however (Figure 13-10).
CT without contrast is the standard screening test when subarachnoid hemorrhage is suspected (see Chapter 12), but almost all aneurysm locations in the circle of Willis can produce diffuse and symmetric subarachnoid hemorrhage and a dedicated vascular study is necessary to define the source of bleeding. Sometimes an aneurysm can be seen as a hypodense region within dense subarachnoid hemorrhage, a finding often referred to as a “ghost sign.” The distribution of the blood can be helpful to predict the location of the ruptured aneurysm, especially when multiple aneurysms are present at the time of hemorrhage. The location of parenchymal hemorrhage is typically adjacent to the point of rupture (Figure 13-11).
Unenhanced CT of the brain has poor sensitivity for vascular malformations but sometimes outlines enlarged draining veins of AVMs (Figure 13-12, A). Administration of contrast significantly increases the sensitivity of CT scans for the diagnosis of vascular abnormalities.
MRI
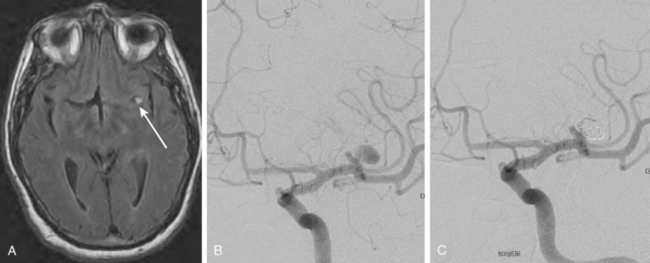
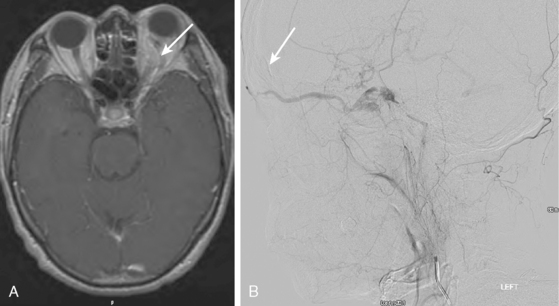
MRI of the brain, although more sensitive than CT, is not adequate to rule out the presence of an aneurysm. However, given the frequent use of this technique as screening test for many neurologic conditions, most of the incidental aneurysms treated in our institution are identified by this technique. Indirect markers are additional flow voids adjacent to vessels or, less commonly, perianeurysmal gliosis (see Figure 13-39 B,).
Rarely, slow flow within the aneurysm can cause high signal of fluid-attenuated inversion recovery (FLAIR)/T2, which can be confused with focal thrombus or hemorrhage (Figure 13-13).
MRI is indispensable in the diagnosis and management planning of AVMs and is part of the routine diagnostic workup preceding the treatment of these lesions (see Figures 13-12 B–C, and 13-43 A–C,).
ANEURYSMS
General Concepts
Types of Aneurysms
Nonsaccular Aneurysms
Fusiform Aneurysms
Transitional Forms
Dolichoectasia
Saccular Aneurysms
TREATMENT
In the past, surgical obliteration (“clipping”) was the main method of treatment of ruptured aneurysms. Since the introduction of detachable coils,50 there has been a progressive increase in the use of coil embolization for the treatment of both ruptured and unruptured aneurysms; nonetheless, the controversy about the optimal management of intracranial aneurysms is far from settled.
Despite various criticisms, the largest trial comparing clipping versus coiling of ruptured aneurysms, the International Subarachnoid Aneurysm Trial (ISAT), has provided Class I evidence indicating that endovascular therapy (EVT) results in superior 1-year outcomes compared with surgical repair of ruptured aneurysms equally amenable to both types of treatment (Figure 13-30).51
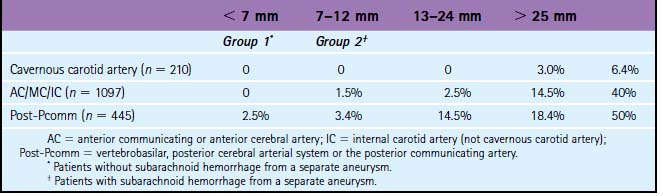
The risk of subarachnoid hemorrhage from unruptured intracranial aneurysms (UIA) as well as the risk of their treatment remains poorly defined. In the ISUIA, the largest study on the natural history of UIA, the cumulative 5-year rupture rates for untreated aneurysms in patients without previous aneurysmal SAH ranged from 0% to 40% in the anterior circulation, and 2.5% to 50% in the posterior circulation depending on aneurysm size26 (Table 13-1).
TABLE 13-1 Five-year cumulative rupture rates according to size and location of unruptured aneurysm.26
