CHAPTER 92 Vascular Anatomy of the Spine, Imaging, and Endovascular Treatment of Spinal Vascular Diseases
Normal Vascular Anatomy of the Spine
Arterial Supply
The spinal blood supply can be divided into macrocirculation, which includes the vasculature of paraspinal structures up to the cord surface, and microcirculation, which involves perforators to the cord beyond the anterior and posterior spinal arteries (ASAs and PSAs).1,2
Macrocirculation (Paraspinal Structures and Spinal Cord Surface)
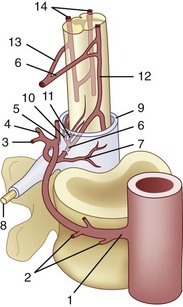
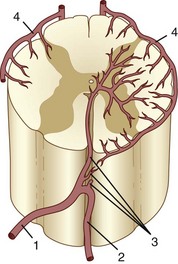
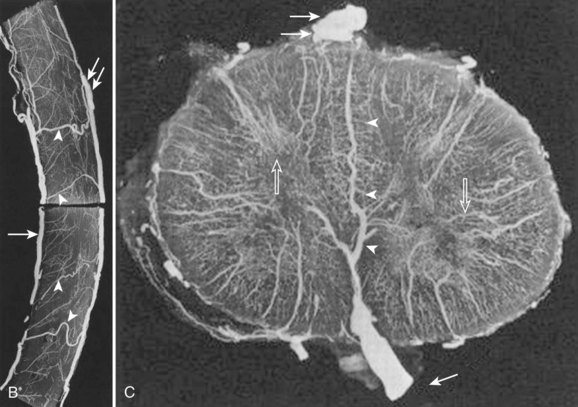
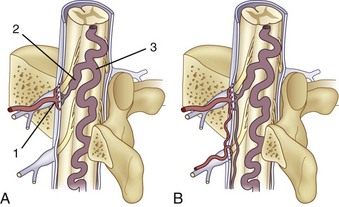
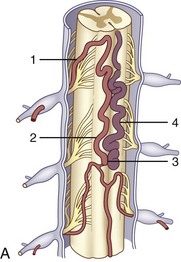
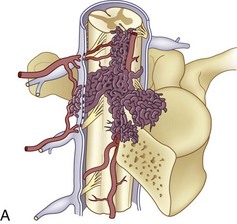
The segmental arteries are numbered according to the nerve they accompany in the neural foramen. Through their branches, they supply all the ipsilateral derivatives of their corresponding metamere: skin, muscle, bone, spinal nerve, dura, and spinal cord (Fig. 92–1). In the embryonic stage of development, each segmental artery has a branch supplying the cord. By the end of the fetal development, most branches have regressed and only a few contribute significantly to spinal cord perfusion. Of the initial 62 metameric arteries (31 pairs), 4 to 8 will end up supplying the ASA (diameter, 0.2 to 0.5 mm) and 10 to 20 will supply the PSA (diameter, 0.1 to 0.4 mm). Most of the segmental arteries end up supplying the related nerves, dura, vertebral body, and paraspinous muscles. The process of regression of the spinal cord blood supply is more pronounced caudad. The dominant artery supplying the spinal cord is named the artery of Adamkiewicz (or radicularis magna).
Radicular arteries originate from the following major arterial trunks1:
The segmental arteries form paraspinal and extradural anastomoses in the craniocaudal extension, which can be subdivided as follows1:
At each level, segmental arteries provide blood supply to the ventral and dorsal nerve roots through radicular arteries (see Fig. 92–1). Some of the radicular branches, however, supply not only the nerve root but also the spinal cord. These branches give rise to (1) the pial/coronal arterial network or the PSA and are called radiculopial arteries or (2) the anterior pial network and to the ASA and are named radiculomedullary arteries.
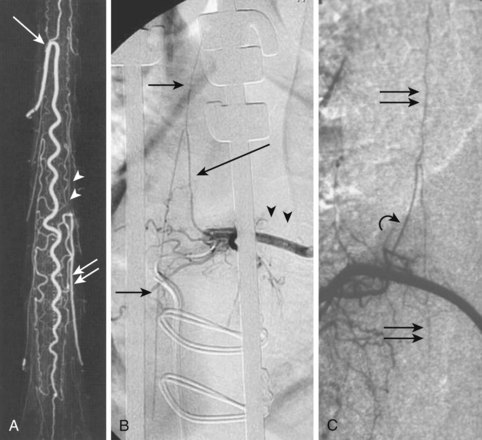
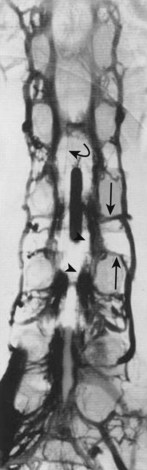
Radiculomedullary arteries are the dominant supply to the ASA. After giving off their radicular branches to the nerve roots, they run along the ventral surface of the nerve root, occasionally give off pial collateral branches, and continue to the ASA. On average, there are two to four radiculomedullary arteries in the cervical region, two to three in the thoracic region, and zero to four in the lumbosacral region. The largest radiculomedullary artery of the thoracolumbar segment is also known as the artery of Adamkiewicz (AKA, diameter, 0.55 to 1.2 mm). In 75% of patients, the AKA arises between T9 and T12, more commonly on the left. When its origin is above T8 or below L2, another major contributor to the ASA can be found either caudad or craniad. In 30% to 50% of cases, it also contributes significantly to the PSA. Generally, a pair of arteries arises in the cervical region from the intradural segment of each vertebral artery that fuse to one “Y”-shaped ASA. The typical hairpin anastomosis between the radiculomedullary arteries and the ASA is found angiographically at the lower thoracic and lumbar levels (Fig. 92–3). There is only one ASA, which continues from its “Y”-shaped origin to the artery of the terminal filum (diameter, 0.5 to 0.8 mm). The ASA is located in the subpial space in the ventral sulcus of the spinal cord, dorsal to the vein; it may be partly absent or not visible angiographically, especially at the thoracic level. Because of a lack of fusion during embryologic development, a short, nonfused cervical segment may be present.
Identifying these small normal spinal arterial vessels has been a significant technical challenge in the development of noninvasive spinal imaging. Recent studies using newer techniques for contrast-enhanced MRA have shown success rates of 82.4% to 100% for detection of the AKA.3,4 Identification of the ASA remains a challenge. Sheehy and colleagues5 reported detection rates of 96% (48 of 50 patients) for the cervical segment of the ASA. Other studies of contrast-enhanced MRA at higher field strength have successfully depicted abnormally dilated ASAs in the setting of spinal vascular malformations, but reliable detection of the normal thoracolumbar ASA remains elusive.6–8
Microcirculation (Spinal Cord Perforators)
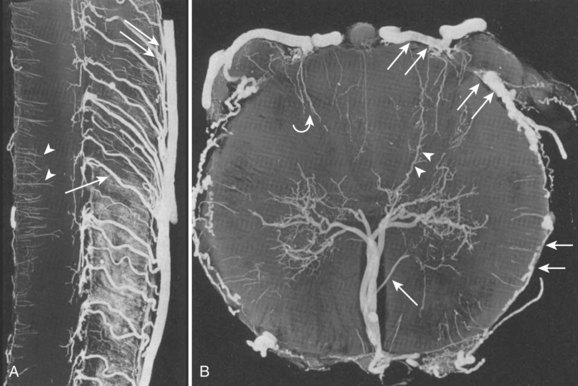
The circulation distal to the subpial ASA, the pial network, and the PSA can be divided into centrifugal (from the center of the cord out toward the surface) and centripetal (from the cord surface toward the center of the cord) systems.1 The centrifugal system, also known as the sulcocommissural system, consists of 200 to 400 sulcocommissural arteries, which are located within the ventral sulcus of the spinal cord and originate from the ASA (Fig. 92–4). These arteries penetrate the sulcus similarly to brain perforators and enter the central gray matter, where they branch into radially oriented small arteries that run toward the white matter. Each sulcocommissural artery usually supplies one half of the cord. The sulcocommissural system supplies most of the spinal cord gray matter and the ventral half of the white matter. Before entering the cord substance, each sulcocommissural artery anastomoses craniad and caudad with neighboring sulcocommissural arteries (see Fig. 92–4). Complex, longitudinally oriented anastomoses are also seen within the white and gray matter. Whereas the sulcocommissural arteries initially run horizontally, they take an ascending course with the growth and disproportionate elongation of the spinal column. The spinal cord territory supplied by the ASA versus the PSAs is comparatively as large as the proportion of a cerebral hemisphere supplied by the internal carotid arteries versus the vertebrobasilar system. An occlusion of the sulcocommissural artery at the lumbar segment in primates can cause severe damage to the ventrolateral two thirds of the cord at the occluded and adjacent levels.9
The centripetal system includes a pial network and the PSAs (Fig. 92–5). At the craniocervical junction, this system receives its blood supply directly from the intradural vertebral arteries or from the posterior inferior cerebellar arteries near their origins. At all other levels, radiculopial arteries provide the blood supply to the centripetal system. Radial branches of the pial network and the PSAs extend around the circumference of the cord and anastomose with the ASA. The radial arteries and the PSAs give off perforating branches to the cord all along their courses. These short perforating branches enter the white matter in a radial fashion and extend to a portion of the gray matter. Intramedullary anastomoses with branches of the sulcocommissural arteries exist. Pial anastomoses also exist, in the longitudinal axis, between the anterior and posterior vascular system. These anastomoses, however, are relatively small and cannot provide adequate craniocaudal supply to the anterior spinal cord in the case of ASA occlusion. Dependent on the location and size of a pial arteriovenous fistula (AVF, anterior or posterior) or a medullary (within the spinal cord) arteriovenous malformation (AVM), the blood supply can originate from the ventral and/or the dorsal vasculature and, therefore, from the centrifugal and/or centripetal system.
Somatic Arteries (Arterial Supply to the Vertebral Body)
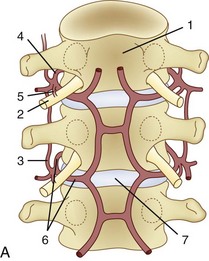
The segmental artery is centered at the level of the intervertebral disc and the corresponding nerve (Fig. 92–6). It gives rise to an ascending somatic branch and a descending somatic branch. Each vertebral body is supplied by the descending somatic branches of the segmental arteries above and the ascending branches of the segmental arteries below. Because of extensive anastomoses, an injection in any one of these four segmental arteries may enhance the entire vertebral body. On angiograms in frontal projection, the arterial network on the posterior surface of the vertebral body has a characteristic hexagonal shape. Tumor blush or vascular bone metastases should not be confused with the normal angiographic blush of the vertebral body.
