Vaginectomy
Partial or total excision of the vagina is performed most often because of vaginal neoplasia. The diagnosis is suspected following an atypical cytology report. Vaginal intraepithelial neoplasia (VAIN) may follow or exist concurrently with cervical intraepithelial neoplasia (CIN) or vulvar intraepithelial neoplasia (VIN), or may occur de novo. A de facto vaginectomy may be performed as a result of treatment for extensive condyloma acuminata. The goal of vaginectomy is twofold: (1) to remove the pathology, and (2) to retain a functioning structure. The latter translates into maintaining the vagina as a supple, nonconstricted, and suitably lengthy structure. The factor most often responsible for vaginal deformity and accompanying dyspareunia is scar formation. As was noted in Chapter 50, neighboring organs are exceedingly close (2 to 4 mm) to the vaginal mucosa. The vagina itself is a rather simple structure—essentially a potential space with its anterior and posterior walls in light contact in vivo. The vagina is attached at its lower margin to the vulva and at its upper margin to the uterus, together with the uterine supports. The vagina is attached laterally to the levator ani and a mass of surrounding connective tissue (endopelvic fascia). The loose peripheral attachments allow movement as well as flexibility between the points of relative fixation. Anteriorly, the vaginal wall and the bladder and urethral walls are in apposition. Similarly, an identical set of circumstances exists between the rectal and vaginal walls posteriorly. When reduced to its lowest common denominator, the vagina is a pleated, lightly muscled, highly vascularized skin tube.
Intraepithelial neoplasia in the absence of glands occupies less than 1 mm of a vaginal wall cross-section. Treating the vagina more deeply to eradicate the disease adds nothing to the cure but may adversely influence the functional outcome. Unfortunately, VAIN is multifocal; therefore, to diminish the chances of persistence or recurrence, very wide excisional margins around visible lesions must be undertaken. This translates into dividing the vagina into thirds and removing a minimum of one third to a maximum of three thirds.
Excision
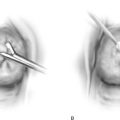
Because the vagina is highly vascular, particularly beneath the urethra and at the bulb of the vestibule, brisk bleeding should be anticipated when it is cut. The sources of much of the bleeding are sinusoidal and cavernous structures. These sites are better sutured as they are encountered rather than clamped. If substantial areas of the vagina are going to be excised, a split-thickness skin graft should be obtained before the vaginal part of the operation is begun (Fig. 63–1). The colposcope will be used throughout the intravaginal operation. Initially, the extent of the lesion is mapped (Fig. 63–2A, B).
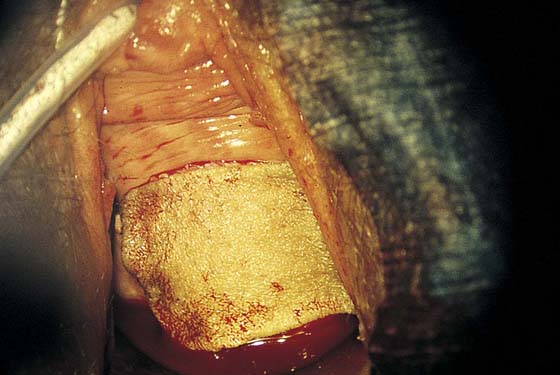
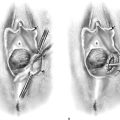
A 1 : 100 vasopressin solution is injected subepithelially into the vaginal stroma (Fig. 63–3A). This provides some hemostasis and a convenient dissection plane (Fig. 63–3B). An axis-oriented incision is made into the anterior or posterior wall, and flaps are created to the right and left of the midline cut as a submucosal plane is created (Fig. 63–4). The dissecting microscope (colposcope) has the great advantage of providing good, bright light as well as variable magnification. Stevens (tenotomy) scissors are ideal for this type of dissection (Fig. 63–5). The lateral wall is divided into two recesses, or sulci, which create an H appearance to the vagina as viewed head on. These are located anterolaterally and posterolaterally on the right and left walls. Between the sulci lies the insertion of the levator ani muscle on the right and left sides, respectively. Above and below the insertion on the muscle is fat, through which course blood vessels, lymphatics, and nerves. The vagina is dissected across the point of levator attachment but superficial to that attachment (i.e., remaining well within the immediate submucosal plane) (Fig. 63–6). Anterior and posterior dissections meet in the anterolateral and posterolateral sulci, and the specimen is removed (Figs. 63–7 and 63–8). Care must be taken at the vaginal fornices to not damage the ureter, which is quite close to the anterior and anterolateral fornices.