No randomized trial has compared elective aneurysm clipping versus coiling. A review of more than 2500 patients with UIA showed adverse outcomes were significantly more common in surgical cases (18.5%) compared with endovascular cases (10.6%) with an in-hospital mortality of 2.3% versus 0.4%.52
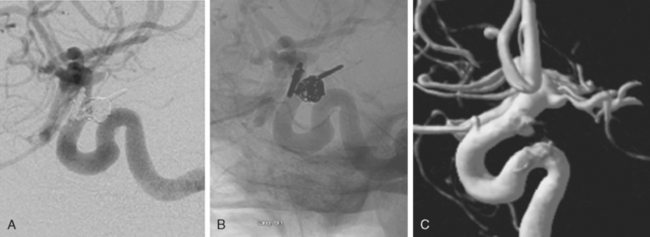
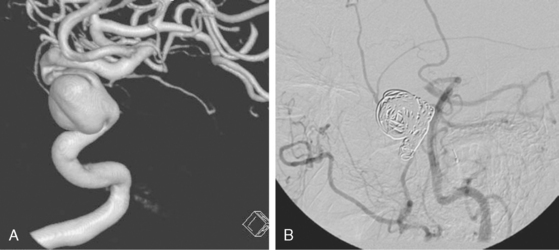
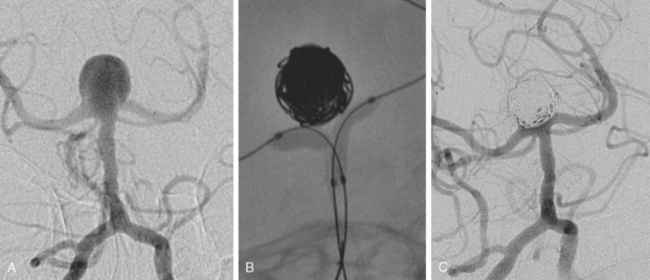
Incomplete occlusion of aneurysms is more common with endovascular therapy (Figure 13-31). Reported rates of initial complete occlusion of ruptured aneurysms treated with EVT are relatively low (40%–68%), and up to 34% require two or more procedures to achieve complete occlusion.46,53,54 However, progressive thrombosis or healing may contribute to occlude the aneurysm over time in approximately 20% of patients with initial remnants.55
Another major criticism of EVT is the possibility of recanalization (ranging from 15% to 30%), most frequently seen in wide-necked and large aneurysms (>10 mm).56,57 In one study, recanalization was not seen in aneurysms with “packing densities” greater than 24% and volumes less than 200 mm3. Aneurysms with volumes greater than 600 mm3 (equivalent to 10.4 mm spherical diameter) could not be densely packed and recanalized in nearly 90% of cases.58 “Bioactive coils” have not yet been shown to reduce the rate of recanalization and may even be detrimental.59,60 A recent study using a liquid embolic agent (Onyx) has shown promising results with no recanalization in aneurysms less than 10 mm and a low rate of recanalization (4%) for stent-assisted embolization of large and giant aneurysms.45
Aneurysm recanalization has been reported to be associated with a significant risk of hemorrhage (7.9% over 5 years).61
The risk of elective retreatment of recanalized aneurysms appears to be much lower than during the acute phase after SAH. Near-complete or complete occlusion can be achieved in more than three quarters of cases, with very low risk of procedural complications.62
Late rebleeding (> 1 month after embolization) occurred at a rate of 0.32% per year in a series of 393 ruptured aneurysms treated between 1995 and 2003 over a mean follow-up of 48 months.63 Rebleeding has been seen as late as 2010 days after treatment.64 If recanalization is detected and treated, the risk of late rerupture is low (0.11%/year).65,66 Follow-up imaging is thus required after aneurysm coiling. MRA may be equivalent to conventional angiography for this purpose.67
After aneurysm remnants are stable for more than 12 months, they are unlikely to rebleed.55,61,68
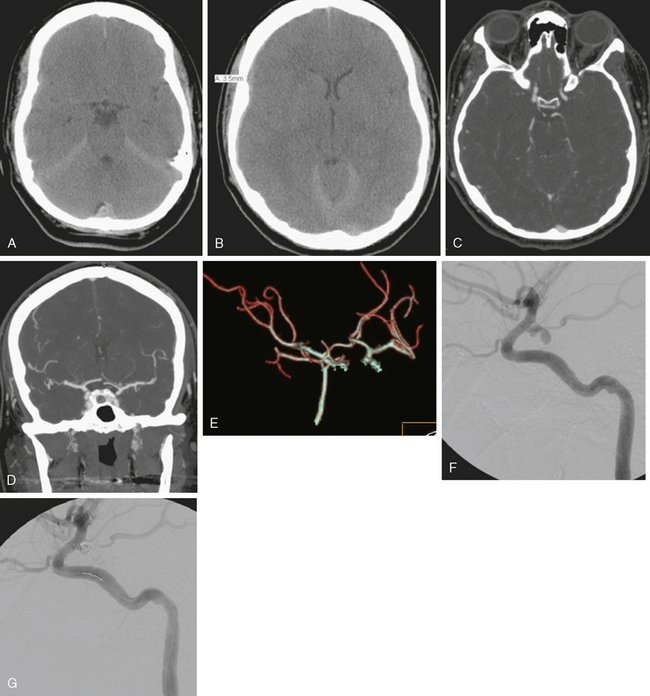
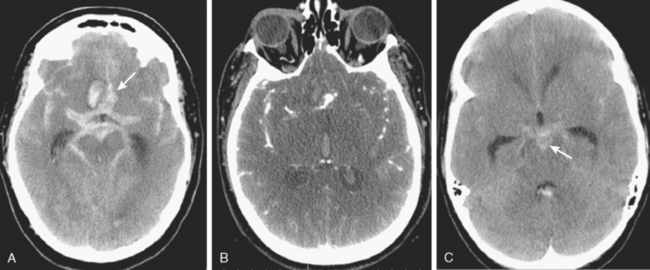
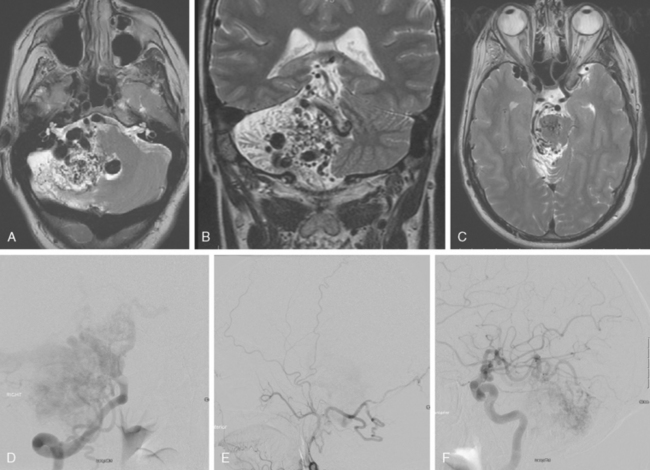
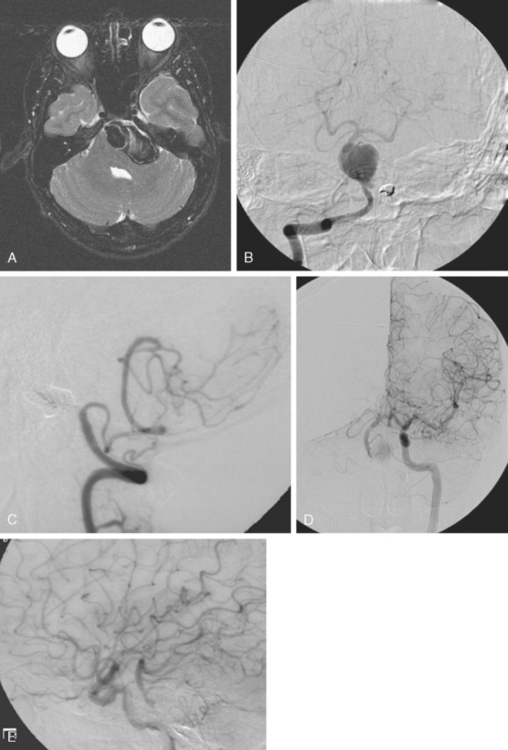
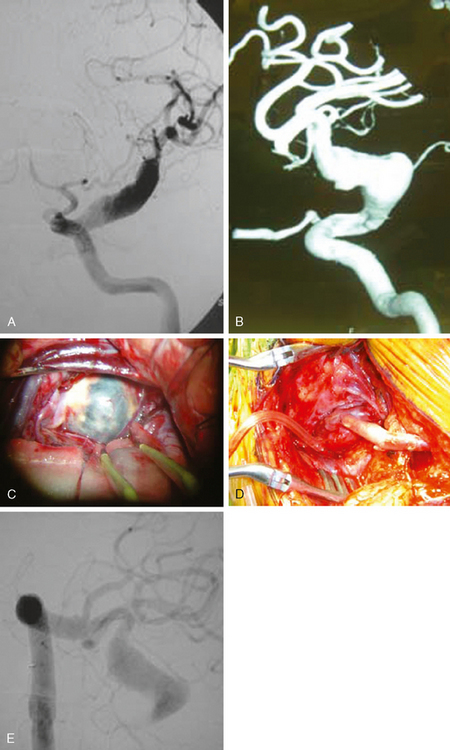
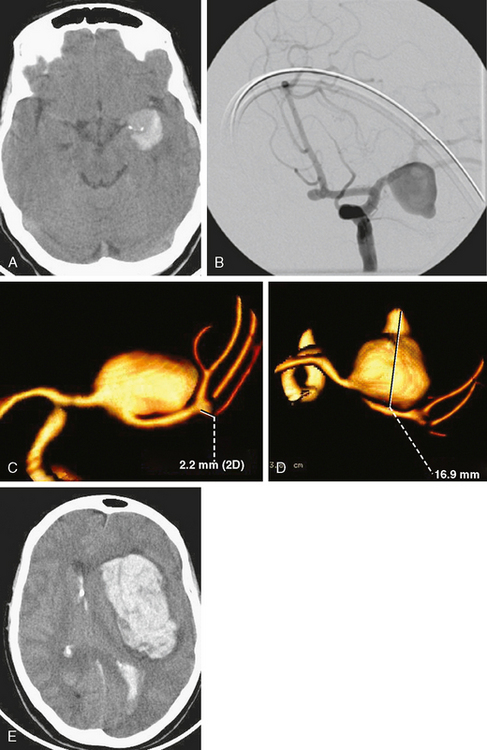
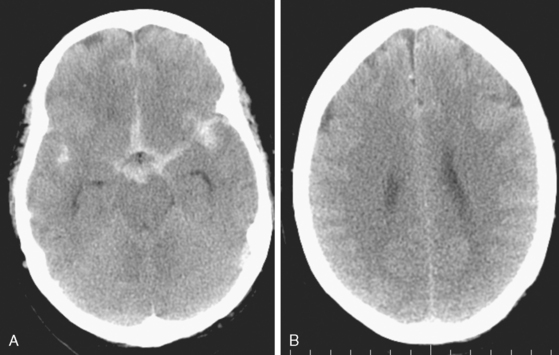
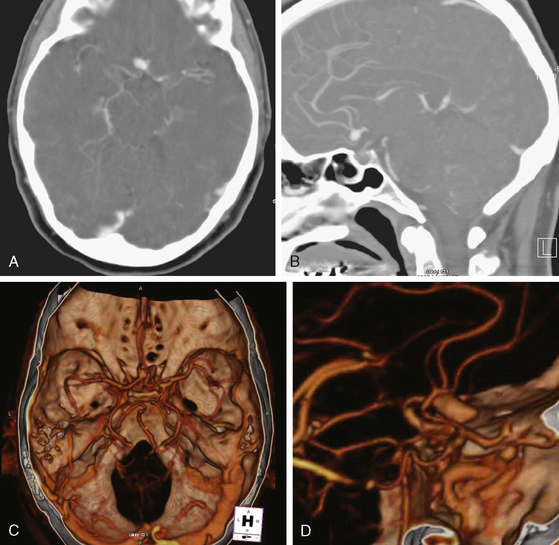
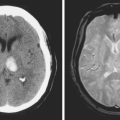
A 62-year-old woman with history of smoking, hypertension, and fibromyalgia presented with acute syncope and severe headache. In the emergency department, the patient was awake but confused (Hess and Hunt Grade III). CT scan of the brain showed an extensive SAH in the basal cisterns (Fisher Grade 3) with predominant extension into the left sylvian fissure. The upper portions of the interhemispheric fissure were largely spared (Figure 13-32 A–B,).
