21 Vascular and endovascular surgery
Pathophysiology of arterial disease
Pathology
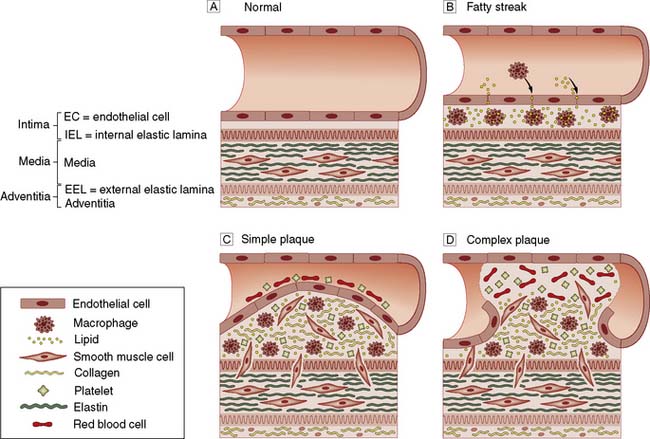
Most patients presenting to vascular specialists in developed countries have atherosclerosis which is characterized histopathologically by endothelial cell injury; sub-endothelial deposition of lipids and inflammatory cells; and smooth muscle cell migration and proliferation, all of which lead to plaque haemorrhage and rupture resulting in thrombosis and embolism (Fig. 21.1).
Clinical features
The clinical manifestations of arterial disease depend upon:
• Whether the artery is an end-artery or well collateralized
• The speed with which the disease develops
• Whether the underlying process is primarily haemodynamic, thrombotic, atheroembolic or thromboembolic, or due to aneurysmal dilatation or dissection
• The presence of other co-morbidity and the general condition of the patient.
Anatomical site
The patient’s symptoms and signs will depend upon the territory supplied by the affected artery:
• Coronary arteries: angina, myocardial infarction (MI)
• Cerebral circulation: stroke, transient ischaemic attack (TIA), amaurosis fugax, vertebrobasilar insufficiency (VBI)
• Renal arteries: hypertension, renal failure
• Mesenteric arteries: mesenteric angina, acute intestinal ischaemia
• Limbs: intermittent claudication (IC), chronic critical limb ischaemia (CLI), acute limb ischaemia.
Chronic lower limb arterial disease
Anatomy
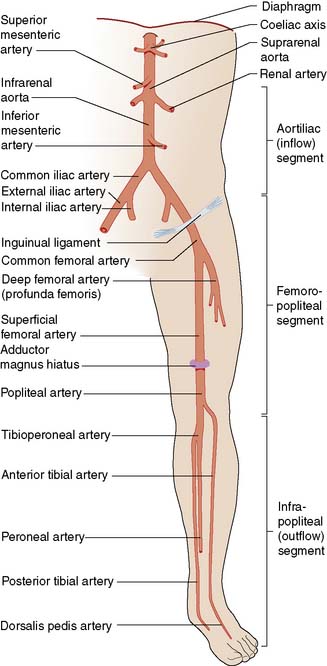
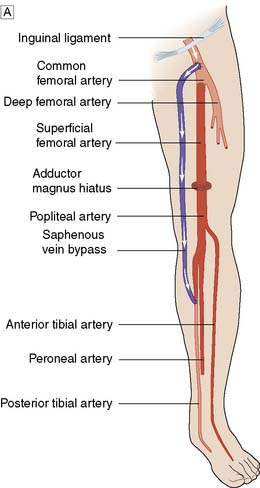
The lower limb arterial tree comprises the aortoiliac segment above the inguinal ligament (‘inflow’), the femoropopliteal segment and the infrapopliteal segment (‘outflow’) (Fig. 21.3).
Clinical features
Examination findings
On examination, the chronically ischaemic limb is usually characterized by:
• Pallor, particularly on elevation. Upon dependency, the foot becomes bright red; this is known as dependent rubor or ‘sunset foot’, and is due to reactive hyperaemia (Buerger’s test)
• Superficial veins that fill sluggishly in the horizontal position and empty upon minimal elevation (venous guttering)
• Nails that are brittle and crumbly
• Pulses that are weak or absent and sometimes associated with thrills on palpation and bruits on auscultation.
Intermittent claudication
Summary Box 21.1 Intermittent claudication (IC)
• IC is the most common manifestation of peripheral arterial disease, affecting up to 1 in 20 people over 60 years of age
• Limb loss is uncommon (up to 1–2% per year), but myocardial infarction and stroke are three times more common than in a non-claudicant population (up to 5–10% per year)
• The mainstays of treatment are risk factor modification (most importantly, complete and permanent smoking cessation), statin and antiplatelet (aspirin, clopidogrel) therapy and (supervised) exercise. This so-called Best Medical Therapy (BMT) leads to a clinically significant improvement in walking distance in the majority of patients; it also improves health related quality of life and increases longevity
• Patients should not normally be considered for surgical or endovascular intervention until they have been compliant with BMT for at least 6 months; intervention in the face of continued smoking is very unlikely to produce meaningful or durable benefit and is cost-ineffective
• Intervention includes angioplasty, stenting and bypass surgery. Long-term results are generally much better in the aorto-iliac segment than below the inguinal ligament.
Clinical features
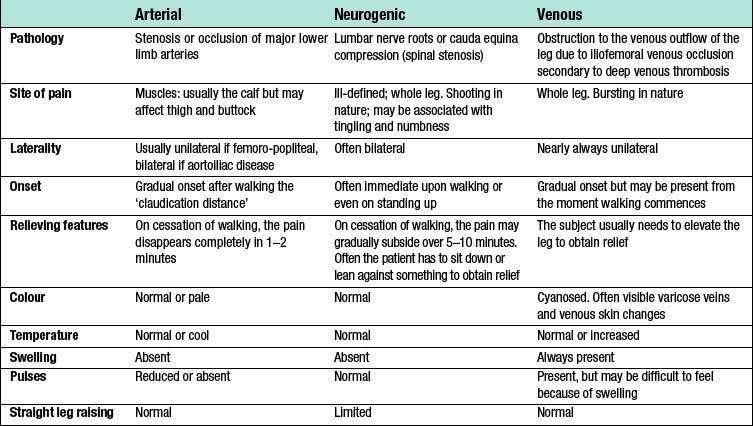
As lower limb arterial disease most frequently affects the SFA (Fig. 21.5), IC is usually characterized by pain on walking in the muscles of one or both calves. If the iliac arteries are affected then that pain may also be felt in the thigh and even the buttock (internal iliac disease). The pain comes on after a reasonably constant ‘claudication distance’, and usually subsides rapidly and completely on cessation of walking. Resumption of walking causes the pain to return. These and other features distinguish it from neurogenic and venous claudication (Table 21.1).