Depending on the size of the removed tissue, the vagina may be closed edge to cut edge or grafted. Generally the latter approach is selected because any substantial excision will lead to constriction, should the vagina be reconstituted by primary closure, particularly if the suture lines are closed under tension.
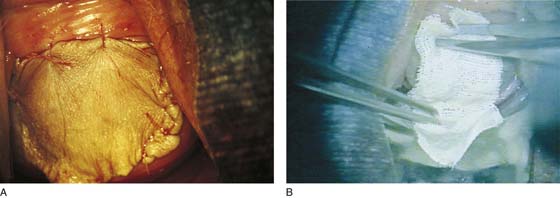
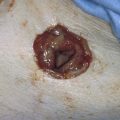
For split-thickness closure, a graft is taken from the buttock or thigh. Upon completion of the vaginectomy, the defect is measured and the graft is cut to fit the defect. The graft is removed from its saline-soaked sponge roll, remoistened with normal saline, and carefully placed to cover the wound (Fig. 63–9). It is sutured into place with multiple 4-0 Vicryl sutures and is covered with fine-mesh gauze (Fig. 63–10A, B). The donor site is covered with a urethane dressing. Although it is obvious, the point must be made that absolute hemostasis must be accomplished before any graft is placed over a surgical bed. This location is preferable to avoid electrosurgical coagulation, which devitalizes tissue and increases the risk of infection. Instead, bleeding areas should be irrigated and suture-ligated with fine absorbable sutures (e.g., 3-0 or 4-0 Vicryl) (Fig. 63–11).
FIGURE 63–1 In preparation for a large vaginal excision (partial vaginectomy) involving the upper and middle thirds of the vagina, a split-thickness skin graft is obtained from the thigh.
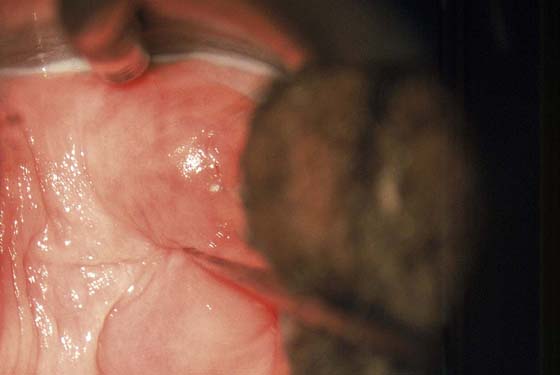
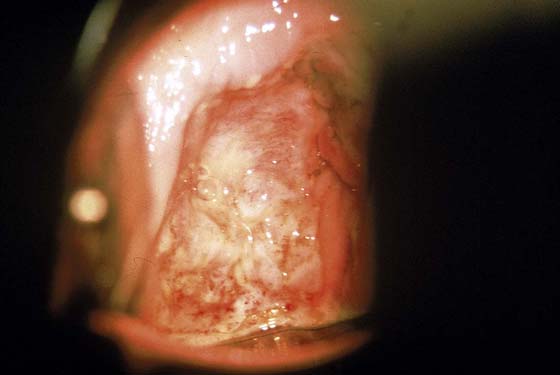
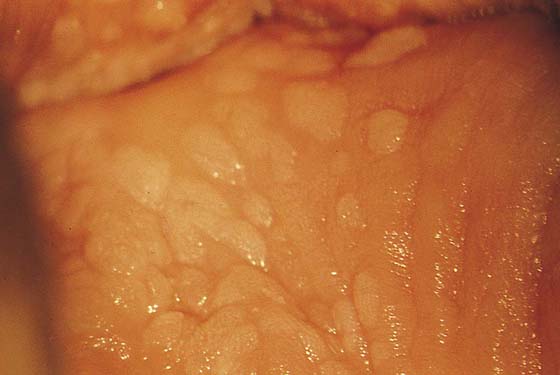
FIGURE 63–2 A. An extensive area of vaginal intraepithelial neoplasia (VAIN) is seen on the anterior and lateral walls of the vagina. B. Close-up view of Figure 63–2A documents the flat, warty pattern of VAIN.
