Vascular Access
A peripheral intravenous (PIV) cannula is the most commonly used device for venous access in children. Although venous access is often achieved in adults with minimal distress, placement of an intravenous catheter in children can be quite traumatic to the child, the parents, and the attendant health care providers. In some situations, it can be a fairly frustrating and time-consuming procedure.1 Particular circumstances of each child may demand specific solutions for vascular access, namely the choice of device and the site chosen for its placement (Table 8-1). Clinicians must be aware of the limitations and potential adverse effects of the various vascular access devices (VADs) that are available.
In an emergency, other options should promptly be considered after a few failed attempts at PIV cannula placement. Historically, the only available options were a venous cutdown or an emergency central venous catheter (CVC) placement. These options take considerable time and frequently require the services of a pediatric surgeon. Intraosseous (IO) needle placement has become the most common contingency method of emergency vascular access in children. The newer mechanical devices allow easier training of emergency medical personnel and have improved the success rate of IO placement in the prehospital setting. In fact, with appropriate training, an IO needle can be placed more quickly than a PIV cannula.2 In sick neonates, umbilical vessels are frequently cannulated, but can only be used for a finite period [maximum of 5 days for an umbilical artery catheter (UAC)] and 14 days for an umbilical venous catheter (UVC).3 Early placement of a peripherally introduced CVC (PICC) is preferable in these infants. Persistence with using PIV cannulas leads to higher complication rates and reduces the number of future PICC placement sites.
In choosing the appropriate VAD for an oncology patient, the requirements of the oncologist, the patient’s age, expected activity level, expected chance of cure, number of previous VADs placed, and patency of the central veins should all be considered. The number of lumens, size of the catheter, type of catheter, and its location can all be tailored to the specific patient.4 Long-term maintenance of central venous access in patients suffering from intestinal malabsorption is particularly challenging. Once the six conventional sites of central venous access—bilateral internal jugular, subclavian, and femoral veins—are exhausted, one must become more creative in gaining central access.
Complications that are common to all types of VADs are extravasation of infusate, hemorrhage, phlebitis, septicemia, thrombosis, and thromboembolism. Multiple studies have shown that catheter-related blood stream infections can be prevented with appropriate education and training utilizing insertion and maintenance bundles.3,5,6
Peripheral Venous Access
Several techniques have been shown to be beneficial in cannulating a peripheral vein, including warming the extremity, transillumination, and epidermal vasodilators.7 Ultrasound (US) guidance has been used to obtain access to basilic and brachial veins in the emergency department.8 Devices utilizing near-infrared imaging of the veins up to a depth of 10 mm are being used routinely in the hospitals, as well as by emergency medical personnel in the field, to find and access peripheral veins in all age groups.9 Unlike ultrasound, there is no physical contact with the overlying skin and hence there is no compression or distortion of the veins. Significant complications associated with PIV catheters include phlebitis, thrombosis, and extravasation with chemical burn or necrosis of surrounding soft tissue.