Venous Drainage
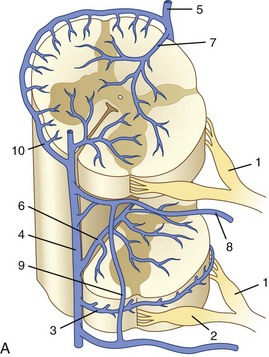
After passing through the capillary network, blood is transported from the deepest parts of the spinal cord toward the surface (Fig. 92–7). Venous drainage of the cord can be divided into an intrinsic system, which runs in proximity to the centrifugal arterial system, and an extrinsic system, which runs in proximity to the centripetal arterial system. Unlike in the arterial blood supply, there is no dominance of the dorsal or the ventral venous return. Dorsal and ventral sulcocommissural veins, which collect the venous outflow from the central gray matter, are a part of the intrinsic venous system. The venous perforators draining into the radial veins, which drain into the dorsal and ventral longitudinal collecting veins, belong to the extrinsic system. These longitudinal collecting veins finally drain into the radicular veins that run along the nerve roots and empty into the ventral epidural venous plexus. In addition to the main dorsal and ventral draining veins, there are short intersegmental lateral longitudinal veins linking adjacent radial veins. However, these lateral longitudinal venous channels are not large enough to create functional dominant craniocaudal drainage like the dorsal and ventral systems.
The radicular (radiculomedullary) veins drain either into spinal nerve venous channels in the neural foramina or into a dural venous pool. Both venous systems eventually empty into the ventral epidural venous plexus (Fig. 92–8). The epidural venous system has a prominent ventral and a smaller dorsal component. The ventral epidural veins receive venous drainage from the vertebral bodies through anterior and posterior venules, the spinal cord via radiculomedullary veins, and the dura. They are also involved in some cerebrospinal fluid resorption via arachnoid granulations along the nerve root sleeves. The ventral epidural venous plexus forms a valveless, retrocorporeal hexagonal anastomotic plexus, which is continuous longitudinally. The direction of flow within this plexus is multidirectional and depends on the location of the contributing veins at each anatomic level.
Imaging and Endovascular Intervention of the Spine
Classification
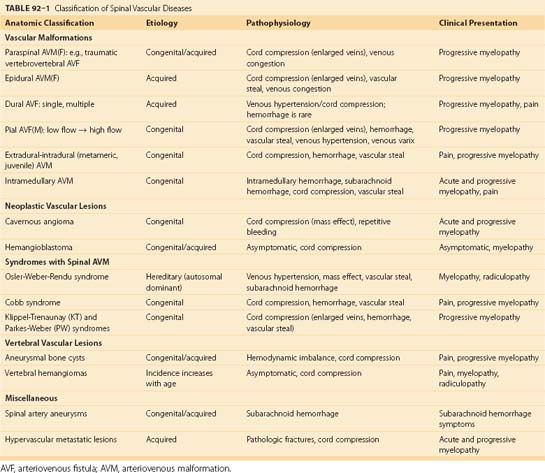
Various classifications have been suggested over the past few decades for spinal vascular malformations on the basis of angiographic features, pathophysiology, and neuroanatomy.10 However, these classifications vary, may create confusion, and can be of little value for the endovascular approach to treating most vascular abnormalities. To assist the understanding of spinal vascular malformations, we address these lesions according to their location with respect to the spinal cord including the paraspinal soft tissue (Table 92–1). Vascular lesions consisting of a single direct connection between the feeding artery and the draining vein are known as AVFs. However, when multiple connections are present at the precapillary level they are called arteriovenous malformations (AVMs). The core of an AVM that appears angiographically and anatomically as a conglomeration of vessels, because of the superimposition of arteries and veins and lack of spatial resolution, is defined as the nidus. Most of the spinal vascular malformations are congenital, may grow over time, and become symptomatic only in adulthood. In this chapter we focus on AVFs, AVMs, spinal artery aneurysms, neoplastic vascular lesions, aneurysmal bone cysts, vertebral hemangiomas, and vascular metastatic disease.
Imaging
Magnetic resonance imaging (MRI) is noninvasive, does not expose the patient to ionizing radiation, and should be the primary diagnostic tool in the evaluation of spinal vascular disease. MRI is able to delineate the spinal cord and paraspinal structures, the flow voids within vascular malformations, and the presence of edema, hemorrhage, venous congestion, and other associated processes. For patients without a known or presumptive diagnosis of spinal vascular disease, MRI remains the initial imaging modality of choice due to its ability to depict the broad range of vascular and nonvascular spinal diseases that may be the cause of a patient’s neurologic symptoms. For instance, a recent study by Germans and colleagues11 found that MRI may be of utility in identifying spinal vascular malformations in patients with cerebral angiogram-negative subarachnoid hemorrhage.
Noninvasive imaging of spinal vascular malformations was initially attempted using phase contrast and time-of-flight MRI techniques.13,14 Later studies with first-pass gadolinium-enhanced MRA show a better definition of the arterial and venous systems of the malformation.7,8,15,16 In the most recent of these studies, Mull and colleagues16 were able to reliably distinguish between spinal dural AVF and spinal AVM, in addition to identifying a large proportion of the clinically relevant vascular anatomy in each case. Further improvement in spatial and temporal resolutions will allow MRA to become the premier diagnostic modality for spinal vascular disease.
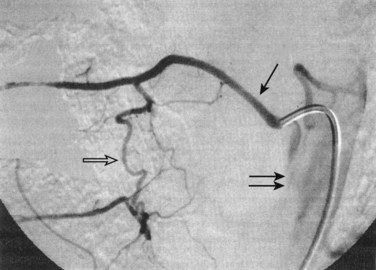
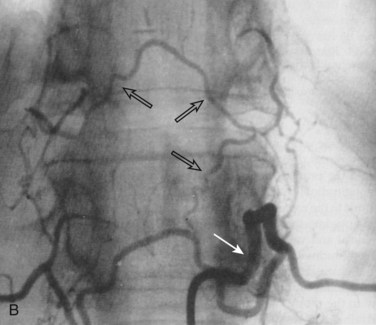
Conventional digital subtraction angiography (DSA) remains the gold standard for evaluation of spinal vascular diseases and is necessary for visualizing the detailed anatomy and vascular architecture of malformations including arterial feeders and venous return. Angiography will also give an estimate of the blood flow velocity within a malformation and help to guide an endovascular intervention. The addition of three-dimensional rotational angiography (3DRA) allows for better delineation of substructures of spinal AVMs such as associated arterial or venous aneurysms and their relationship to the malformation. It is particularly useful in delineating intramedullary from perimedullary malformations and in visualizing nidal and venous aneurysms.12 3DRA helps to assess feeding arteries for planned endovascular procedures, although reduced spatial resolution and limited temporal resolution limit its value for high-flow lesions. Superselective 3DRA may overcome some of these limitations (Fig. 92–9).
With the advancement of multidetector technology, CTA has recently become a reasonable imaging modality for patients in whom MRI is not an option such as those with indwelling ferromagnetic material, those unable to tolerate long imaging times, and those without access to MRI.17,18 CTA may offer several important advantages to MRA. Higher spatial resolution allows for better visualization of submillimeter-sized vessels. Several studies have demonstrated feasibility of imaging the AKA using CTA, even in children.18,19 Unlike MRI, which usually relies on suppressing surrounding tissues to optimize small vessel imaging, CTA can also be useful in the covisualization of anatomic structures (spinal cord, bones) to improve localization of vascular lesions. However, exposure to ionizing radiation and the need for nephrotoxic iodinated contrast agents limit widespread application of CTA for the assessment of spinal vascular disease in the clinical setting.
Catheter-Based Intervention
Unlike often thought, with modern catheter techniques and when performed by a trained physician, spinal diagnostic angiography should not bear a higher rate of complications than a diagnostic angiography of the peripheral system. Infrequently, minor asymptomatic iliac or aortic dissections may be encountered in elderly patients with significant atherosclerotic disease. Recently, MRI studies depicting the level of the vascular malformation or the dilated draining vein have helped to guide the invasive diagnostic workup. Frequently, angiography is used before a planned surgery to locate the artery of Adamkiewicz as the major supply to the anterior spinal cord. If a vascular lesion, especially a dural AVM, is suspected, a more thorough angiography may be required. This includes angiograms of the aortic arch, the descending aorta, the abdominal aorta, and the pelvic system. In cases of brain malformations with drainage into the spine or cervical spinal cord vascular malformations, the vertebral arteries, the thyrocervical trunk, and the deep ascending cervical arteries are also studied. Studies have shown the sensitivity of MRA for depicting dural AVF; defining the level of the blood supply indirectly via enlarged draining veins will help to focus and reduce the time catheter angiography takes.13,15,20
Spinal Vascular Disorders
Paraspinal Arteriovenous Malformations
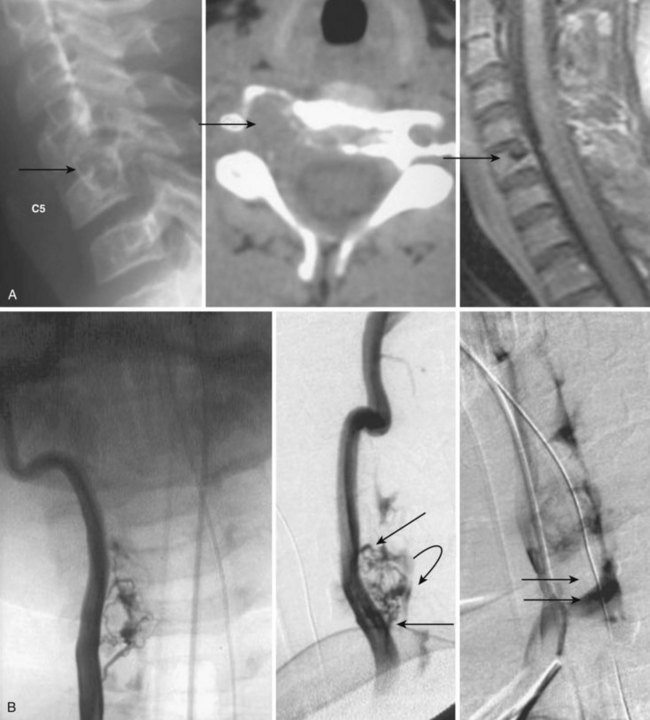
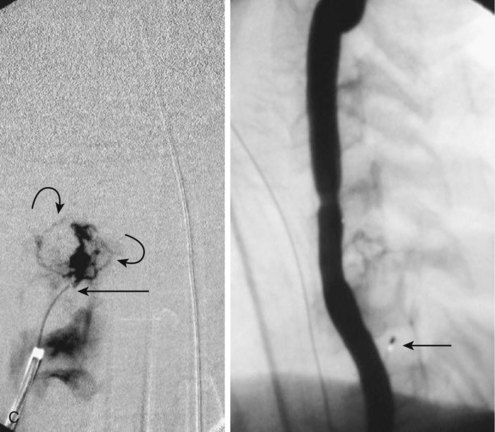
Paraspinal AVMs are rare lesions presenting with a female preponderance. They are mostly found at the thoracic or cervical level, present frequently as a fistula, and drain in enormous ectatic veins located outside the spine (Fig. 92–10). The patient can present with progressive neurologic symptoms and an audible bruit. The pulsatile venous ectasia can erode the bone, enlarge the neuroforamina, invaginate into the spinal canal, and directly compress the cord, thus mimicking an extradural tumor. If paraspinal veins communicate with intradural radicular veins and the perimedullary venous plexus, the pathologic venous drainage can create venous engorgement with congestive myelopathy.21 Paraspinal vertebrojugular fistulas can be traumatic in origin, commonly seen after motor vehicle accidents, or iatrogenic, after placement of transjugular central lines.