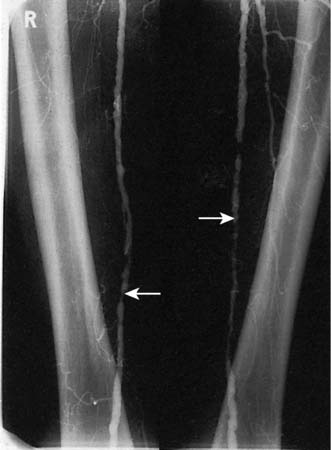
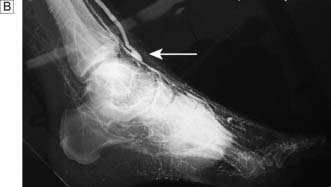
Fig. 21.5 Angiogram showing diffuse disease in both right and left superficial femoral arteries (arrows).
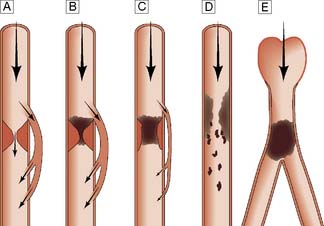
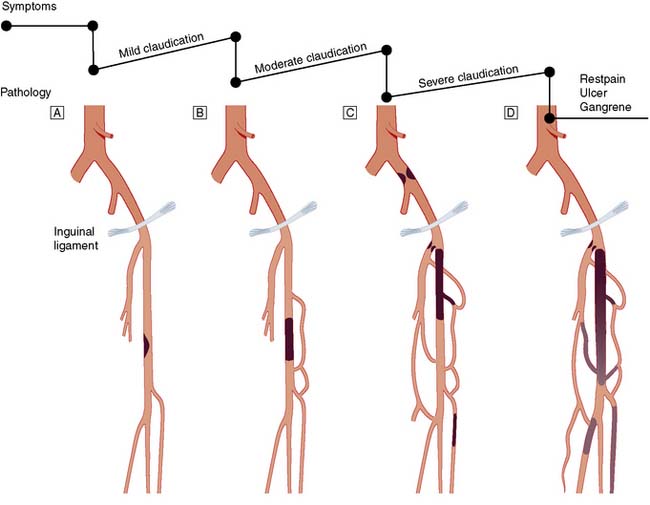
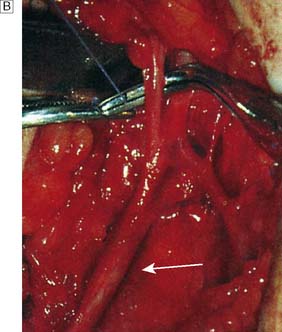
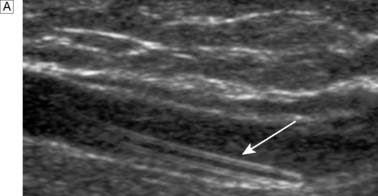
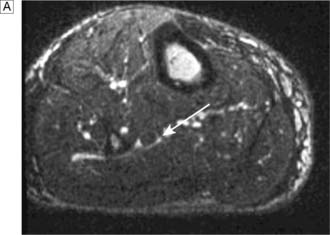
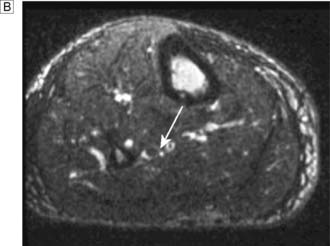
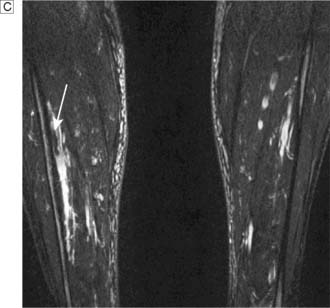
Typically, the superficial femoral artery (SFA) first becomes narrowed at the adductor canal (Fig. 21.6A). Ankle pulses may be palpable but are diminished, and a bruit may be heard at or below the stenosis. The ABPI is often (near) normal at rest but reduced following exercise.
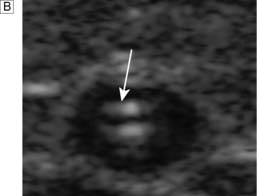
Over the next few months or years, collateral vessels arising from the PFA enlarge so that they carry a higher proportion of the blood flow to the lower leg. As a result, in the majority of patients, symptoms gradually improve or even disappear. But thrombotic occlusion of the SFA (Fig. 21.6B) may lead to a sudden deterioration in walking distance. Ankle and popliteal pulses will be absent at this stage.
Continued development of the collateral circulation may lead to an improvement in symptoms and walking distance. This phase of moderate claudication may remain apparently stable for several years. However, without ‘best medical therapy’ (BMT) (see below) and a change in the patient’s lifestyle, the atherosclerosis will progress to involve other segments, such as the PFA, iliac and tibial vessels (Fig. 21.6C). The IC will progress to become severe, often forcing the patient to stop every 50–100 metres or so, and the scope for spontaneous improvement steadily diminishes. As the disease progresses further in severity and extent, symptoms are likely to worsen to a point where CLI develops due to multilevel disease (Fig. 21.6D). Such patients will often go on to develop night/rest pain and are at risk of tissue loss (see below).
Critical limb ischaemia
Whereas IC is usually due to single-level disease, CLI (sometimes also termed severe limb ischaemia, SLI) is caused by multiple lesions affecting different arterial segments down the leg (Fig. 21.6D). These patients usually have tissue loss (ulceration or gangrene) and/or rest (night) pain; their ankle blood pressure is often 50–70 mmHg or less. Without revascularization, such patients will often lose their limb, and sometimes their life, in a matter of months.
Diabetic vascular disease
• Arteries are often calcified, which makes surgery and angioplasty technically difficult
• Calcification also leads to vessel incompressibility which results in spuriously high ankle pressures and ABPI measurements
• Reduced ability to fight infection
• Severe multisystem arterial disease (coronary, cerebral and peripheral), which increases the risks of intervention
• In the lower limbs, diabetic vascular disease has a predilection for the infrapopliteal vessels. Although vessels in the foot are often spared, the technical challenge of performing a satisfactory bypass or angioplasty to these small vessels is considerable
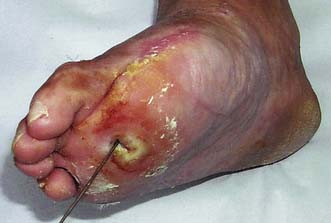
• Frequent coexisting neuropathy may lead to foot ulceration in its own right but may also complicate peripheral ischaemia (see below).
The diabetic foot
Motor neuropathy
The normal structure and function of the foot depends not only upon ligaments, but also upon the long and short flexors and extensors of the calf and foot. The former are affected more than the latter by motor neuropathy, leading to weakness and atrophy. The result is that the long extensors of the toes are unopposed and the toes become increasingly dorsiflexed. This exposes the metatarsal heads to abnormal pressure, and they are a frequent site of callus formation and ulceration (Fig. 21.7).