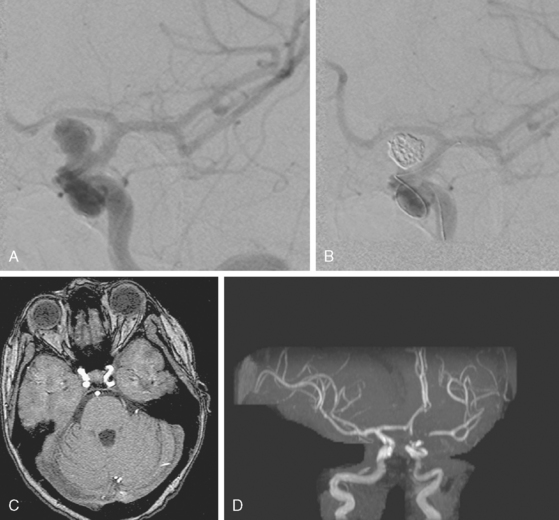
CT angiography (Figure 13-33) showed a 6-mm anterior communicating artery aneurysm. The visualization was limited by irregularities due to motion artifact on the reconstructed 3D images.
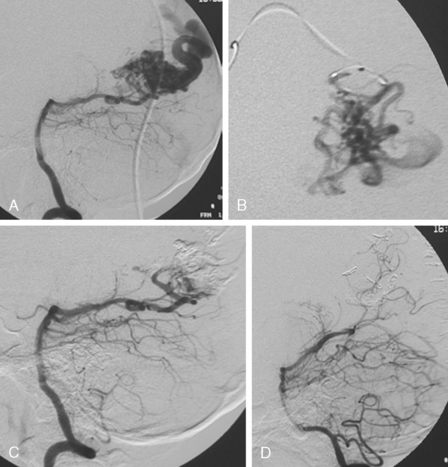
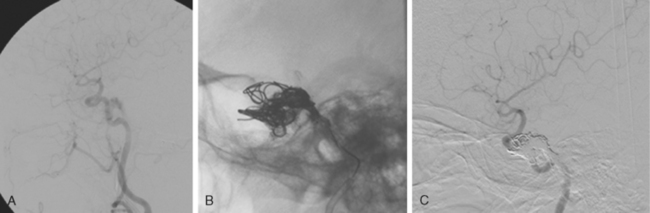
The patient underwent diagnostic catheter angiography with 3D DSA, and the broad-necked anterior communicating artery aneurysm was deemed amenable to coil embolization (Figure 13-34). An incidental 3-mm pericallosal aneurysm was seen, but given the lack of blood in the upper interhemispheric fissure, it was not considered to be the source of bleeding. This aneurysm had not been reported on the CT angiogram, but in retrospect, it was also visible on the reconstruction and, particularly, the source images of this study.
The patient underwent embolization of the anterior communicating artery aneurysm without complications (Figure 13-34 D,), followed in the same session by embolization of the right pericallosal aneurysm, which was primarily filled from the contralateral right carotid artery (Figure 13-35).
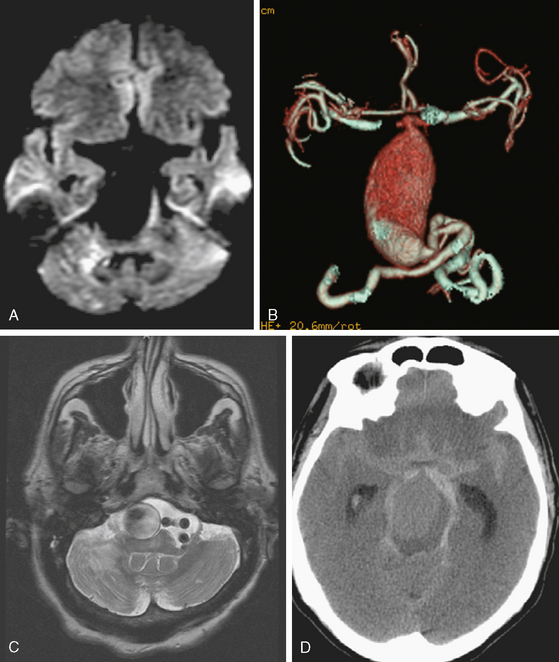
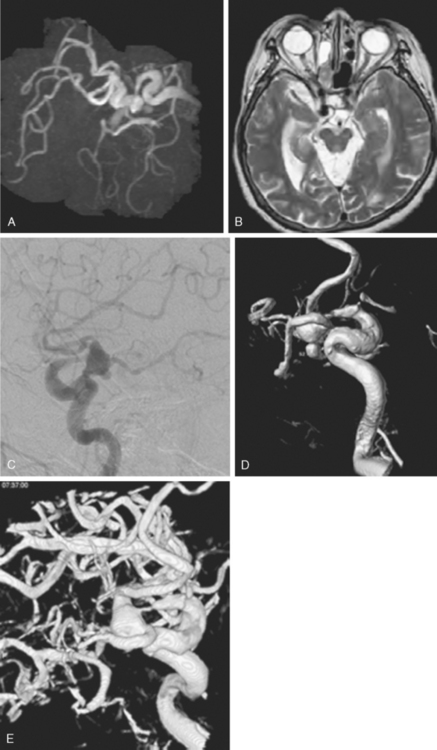
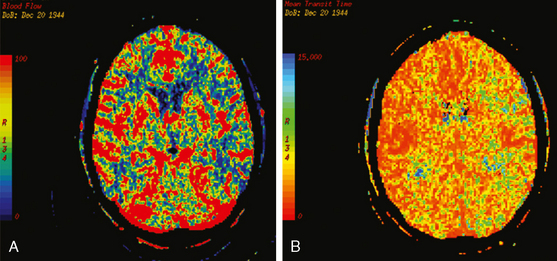
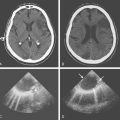
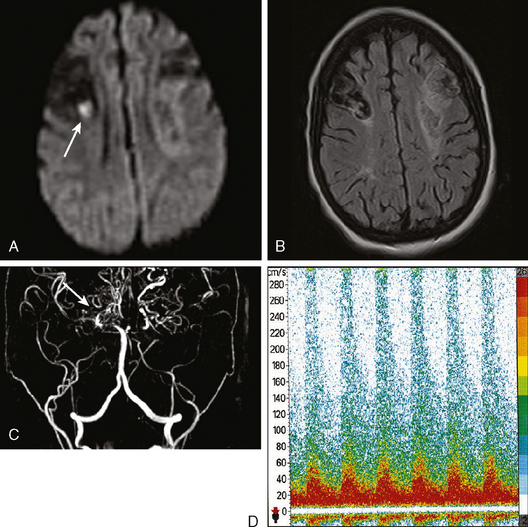
On the fourth day after SAH, transcranial Doppler (TCD) velocities in the left MCA were 62cm/sec with an ICA to MCA ratio of 2.3. On post-bleeding day 5 the patient became increasingly agitated, dysphasic and developed right hemiparesis. She was started on aggressive hemodynamic augmentation therapy. CT perfusion only showed subtle reduction and delay of cerebral blood flow in the left MCA territory (Figure 13-36).