Umbilical Vein and Artery Access
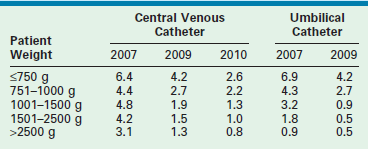
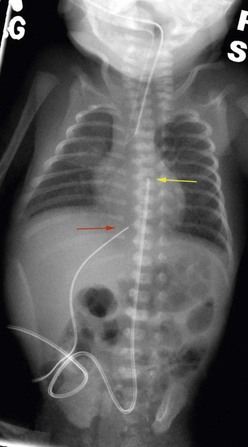
Neonates are often managed with catheters placed either in the umbilical vein and/or one of the umbilical arteries. They can be used for monitoring central venous or arterial pressure, blood sampling, fluid resuscitation, medication administration, and total parenteral nutrition (TPN). To minimize infectious complications, the UVCs are usually removed after a maximum of 14 days.3,10 These catheters are typically placed by neonatal nurse practitioners or neonatologists, and require dissection of the umbilical cord stump within a few hours of birth. It is possible for the pediatric surgeon to cannulate the umbilical vessels after the umbilical stump has undergone early desiccation. A small vertical skin incision is made above or below the umbilical stump to access the umbilical vein or artery, respectively. Once the fascia is incised, the appropriate vessel is identified, isolated, and cannulated. The tip of the UVC should be positioned at the junction of the inferior vena cava (IVC) and the right atrium (RA).11 The xiphisternum is a good landmark for the RA/IVC junction. On the chest radiograph, the tip of the UVC should be at or above the level of the diaphragm. The tip of the UAC is best positioned between the sixth and tenth thoracic vertebrae, cranial to the celiac axis (Fig. 8-1). Various calculations have been proposed to estimate the correct length of the catheter before insertion, based on weight and other biometric measures of the infant.12,13 The long-standing argument about the safety of a ‘high’ versus ‘low’ position for the UAC tip has been laid to rest; the ‘high’ position just described has been shown to be associated with a low incidence of clinically significant aortic thrombosis without any increase in other adverse sequelae.14,15 These umbilical vessel catheters have been associated with various complications. In addition to tip migration, sepsis and thrombosis can occur. UVCs have also been associated with perforation of the IVC, extravasation of infusate into the peritoneal cavity, and portal vein thrombosis.16 UACs are associated with aortic injuries, thromboembolism of aortic branches, aneurysms of the iliac artery and/or the aorta, paraplegia, and gluteal ischemia with possible necrosis.11 Pooled rates of bloodstream infections associated with umbilical catheters and CVCs in level III NICUs has improved over the recent years (Table 8-2).17–19
Peripherally Introduced Central Catheter
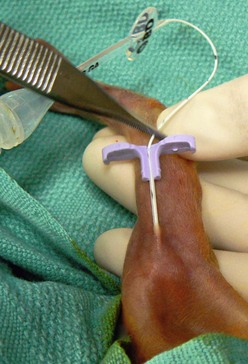
PICC lines provide reliable central venous access in neonates and older children without a need for directly accessing the central veins. PICC lines are suitable for infusion of fluids, medications, TPN, and blood products. Many institutions caring for sick children on a routine basis have developed special teams and protocols for placement of PICC lines to reduce variations in practice and increase availability.20 The modified Seldinger technique is used most frequently. A small peripheral intravenous catheter (about 24 gauge) is first placed, preferably with ultrasound guidance, in a suitable extremity vein such as the basilic, cephalic, or long saphenous vein. A fine guide wire is advanced through the vein, the initial catheter is removed, the track is dilated, and a peel-away PICC introducer sheath is advanced over the guide wire. The guide wire is then removed, and the PICC line is introduced through the sheath (Fig. 8-2).21,22 The tip of the PICC should be placed at the superior vena cava (SVC)/RA junction or the IVC/RA junction. Locations peripheral to these are considered non-central and are associated with higher complications.23 PICC lines are also eminently suitable for short- to medium-term (weeks) home intravenous therapy of antibiotics or TPN.24 The most common complications associated with PICC lines are infections, occlusion, and dislodgement of the catheter.25
Central Venous Catheters
With the development of PICC teams and the increasing use of PICC lines, there has been a decline in the use of CVCs in neonates and older children.20 Nontunneled CVCs are used for short- and medium-term indications, whereas surgically placed tunneled CVCs are used for medium- and long-term indications. In premature neonates, if PICC placement is not successful, tunneled CVCs are preferentially used because of their smaller size and durability as opposed to nontunneled CVCs. The central veins accessed for placement of CVCs are the bilateral internal jugular veins, subclavian veins, and femoral veins. In older children and full-term neonates, the percutaneous Seldinger technique is used. In premature neonates and occasionally in older children, the relevant central vein or one of its tributaries (i.e., common facial vein or external jugular vein in the neck, cephalic vein in the deltopectoral groove, or the long saphenous vein at the groin [Fig. 8-3]), is dissected and cannulated.26–28 In some emergency situations, percutaneous femoral vein access may be preferred as the insertion site is away from the activity centered around the head, neck, and chest. The tip of a CVC placed from the lower body should be positioned at the junction of the IVC and RA, which will ensure prompt dilution of the infusate and likely has a lower chance of thrombosis. Again, the xiphoid process is a good surface landmark to estimate the length of catheter needed. Radiographically, the tip should be positioned just above the diaphragm.
