Chapter 5 Vascular access for percutaneous coronary intervention
 Percutaneous transfemoral intervention is still employed by many cardiologists although this is associated with a significant risk of access site neurovascular complication.
Percutaneous transfemoral intervention is still employed by many cardiologists although this is associated with a significant risk of access site neurovascular complication. The percutaneous transradial approach is the safest route available for percutaneous coronary intervention even in the setting of multiple potent antiplatelet agents.
The percutaneous transradial approach is the safest route available for percutaneous coronary intervention even in the setting of multiple potent antiplatelet agents. Cost benefit, improved short-term quality of life and patient preference all favour the transradial approach.
Cost benefit, improved short-term quality of life and patient preference all favour the transradial approach.INTRODUCTION
The arterial access site chosen for PCI can have an important influence on procedural costs and procedural related morbidity and mortality.1 Access site complications can cause major disability and death. With the exponential rise in PCI procedures and the use of multiple potent antiplatelet agents as standard practice, containing access site complications is an important clinical challenge. Until the late 1980s, most cardiologists utilised the femoral or brachial artery as their access site of choice. The femoral artery has been the preferred route of access ever since the percutaneous Seldinger technique superseded the more technically challenging brachial cut-down approach. There are, however, well documented vascular access complications with the femoral approach especially with concurrent use of glycoprotein (GP) IIb/IIIa inhibitors.2 It has, therefore, become increasingly apparent that a safer route of arterial access would be highly desirable.1
The radial artery has been widely used for many years for haemodynamic monitoring with a low risk of significant neurovascular complications. Two important anatomical features contribute to reducing the risk profile of this access site. First, the vessel is superficial and, in the majority of patients, is not an end artery. Radial artery occlusion does not, therefore, result in major ischaemic complications. Haemostasis is easily achieved by pressure over the point of arterial puncture, while any bleeding is easily recognised allowing prompt action. Second, no major veins or nerves lie close to the radial artery, limiting the risk of neurological damage or arterio-venous fistula formation. The development of small calibre diagnostic catheters facilitated the use of the radial artery for coronary angiography, first described by Campeau in 1989.3 The success of these diagnostic cardiac procedures has led other cardiologists to explore the use of the radial artery as an access site for PCI.4
A COMPARISON OF ARTERIAL ACCESS SITES FOR CORONARY INTERVENTION
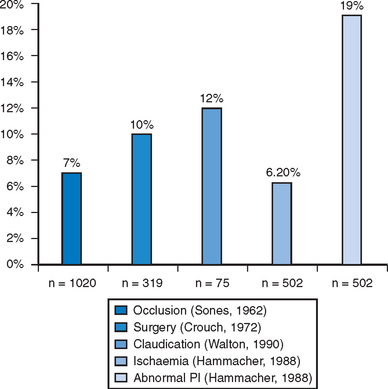
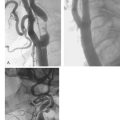
When a surgical cut down approach to the brachial artery is employed for cardiac procedures, operator skill and experience are important factors in limiting the rate of complications associated with this technically demanding approach. Skilled high volume operators can achieve low complication rates even in the setting of intensive antithrombotic therapy.5 For less skilled or infrequent operators, most series consistently reported a 5–10% incidence of major complications1,6–11 (Fig. 5.1). Major neurovascular complications resulting in acute arm ischaemia or median nerve palsy occur in around 5% of patients (Fig. 5.2). An alternative method to this approach employs a percutaneous Seldinger technique to position a sheath in the brachial artery. This technique is technically much simpler than a surgical cut down, but is associated with a similar risk of important neurovascular complications.11 Because of these issues brachial access is now rarely employed.