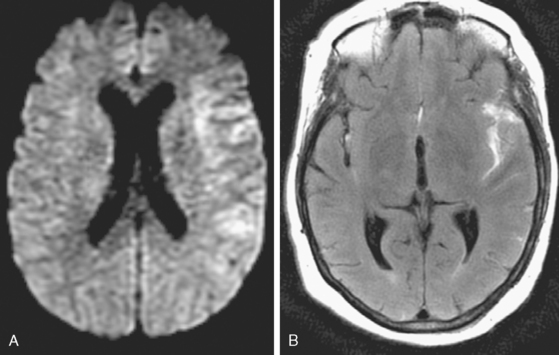
Because of her acute neurological deficits, a diagnostic catheter angiography was performed showing moderately severe distal left MCA vasospasm. The patient was treated with intra-arterial nicardipine. She improved but continued to require very high doses of vasopressors. On Day 6, the patient became abruptly hemiplegic and globally aphasic. MRI showed only spotty areas of restricted diffusion and normal FLAIR signal, suggestive of salvageable left MCA territory (Figure 13-37).
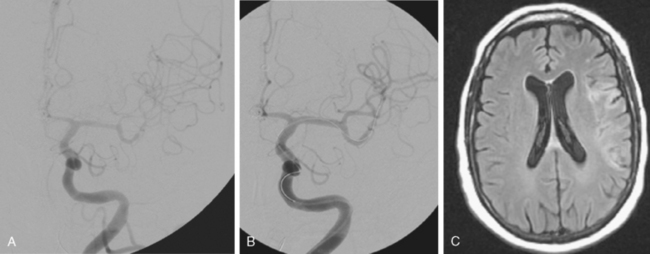
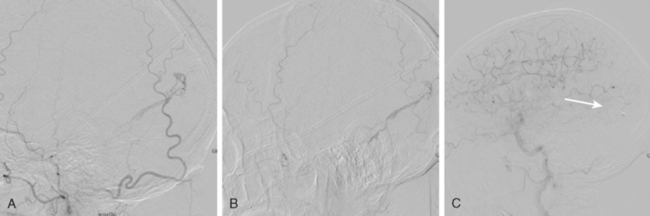
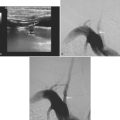
The patient underwent emergent angiography, which revealed global vasospasm, most severe in the left A1 and M1 segments (Figure 13-38A,). Bilateral infusion of nicardipine and angioplasty of the left M1, M2, and A1 segments was performed (Figure 13-38 B,).
After the procedure, the patient recovered speech reception and arm function. On follow-up 3 months later, she only had mild hesitancy and paraphasic errors of speech. MRI displayed focal laminar necrosis and gliosis in the left MCA territory (Figure 13-38 C,
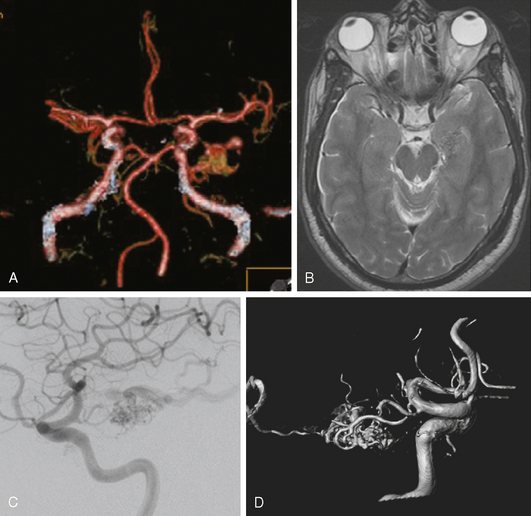
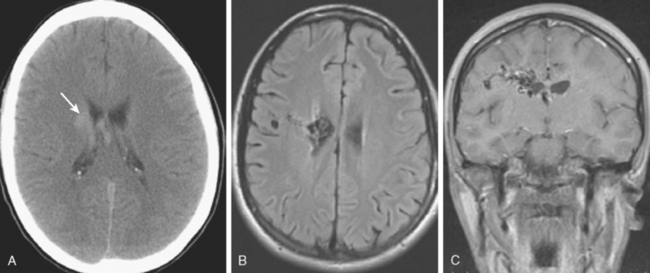
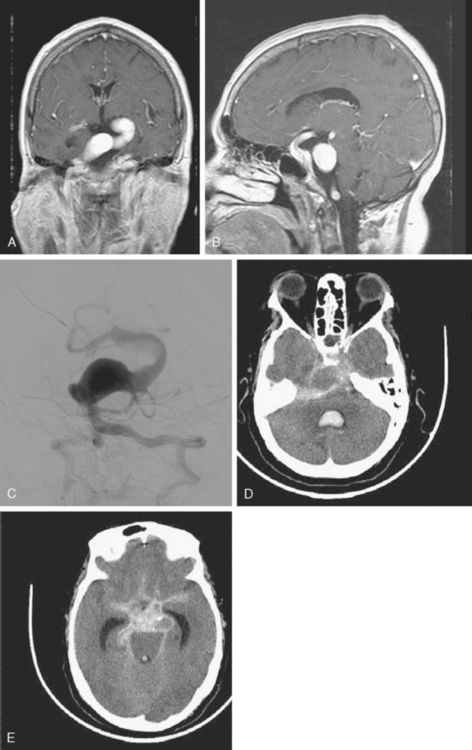
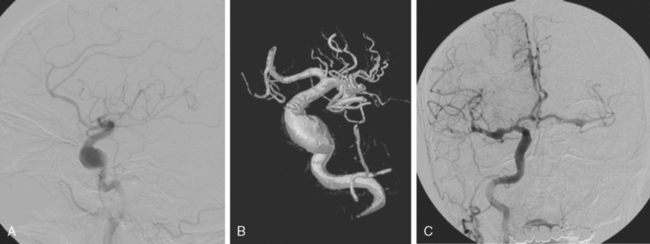
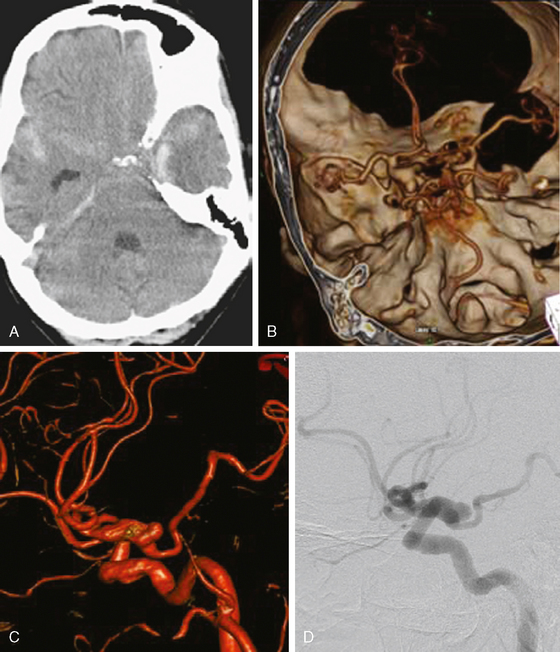
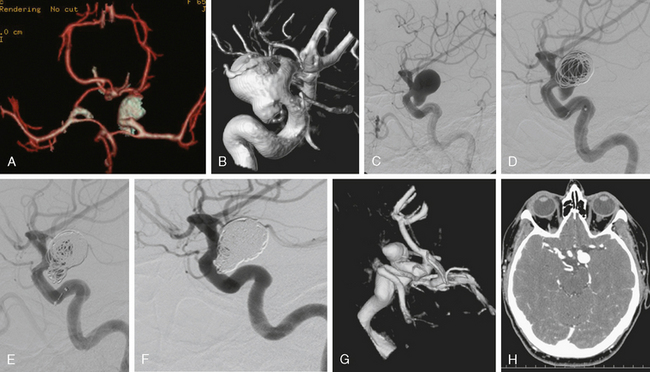
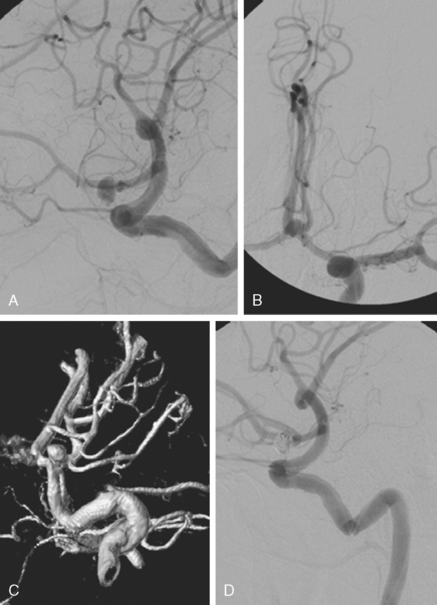
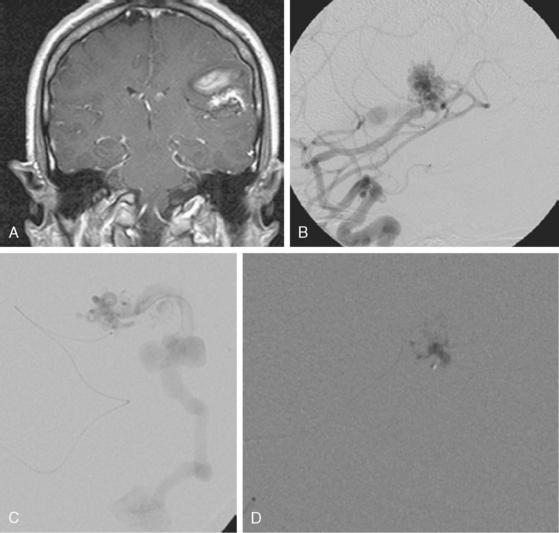
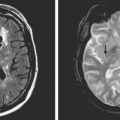
A 42-year-old woman with history of smoking underwent CT of the brain after an assault and was incidentally found to have a small anterior communicating aneurysm (Figure 13-39 A,). On MRI, the aneurysm projected into the right frontal lobe and was surrounded by a small area of gliosis (Figure 13-39 B,).