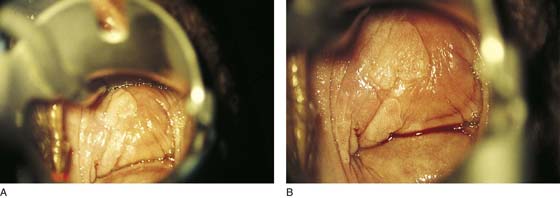
FIGURE 63–3 A. An injection of 1 : 100 vasopressin solution is made into the vagina in preparation for surgery. The vasopressin provides hemostasis, and the solution helps to identify a plane for the vaginectomy. B. Note the extreme blanching produced by the injection of vasopressin. Actually, it is advisable to map the lesion and identify the margins before injection.
FIGURE 63–4 A trace incision is made into the vagina with a 3-mm margin around the visible neoplasia.
FIGURE 63–5 The excision is begun at the distal margin with the use of Stevens tenotomy scissors and with the optical advantage of the dissecting microscope.
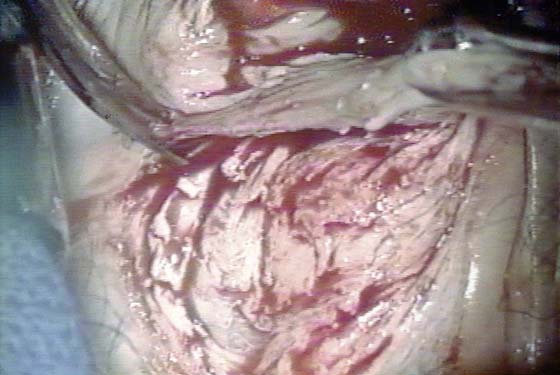
FIGURE 63–6 The full thickness of the vaginal epithelium is dissected from the underlying vaginal stroma. In actuality, a bit of stroma is also excised because the epithelial pegs extend down into the underlying stroma.
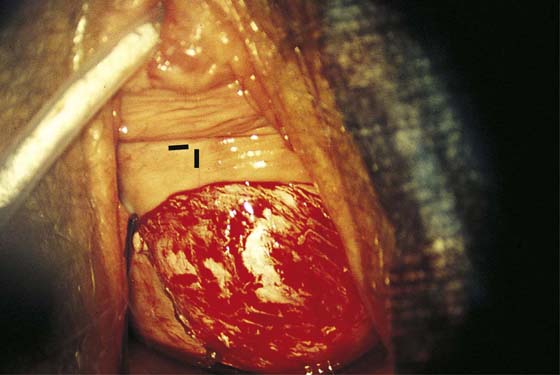
FIGURE 63–7 A large portion of the anterior vaginal wall has been excised. The black markers are beneath the area of the bladder neck (urethrovesical junction).
FIGURE 63–8 The lateral vaginal wall has been primarily sutured, closing the excisional defect. The anterior wall cannot be primarily closed without constricting the vagina.