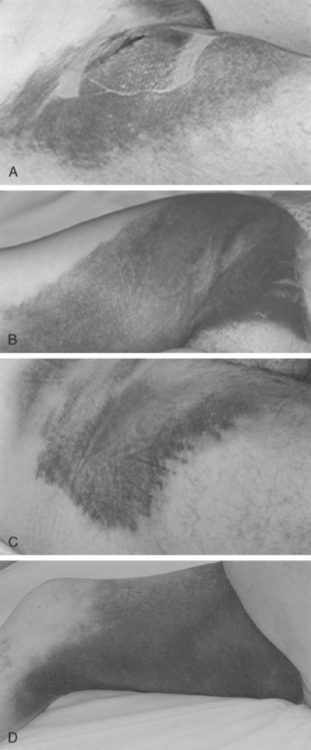
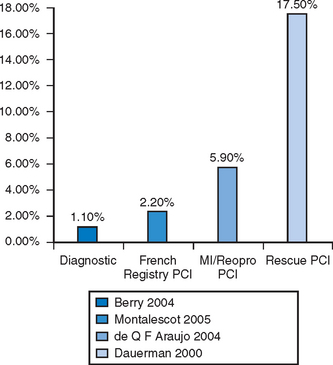
Following the introduction of the Seldinger technique, the percutaneous femoral approach revolutionized the practice of invasive cardiology and remains the access of choice in many institutions.12 The femoral approach facilitates rapid and simple access to the left side of the heart and usually facilitates good catheter support as well as access to large-diameter devices. Such advantages are partially offset by bleeding complications, often mandating prolonged bed rest and further treatment (including compression or thrombin injection for a pseudoaneurysm, blood transfusion or surgical intervention) (Figs. 5.3 and 5.4). These can lead to further discomfort and a longer hospital stay, consuming additional institutional resources. In a minority of patients femoral vascular complications can be severe and lead to death. The incidence of significant neurovascular complications ranges from 1% following a simple diagnostic procedure to 17% when large bore catheters are employed in association with aggressive antithrombotic therapy in PCI (Fig. 5.5).1,13–17 Concealed retroperitoneal bleeding, although uncommon, is an ominous complication that has a reported mortality rate of 15%.18 One-third of patients who sustain an iatrogenic femoral nerve injury related to a cardiac procedure have a permanent neurological deficit.19 These problems have not been resolved with the use of vascular closure device. A meta-analysis of 30 randomised trials (with a total sample size of 4000 patients) indicated that vascular closure device increases the risk of femoral access site complications compared to manual compression.20 In addition to these complications, access via a brachial or femoral access site is impossible in 5–10% of patients, due to anatomical variation, peripheral vascular disease or obesity, and the radial access site may allow such patients to be investigated and treated.21
Multiple studies have compared the radial approach with femoral or brachial access. The best know study, The Access Trial22 examined the relative merits of the percutaneous brachial, femoral and radial access sites in 900 patients undergoing elective PCI. It demonstrates that the radial approach is the safest, with no significant vascular complications occurring, compared to rates of 2% in the femoral group and 2.3% in the brachial group. There was no increase in total procedure duration or radiation exposure when transradial procedures were compared with percutaneous femoral procedures. A recent meta-analysis of 12 randomized control trials further confirmed that the transradial approach is a highly safe technique with comparable procedural duration, radiation exposure and clinical results to that of the transfemoral approach.23 More importantly, vascular access site complications are virtually abolished (0.3%) by the transradial approach.
Given the demonstrated reduction in the risk of vascular complications, the radial artery is a particularly attractive option in the setting of anticoagulation, post thrombolysis or aggressive antiplatelet therapy. Hildick-Smith et al reported low rate of radial access complications in fully anticoagulated patients with INR >2 who had a transradial coronary angiography.24 In a comparison of vascular access site complications in patients undergoing PCI with adjunctive intravenous GP IIbIIIa inhibitor therapy, 7.4% of the transfemoral patients had a major vascular access site complication (despite the use of weight adjusted heparin, small calibre guiding catheters and femoral artery closure devices in the majority of these patients), compared to none of the similarly treated radial patients.25 In the setting of rescue PCI with adjunct GP IIbIIIa inhibitor, the reported rate of major femoral vascular complications ranges from 20–39%2,26–28 and around 10% even if vascular closure device is employed.29 Emerging data assessing the efficacy of transradial PCI in such a setting have all reported near complete elimination of vascular complications and with comparable procedural success rate as transfemoral approach.30
Patient comfort and preference are also important considerations in the comparison of these access sites. Delayed mobilisation after transfemoral procedures is common, due to inguinal pain, while bed rest itself has been shown to have an adverse effect on outcome.31,32 Patients undergoing elective transradial PCI can be mobilised immediately after the completion of these procedures with no adverse effects or risks, which allows PCI to be performed on a day case basis.33,34 Coronary angiography via the radial artery as opposed to the femoral artery is associated with short-term improvements in quality of life, whilst at the same time reducing hospital costs.1,35,36 The radial approach for intervention was preferred by 73% of patients in whom preceding diagnostic films were performed by the femoral route.4 As a result of the shorter hospital stay and reduced complication rates associated with transradial procedures, hospital costs of coronary stent deployment can be reduced by 15% when compared with the femoral route.36 The transradial technique, therefore, fulfils the requirements for a safer access site for interventional procedures with the added advantages of cost savings and improved quality of life.