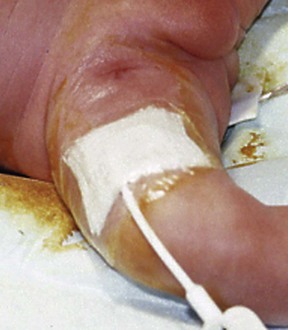
FIGURE 8-3 This broviac catheter was placed through a cutdown at the groin to access the long saphenous vein close to the saphenofemoral junction. The catheter is positioned in a subcutaneous tunnel in the thigh to exit just above the knee on the anteromedial aspect.
A CVC placed through an upper body vein should be positioned such that the tip is at the junction of the SVC and RA. Surface landmarks for this location are less reliable. A point about 1 cm caudal to the manubriosternal junction, at the right sternal border, gives a close estimate to the SVC/RA junction in toddlers and older children. The lower margin of the third right costosternal junction has been shown to be the best surface landmark in adults for placement of the CVC tip at the SVC/RA junction.29 Length recommendations have been made based on a child’s weight for placement of right internal jugular and right subclavian CVCs.30 On a chest radiograph, the tip of the catheter should project about two vertebral bodies caudal to the carina. A tunneled CVC can be left in place for several months to years. The Broviac and Hickman catheters (Bard Access Systems, Salt Lake City, UT) are made of silicone and are available in various sizes, the smallest being a 2.7 French single-lumen catheter. These catheters have a Dacron cuff that promotes tissue ingrowth, resulting in anchoring of the catheter within the subcutaneous tunnel. The cuff can be placed close to the exit site of the catheter to facilitate removal by dissection through the exit site. Placement of the Dacron cuff midway between the venotomy site and the exit site has the advantage of reducing the chance of unintended removal of the catheter.
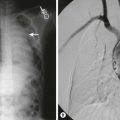
Use of ultrasound guidance for percutaneous central venous access is becoming standard of care. The benefits of ultrasound assistance include higher success rate, faster access, fewer needle passes, and fewer arterial punctures.31–36 Real-time 2D ultrasound guidance is recommended as the preferred method when placing a CVC into the internal jugular vein (IJV) in adults and children in elective situations.31,32,36 In a randomized controlled trial, ultrasound was shown to be beneficial in IJV catheterization in infants weighing less than 7.5 kg when compared with a ultrasound image-based skin surface marking.33 During insertion, the ultrasound transducer is wrapped in a sterile sleeve, and sterile gel is used to obtain real-time images of the vessel being accessed. Either a short-axis view across the diameter of the vessel (Fig. 8-4) or the long axis view along the length of the vessel is used to visualize the needle approaching and entering the vessel. A guide wire is then passed into the vessel, followed by the Seldinger technique for placement of the CVC.36 Because of the proximity of the clavicle, visualization of the subclavian vein is poor with ultrasound. Infraclavicular axillary vein cannulation has been performed with 96% success with ultrasound guidance for tunneled CVC placement into the subclavian vein.37
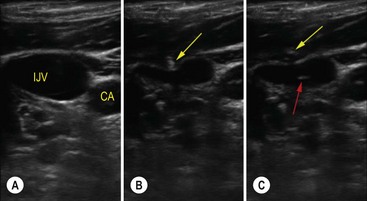
FIGURE 8-4 Ultrasound short-axis image of the right internal jugular vein and carotid artery obtained with SonoSite S Series using a 13–6 MHz transducer (SonoSite Inc., Bothell, Washington) in a child. (A) The internal jugular vein (IJV) and the carotid artery (CA). (B) The yellow arrow is pointing to the needle indenting the IJV. (C) The red arrow points to the target sign caused by the needle in the middle of the IJV confirming correct placement.