Management of lower limb ischaemia
Medical management
• Immediate, absolute and permanent cessation from smoking. This is by far the most important intervention and prognostic factor. In the face of continued smoking, other treatments are rendered largely ineffective and disease progression, limb loss and death (usually from cardiovascular causes) within a few years are the likely outcome.
• Control of hypertension according to current guidelines (for example, see the British Hypertension Society, www.bhsoc.org).
• Control of hypercholesterolaemia according to current guidelines (for example, see the British Heart Foundation, http://www.bhf.org.uk). All vascular patients should be prescribed lipid lowering drugs, usually a statin, so that their baseline total cholesterol is reduced by a third (ideally to below 4 mmol/l). Statins also stabilize atheromatous plaques and prevent the development and progression of aneurysmal disease through as yet incompletely understood anti-inflammatory mechanisms.
• Prescription of an antiplatelet agent (for guidance see National Institute of Clinical and Health Excellence, http://www.nice.org.uk/guidance/index.jsp?action=folder&o=51201). This is normally aspirin (75 mg daily) but clopidogrel (75 mg daily) is a more effective and safer alternative (but is also more expensive). Anticoagulation with warfarin should normally be reserved for patients with AF.
• Regular exercise as possible; it is widely accepted that supervised exercise in a health care environment is more effective than (unsupervised) simple advice to exercise.
• Control of obesity. This will help to bring down blood pressure, cholesterol and the ‘strain’ of walking; diabetes will also be easier to control. Surgical and endovascular intervention is much more difficult and morbid in obese patients.
• The identification and active treatment of patients with diabetes. This includes foot care (for further information see, for example, Diabetes UK, http://www.diabetes.co.uk).
Endovascular management
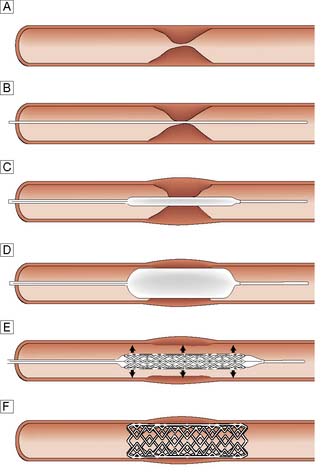
Balloon angioplasty (BAP), with or without stenting, has been used successfully in the iliac, femoral, popliteal and crural arteries and is usually performed under local anaesthesia (Fig. 21.8). The arterial lesion to be treated (stenosis or occlusion) is identified and crossed with a wire. A balloon catheter is introduced over the wire and the balloon inflated. This enlarges the lumen by disrupting the atheromatous plaque. In occlusions and complex disease, metal stents may be deployed across the lesion to improve patency and reduce distal embolic complications. Sometimes these balloons and stents are coated with drugs that reduce the arterial scarring (neo-intimal hyperplasia) that follows such intervention and can lead to restenosis and reocclusion (so-called drug eluting balloons and stents). Endoluminal repair of the aortoiliac segment is the treatment of choice in most vascular units because of its high patency rates, and low morbidity compared to open surgery. Infrainguinal BAP and, less commonly, stenting is also widely used in the management of IC and CLI.
Intermittent claudication
• tend to be younger, so that their symptoms have a greater impact on their quality of life and livelihood
• often have short-segment disease that is amenable to BAP, with or without a stent
• often have (relatively) normal infrainguinal arteries, so that restoring flow in the aortoiliac segment effects a dramatic improvement in the perfusion to their leg(s)
• tend to be more symptomatic, with shorter walking distances and bilateral symptoms
• may not achieve a satisfactory increase in walking distance with BMT alone, because the ability of the body to collateralize around aortoiliac disease is not as good as it is around femoropopliteal disease.
Critical limb ischaemia
The role of BAP and stenting in CLI remains controversial and, with present technology, many such patients remain unsuitable for endovascular therapy. The only published randomized controlled trial to compare BAP and bypass surgery (BSX) (http://basiltrial.com) indicates that although BAP is safer and less expensive than BSX in the short term (12–18 months), BSX (with vein) offers a more durable and complete revascularization in the longer term (3–5 years) (EBM 21.1). At the present time, CLI patients expected to only live 1–2 years and who do not have a suitable vein for the construction of a bypass are probably best treated by BAP where technically possible; all other CLI patients are probably best served by BSX. The role for endovascular therapy may increase in the future as technology improves.
Indications for arterial reconstruction
Intermittent claudication
Many surgeons are reluctant to perform infrainguinal bypass surgery for IC because:
• The risk of limb loss is very low with BMT
• Those patients who fail to comply with BMT (fail to stop smoking) are those most likely to press for surgery because of on-going symptoms. However, they are also those at greatest operative risk and those least likely to gain durable benefit from their bypass
• Surgery is associated with a significant risk of mortality and major morbidity, the size of that risk depending on the procedure and the patient but probably approaching 3–5% for infrainguinal bypass and 5–10% for aorto-bifemoral bypass
• As most patients have bilateral disease, even if they have unilateral symptoms, successful infrainguinal surgery on one side often reveals limiting IC symptoms on the other, requiring a second operation (the same for endoluminal treatment)
• Grafts have a finite patency, especially in those who fail to comply with BMT and, in particular, continue to smoke (the same for endoluminal treatment)
• As soon as a bypass graft is inserted, collaterals circumventing the original lesion shrink down. For this reason, when the graft occludes, usually suddenly, the patient is normally returned to a worse level of IC than before the operation. A patient who was previously a claudicant may now have acute limb-threatening ischaemia, which then forces the surgeon or radiologist to re-intervene. Secondary interventions are technically more difficult, are associated with higher risk and enjoy a lower patency rate.
Principles of arterial reconstruction
Endarterectomy
This involves the direct removal of atherosclerotic plaque and thrombus and is a relatively uncommon operation in modern vascular surgical practice except at the carotid and femoral bifurcations (Fig. 21.9).
Bypass grafting
For a surgical bypass operation (Fig. 21.10) to be successful in the long term, three conditions must be fulfilled:
• There must be high-flow, high-pressure blood entering the graft (inflow)
• The conduit must be suitable
• The blood must have somewhere to go when it leaves the graft (outflow or run-off).
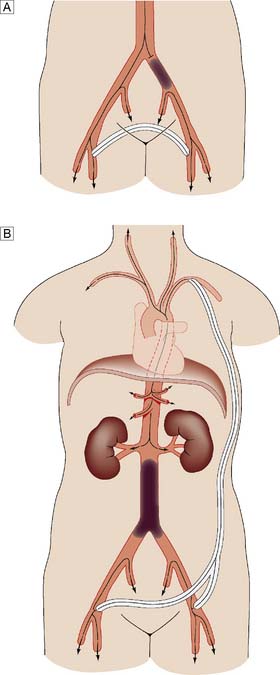
Extra-anatomic bypass
In most bypass operations, the new conduit more or less follows the course of the original artery – so-called anatomic bypass (Fig. 21.11). Where this is not possible and/or desirable, a so-called extra-anatomic bypass can be inserted (Fig. 21.12).