THE TRANSRADIAL APPROACH
Case selection
The radial artery is a superficial vessel palpable in the forearm proximal to the flexor retinaculum of the wrist. Any patient with good radial pulsation and a favourable Allen test may be considered to be suitable for a transradial approach.37 The Allen test is performed by completely occluding the radial and ulnar arterial supply to the hand using digital pressure. The patient is then asked to repeatedly flex and extend their fingers, producing a blanched palmar surface when the hand is opened. With the hand in the open position, the occlusive pressure applied to the ulnar artery is released. If palmar flushing appears within ten seconds the Allen test is regarded as favourable. A favourable Allen test (present in over 90% of the population) confirms that an adequate collateral supply to the hand by the ulnar artery is present, and that occlusion of the radial artery after cannulation will not result in major distal ischaemia. In patients with an unfavourable Allen test, further evaluation with pulse oximetry should be carried out.38 A pulse oximeter is placed on the thumb, demonstrating a normal high saturation phasic pattern. The radial artery is then occluded by manual compression for two minutes. If a normal high saturation phasic pattern persists, a patent ulnar palmer arch is present. If pulsatile flow is initially abolished but returns within two minutes, an adequate recruitable collateral circulation is present. Radial catheterization is safe in both of these situations should radial occlusion occur. If pulsatile flow is abolished by radial compression and does not reappear, there is a risk of thumb ischaemia if radial occlusion occurs. An alternative access should be employed for these patients. Asymptomatic occlusion of the radial artery has been reported to occur in 2–9% of patients following a transradial procedure, with spontaneous recanalisation in half of these cases.39–43 Performing a transradial procedure does not preclude the use of the radial artery as a surgical conduit since the contralateral artery can be utilised
RADIAL ARTERY CANNULATION
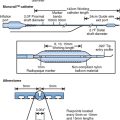
The open needle Seldinger based technique for radial artery cannulation, which most femoral trained operators are familiar with, appears to be easier to master. A specific radial puncture kit which consists of a short, small caliber needle with a flat-sided hub should be used. The guide wire supplied has a soft tip to aid atraumatic entry into the radial artery whilst the shaft is considerably stiffer to facilitate tracking the sheath into the artery. The puncture site and proximal artery are palpated by the non-dominant hand, and the needle is introduced at a 30 degree angle to the skin directly above the planned puncture site. The radial artery usually lies only a few millimetres below the skin surface, and arterial blood will flow back from the needle hub when the anterior wall of the artery is punctured. Because the puncture needle is small caliber, a low volume, less pulsatile bleed back (compared to vigorous pulsatile flow with puncture of femoral artery) is evidence of satisfactory puncture. The needle position is then fixed with the non-dominant hand, and the guide wire is introduced. If the wire will not easily exit from the tip of the needle, it is important not to attempt to forcefully advance it against resistance. The wire should be removed and the needle position adjusted before further attempts at wire introduction. Usually, this difficulty occurs because the needle bevel is partially embedded in the posterior wall of the artery, and slightly withdrawing the needle will facilitate easy guide wire entry. If the wire enters the artery with no difficulty but resistance is encountered in the mid or upper forearm, the wire has usually entered a small side branch. Withdrawal (for 1–2 cm) and rotation of the wire will usually allow satisfactory wire advancement. A scalpel is then used to make a small incision in the skin at the wire entry point. This is to facilitate sheath introduction, and is particularly important when 6 F or larger sheaths are required. Following this the sheath is usually easily inserted over the wire.