Four months later, the patient underwent CT angiography, which confirmed the presence of an aneurysm originating from the left anterior cerebral artery and measuring 11 by 5 mm (Figure 13-40 A–D,).
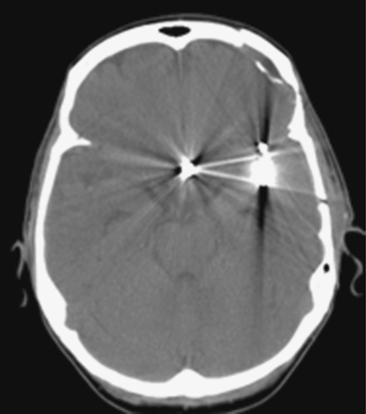
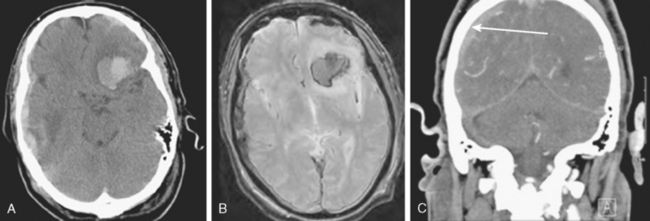
Her treatment was delayed for personal reasons, and 2 months later, she developed a severe headache, nausea, and vomiting associated with mild lethargy. Repeat CT scan of the brain showed a large right frontal hemorrhage and subarachnoid hemorrhage (Figure 13-41).
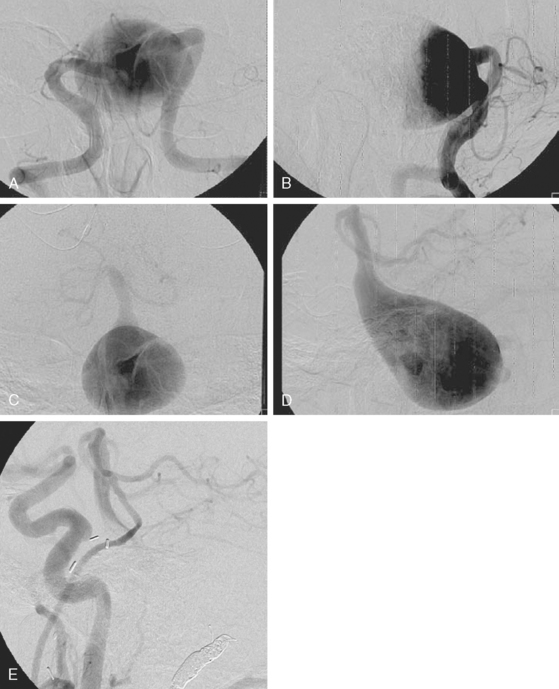
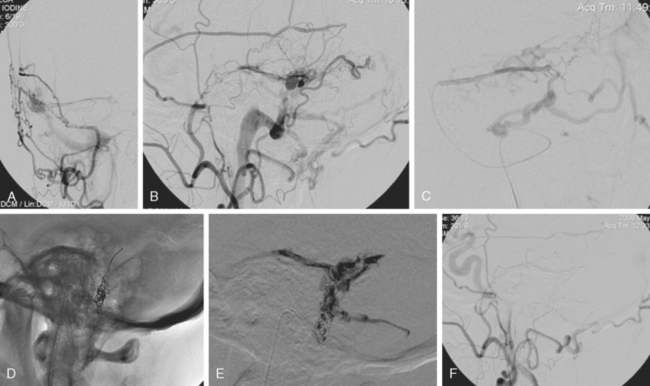
Catheter angiography (Figure 13-42 A,) and 3D DSA (Figure 13-42 B,) showed a number of small branches originating from the base of the left anterior cerebral artery aneurysm. The aneurysm was successfully coiled (Figure 13-42 C,), deliberately leaving a remnant at the base to protect these branches. The patient developed moderate ultrasonographic vasospasm and was treated with hemodynamic augmentation therapy. She was discharged home on postbleed Day 12 with good functional status.
ARTERIOVENOUS MALFORMATIONS
General Concepts
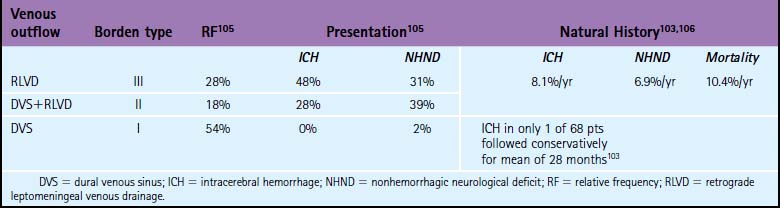
Natural History
105 – patient’s age in years.
TABLE 13-2 Spetzler-Martin arteriovenous malformation grading system.89
| Description | Points |
|---|---|
| Size (cm) | |
| < 3 | 1 |
| 3–6 | 2 |
| > 6 | 3 |
| Eloquence | |
| Yes | 1 |
| No | 0 |
| Deep venous drainage | |
| Yes | 1 |
| No | 0 |
Treatment Options
Current treatment approaches for AVMs include microsurgical resection alone, preoperative endovascular embolization followed by microsurgical resection, stereotactic radiosurgery alone, preprocedural endovascular embolization followed by radiosurgical treatment (Figure 13-44), and endovascular embolization only. However, it is important to remember that observation may be the best approach in some cases.
Decision making is complex and depends on size, location, and drainage. The Spetzler-Martin grade combines these factors to compose a frequently used score (Table 13-2).