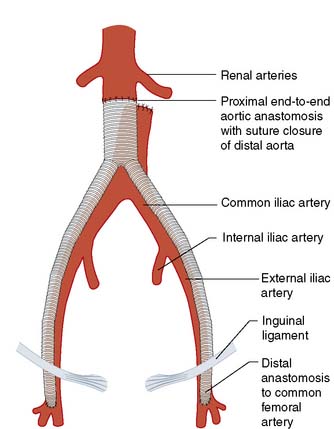
Fig. 21.11 Anatomic aortic bypass.
Reconstruction of an occluded aortoilliac segment by means of aorto-bi-femoral bypass grafting.
Amputation
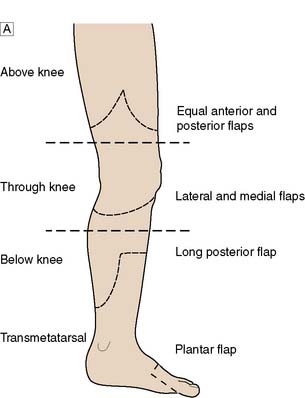
Level of amputation
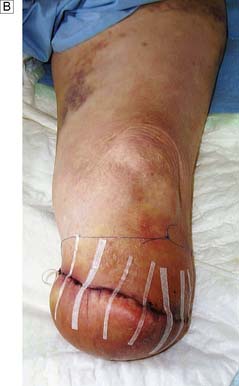
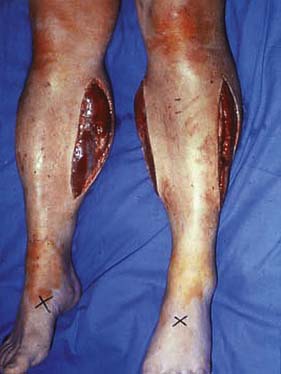
This is determined by local blood supply, the status of the joints, the patient’s general health and his or her age. The broad principle is to amputate at the lowest level consistent with healing (Fig. 21.13). It is important to conserve the knee joint if at all possible, as the energy required to walk on a below-knee prosthesis is much less that required to walk on an above knee prosthesis. However, if the patient has other co-morbidity or disability that would make walking with a prosthesis impossible, there is no point in attempting to conserve the knee joint at the expense of healing. A common situation is where a patient presents with a fixed flexion contracture of the knee. A below-knee amputation in such a patient is usually ill-advised because the contracture will prevent the patient from ever walking and will also result in the stump wound resting on the bed or chair, leading to poor healing and wound breakdown.
Arterial disease of the upper limb
Overview
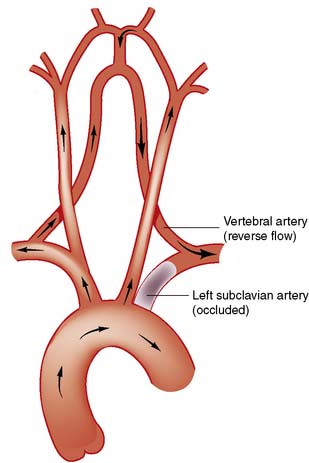
• Arm claudication. This is relatively unusual, even when the subclavian artery is completely occluded, because of collateral supply, mainly from the vertebral artery
• Atheroembolism to the hand. Small emboli lodge in the vessels of the fingers and the hand and lead to symptoms that are often mistaken for Raynaud’s phenomenon, except that in this case the symptoms are unilateral (see below)
• Subclavian steal. In this circumstance, when the arm is used, blood is ‘stolen’ from the brain, with retrograde flow via the vertebral artery. This leads to vertebrobasilar ischaemia (VBI), characterized by dizziness, cortical blindness and/or collapse when the arm is used (Fig. 21.14).
Cerebrovascular disease
Carotid artery disease
Pathophysiology
Summary Box 21.2 Carotid artery disease
• Up to 50% of all ischaemic strokes may be caused by atheroembolism from the carotid bifurcation
• Patients with carotid territory transient ischaemic attacks (TIA) and amaurosis fugax should be assessed by a vascular surgeon with a view to carotid endarterectomy (CEA)
• When compared to BMT alone, CEA in addition to BMT significantly reduces the risk of further ipsilateral ischaemic stroke in patients with high grade symptomatic internal carotid artery stenosis
• CEA for asymptomatic internal carotid artery stenosis is controversial; such patients should be discussed with a vascular surgeon
• Several large trials have shown that with current technology, carotid artery stenting (CAS) can be an effective treatment for carotid stenoses, and should be considered as an option, particularly in patients at high risk for surgery.
Assessment
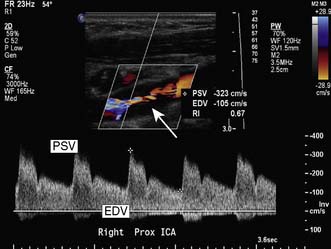
Colour flow Doppler (duplex) ultrasound (CDU) is the initial investigation of choice for imaging the carotid arteries (Fig. 21.15).
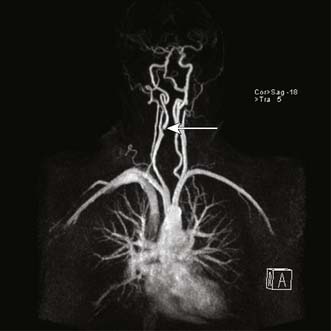
Magnetic resonance (MRA) or computed tomographic (CTA) angiography provide excellent images and are increasingly used to plan treatment (Fig. 21.16).
Management
Carotid endarterectomy (CEA)
• There is a high degree of internal carotid artery stenosis (usually taken as a greater than 60–70% diameter reduction)
• The patient is expected to survive at least 2 years
• The intervention can be undertaken with a stroke and/or death rate of less than 3–5%
• The intervention can be performed soon after the index event. The exact timing remains controversial and is matter of judgment for each patient but, in general, the sooner the better.
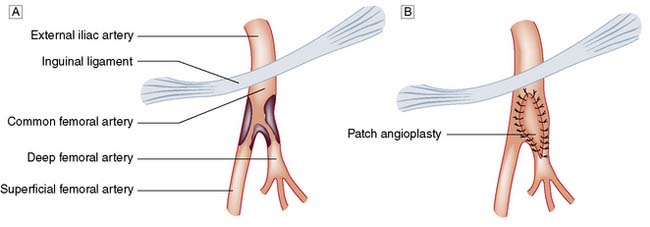
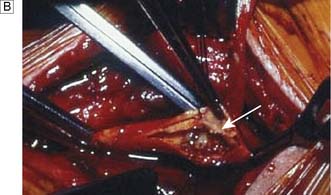
The carotid bifurcation is dissected, heparin is given and the arteries are clamped. If this may lead to cerebral ischaemia, a shunt is inserted. The plaque is shelled out (the endarterectomy) and the artery repaired with direct suture or a patch graft (patch angioplasty) (Fig. 21.17).