GUILDING CATHETER SELECTION AND MANIPULATION
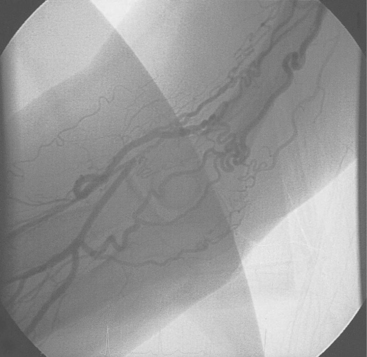
The majority of patients will have an adequate radial lumen to accommodate a 6 F sheath, which is compatible with kissing balloon techniques or the use of adjunctive devices such as intravascular ultrasound and rotablation. The reduction in size of guide catheters from 8 to 6 F can cause problems with the well established techniques of acquiring adequate guiding catheter back-up. The selection of an optimal catheter configuration is, therefore, vitally important when using the transradial technique. The guiding catheter must provide support from the opposite aortic wall, whilst being co-axial with the coronary ostium. Deep intubation of the catheter may be necessary (Fig. 5.6), in which case the guide catheter must have a soft, atraumatic tip and be flexible enough to be inserted into the vessel. The use of coronary stents requires the guide catheter to have gentle, flexible curves to allow the passage of the stent system with a minimum of friction. The guide catheters available include those designed for the femoral approach and dedicated radial catheters.39 The left coronary artery may be engaged with the left Judkins, Extra backup (EBU), left Amplatz (AL) and Multipurpose catheters, the Kimny Radial or MUTA radial left. The right coronary is similarly approached with the right Judkins (JR), Multipurpose, right (or left) Amplatz, the Kimny Radial or MUTA radial right. We normally use an EBU or an AL for PCI to the left coronary system and a JR4 or an AL1 for PCI to the right coronary artery. We only use dedicated radial catheters when these fail.
Some complex PCI procedures require the use of large calibre devices or adjunctive supportive therapy such as balloon pumping and coronary pacing. Published data suggests that many individuals have a radial artery capable of accommodating large calibre sheaths, and 7 F or 8 F sheaths can be used in many patients.44 Where necessary, an antecubital vein can be percutaneously punctured to allow access to the right side of the heart for pacing or pressure measurement. Simultaneous positioning of a radial and femoral sheath will allow PCI to be performed with balloon pump support, without the necessity for bilateral groin punctures.
THE LEARNING CURVE
Performing percutaneous transradial procedures provides new technical challenges and are associated with an important learning curve. During the learning phase, the operator must reliably puncture the relatively small calibre radial artery, successfully manipulate catheters from a distal access site in the right arm, and perform interventions utilising a different range of guiding catheter configurations, calibres and operating techniques. Initial failure rates for radial artery cannulation of 3.8% improving to 1.2% have been reported during a 650 patient series.45
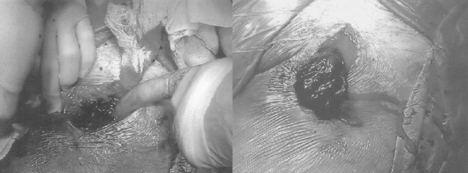
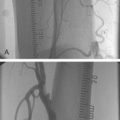
A common problem during the initial part of the learning curve is radial artery spasm (Fig. 5.7) which occurs in up to 10% of cases in an initial series making successful cannulation less likely and catheter manipulation more difficult, as well as causing pain during the procedure and making sheath removal unpleasant.46 The occurrence of spasm is predominantly related to operator experience and to the degree of patient anxiety, such that a successful, painless first pass will be least likely to provoke spasm. The following points may help reduce the occurrence of spasm:
CONCLUSION
Coronary intervention performed via a brachial or femoral access site is associated with a significant risk of important neurovascular complications. The use of aggressive antithrombotic regimes in association with acute unstable patients will increase this risk, which cannot be eliminated by the use of vascular access closure devices. Transradial PCI almost eliminates the risk of arterial access site complications. In addition, patients prefer transradial procedures, and hospital costs can be significantly reduced compared with procedures performed via the femoral or brachial artery. The major limitation to widespread clinical use of this access site is the learning curve associated with reliably puncturing the radial artery, and performing PCI from a distal upper limb access site. With improvements in technique and equipment the learning curve has been considerably shortened, and a committed interventionist should have no difficulty in developing a transradial service.47
1 Eccleshall S, Muthusamy T, Nolan J. The transradial access site for cardiac procedures: A clinical perspective. Stent. 1999;2(3):74-79.
2 Sundlof D, Rerkpattanapitat P, Wongpraparut N, et al. Incidence of bleeding complications associated with abciximab use in conjunction with thrombolytic therapy in patients requiring percutaneous transluminal angioplasty. Am J Cardiol. 1999;83:1569-1571.
3 Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. 1989;16(1):3-7.
4 Kiemeneij F. Transradial artery coronary angioplasty and stenting: History and single centre experience. J Invas Cardiol. 1996;8(Suppl. D):3D-8D.
5 Nolan J, Batin P, Welsh C, et al. Feasibility and applicability of coronary stent implantation with the direct brachial approach: Results of a single-centre study. Am Heart J. 134(939–44), 1997.