Embolization
Radiosurgery
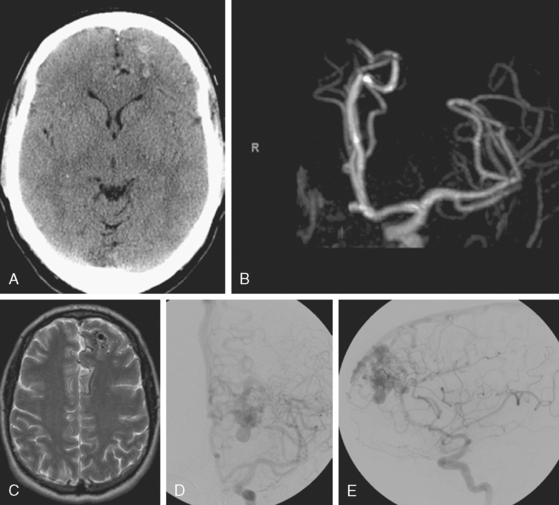
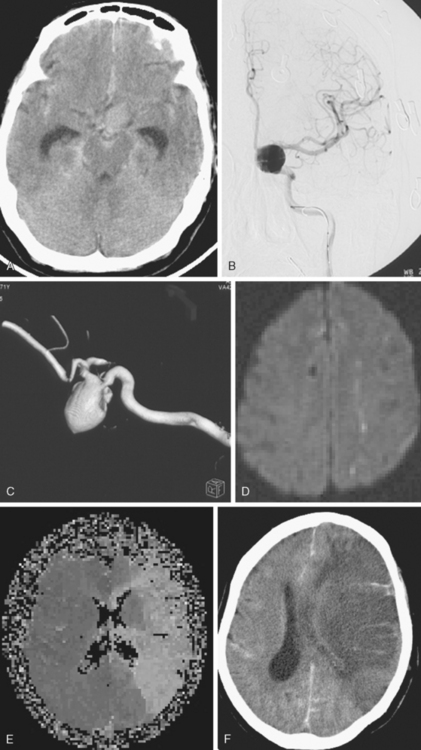
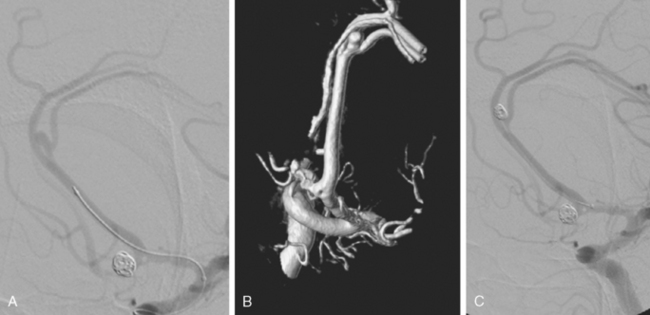
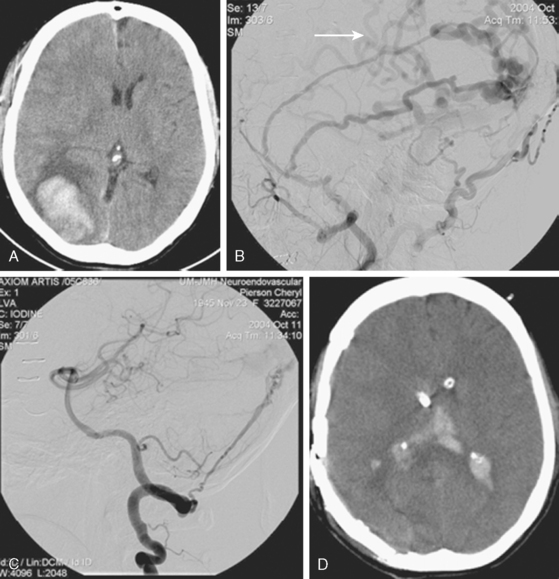
A 24-year-old woman previously evaluated for confusional episodes at age 15 presented to the emergency department with refractory headaches. She was found to harbor a right dorsal frontal AVM measuring 3.5 cm in maximal diameter; the lesion had deep venous drainage. CT scan of the brain and lumbar puncture did not disclose intraparenchymal or subarachnoid hemorrhage. Angiography showed no flow-related aneurysm. Two months later the patient developed rapidly progressive left hemiparesis. Repeat CT scan and CT angiography revealed a right external capsule hemorrhage located caudal to the nidus of the AVM (Figure 13-46). A flow-related aneurysm from a small insular feeder was seen on CT angiogram and subsequently confirmed by 3D angiography.
DURAL ARTERIOVENOUS FISTULAS
General Concepts
Cranial Dural Arteriovenous Fistulas
Cavernous Dural Arteriovenous Fistulas
TABLE 13-3 Barrow classification of carotid-cavernous sinus arteriovenous fistulas104
| Type A | Direct, usually traumatic or iatrogenic, high-flow shunts between the internal carotid artery and the cavernous sinus |
| Type B | Dural shunts between meningeal branches of the internal carotid artery and the cavernous sinus |
| Type C | Dural shunts between meningeal branches of the external carotid artery and the cavernous sinus |
| Type D | Dural shunts between meningeal branches of both the internal and external carotid arteries and the cavernous sinus |
Extracavernous Dural Arteriovenous Fistulas
Classification and Presentation
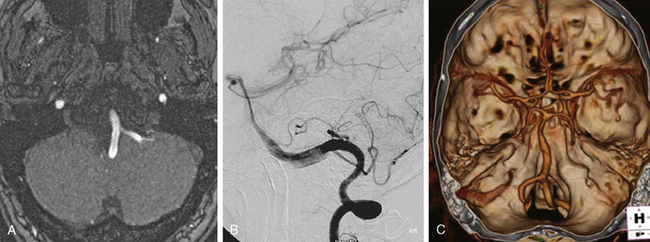
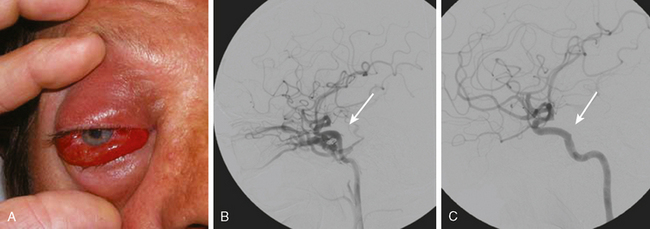
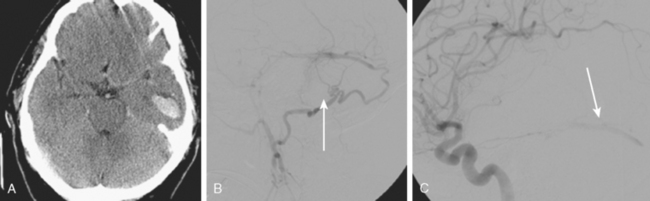
The most common clinical presentation of extracavernous dural arteriovenous fistulas is with intracranial hemorrhage. However, ischemic infarctions may also occur (Figure 13-51).
Hemorrhagic Risk
Treatment
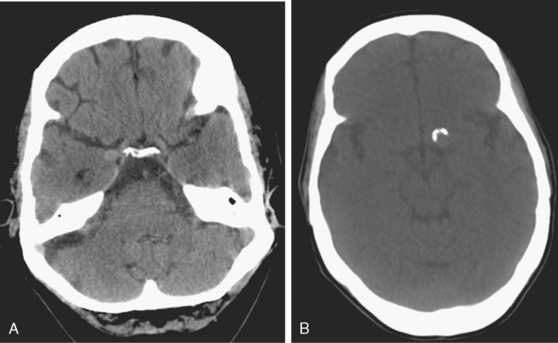
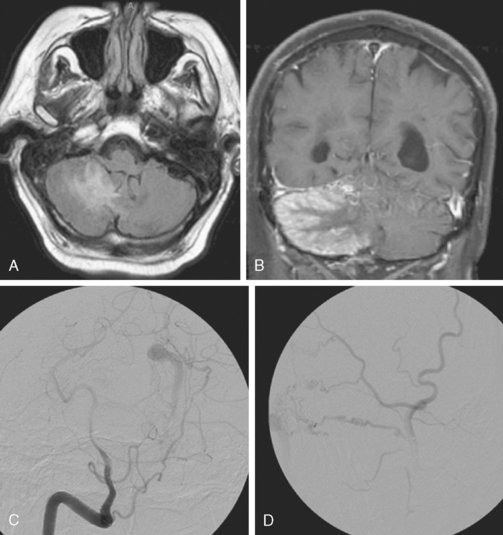
A 60-year-old man was found poorly unresponsive at his house without evidence of trauma, intoxication, or coagulopathy. CT scan of the head revealed a large left frontal hemorrhage, a small right temporal hemorrhage, and a right subdural hematoma (Figure 13-55 A,).
1 Doby T. Cerebral angiography and Egas Moniz. Am J Roentgenol. 1992;159:364.
2 Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke. 1990;21:209-222.
3 Leow K, Murie JA. Cerebral angiography for cerebrovascular disease: the risks. Br J Surg. 1988;75:428-430.
4 Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology. 1992;182:243-246.
5 Gradinscak DJ, Young N, Jones Y, O’Neil D, Sindhusake D. Risks of outpatient angiography and interventional procedures: a prospective study. AJR Am J Roentgenol. 2004;183:377-381.
6 Grzyska U, Freitag J, Zeumer H. Selective cerebral intraarterial DSA. Complication rate and control of risk factors. Neuroradiology. 1990;32:296-299.