Imaging
MRI shows a serpiginous flow void signal corresponding to feeding artery and/or large draining veins, located outside the spine. Spinal angiography shows the supplying artery, which is usually a branch of an intercostal artery at the thoracic level or a branch from the vertebral artery when the AVM is at the cervical level.22,23 Paraspinal shunts may drain into paravertebral, epidural, or intradural venous systems. In case of a high-flow AVM(F), distal flow within the parent artery (e.g., vertebral artery) may be absent owing to the presence of shunt and steal from the contralateral vertebral artery or cervical branches. In traumatic cases the vertebral artery can be involved directly.
Epidural Arteriovenous Shunts
These are fistulas to the ventral epidural venous plexus and are usually slow-flow lesions. Those fistulas that drain only into the epidural venous system usually present as compressive myelopathy or radiculopathy owing to enlarged epidural veins. Lesions that drain primarily into the ventral epidural venous plexus and secondarily into the intradural/ medullary venous system have been reported. These lesions can cause venous hypertension or subarachnoid hemorrhage. Most of the reported cases are sacral, with arterial supply from the lateral sacral arteries.24
Imaging
Using conventional MRI, it is difficult to distinguish dural from epidural AVM. The diagnosis is determined when MRA identifies the draining veins as serpentine, linear, or curved structures around the surface of the cord.21 Intravenous injection of gadolinium-DPTA enhances the dilated veins and allows a better delineation.
Dural Arteriovenous Shunts
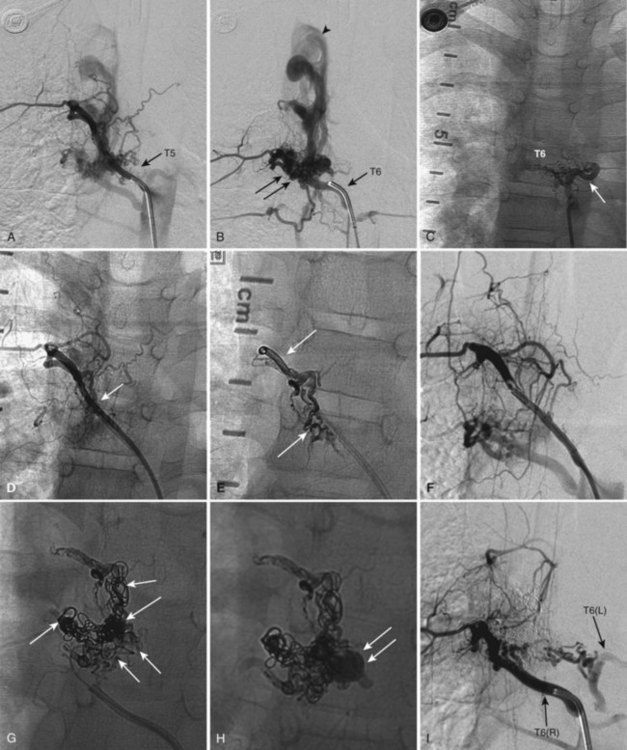
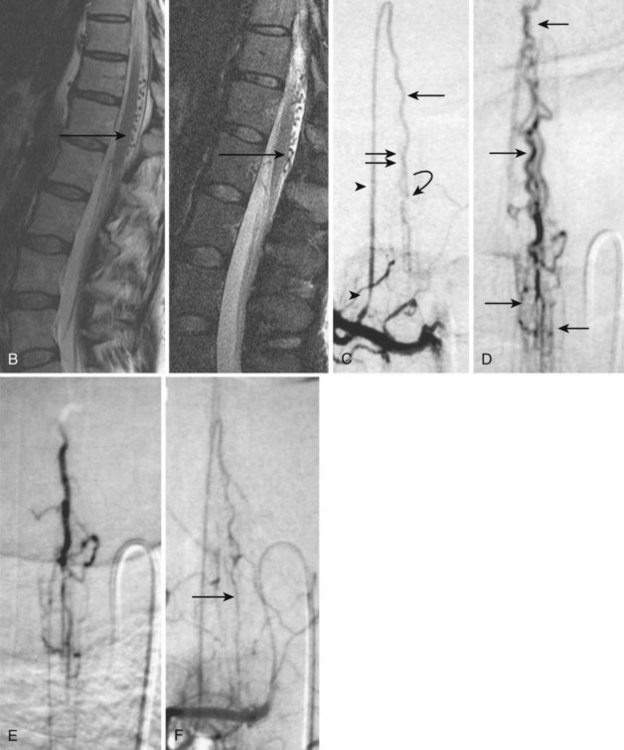
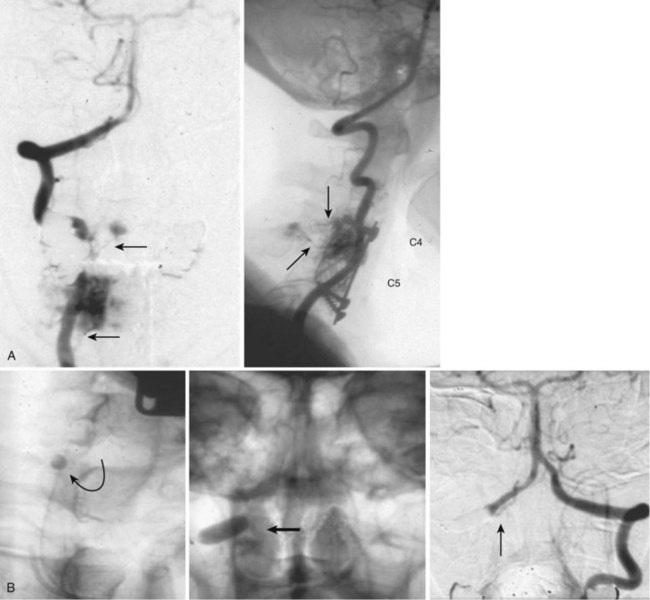
Also known as dorsal intradural AVF or type I spinal AVM, this type represents the most common of spinal vascular malformations and should be in the differential diagnosis in an adult presenting with gradually worsening myelopathy. Most authors have classified this lesion as type A if fed by a single arterial feeder and type B if fed by two or more feeders. The most common location for these shunts is between T4 and L3, with the peak incidence occurring between T7 and T12.25,26 Although reported, dural shunt malformations uncommonly occur above the level of the heart, possibly owing to the helpful effect of gravity on venous drainage above the level of the right atrium. Spinal dural AVFs are composed of tiny arterial connections between the dural branch of a radicular artery (only rarely of a radiculomedullary artery) at the level of the proximal nerve root and a radiculomedullary vein (Fig. 92–11). Branches of adjacent radicular arteries may be involved in blood supply because of an extensive intradural collateral network. The arterialized radiculomedullary vein then transmits the increased flow and pressure to the valveless coronal venous plexus and longitudinal spinal veins. Consequently, the radiculomedullary vein is enlarged and tortuous. The mean intraluminal venous pressure is increased to 74% of the systemic arterial pressure.27,28 The normal venous pressure in the coronal venous plexus is 23 mm Hg and approximately twice that of the epidural venous plexus, which is necessary for venous drainage. In one series the mean venous pressure in the coronal venous plexus was measured at 40 mm Hg.10 Because the venous hypertension affects the normal venous return and extends into venules, it finally causes a venous infarction of the spinal cord. The progressive myelopathy often leads to paraplegia and bowel, bladder, and sexual dysfunction, with gradual worsening over months to a few years. Most of the patients become severely disabled within 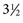 years.10,29–31
years.10,29–31
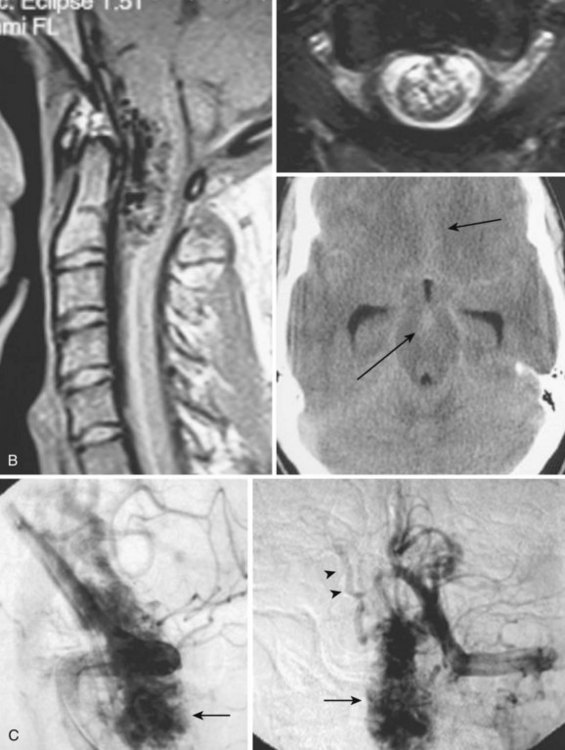
The majority of patients (79% to 85%) are men, and 86% of patients are 41 years of age or older at presentation.25,26,32,33 The mean age at presentation is 55, with patients as young as 26 years reported. The most common presentation is progressive paraparesis, with sensory changes, and complaints of back and leg pain.34 The progression is usually continuous, but it can also present in a stepwise fashion or a waxing-waning course with gradual progression. In 10% to 20% of patients the presentation is an acute exacerbation. The symptoms can be exacerbated by any physical activity that increases intra-abdominal pressure, and thus central venous pressure, as well as by an upright posture (impaired venous drainage due to hydrostatic pressure). A brain dural AVF of the posterior fossa (e.g., tentorial dural AVF) can mimic a spinal dural AVF if the venous drainage takes the pontomedullary path into the spinal cord venous system (Fig. 92–12). These lesions have been classified as type cerebral dural AVFs.35 Thus a diagnostic cerebral workup of the posterior circulation and both internal and external carotid arteries is a part of the spinal angiography. The most common misdiagnosis for these lesions is transverse myelitis, ischemic spinal cord infarction, disc disease, and spinal cord tumors. Normal cerebrospinal fluid pulsations typically dorsal to the cord on T2-weighted images may mimic a dural malformation.