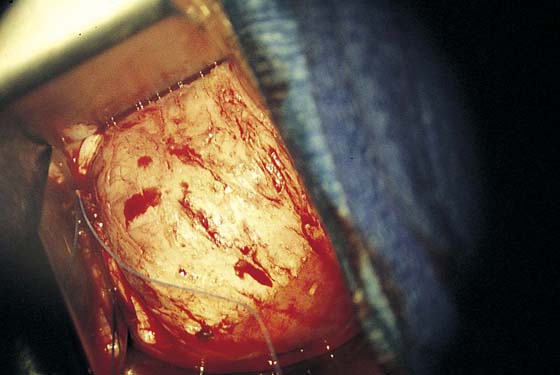
FIGURE 63–9 A split-thickness graft is placed over the defect extending from just cranial to the urethrovesical junction to the anterior vaginal fornix.
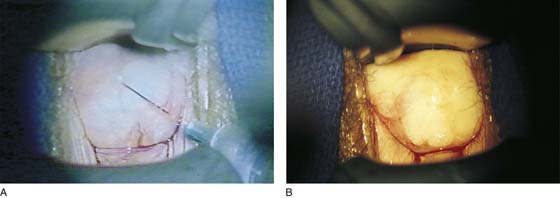
FIGURE 63–10 No electrosurgical devices are used for hemostasis. Instead, bleeding points are suture-ligated with 4-0 Vicryl.
FIGURE 63–11 A. The slightly stretched graft is sutured into place over a dry vaginal bed. B. Fine-mesh gauze is placed over the graft.
Carbon Dioxide Laser
The only practical laser to use in the vagina is the carbon dioxide laser (CO2 laser) delivered via microscope and micromanipulator. The technique for ablation depends on a suitably large laser spot size to avoid deep penetration, and the use of superpulsing to avoid excessive heat conduction. Power should be adjusted so the beam (spot) penetrates no farther than 1 mm.
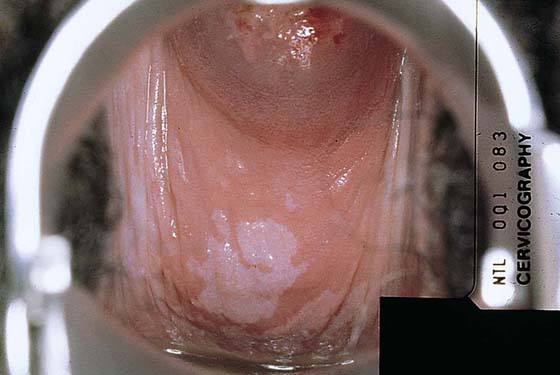
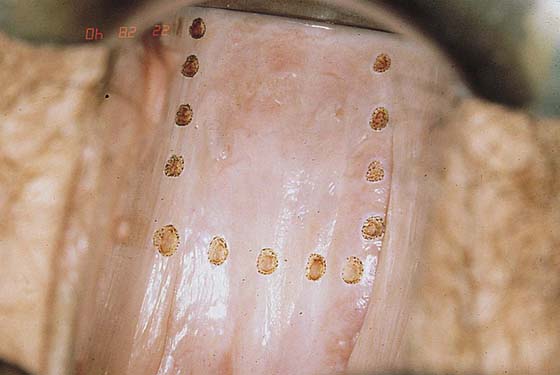
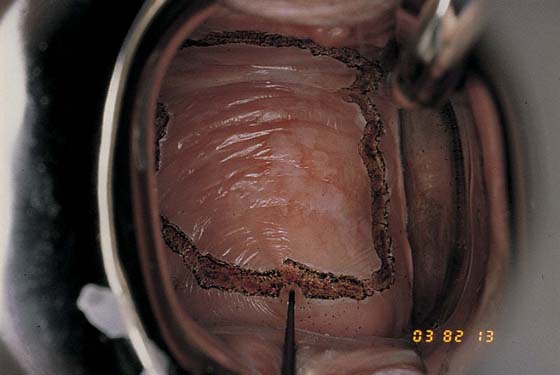
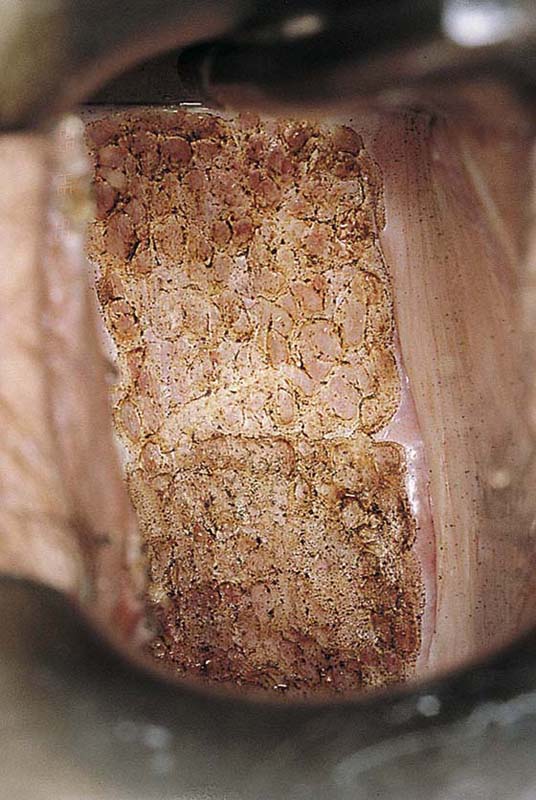
Typically, VAIN appears as white, flat, warty lesions (Fig. 63–12). Neoplastic areas are separated by normal tissue (i.e., multicentricity is the rule for VAIN) (Fig. 63–13). Before treatment, multiple biopsies performed on the lesions have confirmed them to be intraepithelial neoplasia (i.e., the disease has been mapped). For ablative operations, vasopressin is not injected. The margins of the area to be vaporized are outlined by the laser