The majority of bloodstream infections in children are associated with the use of a vascular access device. Tunneled CVCs inserted in the neck veins in neonates in the NICU have been associated with a higher rate of complications than those placed in the inguinal region. Infection rates were 5.8 (neck) versus 0.7 (groin) per 1000 catheter days.28 Urgent CVCs placed in 289 pediatric burn unit patients also resulted in higher infection rates associated with subclavian and internal jugular CVCs compared with the femoral CVCs (10 and 13.6 versus 8.2 per 1000 catheter days).38 This is thought to be due to the higher nursing and respiratory therapy activity around the head and neck in these patients requiring intensive burn care. Ethanol lock, in addition to antibiotic therapy, has been shown to be beneficial in salvaging CVCs that become infected.39 Ethanol lock has also been shown to be superior to heparin lock in preventing catheter infection and loss in children with intestinal failure.40 Retained fragments of CVCs after attempted removal has been reported to occur at a rate of 2%. Leaving a ligated fragment of the catheter within the blood vessel is felt to be safer than the alternative, which involves interventional or operative removal.41
Totally Implanted Central Venous Catheters
Totally implantable intravascular devices (ports) are subcutaneous reservoirs attached to CVCs. The reservoirs are made of a metal or hard plastic shell with a central silicone septum that is penetrated for access. They provide a reliable, long-lasting solution for patients who need intermittent access to their central venous system. They are ideal for patients who desire to be involved in aquatic sports and other physical activities. They are most useful for patients with malignancies, coagulopathies, hemolytic syndromes, and renal failure, all of which require recurrent vascular access. Low profile ports with 5 or 6 French catheters are available for use in infants. Larger ports are available with dual lumens. High-flow ports are available (PowerPort-Bard Access Systems, Salt Lake City, UT) that allow high-pressure injection of intravenous contrast for radiologic imaging. Ports require special noncoring needles to keep the septum from leaking. The reservoir should be implanted in a subcutaneous pocket, over a firm base such as the chest wall. Preferred sites for port placement include the pectoral area, parasternal area, (above and medial to the areola) and the subclavicular area (medial to the anterior axillary fold). In females with a concern for cosmesis, the low presternum area and the lateral chest wall are locations that hide the scar when the port is eventually removed. In determining port placement, consideration should be given to the age of the patient, the intended activities, as well as the convenience of the caregivers. Subclavicular placement will make it easy to access the port with minimal disrobing; in obese girls and young women, it is less likely to get displaced by the highly mobile breast tissue. The presternal location will provide a very stable foundation for the port, even in the obese child. When placed over the lower sternum, it can be accessed with opening the front of a button-down top or shirt. Complications that are unique to a port include an inability to access the port, disconnection of the catheter from the reservoir with extravasation, flipping of the port, fracture and embolization of the catheter, and breakdown of the overlying skin.42
Intraosseous Access
Several studies have been published over the past 60 years establishing the safety and effectiveness of IO access for infusion of fluids and medications in children, including neonates.43,44 IO access has also been shown to be faster than access with a PIV2 and safer than an emergency CVC.