Imaging
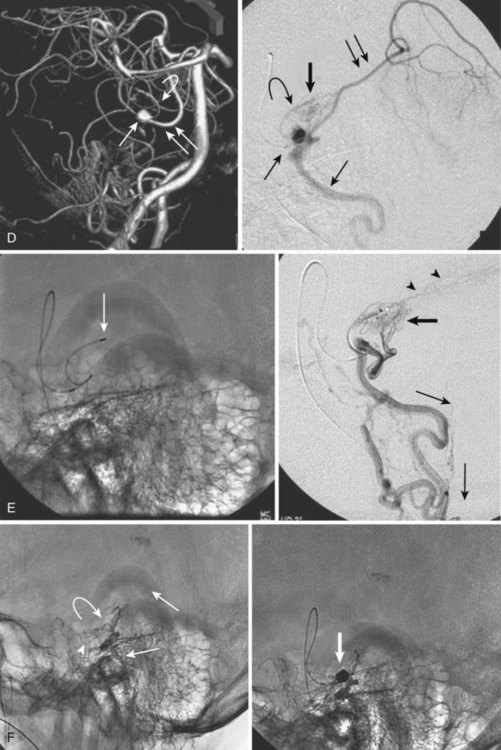
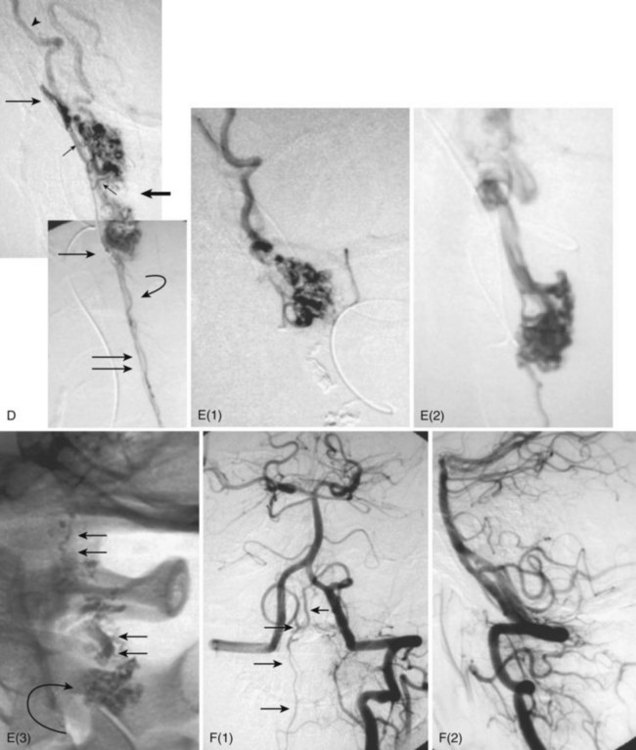
MRI identifies draining vessels as serpentine areas of flow void within the spinal canal on T2-weighted images. In axial T1- and T2-weighted magnetic resonance images, dilated coronal veins with a low signal located around the spinal cord are also visible. Enlarged radiculomedullary veins on dynamic contrast medium-enhanced MRA help to guide catheter angiography and reduce the contrast load for the patient.13 The spinal cord can appear enlarged and hypointense on T1-weighted images; the flame-shaped spinal cord edema that spares the cord periphery can be well appreciated on T2-weighted images. Because it represents venous infarction, contrast medium enhancement within edematous changes is considered prognostically bad for recovery of neurologic function. Spinal angiography shows a direct shunting of contrast agent from the radiculomeningeal artery supply into the extensive and tortuous draining radiculomedullary and perimedullary venous systems. The draining vessels are the coronal venous plexus situated along the dorsal surface of the spinal cord. Angiography also helps to delineate the ASA and guide the embolization. AVF feeding vessels can originate anywhere from vertebral artery to lateral sacral arteries (Fig. 92–13).
Treatment
A cure can be achieved by opening the dura at the affected level and surgically disconnecting the radiculomedullary vein from the dural arterial supply.34 Alternatively, a simple and safe endovascular intervention in experienced hands is effective. Endovascular therapy entails the infusion of acrylate into the radiculomedullary vein after selective microcatheterization of the feeding artery. Complete obliteration has been reported in up to 90% of patients, but with recurrence rates of up to 23%.33 The availability of newer acrylate and liquid embolic agents and more experienced physicians has diminished the recurrence rate significantly. The consensus among interventional neuroradiologists at this time is that the successful treatment of these shunts consists of acrylate penetration of the fistula and the proximal radiculomedullary draining vein without entering the spinal cord draining system (see Fig. 92–11). The protocol used in some centers is an endovascular approach as the first line of treatment because of its noninvasive nature, low complication rate, and the ability to obtain immediate angiographic control and confirmation of obliteration. If the acrylate should not penetrate the radiculomedullary veins, the patient requires a surgical disconnection; the radiopaque acrylate mixture can then be used as a landmark for fluoroscopic-guided intraoperative localization.
Pial Arteriovenous Shunts
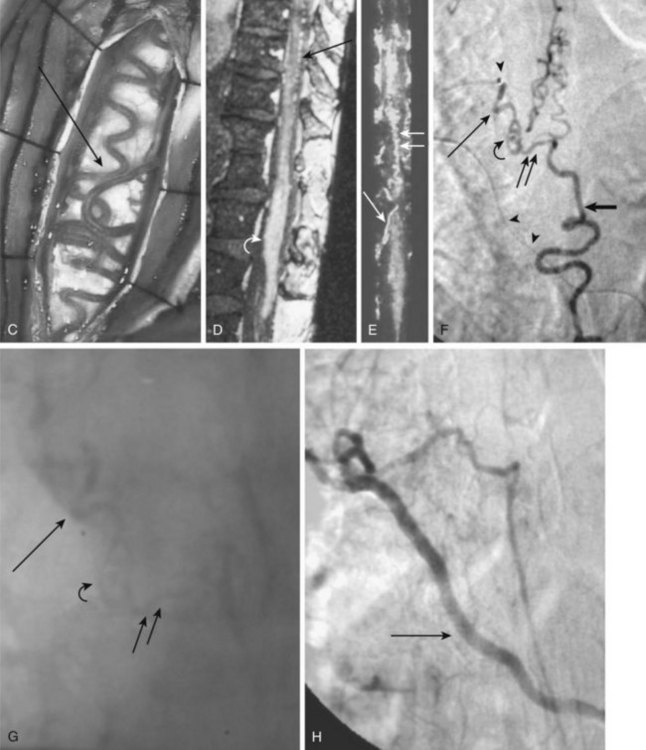
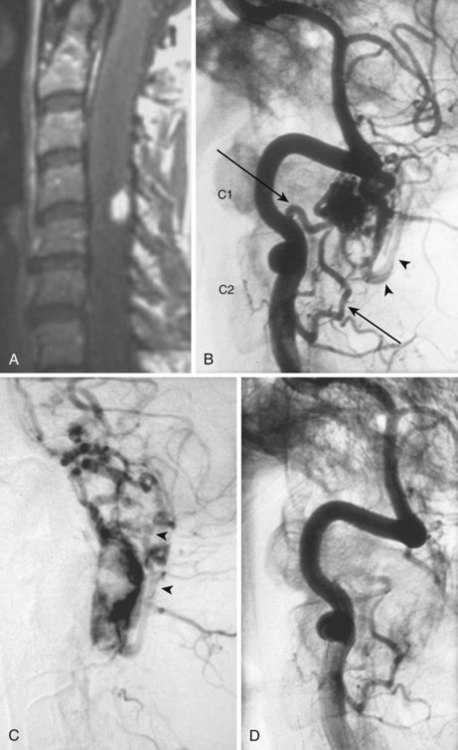
Also called ventral intradural AVF or type IV spinal AVM, pial AVMs represent a direct fistula between the ASA and the coronal venous plexus (Fig. 92–14). Radiculopial supply may also be involved. On the basis of the size of the AVF and blood flow, three different subtypes (A, B, and C) have been described.
Subtype A (Merland subtype I) represents a small shunt, with moderate venous hypertension.36 There is no enlargement of the ASA and only minimal dilatation of the ascending draining vein.10,37 The location of the fistula can be difficult to find, but it is characterized generally by the point at which a vessel caliber change between the smaller artery and the larger ectatic vein is seen.38 Owing to the change from a higher arterial impedance to a lower venous one, a change in blood flow pattern and velocity can be observed on high-speed angiograms.39,40 The ASA is the only feeder, and the AVF is typically located along the anterior aspect of the conus medullaris or proximal filum terminale.36
Subtype B (Merland subtype II) represents a moderate-sized shunt with moderate enlargement of the feeding artery or arteries and draining veins.36 The location of the fistula is marked by venous ectasia.38 Associated flow-related arterial and venous aneurysms may be present. There are several abnormally dilated feeding arteries, composed of the ASA and one or two arteries from the dorsolateral pial network (PSA), all of which converge on the fistula. These are typically located at the level of the conus. Blood returns via tortuous and dilated ascending perimedullary veins.36
Subtype C (Merland subtype III) represents a giant fistula with large arterial feeder(s) from the ASA and dorsolateral pial network (PSA) converging into the fistula and draining directly into a giant venous ectasia, often embedded within the substance of the cord.36 These fistulas are rare, although, in at least one large series, they represented the largest subtype of ventral intradural AVF.36,38 The location of the fistula is more difficult to ascertain because of the giant, ectatic draining vein.38 The giant, ectatic draining vein usually drains into the local metameric efferent veins, which are also dilated.36 These lesions are typically located at the thoracic or cervical levels.
The clinical signs and symptoms may be due to vascular steal, especially with higher flow, venous hypertension, and mass effect with venous enlargement and aneurysm formation, as well as hemorrhage.10 They almost always appear before age 40 and often present during the first decade, with mean age at diagnosis being between 11.5 and 13.5 years. Subarachnoid hemorrhage is the presenting sign in approximately 40% of patients, but according to some authors only subtype C fistulas present as hemorrhage.38,41 Occasionally, hematomyelia has also been reported. Paraparesis or paraplegia is the most common sign, with progressive deterioration over time. Radiculomyelopathy or radiculopathy can also be present, presumably owing to mass effect from dilated venous structures. These lesions can be seen anywhere along the spine. When the fistula is located ventrolaterally or posterolaterally, a significant involvement of the dorsolateral pial network (PSA) is present.
Imaging
MRI reveals low-signal feeding and draining vessels within the spinal cord by identifying flow voids on T1 and T2 weighting (see Fig. 92–14). Additionally, sagittal and coronal T1-weighted images characterize the malformation by revealing the low-signal nidus and enlarged ASA. On contrast medium–enhanced scans, pial vessels and epidural plexus can be appreciated. The spinal cord can appear enlarged and may show patchy enhancement on T1 weighting. It will appear hyperintense on T2 weighting, which corresponds to venous infarction, edema, or gliosis. Lumbar canal stenosis with engorged venous plexus or tortuous intradural roots may mimic a pial AVM. Other enhancing spinal cord masses such as ependymoma, astrocytoma, or paraganglioma must be considered.