on the basis of prior colposcopically directed mapping (Fig. 63–14). The dots are connected, thereby clearly outlining the area to be vaporized and permitting a ready reference for orientation (Fig. 63–15). Next, the laser spot size is increased to 2.5 mm, and the area within the outlined margins is vaporized (Fig. 63–16). Power settings depend on the surgeons’ skill and experience with laser technology and range from 15 to 40 W. The goal is to vaporize the tissue to a depth not to exceed 1 mm. All char is washed away with 4% acetic acid (Fig. 63–17). When the fornices are to be vaporized, a titanium hook is utilized to manipulate the cervix so as to expose completely the vaginal recesses (Fig. 63–18A through C).
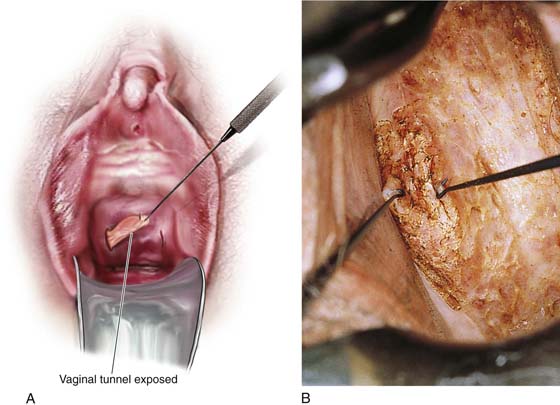
For women who have undergone hysterectomy and who have upper-third VAIN, the vaginal vault must be vaporized to effectively treat the disease. This in fact constitutes a high-risk group for invasive disease. Therefore, in the pretreatment and intraoperative phases, particular attention to detail is a requisite. The vault and tunnels must be multiply sampled and mapped. During the treatment phase, the vault recesses (tunnels) must be drawn out by means of a titanium hook and completely exposed and vaporized (Fig. 63–19A, B). Postoperatively, the vaginal walls can agglutinate and must be separated by application of a vaginal cream daily or twice daily. A sulfa-based vaginal cream or clindamycin (Cleocin) cream is suitable.