Renal artery disease
Management
The indications for intervention remain controversial and, especially since the ASTRAL trial results were published (EBM 21.4), most patients with renovascular disease are treated medically in the UK. Although the evidence for benefit is weak, primary stenting for atherosclerotic renal artery disease may be considered in selected patients to:
Mesenteric artery disease
Summary Box 21.3 Acute limb ischaemia
• Acute limb ischaemia is the most common vascular emergency
• The most common causes are embolus from the left atrium in association with atrial fibrillation and acute thrombosis at a site of long-standing atherosclerotic narrowing
• It is often but not always possible to distinguish these two conditions on clinical grounds alone
• The treatment of embolism is urgent embolectomy, usually without prior angiography
• Patients with thrombosis in situ should undergo angiography if possible; treatment comprises a combination of medical therapy, thrombolysis, angioplasty and bypass
• Paralysis, paraesthesia and muscle tenderness are the cardinal signs of complete acute ischaemia; when they are present, the limb must be revascularized within 4-6 hours if it is to be saved and full function restored. Fasciotomy should always be considered upon successful reperfusion to avoid compartment syndrome.
Acute limb ischaemia
Aetiology
Acute limb ischaemia is caused most frequently by acute thrombotic occlusion of a pre-existing stenotic arterial segment (60%), thromboembolism (30%) and trauma, which may be iatrogenic. Distinguishing between thrombosis and embolism is important because investigation, treatment and prognosis are different (Table 21.2).
| Clinical features | Embolus | Thrombosis |
|---|---|---|
| Severity | Complete ischaemia (no collaterals) | Incomplete ischaemia (collaterals) |
| Onset | Seconds or minutes | Hours or days |
| Limb | Leg 3:1 arm | Leg 10:1 arm |
| Multiple sites | Up to 15% | Rare |
| Embolic source | Present (usually AF) | Absent |
| Previous claudication | Absent | Present |
| Palpation of artery | Soft; tender | Hard/calcified |
| Bruits | Absent | Present |
| Contralateral leg pulses | Present | Absent |
| Diagnosis | Clinical | Angiography |
| Management | Embolectomy, warfarin | Medical, bypass, thrombolysis |
| Prognosis | Loss of life > loss of limb | Loss of limb > loss of life |
Classification
Limb ischaemia is classified on the basis of onset and severity (Table 21.3). Incomplete acute ischaemia (usually due to thrombosis in situ) can often be treated medically, at least in the first instance. Complete ischaemia (usually due to embolus) will normally result in extensive irreversible tissue injury within 6 hours unless the limb is revascularized. Irreversible ischaemia mandates early amputation or, if the patient is elderly and unfit, end-of-life care.
| Terminology | Definition/comment |
|---|---|
| Onset | |
| Acute | Ischaemia < 14 days |
| Acute-on-chronic | Worsening symptoms and signs (< 14 days) |
| Chronic | Ischaemia stable for > 14 days |
| Severity (acute, acute- on-chronic) | |
| Incomplete | Limb not threatened |
| Complete | Limb threatened |
| Irreversible | Limb non-viable |
| Severity (chronic) | |
| Non-critical | Intermittent claudication |
| Subcritical | Night/rest pain |
| Critical | Tissue loss (ulceration ± gangrene) |
Clinical features
Apart from those that indicate ‘loss of function’, namely paralysis (inability to wiggle toes/fingers) and paraesthesia (loss of light touch over the dorsum of the foot/hand), the so-called Ps of acute ischaemia are non-specific and/or inconsistently related to its severity and should not be relied upon (Table 21.4).
| Symptoms/signs | Comment |
|---|---|
| Pain | May be absent in complete acute ischaemia; severe pain is also a feature of chronic ischaemia |
| Pallor | Also a feature of chronic ischaemia |
| Pulseless | Also a feature of chronic ischaemia |
| Perishing cold | Unreliable, as the ischaemic limb takes on the ambient temperature |
| Paraesthesia and paralysis | Loss of function is the most important feature of acute limb ischaemia and denotes a threatened limb that is likely to be lost unless it is revascularized within a few hours |
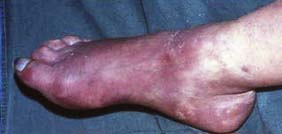
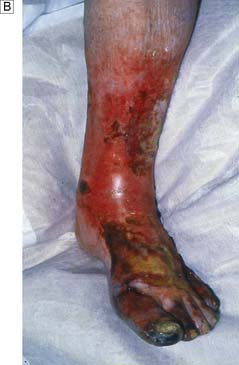
At first, acute complete ischaemia is associated with intense distal arterial spasm and the limb is ‘marble’ white. As the spasm relaxes over the next few hours and the skin fills with deoxygenated blood, mottling appears. This is light blue or purple, has a fine reticular pattern and blanches on pressure: so-called ‘non-fixed mottling’. At this stage, the limb is still salvageable. As ischaemia progresses, blood coagulates in the skin, leading to mottling that is darker in colour, coarser in pattern and does not blanch. Finally, large patches of fixed staining progress to blistering and liquefaction (Fig. 21.18). Attempts at revascularization at this late stage are futile and will lead to life-threatening reperfusion injury (see below).
Management
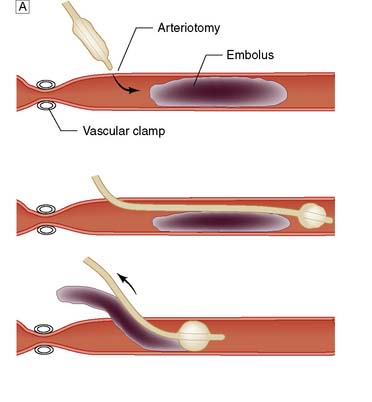
Acute embolus
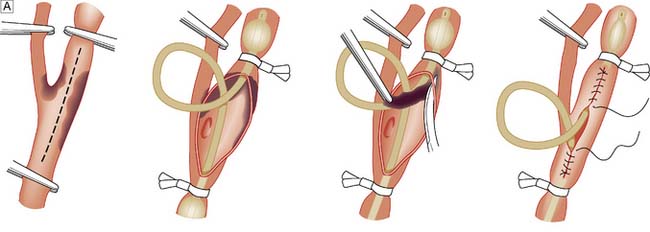
A leg affected by embolus is nearly always threatened and requires immediate surgical revascularization. Femoral embolus is usually associated with profound ischaemia to the level of the upper thigh because the deep femoral artery is also affected. Acute embolic occlusion of the aortic bifurcation (saddle embolus) leads to absent femoral pulses and a patient who is ‘marble’ white or mottled to the waist. Such patients may also present with paraplegia due to ischaemia of the cauda equina, which may be irreversible. Embolectomy can be performed under local/regional or general anaesthetic (Fig. 21.19).