Bone marrow consists of rich lattice network of vessels. Whereas the peripheral veins collapse in patients in shock, the vascular spaces in the bone marrow do not.45 The bioavailability of resuscitative drugs administered through IO access has been well established and shown to be better than that of those administered through an endotracheal tube.46,47 The current Pediatric Advanced Life Support (PALS) recommendation is to establish IO access promptly if PIV access cannot be attained rapidly in neonates and children of all ages who need intravenous drugs or fluids urgently.48 In children, the long bones of the lower extremities are used preferentially for IO placement. The proximal tibia is the most common site used, followed by the distal femur. With full sterile precautions, a needle designed for bone marrow aspiration is advanced through the cortical bone to access the bone marrow. In an infant, a spinal needle may be used, but in an older child, a generic bone marrow needle or a purpose designed IO needle such as the widely used Jamshidi needle (Cardinal Health, McGraw Park, IL) is used.47,49
Contraindications to IO placement include injury or suspected injury to the bone or soft tissue overlying the placement site. Mechanical devices such as the Bone Injection Gun (BIG, Wasimed, Caesarea, Israel), FAST1 System (Pyng Medical Corporation, Vancouver, Canada), and EZ-IO (Vidacare, San Antonio, TX) have helped to expand the use of IO access.2,47 The first two are spring-loaded devices. The FAST1 System is designed for sternal use in adults. The EZ-IO is a power drill-assisted device that makes IO placement easier in older children and adults.
Early concerns about potential adverse effects on the growth plates of long bones used for IO access have been allayed by animal studies.49 The overall complication rate is estimated to be about 1%.47 Extravasation of fluid is the most common adverse event. Compartment syndrome, osteomyelitis, skin and soft tissue infection, bone fractures, and fat embolism, although rare, have also been reported.47
Venous Cutdown
Although the advent and eventual broader acceptance of IO infusion has almost eliminated the need for venous cutdown and reduced the role of emergency placement of CVCs, pediatric surgeons should maintain the knowledge and skills required to perform this procedure.7 The vessel of choice is the long saphenous vein near the medial malleolus. The vein is superficial, is of satisfactory size, and there is minimal subcutaneous fat in this location. A transverse incision is made anterior and cephalad to the medial malleolus. The vein is readily identified by dissecting through the thin subcutaneous tissue and is stabilized by placing proximal and distal stay ligatures. The vein is then directly cannulated using a venous catheter of appropriate size relative to the vein, and the catheter is anchored to the adjacent skin.
Alternate Routes for Central Venous Access
Patients who have had multiple previous CVCs can have thrombosis or stenosis of the central veins that precludes successful placement of a new catheter. Doppler ultrasound or magnetic resonance angiography can be used to survey the central veins, including the brachiocephalic vein, the SVC, and the IVC.50 When necessary, percutaneous access can be gained via a patent IVC by either by a translumbar or a transhepatic approach.51–53 A tunneled catheter can then be inserted to reach the IVC/RA junction. Translumbar IVC catheters are quite durable while transhepatic catheters tend to get withdrawn from the vascular space owing to the constant respiratory excursions of the diaphragm. The brachiocephalic vein, if patent, can similarly be accessed through a suprasternal route. The azygos vein may be accessed through one of the intercostal veins percutaneously or surgically using a thoracotomy or thoracoscopic assistance.54–56 Direct RA access has been used to manage patients with intestinal failure and occluded central veins for long periods, and in cardiac patients in an acute situation.53,57
Arterial Catheter
Intra-arterial catheters allow continuous hemodynamic monitoring and blood sampling. The radial artery at the wrist is most commonly used for intra-arterial access due to the excellent collateral circulation. The dorsalis pedis and posterior tibial arteries are other peripheral sites that may sometimes be used. Femoral arteries are frequently used by cardiologists for catheter-based cardiac interventions and occasionally for monitoring. However, in general, it is advisable not to use the main artery of an extremity for chronic arterial catheter placement to avoid thromboembolic and ischemic complications.3 Adequacy of collateral arterial supply through the ulnar artery should be confirmed before placement of a radial arterial line by using the Allen test.58 The right radial artery allows preductal monitoring and sampling. Percutaneous cannulation is usually successful. If unsuccessful, an arterial cutdown can be performed. Digital ischemia can result from radial artery catheters. Thromboembolism can result in limb loss if the axillary, brachial, or femoral artery is catheterized. Local infections can occur when the catheters are left for several days. A pseudoaneurysm can result from injury to the adjacent vein during placement.