Treatment
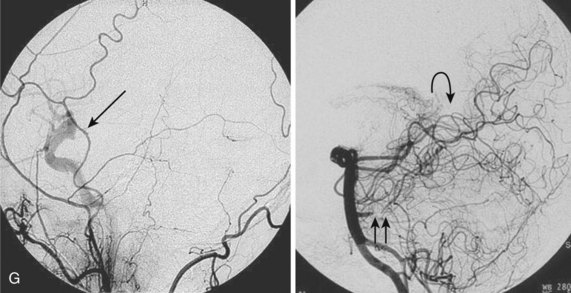
In subtype A surgical obliteration is the treatment of choice. The blood supply occurs through a minimally dilated ASA with slow flow; thus endovascular treatment is difficult to achieve and microcatheterization might be hazardous. In select cases a polyvinyl alcohol (PVA) particle embolization from a catheter positioned proximally may be considered if a coil- or acrylate-assisted occlusion of the fistula is not feasible. In subtype B a higher flow and a larger feeding artery facilitate the endovascular catheterization of the fistula and a curative acrylate infusion. Because of a high flow in subtype C, detachable balloons or fibered coils have been used in the past for a permanent obliteration of the fistula. N-butyl-2-cyanoacrylate (NBCA) may be used safely for a complete closure either alone or in conjunction with coils (see Fig. 92–14).36,41,42 In case of a complex AVF, an intraoperative transvenous embolization has been described.36,43
Conus Medullaris Arteriovenous Malformations
A new category of spinal AVMs proposed recently is characterized by multiple feeding arteries, multiple niduses, and a complex venous drainage. These lesions are composed of multiple direct arteriovenous shunts with feeders from the ASA and PSA, as well as a glomus-type nidus that is usually extramedullary/pial. The conus medullaris AVM can, however, occasionally be intramedullary.10 They are always located in the conus medullaris and cauda equina and can extend along the entire extent of the filum terminale. Symptoms can be caused by venous hypertension, venous compression of the cord/cauda equina, or hemorrhage. Because of its location, this type of spinal vascular malformation is frequently associated with radiculopathy in addition to myelopathy.
Extradural/Intradural Arteriovenous Malformations
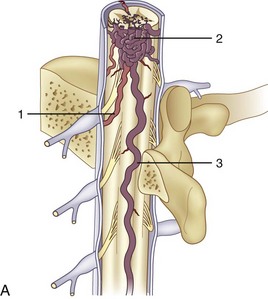
The rare form of an extradural and intradural AVM is also known as metameric or juvenile AVM (Fig. 92–15). If all derivatives of the metamere (i.e., skin, muscle, bone, dura, and cord) are involved, it is known as Cobb syndrome.
Intramedullary Arteriovenous Malformations
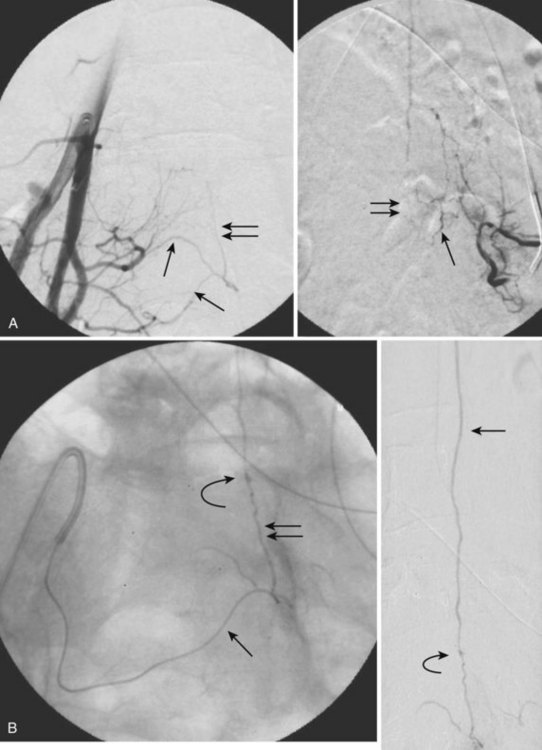
Also known as type II or classic spinal cord AVM, intramedullary AVMs are the second most common type of spinal vascular malformation. The angioarchitecture of these lesions is similar to that of the classic brain AVM, with multiple arterial feeders, a nidus, and draining veins. The nidus can be compact, known as glomus type, or diffuse, known as the juvenile type, which should not be confused with the metameric type of AVM.10 The arterial feeders are usually multiple branches of the ventral spinal axis (ASA) and/or dorsolateral pial network (PSA). Medullary AVMs can be of high flow, high pressure, and low resistance.10
The natural history of intramedullary AVMs is difficult to ascertain, but the majority of patients present before the age of 40.38 The most common presentation is an acute myelopathy due to intramedullary and/or subarachnoid hemorrhage.10,38 A proportion of patients present with intermittent or progressive myelopathy with deterioration of limb function or bowel and bladder function. The progressive myelopathy can be due to vascular steal, venous hypertension, or venous compression.10 Pain can also be a common presenting symptom in these patients. If left untreated, patients can be expected to experience an episodic but progressive deterioration due to repetitive bleeding. In an 8-year study of 60 patients, 36% of patients younger than 41 years of age and 48% of patients aged 41 to 61 were wheelchair bound within 3 years of diagnosis.44 On the basis of Djindjian’s original series of 150 patients, 13% of patients at the 5-year follow-up, 20% of patients at the 10-year follow-up, and 57% of patients at the 20-year follow-up had experienced clinical deterioration.45
Imaging
Similar to brain AVM, spinal cord AVM appears on MRI as a conglomerate of dilated, perimedullary, and intramedullary located vessels that are shown on T2-weighted sequences as flow voids and on T1-weighted images, depending on their flow velocity and direction, as mixed hyperintense/hypointense tubular structures. A venous congestive edema within the spinal cord with concomitant swelling of the cord may be present on T2-weighted images. Intraparenchymal hemorrhage, as well as subarachnoid hemorrhage, may appear as hyperintense spinal cord signal on T2 weighting, representing ischemia, edema, or gliosis.46 Selective spinal angiography is necessary to define the exact type of AVM and to plan subsequent treatment (Fig. 92–16).
Treatment
The endovascular approach is the treatment of choice because a surgical resection is difficult to achieve owing to the intramedullary location and the blood supply via tiny perforators arising from sulcocommissural branches or PSA. A staged occlusion of the proximal draining vein with acrylate as a more permanent embolic agent may be curative (see Fig. 92–16). PVA has been described as safer for palliative embolization to obtain a temporary symptomatic effect, but it may not provide a permanent cure.47–49 If a surgical resection is planned, embolization before surgery may be helpful.
Spinal Artery Aneurysms
Aneurysms of the spinal arteries are extremely rare. In more than 3000 spinal angiograms reviewed by Djindjian, only one isolated aneurysm was found.50 Subarachnoid hemorrhage of spinal origin is a rare event and accounts for less than 1% of all cases reported in the literature. Because of spontaneous obliteration of the aneurysm, the spinal angiography may remain negative.51
Neoplastic Vascular Lesions
Cavernous Malformations
Spinal cord cavernous malformations are uncommon vascular lesions and occur more frequently in females than in males, with a mean age of 36.4 years at diagnosis.52 Cavernoma or cavernous angioma is a well-circumscribed, red to purple mulberry-like lesion. Microscopically, it is composed of dilated capillaries consisting of a simple endothelial layer with thin fibrous adventitia. Typically, there is no neural parenchyma centrally and the lesion is surrounded by a variable degree of gliosis and a pseudocapsule.53 Within the lesion, there is often evidence for hyalinization, thrombosis in various stages of organization, calcification, cholesterol crystals, and cysts.54 The clinical course is variable, ranging from slowly progressive symptoms caused by a small hemorrhage or progressive symptoms due to repeated bleeding or mass effect due to capillary proliferation and vessel dilatation. Diagnosis is made by typical findings on MRI, whereas angiography is negative. Thus cavernomas are often called angiographically occult vascular malformations.
Imaging
Cavernomas of the spine have a characteristic appearance on MRI, resembling those of the brain. They appear as well-defined lesions, with a hypointense rim on T2- or proton density-weighted images and often a hyperintense center on T2-weighted images. The hypointense rim is due to magnetic susceptibility artifacts from hemosiderin deposits.46,55 The complex reticulated core with its typical mulberry-like appearance represents hemorrhage in different stages of evolution. T2-weighted, gradient-recalled-echo images will show intense blooming due to susceptibility effects. Enhancement after instillation of a contrast agent may be present.
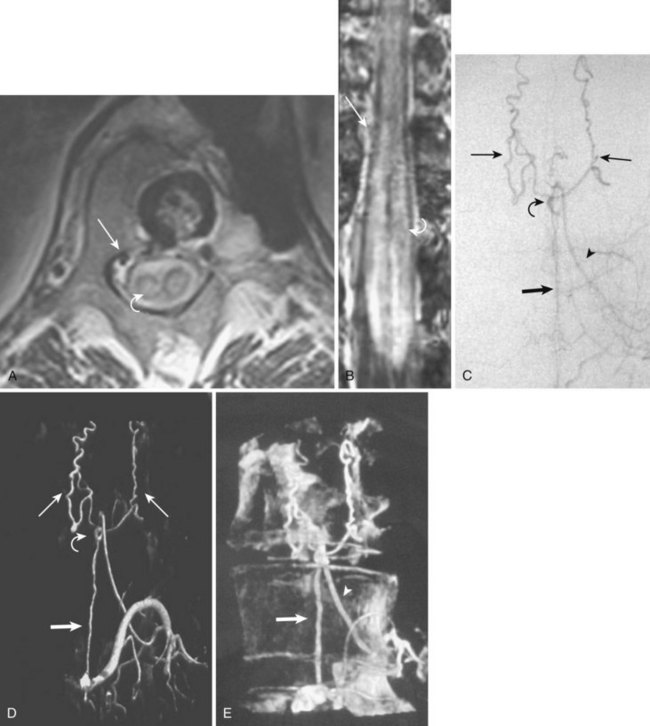
Hemangioblastoma
A hemangioblastoma is a true neoplasm of blood vessels, usually benign. It can either arise spontaneously or be associated with von Hippel-Lindau syndrome.56 In the spinal cord, hemangioblastomas constitute 3.3% of all intramedullary tumors and most commonly present in the fourth decade.57 Up to 30% of patients with spinal cord hemangioblastomas have von Hippel-Lindau syndrome. The majority of spinal hemangioblastomas (79%) are singular. The thoracic cord is the most common site, followed by the cervical cord.57
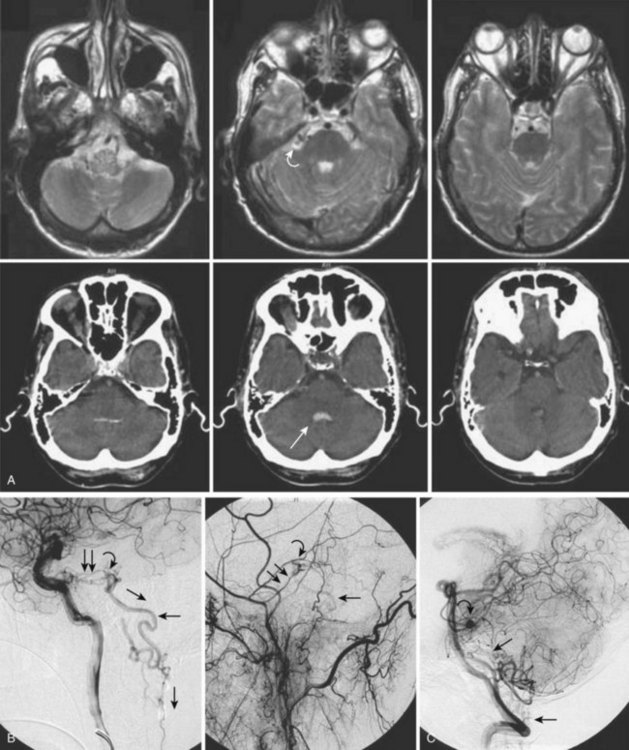
Imaging
Because these tumors are typically quite vascular, serpentine areas of signal void may be seen that represent feeding arteries or draining veins associated with the tumor nidus. The administration of paramagnetic contrast agent dramatically improves visualization of the tumor nidus, often allowing its differentiation from the adjacent edematous spinal cord (see Fig. 92–17). Diagnostic angiography shows a hypervascular mass supplied by medullary arterial feeders. In case of high blood flow velocity, they may resemble AVMs of the cord. However, unlike in spinal AVMs, there is no direct arteriovenous shunting and no individual vessels within the lesion that would be characteristic of a nidus (Fig. 92–17).
