Chapter 6 Validity of sleep nasendoscopy in the investigation of sleep-related breathing disorder
1 INTRODUCTION
Croft and Pringle introduced the technique of sleep nasendoscopy for use in the assessment of snoring to aid proper case selection for surgical intervention.1
The attraction of sleep nasendoscopy lies with its ability to provide a dynamic visualization of the anatomical areas responsible for the generation of noise (snoring) or obstruction, under conditions which mimic sleep. Prior to the introduction of sleep nasendoscopy various methods including lateral cephalometry, computerized tomography and the Mueller maneuver had been used in an attempt to achieve the above objective.2
Sleep nasendoscopy has been criticized for not being a true reflection of normal physiological sleep in view of the sedation process involved. Various techniques of sedation have been used.3–5 Bolus injections of sedatives are commonly used and may lead to fluctuating blood, plasma and tissue levels leading in turn to fluctuating drug effects. The correct level of sedation is crucial to produce sufficient muscle relaxation to recreate snoring but not cause respiratory depression.3 Roblin et al. adopted a computer-controlled infusion system that employs the concept of target controlled infusion (TCI)2 using propofol as the sedating agent. Propofol has the attraction of possessing a rapid onset of action and recovery period, with minimal side effects.6 In addition it allows for standardization and reproducibility between different operators.
2 PATIENT SELECTION
Sleep nasendoscopy has the potential to be a valuable investigation for making an accurate dynamic anatomical assessment in patients with snoring and obstructive features. Determination of the anatomical site of the obstruction in this way allows an appropriate or targeted choice of treatment options to be made.1 Although commonly used, questions have been raised regarding the potential for false-positive results; that is, the production of symptoms (snoring/obstruction) in individuals with no history of sleep-disordered breathing.
During sleep endoscopy the correct level of sedation is vital to induce symptoms of snoring with or without obstruction but not cause respiratory depression.3 This window of sedation can be narrow, may differ from patient to patient and be difficult to maintain for any length of time.
3 TECHNIQUE OF SLEEP NASENDOSCOPY
The blood concentration level is increased in 2 μg/ml incremental doses and the system automatically adjusts the rate of propofol infusion to achieve the required blood concentration level. The flexible nasendoscope (Olympus P4) is introduced when the patient begins to snore; if the patient obstructs the target concentration level is reduced. In the location of obstructive sites, attention is paid to the following levels:
A video and sound recording of the sleep nasendoscopy is carried out during the procedure.
The degree of obstruction is categorized as follows.
3.1 PHARMACOKINETICS OF PROPOFOL
Propofol is a white, sterile, odorless and isotonic oil in water emulsion, prepared by chemical synthesis. The emulsion vehicle contains soybean oil and purified egg phosphatide. Propofol is used as the sedating agent for target controlled infusion as it has a rapid onset of action, is metabolized quickly, giving a fast recovery phase, and has a low incidence of postoperative nausea, vomiting and headache. Propofol has the attraction of possessing a rapid onset of action and recovery period, with minimal side effects.6 In addition, it allows for standardization and reproducibility between different operators.
4 SPECIFICITY AND SENSITIVITY OF SLEEP NASENDOSCOPY
Previous studies have questioned the value of sleep nasendoscopy because asymptomatic subjects could be induced to snore and obstruct. Marais3 stated that although sleep nasendoscopy is a widely used investigation in patients with sleep-disordered breathing despite its non-physiological basis, there still remains a doubt of its validity as an investigative tool. Marais compared the presence or absence of snoring and its site of generation between a group of 205 snorers and another of 126 non-snorers. Snoring was produced at nasendoscopy in 45.3% of non-snorers but could not be produced in 18.1% of snorers. There was no significant difference in the site of sound production between the two groups and although the noise produced by the non-snoring group was quieter, this difference was not significant. Abdullah et al.7 suggested that a clear establishment of the site of obstruction is crucial for subsequent treatment planning. Video sleep nasendoscopy (VSE) is probably the most accurate assessment of the situation and also helps in identifying the situation that needs correction.