Hemodialysis Catheters
The current recommendation is to use an autologous arteriovenous fistula (AVF) as the route of choice for hemodialysis.59 AVFs permit high-flow rates that facilitate effective dialysis. They also are reliable, durable, and, once established, have low complication rates. Because patients are often referred late, and AVFs take time to mature, there is frequently a need for CVCs for immediate dialysis. Temporary and tunneled long-term double-lumen hemodialysis catheters are placed preferentially through the right IJV, either percutaneously or by cutdown. The larger size of the vein and the straight internal path of the catheter allow a larger catheter to be placed safely through the right IJV. Additionally, use of the IJV avoids possible injury or thrombosis to the SCV which must be patent to develop a functioning AVF at the wrist. The long-term, cuffed hemodialysis catheters are precurved to allow right internal jugular placement with tunneling to the pectoral area. Flow rates achieved through hemodialysis catheters tend to be lower and they last a relatively short time. AVF is also an option for management of young children with severe hemophilia.60
References
1. Stovroff, M, Teague, WG. Intravenous access in infants and children. Pediatr Clin North Am. 1998; 45:1373–1393.
2. de Caen, A. Venous access in the critically ill child: When the peripheral intravenous fails!. Pediatr Emerg Care. 2007; 23:422–424.
3. O’Grady, N, Alexander, M, Burns, LA, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1). Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Inf Dis. 2011; 52:1087–1099.
4. Alexander, N. Question 3. Do Portacaths or Hickman lines have a higher risk of catheter-related bloodstream infections in children with leukaemia? Arch Dis Child. 2010; 95:239–241.
5. Stevens, TP, Schulman, J. Evidence-based approach to preventing central line-associated bloodstream infection in the NICU. Acta Paediatrica Supplement. 2012; 101:11–16.
6. Holzmann-Pazgal, G, Kubanda, A, Davis, K, et al. Utilizing a line maintenance team to reduce central-line-associated bloodstream infections in a neonatal intensive care unit. J Perinatol. 2012; 32:281–286.
7. Haas, NA. Clinical review: Vascular access for fluid infusion in children. Crit Care. 2004; 8:478–484.
8. Keyes, LE, Frazee, BW, Snoey, ER, et al. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999; 34:711–714.
9. Miyake, RK, Zeman, HD, Duarte, FH, et al. Vein imaging: A new method of near infrared imaging, where a processed image is projected onto the skin for the enhancement of vein treatment. Dermatol Surg. 2006; 32:1031–1038.
10. Butler-O’Hara, M, Buzzard, CJ, Reubens, L, et al. A randomized trial comparing long-term and short term use of umbilical venous catheters in premature infants with birth weights of less than 1251 grams. Pediatrics. 2006; 118:e25–e35.
11. Ramasethu, J. Complications of vascular catheters in the neonatal intensive care unit. Clin Perinatol. 2008; 35:199–222.
12. Sritipsukho, S, Sritipsukho, P. Simple and accurate formula to estimate umbilical arterial catheter length of high placement. J Med Assoc Thai. 2007; 90:1793–1797.
13. Lin, MS, Lim, YJ, Ho, NK. A quicker simpler method of pre-determination of the length of umbilical artery catheter insertion in Asian babies. J Singapore Paediatr Soc. 1989; 31:79–81.
14. Green, C, Yohannan, MD. Umbilical arterial and venous catheters: Placement, use, and complications. Neonatal Netw. 1998; 17:23–28.
15. Barrington, KJ. Umbilical artery catheters in the newborn: Effects of position of the catheter tip. Cochrane Database Syst Rev. 2000; 000505.
16. Kim, JH, Lee, YS, Kim, SH, et al. Does umbilical vein catheterization lead to portal venous thrombosis? Prospective ultrasound evaluation in 100 neonates. Radiology. 2001; 219:645–650.
17. Edwards, JR, Peterson, KD, Andrus, ML, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007; 35:290–301.
18. Dudeck, MA, Horan, TC, Peterson, KD, et al. National Healthcare Safety Network (NHSN) report, data summary for 2009, device-associated module. Am J Infect Control. 2011; 39:349–367.