Systemic Syndromes Associated with Spinal Vascular Malformations
Osler-Weber-Rendu Syndrome (Hereditary Hemorrhagic Telangiectasia)
This autosomal dominant syndrome consists of two genotypes (types I and II). Type I is associated with mucocutaneous telangiectasia, pulmonary AVF, and arteriovenous shunts of the central nervous system. The associated spinal arteriovenous shunts are most often seen in the pediatric population and are always pial AVF (subtype C, ventral intradural AVF, or type IV). The endothelial cells in this syndrome lack the molecule endoglin and form abnormal vessels, especially after injury.60
Cobb Syndrome
This is the synonym for the complete manifestation of the metameric type of spinal vascular malformation (see also extradural/intradural AVM), where skin, muscle, bone, dura, and spinal cord are involved.61
Klippel-Trenaunay and Parkes-Weber Syndromes
These syndromes consist of vascular malformations involving primarily the lower limbs, with the following dominant features: cutaneous capillary malformation, varicose veins, and limb hypertrophy. Klippel-Trenaunay syndrome is composed mainly of venous anomalies; Parkes-Weber syndrome has more arteriovenous shunts.61 A spinal cord involvement with pial AVF or AVM can be present.
Miscellaneous Vascular Lesions of the Vertebrae
Vertebral Hemangiomas
Vertebral hemangiomas are benign vascular malformations of the bone, with a well-known and well-described appearance on conventional radiography, CT, and MRI. The incidence of hemangiomas is variable, depending on age, but has been reported at around 11% with increasing age. Up to 30% of patients have multiple lesions. Pathologically, they are considered to be postcapillary vascular dysembryogenetic malformations. Microscopically, they are divided into capillary, cavernous, and mixed types.62,63 Most of these lesions are asymptomatic and are incidental findings on MRI. Less than 1% of hemangiomas become symptomatic.62 They may present as neurologic symptoms associated with cord compression or radiculopathy. A complete involvement of the vertebral body including the body, pedicles, and laminae is present in up to 65% of cases. A smaller number of patients show a partial body and pedicle/posterior element involvement (23.2%), and 11.8% have involvement of the body only. There is a female predominance (3 : 1), and young affected adults often present with cord compression and/or radiculopathy. Most of the lesions are in the thoracic spine (>70%).62–64
Fox and colleagues63 noted that neck or back pain often preceded the neurologic symptoms, and thoracic myelopathy was the most common neurologic presentation. An additional known risk factor for development of neurologic symptoms is pregnancy, with symptoms developing in the third trimester, possibly owing to the role of estrogen and/or increased venous pressure from abdominal distention and pressure of the growing uterus on the venous structures.63 The mechanism for cord compression can be epidural extension of the lesion from the bone into the spinal canal, expansion of the bony vertebra by the hemangioma, a pathologic fracture of the vertebra, epidural hematoma from bleeding from the lesion, or compression by enlarged feeding arteries or draining veins.63 Djindjian and colleagues61 characterized vertebral hemangiomas into three groups (types A, B, and C) on the basis of clinical and imaging characteristics.
Imaging
The lesions are commonly seen incidentally on imaging studies, particularly on MRI. On T1-weighted images they present as increased signal intensity because of adipose tissue interspersed among the bone trabeculae.65 An intense contrast medium enhancement is seen in the frequently mottled-appearing vertebral body. Extraosseous components, which contain only small amounts of adipose tissue, often display a lower signal on T1-weighted images. On T2-weighted images, both intraosseous and extraosseous components present increased signal intensity, which may be difficult to differentiate from metastasis. Pathologic fracture may be present.
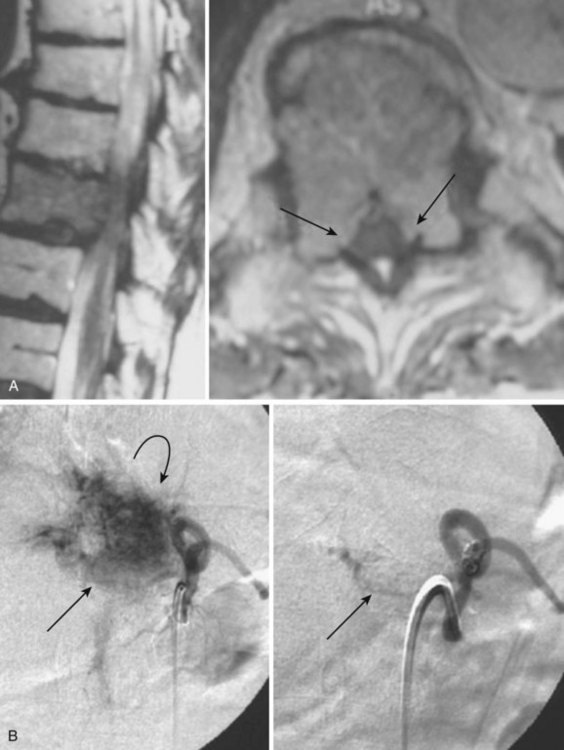
Type A
These lesions present as signs and symptoms of cord compression. Imaging shows an extraosseous extension of the lesion, usually related to an insufficiency fracture associated with the lesion weakening the vertebral body (Fig. 92–18). Angiography shows dense opacification of the vertebral body via enlarged somatic branches of a normal-sized intercostal/segmental artery. The appearance of the lesion in the vertebral body is described as dense “pools” of contrast agent appearing in the midarterial phase and persisting into the venous phase.
Treatment
The usual treatment for type A hemangiomas consists of a preoperative embolization of the lesion with particles and/or NBCA infusion directly into the hemangioma and operative decompression of the spinal cord/canal, possibly with a resection of the lesion and spinal reconstruction and stabilization. Doppman and colleagues62 made the important observation that even in these lesions with epidural extension, the lesion does not penetrate the dura but is confined by the periosteum, which results in the characteristic bilobed posterior margin of these lesions, indented centrally by the posterior longitudinal ligament. An additional treatment option for these patients for whom timely treatment is a medical necessity is the technique of percutaneous transpedicular injection of ethanol.63 With current CT or MRI technology, a percutaneous approach through the pedicle can be accomplished. The tip of the needle is usually positioned at the vertebropedicular junction. Contrast medium–enhanced CT or MRI may be helpful for the intervention. After needle placement, contrast agent is injected to show the opacification of the lesion. Subsequently, dehydrated ethanol opacified with metrizamide powder is forcefully injected. Anesthesia is recommended because of the pain associated with ethanol injection. For lower thoracic and upper lumbar lesions, the artery of Adamkiewicz must be delineated on arterial angiograms before an intervention to prevent inadvertent cord ischemia. An improvement of symptoms can be seen within 1 to 2 days after the treatment. Follow-up MRI shows the shrinkage of lesions.
Type B
These lesions are associated with local pain and tenderness over the involved vertebral body and/or radicular signs. Imaging does not reveal any extraosseous extension. The angiographic appearance is similar to type A lesions, which are generally large. The first step in the evaluation of these lesions is to exclude the more common causes of back pain, with the help of imaging and physical examination.63 Imaging further helps to exclude involvement of the posterior element, cortical disruption, and epidural spread of the lesion. In the absence of these findings, percutaneous vertebroplasty with polymethyl methacrylate (PMMA) is the treatment of choice. Other treatment options include the endovascular transarterial embolization of the lesion using particles or NBCA or ethanol. Embolization can be effective in as high as 60% to 100% of patients treated.63 Reizine and colleagues66 suggested that if a painful lesion is located in the cervical or lumbar spine, without involvement of the posterior elements or cortical disruption, these lesions could be considered as nonevolutive (without potential for future growth causing cord compression). On the other hand, if a painful lesion is located in the thoracic spine, especially in a young patient, and shows involvement of the posterior elements, cortical disruption, or soft tissue extension, it should be considered for potential growth and future cord compression.
Type C
These lesions represent the vast majority of hemangiomas, which are incidental findings and not associated with any symptoms. The angiogram only shows normal vertebral body enhancement.38 Unless the patient develops symptoms (i.e., pain and/or neurologic deficits), follow-up imaging or additional studies are not necessary.63 An exception can be made for large lesions in a young patient, with the chance of further growth. A yearly follow-up may be considered, but no treatment is necessary unless symptoms develop.