6 McCollum CH, Mavor E. Brachial artery injury after cardiac catheterization. J Vasc Surg. 1986;4:355-359.
7 Hammacher ER, Eikelboom BC, van Lier HJ, Skotnicki SH, Wijn PF. Brachial artery lesions after cardiac catheterisation. Eur J Vasc Surg. 1988;3:145-149.
8 Adams DF, Fraser DB, Abrams HL. The complications of coronary arteriography. Circulation. 1973;48(3):609-618.
9 Walton J, Greenhalgh RM. Brachial artery damage following cardiac catheterisation. When to re-explore. Eur J Vasc Surg. 1990;4(3):219-222.
10 Sones FMJr. Complications of coronary arteriography and left heart catheterization. Cleve Clin Q. 1978;45(1):21-23.
11 Hildick-Smith DJ, Khan ZI, Shapiro LM, Petch MC. Occasional-operator percutaneous brachial coronary angiography: first, do no arm. Catheter Cardiovasc Interv. 2002;57(2):161-165.
12 Noto TJJr, Johnson LW, Krone R, Weaver WF, Clark DA, Kramer JRJr, Vetrovec GW. Cardiac catheterization 1990: a report of the Registry of the Society for Cardiac Angiography and Interventions. Cathet Cardiovasc Diagn. 1991;24(2):75-83.
13 Oweida SW, Roubin GS, Smith RB3rd, Salam AA. Postcatheterization vascular complications associated with percutaneous transluminal coronary angioplasty. J Vasc Surg. 1990;12(3):310-315.
14 Berry C, Kelly J, Cobbe SM, Eteiba H. Comparison of femoral bleeding complications after coronary angiography versus percutaneous coronary intervention. Am J Cardiol. 2004;94(3):361-363.
15 Montalescot G, Chevalier B, Dalby MC, Steg PG, Morice M-C, Cribier A, Meyer P, Alor F. Description of modern practices of percutaneous coronary intervention and identification of risk factors for adverse outcome in the French nationwide OPEN registry. Heart. 2005;91:89-90.
16 Dauerman HL, Prpic R, Andreou C, Vu MA, Popma JJ. Angiographic and clinical outcomes after rescue coronary stenting. Catheter Cardiovasc Interv. 2000;50(3):269-275.
17 de Queiroz Fernandes Araujo JO, Veloso HH, Braga De Paiva JM, Filho MW, Vincenzo De Paola AA. Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta-analysis of randomized, controlled trials. Am Heart J. 2004;148(6):937-943.
18 Sreeram S, Lumsden AB, Miller JS, Salam AA, Dodson TF, Smith RB. Retroperitoneal hematoma following femoral arterial catheterization: a serious and often fatal complication. Am Surg. 1993;59(2):94-98.
19 Kent KC, Moscucci M, Gallagher SG, DiMattia ST, Skillman JJ. Neuropathy after cardiac catheterization: incidence, clinical patterns, and long-term outcome. J Vasc Surg. 1994;19(6):1008-1013.
20 Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291(3):350-357.
21 Al-Allaf K, Eccleshall S, Muthusamy T, Nolan J. Selection of arterial access sites for cardiac procedures. Br J Cardiol. 2000;7:422-425.
22 Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the Access study. J Am Coll Cardiol. 1997;29(6):1269-1275.
23 Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44(2):349-356.
24 Hildick-Smith DJR, Walsh JT, Lowe MD, Petch MC. Coronary angiography in the fully anticoagulated patient: the transradial route is successful and safe. Catheter Cardiovasc Interv. 2003;58(1):8-10.
25 Choussat R, Black A, Bossi I, Fajadet J, Marco J. Vascular complications and clinical outcome after coronary angioplasty with platelet IIb/IIIa receptor blockade. Eur Heart J. 2000;21:662-667.
26 Ellis SG, Da Silva ER, Spaulding CM, Nobuyoshi M, Weiner B, Talley JD. Review of immediate angioplasty after fibrinolytic therapy for acute myocardial infarction: insights from the RESCUE I, RESCUE II, and other contemporary clinical experiences. Am Heart J. 2000;139(6):1046-1053.