19. Dudeck, MA, Horan, TC, Peterson, KD, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control. 2011; 39:798–816.
20. Linck, DA, Donze, A, Hamvas, A. Neonatal peripherally inserted central catheter team. Evolution and outcomes of a bedside-nurse-designed program. Adv Neonat Care. 2007; 7:22–29.
21. Pettit, J. Technological advances for PICC placement and management. Adv Neonat Care. 2007; 7:122–131.
22. Braswell, LE. Peripherally inserted central catheter placement in infants and children. Tech Vasc Interv. Radiol. 2011; 14:204–211.
23. Racadio, JM, Doellman, DA, Johnson, ND, et al. Pediatric peripherally inserted central catheters: Complication rates related to catheter tip location. Pediatrics. 2001; 107:E28.
24. Earhart, A, Jorgensen, C, Kaminski, D. Assessing pediatric patients for vascular access and sedation. J Infus Nurs. 2007; 30:226–231.
25. Pettit, J. Assessment of infants with peripherally inserted central catheters: Part 1. Detecting the most frequently occurring complications. Adv Neonat Care. 2002; 2:304–315.
26. Zumbro, GL, Jr., Mullin, MJ, Nelson, TG. Catheter placement in infants needing total parenteral nutrition utilizing common facial vein. Arch Surg. 1971; 102:71–73.
27. Meland, NB, Wilson, W, Soontharotoke, CY, et al. Saphenofemoral venous cutdowns in the premature infant. J Pediatr Surg. 1986; 21:341–343.
28. Vegunta, RK, Loethen, P, Wallace, LJ, et al. Differences in the outcome of surgically placed long-term central venous catheters in neonates: Neck vs. groin placement. J Pediatr Surg. 2005; 40:47–51.
29. Hsu, JH, Wang, SS, Lu, DV, et al. Optimal skin surface landmark for the SVC-RA junction in cancer patients requiring the implantation of permanent central venous catheters. Anaesthesia. 2007; 62:818–823.
30. Andropoulos, DB, Bent, ST, Skjonsby, B, et al. The optimal length of insertion of central venous catheters for pediatric patients. Anesth Analg. 2001; 93:883–886.
31. Hind, D, Calvert, N, McWilliams, R, et al. Ultrasonic locating devices for central venous cannulation: Meta-analysis. BMJ. 2003; 327:361.
32. Abboud, PA, Kendall, JL. Ultrasound guidance for vascular access. Emerg Med Clin North Am. 2004; 22:749–773.
33. Hosokawa, K, Shime, N, Kato, Y, et al. A randomized trial of ultrasound image-based skin surface marking versus real-time ultrasound-guided internal jugular vein catheterization in infants. Anesthesiology. 2007; 107:720–724.
34. Maecken, T, Grau, T. Ultrasound imaging in vascular access. Crit Care Med. 2007; 35:S178–S185.
35. Wigmore, TJ, Smythe, JF, Hacking, MB, et al. Effect of the implementation of NICE guidelines for ultrasound guidance on the complication rates associated with central venous catheter placement in patients presenting for routine surgery in a tertiary referral centre. Br J Anaesth. 2007; 99:662–665.
36. Pirotte, T. Ultrasound-guided vascular access in adults and children: Beyond the internal jugular vein puncture. Acta Anaesthesiol Belg. 2008; 59:157–166.
37. Sharma, A, Bodenham, AR, Mallick, A. Ultrasound-guided infraclavicular axillary vein cannulation for central venous access. Br J Anaesth. 2004; 93:188–192.
38. Sheridan, RL, Weber, JM. Mechanical and infectious complications of central venous cannulation in children: Lessons learned from a 10-year experience placing more than 1000 catheters. J Burn Care Res. 2006; 27:713–718.
39. Valentine, KM. Ethanol lock therapy for catheter-associated blood stream infections in a pediatric intensive care unit. Pediatr Crit Care Med. 2011; 12:e292–e296.