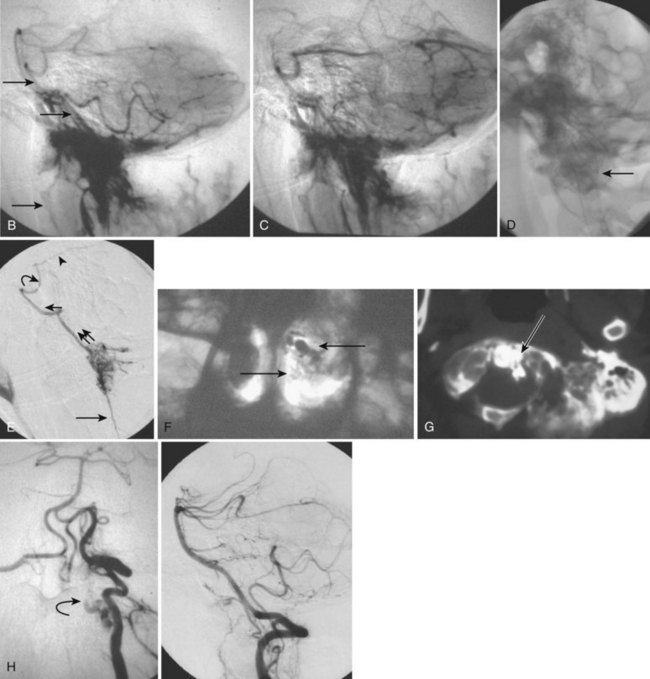
Aneurysmal Bone Cysts
Aneurysmal bone cysts are benign lesions of bones that primarily affect young people; 80% of patients present when younger than the age of 20. There is no sex predilection. Although aneurysmal bone cysts can occur at any location, 90% are seen in the spine. Within the spine, most lesions involve the posterior elements; however, the vertebral body can also be involved. Additionally, aneurysmal bones cysts can involve two contiguous vertebral bodies.67 With regard to pathogenesis, most authors believe that a hemodynamic imbalance or abnormality within the bone is the etiologic factor, especially with regard to impaired venous drainage.68,69 Some have suggested the presence of a congenital vascular abnormality in cases of de novo aneurysmal bone cysts and an impairment of venous drainage by a secondary factor (associated lesions or trauma) in other cases.70
Imaging
The radiographic appearance of aneurysmal bone cysts is characteristic (Fig. 92–19). Pathologically, the lesions consist of enlarged, septated, but communicating spaces within the bone, containing venous blood under higher than normal venous pressure. Fluid/fluid levels may be present on T1- and T2-weighted images resulting from blood products. Because of a rim of periosteum, a hypointense rim around the mass may be seen. The expansile remodeling of the vertebral body and the thinned cortex with absent tumor matrix can be appreciated. CT is excellent for finalizing the diagnosis. The lining of the spaces consists of a fibro-osseous patchwork and some giant cells.67 Other differential diagnoses include osteoblastoma, metastases, and telangiectatic osteogenic sarcoma. Interestingly, up to one third of aneurysmal bone cysts are found in conjunction with other lesions such as fibrous dysplasia, osteoblastoma, or chondrosarcoma,70 whereas others may be associated with previous trauma.69 Angiographically, there is no pathognomonic pattern for aneurysmal bone cysts (see Fig. 92–19). Findings can vary from a faint or moderate vascularity to a dense vascularity with a rich network of dilated, tortuous feeding vessels and a dense stain of the lesion within the vertebral body.67 Djindjian described arteriovenous shunting in some lesions, whereas others have described patchy collections of contrast agent within the cystic spaces, persisting into the late venous phase.71
Treatment
The most common approach to symptomatic aneurysmal bone cysts is surgery, whether with curettage or resection of the lesion and reconstruction of the spine, if necessary. In many cases, owing to the vascularity of the lesion, the operating surgeon will request a preoperative angiography and embolization of the lesion to decrease intraoperative blood loss, which can be significant (see Fig. 92–19). An endovascular embolization can be the sole therapy for aneurysmal bone cysts.72 Long-term follow-up data after embolization show an almost complete healing of the lesion and restoration of the normal shape of the affected bone. Thus the patient may not require any subsequent surgery. If present, an endovascular embolization of an aneurysmal bone cyst can, besides reduce the size, relieve the primary pain symptom.73 More recently, percutaneous injection of PMMA under fluoroscopic guidance has been advocated for cure. Resection of the lesion and resection of the spine is the treatment of choice when the lesion is symptomatic and if interventional treatment is not considered. In many cases, to decrease intraoperative blood loss, preoperative angiography and embolization may be necessary.
Spinal Metastasis
Neoplastic and metastatic lesions can involve the vertebral bodies, as well as intramedullary and extramedullary structures. The goal of an endovascular treatment remains a devascularization before a planned surgery or biopsy (Fig. 92–20). This significantly reduces the blood loss and improves the surgical resection.74–77 Because the embolization is performed using Gelfoam, PVA, or, occasionally dehydrated ethanol, attention must be paid to a potential supply of radiculomedullary/pial arteries to the ASA or PSA. An embolization can, rarely, lead to tumor necrosis with subsequent swelling and spinal cord compression; a preprocedural high-dose corticosteroid medication has been suggested.78 On rare occasions and in nonsurgical patients, embolization helps to reduce pain and treat radicular compression.79 Although a reduction of tumor growth may be seen, embolization for spinal metastasis and malignant spinal tumors is not curative.
Treatment
An endovascular or direct percutaneous embolization of a vertebral body metastasis or malignant tumor can be achieved. The latter can be performed under CT or fluoroscopic guidance using NBCA,80 PMMA, or dehydrated ethanol.81,82 Use of PMMA can also provide biomechanical stability of the vertebral body.83
Pearls
Key Points
1 Berenstein A, Lasjaunias P. Surgical Neuro-angiography: Endovascular Treatment of Spine and Spinal Cord Lesions, vol 5. New York: Springer-Verlag. 1992.
The authors summarize the endovascular approach to spinal vascular disorders.
2 Biondi A, Merland JJ, Hodes JE, et al. Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations: II. Results of AVM endovascular treatment and hemodynamic consideration. AJNR Am J Neuroradiol. 1992;13:923-931.
This article is a summary of a vast experience in endovascular treatment of spinal AVMs.
3 Djindjian R. Clinical symptomatology and natural history of arteriovenous malformations of the spinal cord: A study of the clinical aspects and prognosis based on 150 cases. In: Pia H, Djindjian R, editors. Spinal Angiomas: Advances in Diagnosis and Therapy. Berlin: Springer-Verlag, 1978.
This is an excellent summary of spinal AVMs from one of the pioneers in this field.
4 Lasjaunias P, Berenstein A, ter Brugge KG. Surgical Neuroangiography, 2nd ed. Berlin: Springer-Verlag; 2001.
This is an excellent reference book for spinal vascular anatomy.
5 Merland JJ, Reizine D, Laurent A, et al. Embolization of spinal cord vascular lesions. In: Viñuela F, Halbach VV, Dion JE, editors. Interventional Neuroradiology: Endovascular Therapy of the Central Nervous System. New York: Raven Press; 1992:153-165.
This reflects the experience from one of the leading schools in endovascular treatment of the spine.
6 Saraf-Lavi E, Bowen BC, Quencer RM, et al. Detection of spinal dural arteriovenous fistulae with MR imaging and contrast-enhanced MR angiography: Sensitivity, specificity, and prediction of vertebral level. AJNR Am J Neuroradiol. 2002;23:858-867.
7 Spetzler RF, Detwiler PW, Riina HA, et al. Modified classification of spinal cord vascular lesions. J Neurosurg Spine. 2002;96(Suppl):145-156.
1 Lasjaunias P, Berenstein A, ter Brugge KG. Surgical Neuroangiography, 2nd edition (vol 1: Clinical Vascular Anatomy and Variations). Berlin: Springer-Verlag; 2001.
2 Krauss WE. Vascular anatomy of the spinal cord. Neurosurg Clin N Am. 1999;10(1):9-15.
3 Hyodoh H, Shirase R, Akiba H, et al. Double-subtraction maximum intensity projection MR angiography for detecting the artery of Adamkiewicz and differentiating it from the drainage vein. Journal of Magnetic Resonance Imaging: JMRI. 2007;26:359-365.
4 Nijenhuis RJ, Jacobs MJ, Schurink GW, et al. Magnetic resonance angiography and neuromonitoring to assess spinal cord blood supply in thoracic and thoracoabdominal aortic aneurysm surgery. Journal of Vascular Surgery. 2007;45:71-77. discussion 7-8–7; discussion 7-8 Official Publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter
5 Sheehy NP, Boyle GE, Meaney JFM. Normal anterior spinal arteries within the cervical region: high-spatial-resolution contrast-enhanced three-dimensional MR angiography. Radiology. 2005;236:637-641.
6 Bowen BC. MR angiography of spinal vascular disease: what about normal vessels? AJNR Am J Neuroradiol. 1999;20:1773-1774.
7 Farb RI, Kim JK, Willinsky RA, et al. Spinal dural arteriovenous fistula localization with a technique of first-pass gadolinium-enhanced MR angiography: initial experience. Radiology. 2002;222:843-850.
8 Binkert CA, Kollias SS, Valavanis A. Spinal cord vascular disease: characterization with fast three-dimensional contrast-enhanced MR angiography. AJNR Am J Neuroradiol. 1999;20:1785-1793.
9 Yoss RE. Vascular supply of the spinal cord: The production of vascular syndromes. Med Bull (Ann Arbor). 1950;16:333-345.
10 Spetzler RF, Detwiler PW, Riina HA, et al. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(2 Suppl):145-156.
11 Germans MR, Pennings FA, Sprengers MES, et al. Spinal vascular malformations in non-perimesencephalic subarachnoid hemorrhage. J Neurol. 2008;255:1910-1915.
12 Prestigiacomo CJ, Niimi Y, Setton A, et al. Three-dimensional rotational spinal angiography in the evaluation and treatment of vascular malformations. AJNR Am J Neuroradiol. 2003;24:1429-1435.
13 Saraf-Lavi E, Bowen BC, Quencer RM, et al. Detection of spinal dural arteriovenous fistulae with MR imaging and contrast-enhanced MR angiography: sensitivity, specificity, and prediction of vertebral level. AJNR Am J Neuroradiol. 2002;23:858-867.
14 Mascalchi M, Ferrito G, Quilici N, et al. Spinal vascular malformations: MR angiography after treatment. Radiology. 2001;219:346-353.
15 Luetmer PH, Lane JI, Gilbertson JR, et al. Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR Angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol. 2005;26:711-718.
16 Mull M, Nijenhuis RJ, Backes WH, et al. Value and limitations of contrast-enhanced MR angiography in spinal arteriovenous malformations and dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2007;28:1249-1258.
17 Backes WH, Nijenhuis RJ. Advances in spinal cord MR angiography. AJNR Am J Neuroradiol. 2008;29(4):619-631.
18 Ou P, Schmit P, Layouss W, et al. CT angiography of the artery of Adamkiewicz with 64-section technology: first experience in children. AJNR Am J Neuroradiol. 2007;28:216-219.
19 Yamaguchi S, Eguchi K, Kiura Y, et al. Multi-detector-row CT angiography as a preoperative evaluation for spinal arteriovenous fistulae. Neurosurg Rev. 2007;30:321-326. discussion 7–6; discussion 7
20 Pattany PM, Saraf-Lavi E, Bowen BC. MR angiography of the spine and spinal cord. Top Magn Reson Imaging. 2003;14:444-460.
21 Rodesch G, Lasjaunias P. Spinal cord arteriovenous shunts: from imaging to management. Eur J Radiol. 2003;46:221-232.
22 Chen CJ, Huang CC, Hsu YY, et al. Small isolated paraspinal arteriovenous fistula. AJNR Am J Neuroradiol. 1997;18:359-361.