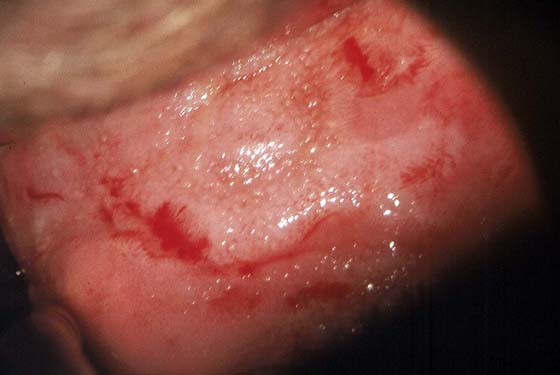
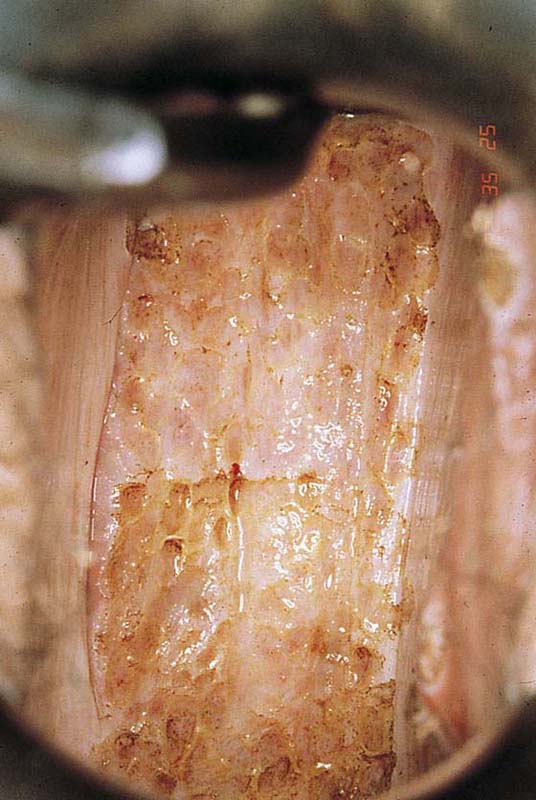
FIGURE 63–12 Extensive, white condylomatous lesions characteristic of VAIN are seen on the posterior wall of the vagina.
FIGURE 63–13 Magnified detail of VAIN illustrates the multifocal nature of the disease.
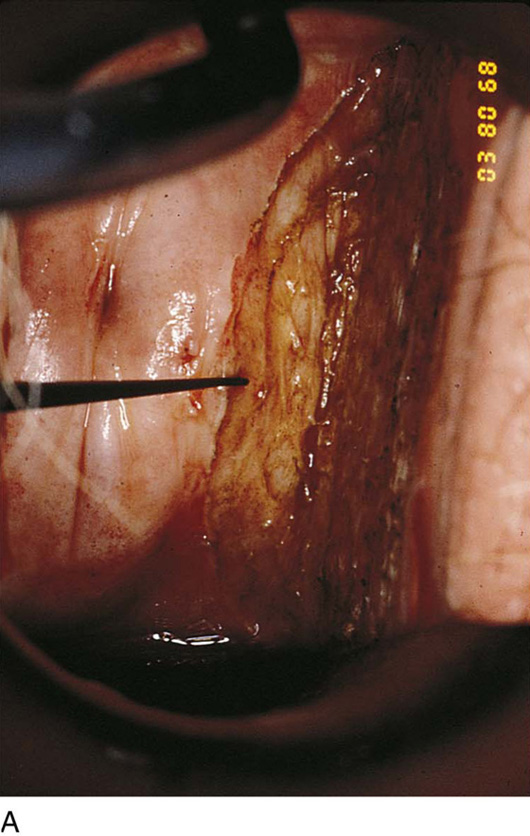
FIGURE 63–14 Initially, the power density of the carbon dioxide (CO2) laser (coupled to the operating microscope) is reduced to map the area to be vaporized by means of multiple trace spots.
FIGURE 63–15 The dots are connected by a superficial incision.