Berry et al.8 conducted a prospective cohort study involving 107 patients divided into two groups. The two groups of patients were matched for their Body Mass Index (BMI). The first group consisted of 53 patients with a history suggestive of obstructive sleep apnea. The second group consisted of 54 patients with a partner-confirmed history of no snoring. These patients were undergoing anesthesia for other reasons. Both groups of patients were free of associated otorhinolaryngological symptoms. The main outcome measure was assessment of production of snoring or obstruction in patients with no documented history of snoring when sedation was administered as part of general anesthesia using TCI with propofol.
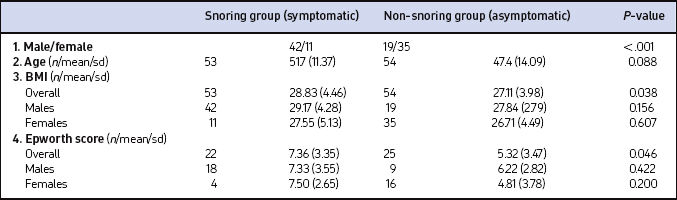
The symptomatic group comprised 53 patients whereas 54 patients made up the non-symptomatic group. The various relevant characteristics are summarized in Table 6.1. Categorical variables (gender) were assessed using the chi-squared test whereas continuous variables (BMI and Epworth score) were analyzed using tests for normality; differences were compared by the paired t-test. Taking gender into account, both the groups are reasonably matched.
Both groups contained similar numbers of males and females but there was a predominance of males in the snoring group whereas females were predominant in the non- snoring group (Table 6.1). The mean age of the patients in the second group was 47.4 years whereas in the first group, it was 51.7 years.
There was a difference (P=0.046) between the means of the Epworth score in the two groups: non-snorers 5.32 (with SD of 3.473) against snorers 7.36 (SD of 3.346). However, gender difference, whether considered alone or as a second factor, was not significantly different (Table 6.1).
Body Mass Index was effectively matched between the two groups overall, and between males and females within these groups. A difference (P=0.038) between the means of the BMI values was observed in the two groups: non-snorers 27.11 (with SD 3.984) against snorers 28.83 (SD 4.462) and also, there was a difference (P=0.027) between the means of the BMI values for men (mean 28.75, SD 3.902) and women (mean 26.91, SD 4.604) (Table 6.1).
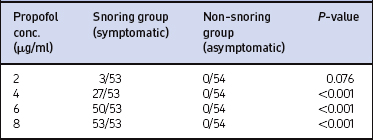
All patients in the symptomatic (snoring) group snored or obstructed at different concentrations of propofol (Table 6.2). There was no statistically significant difference (P=0.401) in the distributions of concentrations of propofol at which snoring started between men and women.
Earlier authors who have noted false-positive results with sleep endoscopy did not use this technique. Different individuals may have administered the sedating agent or agents used in these studies. The sedating agents indeed differed between operators in such studies, and no measure of sedation effect was used.3–5 This difference in technique is perhaps an explanation for the encouraging and obvious differentiation of symptomatic from asymptomatic patients demonstrated in this study.
1. Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnea. Clin Otolaryngol. 1991;16:377-382.
2. Roblin G, Williams A, Whittet HB. Target controlled infusion in sleep endoscopy. Laryngoscope. 2001;111:175-176.
3. Marais J. The value of sleep nasendoscopy: a comparison between snoring and non snoring patients. Clin Otolaryngol. 1998;23:74-76.
4. Quin SJ, Huang L, Ellis PDM. Observation of the mechanism of snoring using sleep nasendoscopy. Clin Otolaryngol. 1995;20:360-374.
5. Sadoka T, Kakitsuba N, Fujiwara Y, Kanai R, Takahashi H. The value of sleep nasendoscopy in he evaluation of patients with suspected sleep related breathing disorders. Clin Otolaryngol. 1986;21:485-489.
6. Glen JB. The development of ‘Diprifusor’: a TCI system for Propofol. Anaesthesia. 1985;53(Suppl 1):13-21.
7. Abdullah VJ, Wing YK, Van Hasselt CA. Video sleep nasendoscopy: the Hong Kong experience. Otolaryngol Clin North Am. 2003;23:461-471.
8. Berry S, Roblin G, Williams A, Watkins A, Whittet HB. Validity of sleep nasendoscopy in the investigation of sleep related breathing disorders. Laryngoscope. 2005;115(3):538-540.