23 Cognard C, Semaan H, Bakchine S, et al. Paraspinal arteriovenous fistula with perimedullary venous drainage. AJNR Am J Neuroradiol. 1995;16:2044-2048.
24 Niimi Y, Berenstein A. Endovascular treatment of spinal vascular malformations. Neurosurg Clin N Am. 1999;10:47-71.
25 Oldfield EH, Bennett A, Chen MY, et al. Successful management of spinal dural arteriovenous fistulas undetected by arteriography. Report of three cases. J Neurosurg. 2002;96(2 Suppl):220-229.
26 Oldfield EH, Di Chiro G, Quindlen EA, et al. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg. 1983;59:1019-1030.
27 Eskandar EN, Borges LF, Budzik RF, et al. Spinal dural arteriovenous fistulas: experience with endovascular and surgical therapy. J Neurosurg. 2002;96(2 Suppl):162-167.
28 Hassler W, Thron A, Grote EH. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg. 1989;70:360-370.
29 Merland JJ, Reizine D. Treatment of Arteriovenous Spinal-Cord Malformations. Semin Interv Radiol. 1987;4:281-290.
30 Merland JJ, Reizine D, Laurent A, et al. Embolization of spinal cord vascular lesions. Interventional Neuroradiology: Endovascular Therapy of the Central Nervous System. New York: Raven Press; 1992. p. 153-165
31 Merland JJ, Riche MC, Chiras J. Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol. 1980;7:271-320.
32 Niimi Y, Berenstein A, Setton A, et al. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997;40:675-682. discussion 682-683
33 Van Dijk JMC, TerBrugge KG, Willinsky RA, et al. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke. 2002;33:1578-1583.
34 Atkinson JL, Miller GM, Krauss WE, et al. Clinical and radiographic features of dural arteriovenous fistula, a treatable cause of myelopathy. Mayo Clin Proc. 2001;76:1120-1130.
35 Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
36 Mourier KL, Gobin YP, George B, et al. Intradural perimedullary arteriovenous fistulae: results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993;32:885-891. discussion 891
37 Bao YH, Ling F. Classification and therapeutic modalities of spinal vascular malformations in 80 patients. Neurosurgery. 1997;40:75-81.
38 Hodes JE, Merland JJ, Casasco A, et al. Spinal vascular malformations: endovascular therapy. Neurosurg Clin N Am. 1999;10:139-152.
39 Wakhloo AK, Lieber BB, Rudin S, et al. A novel approach to flow quantification in brain arteriovenous malformations prior to enbucrilate embolization: use of insoluble contrast (Ethiodol droplet) angiography. J Neurosurg. 1998;89:395-404.
40 Rudin S, Wakhloo AK, Lieber BB, et al. Microdroplet tracking using biplane digital subtraction angiography for cerebral arteriovenous malformation blood flow path and velocity determinations. AJNR Am J Neuroradiol. 1999;20:1110-1114.
41 Ricolfi F, Gobin PY, Aymard A, et al. Giant perimedullary arteriovenous fistulas of the spine: clinical and radiologic features and endovascular treatment. AJNR Am J Neuroradiol. 1997;18:677-687.
42 Cho KT, Lee DY, Chung CK, et al. Treatment of spinal cord perimedullary arteriovenous fistula: embolization versus surgery. Neurosurgery. 2005;56:232-241.
43 Touho H, Monobe T, Ohnishi H, et al. Treatment of type II perimedullary arteriovenous fistulas by intraoperative transvenous embolization: case report. Surg Neurol. 1995;43:491-496.
44 Aminoff MJ, Logue V. Clinical features of spinal vascular malformations. Brain. 1974;97:197-210.
45 Djindjian R. Clinical symptomatology and natural history of arteriovenous malformations of the spinal cord: A study of the clinical aspects and prognosis based on 150 cases. In: Djindjian R, Pia H, editors. Spinal Angiomas: Advances in Diagnosis and Therapy. Berlin: Springer-Verlag, 1978.
46 Krings T, Mull M, Gilsbach JM, et al. Spinal vascular malformations. Eur Radiol. 2005;15:267-278.
47 Biondi A, Merland JJ, Hodes JE, et al. Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations. II. Results of AVM endovascular treatment and hemodynamic considerations. AJNR Am J Neuroradiol. 1992;13:923-931.
48 Biondi A, Merland JJ, Hodes JE, et al. Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations. I. Angiographic and clinical aspects. AJNR Am J Neuroradiol. 1992;13:913-922.
49 Biondi A, Merland JJ, Reizine D, et al. Embolization with particles in thoracic intramedullary arteriovenous malformations: long-term angiographic and clinical results. Radiology. 1990;177:651-658.
50 Berenstein A, Lasjaunias P, terBrugge KG. 1st edition. Surgical Neuroangiography: Endovascular Treatment of Spine and Spinal Cord Lesions, vol 5. Berlin: Springer-Verlag. 1992. p. 33
51 Berlis A, Scheufler K-M, Schmahl C, et al. Solitary spinal artery aneurysms as a rare source of spinal subarachnoid hemorrhage: potential etiology and treatment strategy. AJNR Am J Neuroradiol. 2005;26:405-410.
52 Canavero S, Pagni CA, Duca S, et al. Spinal intramedullary cavernous angiomas: a literature meta-analysis. Surg Neurol. 1994;41:381-388.
53 Rigamonti D, Johnson PC, Spetzler RF, et al. Cavernous malformations and capillary telangiectasia: a spectrum within a single pathological entity. Neurosurgery. 1991;28:60-64.
54 Maraire JN, Awad IA, Batjer HH, et al. Cavernous malformations: Natural history and indications for treatment. Cerebrovascular Disease. Philadelphia: Lippincott-Raven; 1997. p. 669-690
55 Zevgaridis D, Medele RJ, Hamburger C, et al. Cavernous haemangiomas of the spinal cord. A review of 117 cases. Acta Neurochir (Wien). 1999;141:237-245.
56 Neumann HP, Eggert HR, Scheremet R, et al. Central nervous system lesions in von Hippel-Lindau syndrome. J Neurol Neurosurg Psychiatry. 1992;55:898-901.
57 Sze G. Neoplastic disease of the spine and spinal cord. In: Atlas SW, editor. Magnetic Resonance Imaging of the Brain and Spine. 2nd edition. Philadelphia: Lippincott-Raven; 1996:1377-1378.
58 Tampieri D, Leblanc R, TerBrugge K. Preoperative embolization of brain and spinal hemangioblastomas. Neurosurgery. 1993;33:502-505. discussion 505
59 Eskridge JM, McAuliffe W, Harris B, et al. Preoperative endovascular embolization of craniospinal hemangioblastomas. AJNR Am J Neuroradiol. 1996;17:525-531.
60 Rodesch G, Hurth M, Alvarez H, et al. Classification of spinal cord arteriovenous shunts: proposal for a reappraisal—the Bicêtre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery. 2002;51:374-379. discussion 379-380
61 Djindjian R, Merland JJ, Djindjian M, et al. Vertebral hemangiomas and metameric angiomatosis (Cobb’s syndrome. In: Nadjmi M, Piepgras U, Vogelsang H, editors. Angiography of Spinal Column and Spinal Cord Tumors. Stuttgart: Georg Thieme, 1981.
62 Doppman JL, Oldfield EH, Heiss JD. Symptomatic vertebral hemangiomas: treatment by means of direct intralesional injection of ethanol. Radiology. 2000;214:341-348.
63 Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78:36-45.
64 Jayakumar PN, Vasudev MK, Srikanth SG. Symptomatic vertebral haemangioma: endovascular treatment of 12 patients. Spinal Cord. 1997;35:624-628.
65 Ross JS, Masaryk TJ, Modic MT, et al. Vertebral hemangiomas: MR imaging. Radiology. 1987;165:165-169.
66 Reizine D, Laouiti M, Guimaraens L, et al. Vertebral arteriovenous fistulas. Clinical presentation, angiographical appearance and endovascular treatment. A review of twenty-five cases. Ann Radiol (Paris). 1985;28:425-438.
67 Berenstein A, Lasjaunias P, terBrugge KG. 1st edition. Surgical Neuroangiography: Endovascular Treatment of Spine and Spinal Cord Lesions, vol 5. Berlin: Springer-Verlag. 1992. p. 125-127
68 Lichtenstein L. Aneurysmal bone cyst. A pathological entity commonly mistaken for giant-cell tumor and occasionally for hemangioma and osteogenic sarcoma. Cancer. 1950;3:279-289.
69 Ameli NO, Abbassioun K, Saleh H, et al. Aneurysmal bone cysts of the spine. Report of 17 cases. J Neurosurg. 1985;63:685-690.
70 Bonakdarpour A, Levy WM, Aegerter E. Primary and secondary aneurysmal bone cyst: a radiological study of 75 cases. Radiology. 1978;126:75-83.
71 Lum C, terBrugge KG. Intervention in vascular lesions of the vertebrae. Semin Interv Radiol. 2002;19:245-256.
72 Cigala F, Sadile F. Arterial embolization of aneurysmal bone cysts in children. Bull Hosp Jt Dis. 1996;54:261-264.
73 Radanovic B, Simunic S, Stojanovic J, et al. Therapeutic embolization of aneurysmal bone cyst. Cardiovasc Intervent Radiol. 1989;12:313-316.
74 Broaddus WC, Grady MS, Delashaw JB, et al. Preoperative superselective arteriolar embolization: a new approach to enhance resectability of spinal tumors. Neurosurgery. 1990;27:755-759.
75 King GJ, Kostuik JP, McBroom RJ, et al. Surgical management of metastatic renal carcinoma of the spine. Spine. 1991;16:265-271.
76 Gellad FE, Sadato N, Numaguchi Y, et al. Vascular metastatic lesions of the spine: preoperative embolization. Radiology. 1990;176:683-686.
77 Hilal SK, Michelsen JW. Therapeutic percutaneous embolization for extra-axial vascular lesions of the head, neck, and spine. J Neurosurg. 1975;43:275-287.
78 Jensen ME, Hendrix LE, Dion JE, et al. Preoperative and palliative embolization of vertebral body metastases. In: Proceedings of the 31st Annual Meeting of the American Society of Neuroradiology. Vancouver: British Columbia; 1993.
79 Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology. 2003;226:366-372.
80 Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol. 1994;15(1):83-86.
81 Chiras J, Cognard C, Rose M, et al. Percutaneous injection of an alcoholic embolizing emulsion as an alternative preoperative embolization for spine tumor. AJNR Am J Neuroradiol. 1993;14:1113-1117.
82 Heiss JD, Doppman JL, Oldfield EH. Brief report: relief of spinal cord compression from vertebral hemangioma by intralesional injection of absolute ethanol. N Engl J Med. 1994;331:508-511.
83 Cotten A, Deramond H, Cortet B, et al. Preoperative percutaneous injection of methyl methacrylate and N-butyl cyanoacrylate in vertebral hemangiomas. AJNR Am J Neuroradiol. 1996;17:137-142.