7 Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995;37:851-855.
8 Walsh M, Adams WM, Mukonoweshuro W. CT angiography of intracranial aneurysms related to arteriovenous malformations: a cautionary tale. Neuroradiology. 2006;48:255-258.
9 van Rooij WJ, Sprengers ME, de Gast AN, Peluso JP, Sluzewski M. 3D rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. Am J Neuroradiol. 2008;29:976-979.
10 Piotin M, Gailloud P, Bidaut L, Mandai S, Muster M, Moret J, et al. CT angiography, MR angiography and rotational digital subtraction angiography for volumetric assessment of intracranial aneurysms. An experimental study. Neuroradiology. 2003;45:404-409.
11 Schwartz RB, Tice HM, Hooten SM, Hsu L, Stieg PE. Evaluation of cerebral aneurysms with helical CT: correlation with conventional angiography and MR angiography. Radiology. 1994;192:717-722.
12 Villablanca JP, Jahan R, Hooshi P, Lim S, Duckwiler G, Patel A, et al. Detection and characterization of very small cerebral aneurysms by using 2D and 3D helical CT angiography. Am J Neuroradiol. 2002;23:1187-1198.
13 Colen TW, Wang LC, Ghodke BV, Cohen WA, Hollingworth W, Anzai Y. Effectiveness of MDCT angiography for the detection of intracranial aneurysms in patients with nontraumatic subarachnoid hemorrhage. Am J Roentgenol. 2007;189:898-903.
14 Romijn M, Gratama van Andel HA, van Walderveen MA, Sprengers ME, van Rijn JC, van Rooij WJ, et al. Diagnostic accuracy of CT angiography with matched mask bone elimination for detection of intracranial aneurysms: comparison with digital subtraction angiography and 3D rotational angiography. Am J Neuroradiol. 2008;29:134-139.
15 Teksam M, McKinney A, Cakir B, Truwit CL. Multi-slice CT angiography of small cerebral aneurysms: is the direction of aneurysm important in diagnosis? Eur J Radiol. 2005;53:454-462.
16 Taschner CA, Thines L, Lernout M, Lejeune JP, Leclerc X. Treatment decision in ruptured intracranial aneurysms: comparison between multi-detector row CT angiography and digital subtraction angiography. J Neuroradiol. 2007;34:243-249.
17 van der Schaaf IC, Velthuis BK, Wermer MJ, Frenkel NJ, Majoie CB, Witkamp TD, et al. Multislice computed tomography angiography screening for new aneurysms in patients with previously clip-treated intracranial aneurysms: Feasibility, positive predictive value, and interobserver agreement. J Neurosurg. 2006;105:682-688.
18 Aygun N MR angiography: techniques and clinical applications. Atlas SW, editor. Magnetic Resonance Imaging of Brain and Spine, 3rd ed., Vol. 1. Philadelphia: Lippincott, Williams and Wilkins, 2002;981-1057.
19 Pierot L, Delcourt C, Bouquigny F, Breidt D, Feuillet B, Lanoix O, et al. Follow-up of intracranial aneurysms selectively treated with coils: Prospective evaluation of contrast-enhanced MR angiography. Am J Neuroradiol. 2006;27:744-749.
20 Anzalone N, Scomazzoni F, Cirillo M, Righi C, Simionato F, Cadioli M, et al. Follow-up of coiled cerebral aneurysms at 3T: comparison of 3D time-of-flight MR angiography and contrast-enhanced MR angiography. Am J Neuroradiol. 2008;29:1530-1536.
21 Nael K, Villablanca JP, Mossaz L, Pope W, Juncosa A, Laub G, et al. 3-T contrast-enhanced MR angiography in evaluation of suspected intracranial aneurysm: comparison with MDCT angiography. Am J Roentgenol. 2008;190:389-395.
22 Hadizadeh DR, von Falkenhausen M, Gieseke J, Meyer B, Urbach H, Hoogeveen R, et al. Cerebral arteriovenous malformation: Spetzler-Martin classification at subsecond-temporal-resolution four-dimensional MR angiography compared with that at DSA. Radiology. 2008;246:205-213.
23 Taschner CA, Gieseke J, Le Thuc V, Rachdi H, Reyns N, Gauvrit JY, et al. Intracranial arteriovenous malformation: time-resolved contrast-enhanced MR angiography with combination of parallel imaging, keyhole acquisition, and k-space sampling techniques at 1.5 T. Radiology. 2008;246:871-879.
24 Jordan LC, Jallo GI, Gailloud P. Recurrent intracerebral hemorrhage from a cerebral arteriovenous malformation undetected by repeated noninvasive neuroimaging in a 4-year-old boy. Case report. J Neurosurg Pediatr. 2008;1:316-319.
25 Meckel S, Maier M, Ruiz DS, Yilmaz H, Scheffler K, Radue EW, et al. MR angiography of dural arteriovenous fistulas: diagnosis and follow-up after treatment using a time-resolved 3D contrast-enhanced technique. Am J Neuroradiol. 2007;28:877-884.
26 Wiebers DO, Whisnant JP, Huston J3rd, Meissner I, Brown RDJr., Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
27 Mueller-Kronast N, Rabinstein A, Quintero S, Aziz-Sultan M, Morcos J, Heros R, Zauner A. Aneurysm size and associated risk factors for rupture. 8th Annual Meeting of the Joint Section of Cerebrovascular Surgery/ASITN, New Orleans, Louisiana, February 2005.
28 Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
29 Wermer MJ, van der Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ. Follow-up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain. 2005;128:2421-2429.
30 Brown RDJr, Huston J, Hornung R, Foroud T, Kallmes DF, Kleindorfer D, et al. Screening for brain aneurysm in the Familial Intracranial Aneurysm study: frequency and predictors of lesion detection. J Neurosurg. 2008;108:1132-1138.
31 Kissela BM, Sauerbeck L, Woo D, Khoury J, Carrozzella J, Pancioli A, et al. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke. 2002;33:1321-1326.
32 Teasdale GM, Wardlaw JM, White PM, Murray G, Teasdale EM, Easton V. The familial risk of subarachnoid haemorrhage. Brain. 2005;128:1677-1685.
33 Wermer MJ, Koffijberg H, van der Schaaf IC. Effectiveness and costs of screening for aneurysms every 5 years after subarachnoid hemorrhage. Neurology. 2008;70:2053-2062.
34 Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H, et al. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: insight on the mechanism of growth. Stroke. 2000;31:896-900.
35 Flemming KD, Wiebers DO, Brown RDJr, Link MJ, Nakatomi H, Huston J3rd, et al. Prospective risk of hemorrhage in patients with vertebrobasilar nonsaccular intracranial aneurysm. J Neurosurg. 2004;101:82-87.
36 Mangrum WI, Huston J3rd, Link MJ, Wiebers DO, McClelland RL, Christianson TJ, et al. Enlarging vertebrobasilar nonsaccular intracranial aneurysms: frequency, predictors, and clinical outcome of growth. J Neurosurg. 2005;102:72-79.
37 Fiorella D, Woo H, Albuquerque F, Nelson PK. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1120-1121.
38 Mount LA. Results of treatment of intracranial aneurysms using the Selverstone clamp. J Neurosurg. 1959;16:611-618.
39 Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141-162.
40 al-Rodhan NR, Piepgras DG, Sundt TMJr. Transitional cavernous aneurysms of the internal carotid artery. Neurosurgery. 1993;33:993-996. discussion 997–998.
41 Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology. 2008;70:66-72.
42 Thorell WE, Chow MM, Woo HH, Masaryk TJ, Rasmussen PA. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005;56:1035-1040. discussion 1035–1040.
43 Horowitz M, Levy E, Sauvageau E, Genevro J, Guterman LR, Hanel R, et al. Intra/extra-aneurysmal stent placement for management of complex and wide-necked- bifurcation aneurysms: eight cases using the waffle cone technique. Neurosurgery. 2006;58:ONS-258-262. discussion ONS-262.
44 Molyneux AJ, Cekirge S, Saatci I, Gal G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. Am J Neuroradiol. 2004;25:39-51.
45 Cekirge HS, Saatci I, Ozturk MH, Cil B, Arat A, Mawad M, et al. Late angiographic and clinical follow-up results of 100 consecutive aneurysms treated with Onyx reconstruction: largest single-center experience. Neuroradiology. 2006;48:113-126.