40. Oliveira, C, Nasr, A, Brindle, M, et al. Ethanol locks to prevent catheter-related bloodstream infections in parenteral nutrition: A meta-analysis. Pediatrics. 2012; 129:318–329.
41. Milbrandt, K, Beaudry, P, Anderson, R, et al. A multiinstitutional review of central venous line complications: Retained intravascular fragments. J Pediatr Surg. 2009; 44:972–976.
42. Dillon, PA, Foglia, RP. Complications associated with an implantable vascular access device. J Pediatr Surg. 2006; 41:1582–1587.
43. DeBoer, S, Russell, T, Seaver, M, et al. Infant intraosseous infusion. Neonatal Netw. 2008; 27:25–32.
44. Engle, WA. Intraosseous access for administration of medications in neonates. Clin Perinatol. 2006; 33:161–168.
45. Calkins, MD, Fitzgerald, G, Bentley, TB, et al. Intraosseous infusion devices: A comparison for potential use in special operations. J Trauma. 2000; 48:1068–1074.
46. Buck, ML, Wiggins, BS, Sesler, JM. Intraosseous drug administration in children and adults during cardiopulmonary resuscitation. Ann Pharmacother. 2007; 41:1679–1686.
47. Blumberg, SM, Gorn, M, Crain, EF. Intraosseous infusion: A review of methods and novel devices. Pediatr Emerg Care. 2008; 24:50–56.
48. de Caen, AR, Kleinman, ME, Chameides, L, et al. Part 10: Paediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2010; 81:e213–e259.
49. Boon, JM, Gorry, DL, Meiring, JH. Finding an ideal site for intraosseous infusion of the tibia: An anatomical study. Clin Anat. 2003; 16:15–18.
50. Gupta, H, Araki, Y, Davidoff, AM, et al. Evaluation of pediatric oncology patients with previous multiple central catheters for vascular access: Is Doppler ultrasound needed? Pediatr Blood Cancer. 2007; 48:527–531.
51. Azizkhan, RG, Taylor, LA, Jaques, PF, et al. Percutaneous translumbar and transhepatic inferior vena caval catheters for prolonged vascular access in children. J Pediatr Surg. 1992; 27:165–169.
52. Mortell, A, Said, H, Doodnath, R, et al. Transhepatic central venous catheter for long-term access in paediatric patients. J Pediatr Surg. 2008; 43:344–347.
53. Rodrigues, AF, van Mourik, ID, Sharif, K, et al. Management of end-stage central venous access in children referred for possible small bowel transplantation. J Pediatr Gastroenterol Nutr. 2006; 42:427–433.
54. Solomon, BA, Solomon, J, Shlansky-Goldberg, R. Percutaneous placement of an intercostal central venous catheter for chronic hyperalimentation guided by transhepatic venography. JPEN J Parenter Enteral Nutr. 2001; 25:42–44.
55. Tannuri, U, Tannuri, AC, Maksoud, JG. The second and third right posterior intercostal veins: An alternate route for central venous access with an implantable port in children. J Pediatr Surg. 2005; 40:e27–e30.
56. Sola, JE, Thompson, WR. Thoracoscopic-assisted placement of azygos vein central venous catheter in a child. Am J Transplant. 2008; 8:715–718.
57. Oram-Smith, JC, Mullen, JL, Harken, AH, et al. Direct right atrial catheterization for total parenteral nutrition. Surgery. 1978; 83:274–276.
58. Kohonen, M, Teerenhovi, O, Terho, T, et al. Is the Allen test reliable enough? Eur J Cardiothorac Surg. 2007; 32:902–905.
59. D’Cunha, PT, Besarab, A. Vascular access for hemodialysis: 2004 and beyond. Curr Opin Nephrol Hypertens. 2004; 13:623–629.
60. Mancuso, ME, Berardinelli, L. Arteriovenous fistula as stable venous access in children with severe haemophilia. Haemophilia. 2010; 16:25–28.