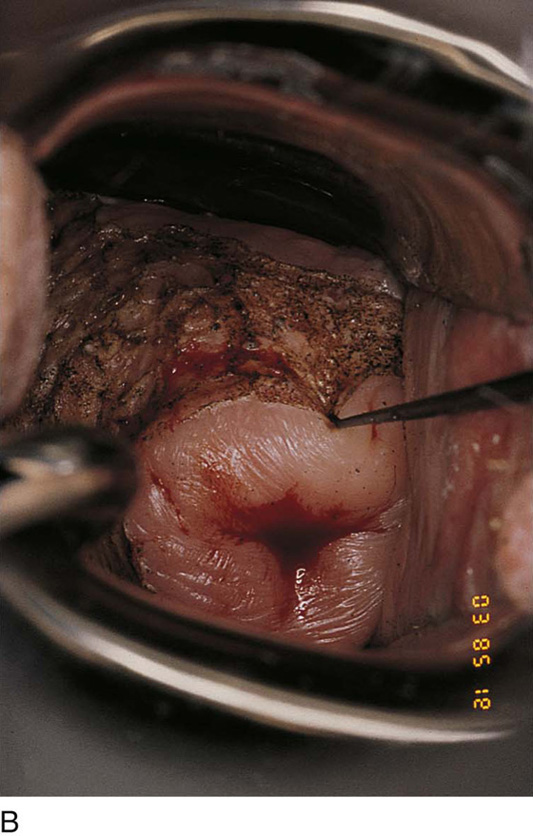
FIGURE 63–16 The entire posterior wall of the vagina is vaporized to a depth of no more than 1 mm.
FIGURE 63–17 The superpulsed laser creates minimal char formation. The laser wound is swabbed clean of all debris with the use of sodium chloride solution.
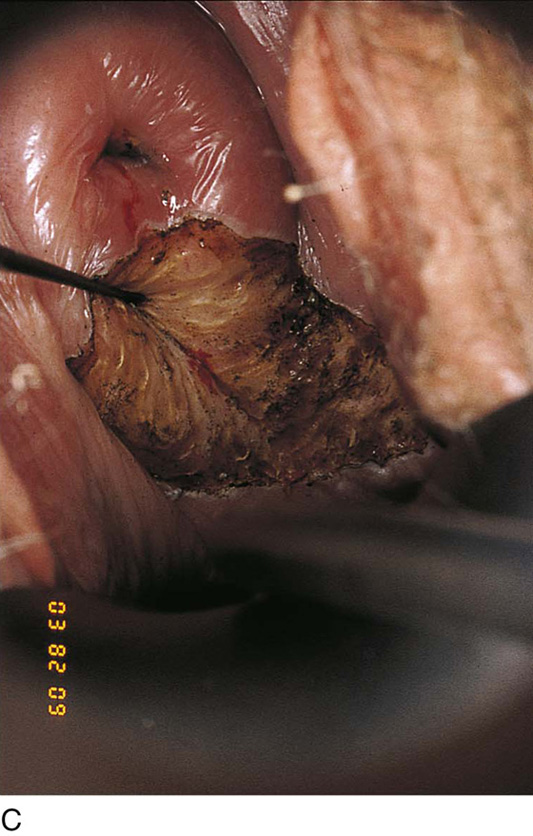
FIGURE 63–18 A. The cervix is placed on traction with a titanium hook to expose the lateral fornix. The lateral fornix is vaporized. B. Next, the cervix is pulled downward and posteriorly by manipulating hook to expose the anterior fornix. This is also vaporized. C. The posterior fornix is exposed by pulling the cervix downward and anteriorly. The posterior fornix is vaporized.
FIGURE 63–19 A. The exposure and vaporization of vaginal tunnels created as the result of hysterectomy are vitally important when VAIN is treated. B. This vaginal tunnel has been exposed with the use of two titanium hooks. Note that the epithelium has been completely destroyed by laser vaporization.