46 Henkes H, Fischer S, Weber W, Miloslavski E, Felber S, Brew S, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54:268-280. discussion 280–265.
47 Fiorella D, Albuquerque FC, Deshmukh VR, McDougall CG. In-stent stenosis as a delayed complication of neuroform stent-supported coil embolization of an incidental carotid terminus aneurysm. Am J Neuroradiol. 2004;25:1764-1767.
48 Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Balloon-assisted coil embolization of intracranial aneurysms: incidence, complications, and angiography results. J Neurosurg. 2006;105:396-399.
49 Ohashi Y, Horikoshi T, Sugita M, Yagishita T, Nukui H. Size of cerebral aneurysms and related factors in patients with subarachnoid hemorrhage. Surg Neurol. 2004;61:239-245. discussion 245–237.
50 Guglielmi G, Vinuela F, Duckwiler G, Dion J, Lylyk P, Berenstein A, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg. 1992;77:515-524.
51 Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
52 Johnston SC, Dudley RA, Gress DR, Ono L. Surgical and endovascular treatment of unruptured cerebral aneurysms at university hospitals. Neurology. 1999;52:1799-1805.
53 Ng P, Khangure MS, Phatouros CC, Bynevelt M, ApSimon H, McAuliffe W. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke. 2002;33:210-217.
54 Friedman JA, Nichols DA, Meyer FB, Pichelmann MA, McIver JI, Toussaint LG3rd, et al. Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. AJNR Am J Neuroradiol. 2003;24:526-533.
55 Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50:239-249. discussion 249–250.
56 Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Vinuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561-568.
57 Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398-1403.
58 Sluzewski M, van Rooij WJ, Slob MJ, Bescos JO, Slump CH, Wijnalda D. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology. 2004;231:653-658.
59 Fiorella D, Albuquerque FC, McDougall CG. Durability of aneurysm embolization with matrix detachable coils. Neurosurgery. 2006;58:51-59. discussion 51–59.
60 Niimi Y, Song J, Madrid M, Berenstein A. Endosaccular treatment of intracranial aneurysms using matrix coils: early experience and midterm follow-up. Stroke. 2006;37:1028-1032.
61 Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656-663.
62 Slob MJ, Sluzewski M, van Rooij WJ, Roks G, Rinkel GJ. Additional coiling of previously coiled cerebral aneurysms: clinical and angiographic results. Am J Neuroradiol. 2004;25:1373-1376.
63 Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. Am J Neuroradiol. 2005;26:2542-2549.
64 Kremer C, Groden C, Lammers G, Weineck G, Zeumer H, Hansen HC. Outcome after endovascular therapy of ruptured intracranial aneurysms: morbidity and impact of rebleeding. Neuroradiology. 2002;44:942-945.
65 Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34-42. discussion 34–42.
66 Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006;37:1437-1442.
67 Yamada N, Hayashi K, Murao K, Higashi M, Iihara K. Time-of-flight MR angiography targeted to coiled intracranial aneurysms is more sensitive to residual flow than is digital subtraction angiography. Am J Neuroradiol. 2004;25:1154-1157.
68 Sluzewski M, van Rooij WJ, Rinkel GJ, Wijnalda D. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long-term clinical and serial angiographic results. Radiology. 2003;227:720-724.
69 Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. J Hematother Stem Cell Res. 2002;11:5-7.
70 Kamiryo T, Nelson PK, Bose A, Zalzal P, Jafar JJ. Familial arteriovenous malformations in siblings. Surg Neurol. 2000;53:255-259.
71 Herzig R, Burval S, Vladyka V, Janouskova L, Krivanek P, Krupka B, et al. Familial occurrence of cerebral arteriovenous malformation in sisters: case report and review of the literature. Eur J Neurol. 2000;7:95-100.
72 Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly TJr., Stewart CL, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54:410-423. discussion 423–415.
73 Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812-1818.
74 Brown RDJr., Wiebers DO, Torner JC, O’Fallon WM. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology. 1996;46:949-952.
75 Hofmeister C, Stapf C, Hartmann A, Sciacca RR, Mansmann U, terBrugge K, et al. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke. 2000;31:1307-1310.
76 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
77 Crawford PM, West CR, Chadwick DW, Shaw MD. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
78 Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350(9084):1065-1068.
79 Jane JA, Kassell NF, Torner JC, Winn HR. The natural history of aneurysms and arteriovenous malformations. J Neurosurg. 1985;62:321-323.
80 Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995;37:851-855.
81 Fleetwood IG, Marcellus ML, Levy RP, Marks MP, Steinberg GK. Deep arteriovenous malformations of the basal ganglia and thalamus: natural history. J Neurosurg. 2003;98:747-750.
82 Itoyama Y, Uemura S, Ushio Y, Kuratsu J, Nonaka N, Wada H, et al. Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg. 1989;71:805-809.
83 Kader A, Young WL, Pile-Spellman J, Mast H, Sciacca RR, Mohr JP, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801-807. discussion 807–808.
84 Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539-546.
85 Nataf F, Meder JF, Roux FX, Blustajn J, Merienne L, Merland JJ, et al. Angioarchitecture associated with haemorrhage in cerebral arteriovenous malformations: a prognostic statistical model. Neuroradiology. 1997;39:52-58.
86 Brown RDJr., Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859-863.
87 Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin Grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.
88 Miyamoto S, Hashimoto N, Nagata I, Nozaki K, Morimoto M, Taki W, et al. Posttreatment sequelae of palliatively treated cerebral arteriovenous malformations. Neurosurgery. 2000;46:589-594. discussion 594–585.
89 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
90 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6. discussion 6–7.
91 Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery. 1990;26:570-577. discussion 577–578.
92 Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: Part I—Technique, morphology, and complications. Neurosurgery. 1996;39:448-457. discussion 457–449.
93 Jayaraman MV, Marcellus ML, Hamilton S, Do HM, Campbell D, Chang SD, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. Am J Neuroradiol. 2008;29:242-246.
94 Fiorella D, Albuquerque FC, Woo HH, McDougall CG, Rasmussen PA. The role of neuroendovascular therapy for the treatment of brain arteriovenous malformations. Neurosurgery. 2006;59:S163-177. discussion S163–113.
95 Kim EJ, Halim AX, Dowd CF, Lawton MT, Singh V, Bennett J, et al. The relationship of coexisting extranidal aneurysms to intracranial hemorrhage in patients harboring brain arteriovenous malformations. Neurosurgery. 2004;54:1349-1357. discussion 1357–1348.
96 Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
97 Yu SC, Chan MS, Lam JM, Tam PH, Poon WS. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. Am J Neuroradiol. 2004;25:1139-1143.
98 Liscak R, Vladyka V, Simonova G, Urgosik D, Novotny JJr., Janouskova L, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1014. discussion 1015–1006.
99 Maruyama K, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg. 2004;100:407-413.
100 Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg. 1995;82:180-189.
101 Houser OW, Campbell JK, Campbell RJ, Sundt TMJr. Arteriovenous malformation affecting the transverse dural venous sinus—an acquired lesion. Mayo Clin Proc. 1979;54:651-661.
102 Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93:1071-1078.
103 Satomi J, van Dijk JM, Terbrugge KG, Willinsky RA, Wallace MC. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002;97:767-770.
104 Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
105 Davies MA, TerBrugge K, Willinsky R, Coyne T, Saleh J, Wallace MC. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
106 van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
107 Tummala RP, Jahromi BS, Hopkins LNH. Intracranial arterial disease. In: Dieter RS, Dieter RAJr, Dieter RAIII, editors. Peripheral Arterial Disease. New York: McGraw-Hill, 2009.
108 Cognard C, Januel AC, Silva NAJr, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. Am J Neuroradiol. 2008;29:235-241.
109 Rezende MT, Piotin M, Mounayer C, Spelle L, Abud DG, Moret J. Dural arteriovenous fistula of the lesser sphenoid wing region treated with Onyx: technical note. Neuroradiology. 2006;48:130-134.
110 Carlson AP, Taylor CL, Yonas H. Treatment of dural arteriovenous fistula using ethylene vinyl alcohol (Onyx) arterial embolization as the primary modality: short-term results. J Neurosurg. 2007;107:1120-1125.