Postischaemic syndromes
Compartment syndrome
Endothelial cell injury during ischaemia leads to increased capillary permeability and oedema on reperfusion. In the calf, where muscles are confined within tight fascial boundaries, the increase in interstitial tissue pressure can lead to continuing muscle necrosis despite apparently adequate arterial inflow: the so-called compartment syndrome. There is swelling and pain on squeezing the calf muscle or moving the ankle or toes. It is very important to remember that palpable pedal pulses do not exclude compartment syndrome. The key to management is prevention through expeditious revascularization and a low threshold for fasciotomy to relieve the pressure (Fig. 21.20).
Aneurysmal disease
Classification
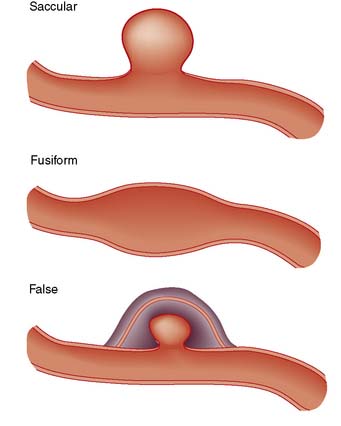
True aneurysms
All three layers of the arterial wall enclose a true aneurysm, which may be saccular or fusiform (Fig. 21.21).
Abdominal aortic aneurysm (AAA)
Summary Box 21.4 Abdominal aortic aneurysm (AAA)
• AAAs are present in up to 5% of men aged over 70 years; more than half are asymptomatic and go undetected until they rupture
• Ruptured AAA is the 10th most common cause of death in men. Only a third of patients with ruptured AAA reach hospital alive and, of these, only about half survive surgery. The overall mortality for the condition therefore exceeds 80%. Population screening of men over the age of 65 years with ultrasound may reduce the number of ruptured AAAs by up to 50%
• Patients with asymptomatic AAA should be considered for repair if the maximum diameter reaches 5.5 cm and the surgeon believes the operation will be associated with a mortality of less than 5%
• An increasingly large number of AAAs are being treated by endovascular aneurysm repair (EVAR), which is associated with a significant reduction in peri-operative morbidity and mortality although questions still remain around long-term durability and protection from rupture.
Clinical features
An AAA may present in the following ways:
• Asymptomatic (60%). The AAA may be detected incidentally on routine physical examination, plain X-ray or, most commonly, abdominal ultrasound scan conducted for another reason. Even a large AAA can be difficult to feel. Patients in whom an incidental finding of an AAA is made should be considered for treatment or surveillance.
• Symptomatic (10%). AAA may cause pain in the central abdomen, back, loin, iliac fossa or groin. Thrombus within the aneurysm sac may be a source of emboli to the lower limbs. Much less commonly, the aneurysm may undergo thrombotic occlusion. AAA may also become inflamed and then compress surrounding structures such as the duodenum, ureter and the inferior vena cava.
• Rupture (30%). AAA may rupture, usually into the retroperitoneum, but sometimes into the peritoneal cavity or rarely into surrounding structures, most commonly the inferior vena cava, leading to an aorto–caval fistula.
Studies have shown that screening for AAA by means of ultrasound results in a reduction in the number of deaths from rupture (EBM 21.5). The UK has begun introducing a national screening programme for AAA (http://aaa.screening.nhs.uk).
Investigations
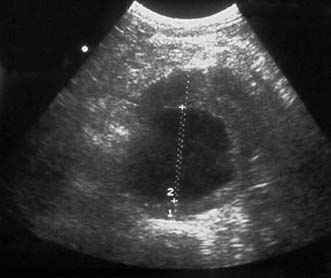
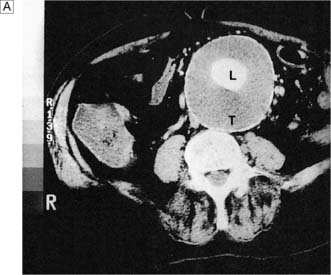
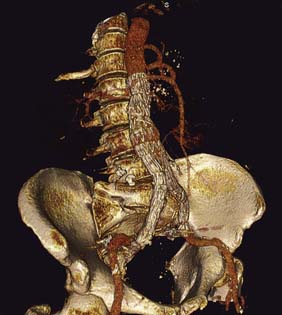
Ultrasound is the best way of establishing the diagnosis, of obtaining an approximate size, and of following up patients with asymptomatic AAAs that are not yet large enough to warrant surgical repair (Fig. 21.22). CT angiography (CTA) will provide much more accurate information about the size and extent of the aneurysm, involvement of visceral arteries and the surrounding structures, and whether there is any other intra-abdominal pathology. It is the standard preintervention investigation but is not suitable for surveillance because of the ionizing radiation and cost (Fig. 21.23).
Ruptured AAA
• The rupture is usually into the retroperitoneum, which tamponades (restricts the extent of) the leak
• There is intense vasoconstriction of non-essential circulatory beds
• The patient develops an intensely prothrombotic state
• The patient’s blood pressure drops, which helps to limit the blood loss.
Any medical intervention that upsets this delicate balance will convert a relatively stable, potentially salvageable patient into one unlikely to reach the operating theatre or to survive intervention. Specifically, large volumes of intravenous fluid (saline or plasma expander) increase the patient’s blood pressure, impair haemostasis and abolish vasoconstriction, and must therefore not be given. The only way of saving the patient is to either clamp and graft the aorta or insert a stent graft (EVAR) (if necessary having controlled the bleeding through angioplasty balloon occlusion of the thoracic aorta); there must be absolutely no delay in getting the patient to a hospital where this can be done (Fig. 21.24).
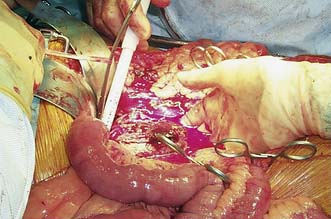
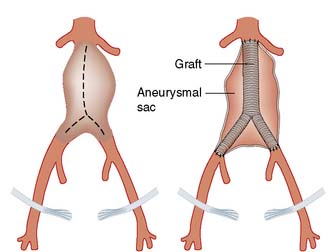
Open AAA repair (OR)
This entails replacing the aneurysmal segment with a prosthetic graft (Fig. 21.25). The 30-day major morbidity and mortality for this procedure is approximately 5–10% for elective asymptomatic AAA, 10–20% for emergency symptomatic AAA and up to 50% for ruptured AAA.