27 Sutton AG, Campbell PG, Graham R, Price DJ, Gray JC, Grech ED, Hall JA, Harcombe AA, Wright RA, Smith RH, Murphy JJ, Shyam-Sundar A, Stewart MJ, Davies A, Linker NJ, de Belder MA. A randomized trial of rescue angioplasty versus a conservative approach for failed fibrinolysis in ST-segment elevation myocardial infarction: the Middlesbrough Early Revascularization to Limit INfarction (MERLIN) trial. J Am Coll Cardiol. 2004;44(2):287-296.
28 O’Neill WW, Weintraub R, Grines CL, Meany TB, Brodie BR, Friedman HZ, Ramos RG, Gangadharan V, Levin RN, Choksi N. A prospective, placebo-controlled, randomized trial of intravenous streptokinase and angioplasty versus lone angioplasty therapy of acute myocardial infarction. Circulation. 1992;86(6):1710-1717.
29 Boccalandro F, Assali A, Fujise K, Smalling RW, Sdringola S. Vascular access site complications with the use of closure devices in patients treated with platelet glycoprotein IIb/IIIa inhibitors during rescue angioplasty. Catheter Cardiovasc Interv. 2004;63(3):284-289.
30 Kassam S, Cantor WJ, Patel D, Gilchrist IC, Winegard LD, Rea ME, Bowman KA, Chisholm RJ, Strauss BH. Radial versus femoral access for rescue percutaneous coronary intervention with adjuvant glycoprotein IIb/IIIa inhibitor use. Can J Cardiol. 2004 Dec;20(14):1439-1442.
31 Allen C, Glasziou P, Del Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet. 1999;354:1233-1299.
32 Foulger V. Patients’ views of day-case cardiac catheterisation. Prof Nurse. 1997;12:478-480.
33 Laarman GJ, Kiemeneij F, van der Wieken LR, Tijssen JG, Suwarganda JS, Slagboom T. A pilot study of coronary angioplasty in outpatients. Br Heart J. 1994;72(1):12-15.
34 Kumar S, Anantharaman R, Das P, Hobbs J, Densem C, Ansell J, Roberts DH. Radial approach to day case intervention in coronary artery lesions (RADICAL): a single centre safety and feasibility study. Heart. 2004;90(11):1340-1341.
35 Mann JT3rd, Cubeddu MG, Schneider JE, Arrowood M. Right Radial Access for PTCA: A Prospective Study Demonstrates Reduced Complications and Hospital Charges. J Invasive Cardiol. 1996;8(Suppl D):40D-44D.
36 Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, Moore JA. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J. 1999;138(3 Pt 1):430-436.
37 AlAllaf K, Eccleshall S, Kaba R, Nolan J. Arterial access for cardiac procedures utilising the percutaneous transradial approach. Br J Cardiol. 2000;7:548-552.
38 Barbeau GR, Arsenault F, Dugas L, Simard S, Lariviere MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen’s test in 1010 patients. Am Heart J. 2004;147(3):489-493.
39 Kiemeneij F. Transradial approach for coronary angioplasty and stenting. Stent. 1998;1:83-88.
40 Stella PR, Kiemeneij F, Laarman GJ, Oderkerken D, Slagboom T, van der Wieken R. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997;40(2):156-158.
41 Bertrand B, Sene Y, Huygue O, Monségu J. Doppler ultrasound imaging of the radial artery after catheterization. Ann Cardiol Angeiol (Paris). 2003;52(3):135-138.
42 Lotan C, Hasin Y, Mosseri M, Rozenman Y, Admon D, Nassar H, Gotsman MS. Transradial approach for coronary angiography and angioplasty. Am J Cardiol. 1995;76(3):164-167.
43 Nagai S. Ultrasonic assessment of vascular complications in coronary angiography and angioplasty after transradial approach. Am J Cardiol. 1999;83(2):180-186.
44 Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Cathet Cardiovasc Intervent. 1999;46:173-178.
45 Louvard Y, Harvey R, Pezzano M, Bradai R, Benaim R, Morice M. Transradial complex coronary angioplasty: The influence of a single operator’s experience. J Invas Cardiol. 1997;9(Suppl. C):647-649.
46 Barbeau G, Carrier G, Ferland S, Létourneau L, Gleeton O, Larivière M. Right transradial approach for coronary procedures: Preliminary results. J Invas Cardiol. 1996;8(Suppl. D):19D-21D.
47 Eccleshall SC, Banks M, Carroll R, Jaumdally R, Fraser D, Nolan J. Implementation of a diagnostic and interventional transradial programme: resource and organisational implications. Heart. 2003;89(5):561-562.