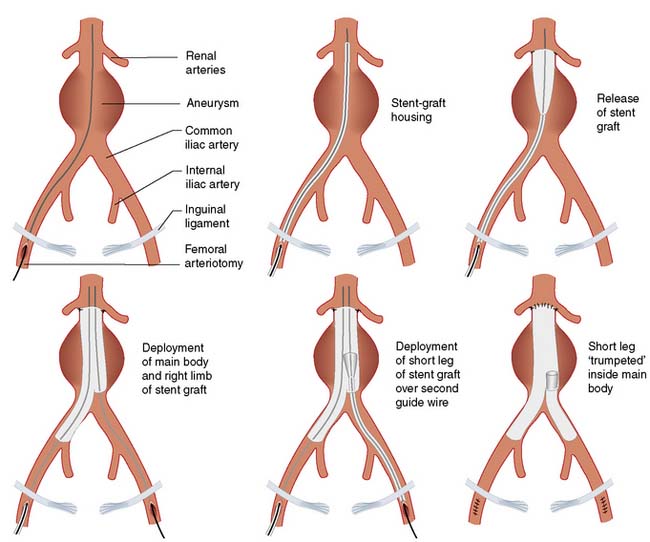
Endovascular aneurysm repair (EVAR)
EVAR involves placing a covered stent graft inside the aneurysm via a femoral arteriotomy, or percutaneously, under radiological guidance (Figs 21.26 and 21.27). The procedure can be performed under regional (epidural) or even local anaesthesia. Laparotomy and cross-clamping of the aorta are avoided. The patient is often fit to go home within 48 hours, as opposed to the 7–10 days that are typical following OR. Patients also usually make a rapid return (4–6 weeks) to their preoperative functional status, whereas those who have undergone OR often take 4–6 months to feel as well as they did before their operation. Not surprisingly therefore, several trials have shown that EVAR is associated with a marked reduction in hospital mortality and morbidity, reduced hospital stay and improved early postoperative quality of life (EBM 21.6). There are, however, downsides; specifically, the devices are expensive (£5000 or more); a significant proportion of AAAs are unsuitable for the procedure with present technology; and there are still questions over durability in that the secondary intervention rate following EVAR is much higher than it is following OR, and EVAR appears to afford less protection from rupture than OR in the long term. In patients unfit for open surgery, the addition of EVAR to BMT is of no benefit when compared to BMT alone (EBM 21.6).
Peripheral aneurysms
Iliac aneurysms
affected. Isolated iliac aneurysms can also occur. The bifurcation of the aorta is at the level of the umbilicus, so that a pulsatile mass felt below that level is likely to be iliac in origin. Iliac aneurysms are most often treated in the course of AAA repair. Isolated iliac aneurysms should be considered for EVAR or surgical repair if they are causing symptoms or have reached twice the normal diameter of the native artery.
Raynaud’s phenomenon
Secondary Raynaud’s phenomenon
This is also known as Raynaud’s syndrome and tends to occur in older people in association with:
Pathophysiology of venous disease
Anatomy
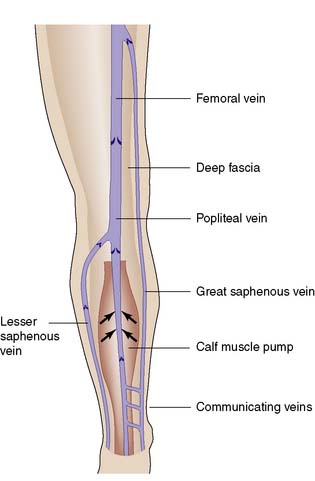
The deep venous system comprises intramuscular veins and axial veins that accompany the main arteries; they are usually paired in the calf. Communicating veins perforate the deep fascia to connect the superficial and deep systems (Fig. 21.28).
Physiology
Summary Box 21.5 Venous disease
• Varicose veins are present in over 50% of the adult population; approximately 10% have skin changes of chronic venous insufficiency; and the life time risk of chronic venous ulceration is about 1%
• Compression therapy is the mainstay of treatment for chronic venous insufficiency, but should never be implemented unless the arterial status of the leg is known to be satisfactory (palpable pedal pulses and/or an ABPI > 0.8)
• If patients with lower limb venous disease are being considered for surgical or endovenous treatment, they should undergo duplex ultrasonography to delineate the pattern of superficial and deep venous reflux and obstruction
• DVT in postoperative patients is often asymptomatic. Most postoperative patients developing (fatal) PE have normal legs on clinical examination. Duplex ultrasound or venography should be used to confirm the diagnosis of DVT; V/Q scan or CT pulmonary angiography can be used to diagnose PE
• All hospital patients, medical and surgical, should have their thromboembolic risk assessed and receive prophylaxis accordingly. PE remains the most common cause of potentially preventable death in hospital
Varicose veins
Classification
Trunk varices
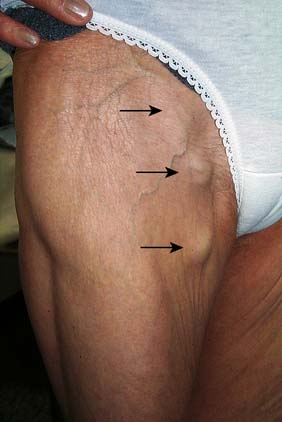
These involve the main stem and/or major tributaries of the GSV and LSV, are usually > 4 mm in diameter (and may be much larger); lie subcutaneously; are palpable; do not usually discolour the overlying skin; and are present in about a third of the adult population (Fig. 21.29).
Management
Surgery
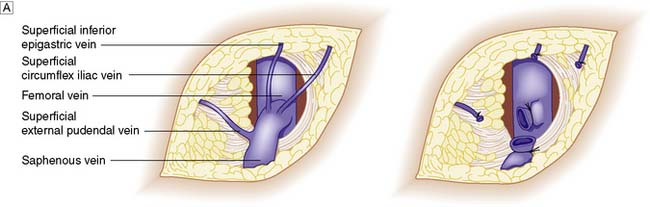
VV surgery aims to remove varices and intercept incompetent connections between deep and superficial veins so that further varices do not form. In patients with GSV disease, the SFJ is ligated flush with the femoral vein (Fig. 21.30). Recurrence is very much less likely if the LSV is stripped out from knee to groin. Care must be taken not to damage the saphenous nerve, which joins and runs with the GSV below the knee. In patients with LSV disease, the SPJ is dealt with in a similar fashion. However, the LSV is not normally stripped for fear of injuring the sural nerve with which it runs. Remaining varices are avulsed through multiple tiny incisions.
Chronic venous insufficiency
Pathophysiology
• Venous reflux due to valvular incompetence (90%). This may affect the superficial veins, the deep veins or both, and may be due to primary valvular insufficiency (as in VV) or to post-thrombotic damage.
• Venous obstruction (10–20%). This is usually post-thrombotic in nature and coexists with reflux.
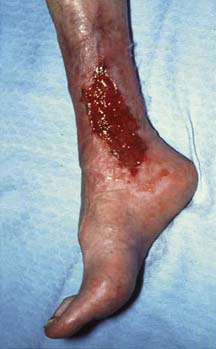
Most patients with CVI and CVU (Fig. 21.32) are over 50 years of age and the incidence increases exponentially with advancing years. In 1992 it was estimated that the treatment of lower limb venous disease accounted for about 1–2% of health-care spending in the UK (£400–600 million per annum at the time). The female to male ratio is about 3:1. Approximately 70% of all leg ulcers are venous in aetiology and 20% are due to mixed arterial and venous disease. In many cases, the situation is aggravated by old age, poor social circumstances, obesity, trauma, immobility, osteoarthritis, rheumatoid arthritis, diabetes and neurological problems. It is usually possible to differentiate venous from arterial ulceration on clinical examination alone (Table 21.5).
| Clinical features | Arterial ulcer | Venous ulcer |
|---|---|---|
| Gender | Men > women | Women > men |
| Age | Usually presents > 60 years | Typically develops at 40–60 years but patient may not present for medical attention until much older; multiple recurrences are the norm |
| Risk factors | Smoking, diabetes, hyperlipidaemia and hypertension | Previous DVT, thrombophilia, varicose veins |
| Past medical history | Most have a clear history of peripheral, coronary and cerebrovascular disease | More than 20% have a clear history of DVT; many more have a history suggestive of occult DVT, i.e. leg swelling after childbirth, hip/knee replacement or long bone fracture |
| Symptoms | Severe pain is present unless there is (diabetic) neuropathy; pain may be relieved by dependency | About a third have pain, but it is not usually severe and may be relieved on elevation |
| Site | Normal and abnormal (diabetics) pressure areas (malleoli, heel, metatarsal heads, 5th metatarsal base) | Medial (70%), lateral (20%) or both malleoli and gaiter area |
| Edge | Regular, ‘punched-out’, indolent | Irregular, with neo-epithelium (whiter than mature skin) |
| Base | Deep, green (sloughy) or black (necrotic) with no granulation tissue; may involve tendon, bone and joint | Pink and granulating but may be covered in yellow-green slough |
| Surrounding skin | Features of severe limb ischaemia | Lipodermatosclerosis, varicose eczema, atrophe, blanche |
| Veins | Empty, ‘guttering’ on elevation | Full, usually varicose |
| Swelling | Usually absent | Often present |
Assessment
Examination
This should include a description of the ulcer, concentrating on the features outlined in Table 21.5. Pulse status and ABPI should be recorded. Gait, particularly ankle mobility which is vital for the proper functioning of the calf muscle pump, should be assessed.
Management
Compression therapy
Although it is still unclear exactly how compression therapy works, it continues to be the mainstay of treatment and, correctly applied, is highly effective in healing the majority of venous ulcers and preventing recurrence (Fig. 21.33A).
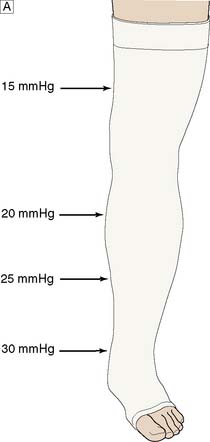
To be maximally effective, compression should be:
• elastic, as this achieves the best and most durable pressure profile
• multilayer, as using many layers evens out the high- and low-pressure areas found under any bandage; the ‘four-layer bandage’ is a popular system
• graduated, with the pressure greatest at the ankle (c. 30–40 mmHg) and least at the knee (c. 15–20 mmHg).
It is vitally important to exclude arterial disease before compression is applied (Fig. 21.33B).
Surgical and endovenous therapy
Eradication of superficial venous reflux by means of surgery or endovenous treatment in addition to compression therapy definitely reduces CVU recurrence and probably increases CVU healing rates when compared to compression alone (EBM 21.7).
Venous thromboembolism (VTE)
Diagnosis
Colour flow duplex ultrasound
Colour duplex ultrasound imaging has largely replaced conventional venography in the diagnosis of DVT. It is non-invasive, avoids ionizing radiation and contrast, and is as accurate as venography in most cases. At times of doubt, MR (Fig. 21.34) or CT venography may be useful.
Prevention
Rationale
Because of our inability to diagnose DVT easily in its early asymptomatic but dangerous phase, prevention is very important. In this respect, it is helpful to determine which patients are at the highest risk and thus have the most to gain from prophylactic measures. The most important risk factors are a history of previous DVT or embolism, advanced age, malignant disease, obesity, and congenital or acquired thrombophilia. However, even patients at apparently low risk do develop DVT and PE, and prophylactic measures/anticoagulation must be considered for all surgical patients (for further guidance see the National Institute of Clinical and Health Excellence, NICE, http://www.nice.org.uk/CG46).
Management
Uncomplicated DVT
Complicated DVT
Surgical thrombectomy
In the UK, surgical thrombectomy to clear iliofemoral thrombus is very rarely performed nowadays.
Lymphoedema
Pathophysiology
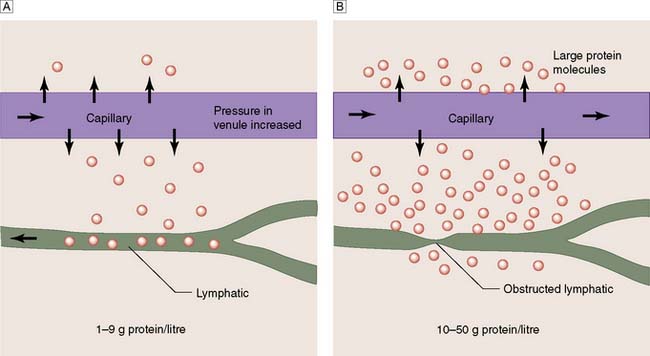
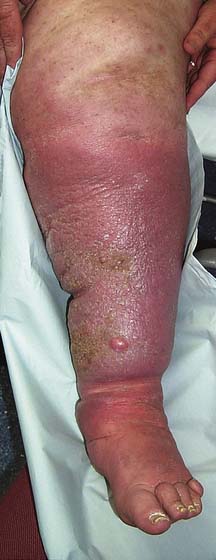
The lymphatic system removes excess water and protein from the interstitial space. Flow is directed centrally by intrinsic lymphatic contractions and endothelial valves, and is increased by muscle contraction. Lymph passes through lymph nodes before it re-enters the venous system, mainly through the thoracic duct. The total daily lymph flow is only 2–4 litres. Failure of this mechanism leads to the accumulation of protein-rich oedema fluid in the tissues (lymphoedema) (Fig. 21.35). Lymphoedema may be primary or secondary, and must be differentiated from other causes of leg swelling (Fig. 21.36, Table 21.6).
| Non-Vascular or Lymphatic General disease states |
Deep venous thrombosis
Primary lymphoedema
• Lymphoedema congenita. Swelling is present at birth or develops within the first year of life
• Lymphoedema praecox. Swelling develops between 1 and 35 years, usually during adolescence. It affects predominantly females, and may be unilateral or bilateral.
• Lymphoedema tarda. In patients developing lymphoedema after the age of 35, an underlying pelvic tumour (benign or malignant) compressing the proximal lymphatic (and venous) systems must be excluded.
Clinical features
Investigations
Lymphoedema is essentially a clinical diagnosis and most patients require no further investigation.