Vaccine-Preventable Diseases
Learning Objectives
• Identify the etiologic agent of diphtheria
• Recognize the most virulent strain of diphtheria
• Explain the relationship between iron and diphtheria exotoxin production
• Restate the diphtheria exotoxin mechanism of action
• Design a therapeutic regimen to treat diphtheria
• Identify the etiologic agent of whooping cough
• Restate the pertussis toxin mechanism of action
• Design a therapeutic regimen to treat whooping cough
• Compare and contrast whole-cell and acellular pertussis vaccines
• Identify the etiologic agent of tetanus
• Restate the tetanus exotoxin mechanism of action
• Identify the four different types of tetanus
• Design a therapeutic regimen to treat tetanus
• Identify the most common etiologic strain of Haemophilus infections
• Identify the major Haemophilus virulence factors
• Identify the factors that increase the risk of Haemophilus infections in children
• Explain the difference between T cell–dependent antigens and T cell–independent antigens
• Compare and contrast type I and II T cell–independent antigens
• Explain the rationale for conjugating capsular antigens to proteins
• Design a therapeutic regimen to treat Haemophilus infections
• Identify the major virulence factor associated with Streptococcus pneumoniae
• Identify the four different diseases caused by pneumococcus
• Identify therapeutic agents used to treat pneumococcal diseases
• Discuss the advantages and disadvantages of the licensed pneumococcal vaccines
• Identify the major strains of Neisseria meningitidis that causes infections in the United States
• Identify the major virulence factors associated with N. meningitidis
• Identify the three sequelae associated with meningitis
• Identify the role of lipo-oligosaccharide (LOS) in the pathophysiology of meningitis
• Understand the advantages and disadvantages of the three meningitis vaccines
• Recognize the four different types of polio infections and the three types of paralytic polio
• Discuss the mechanisms involved in post-polio syndrome (PPS)
• Compare and contrast the Sabin vaccine and the Salk vaccine for polio
• Identify the complications of rubeola infections
• Identify the two types of rubeola vaccines
• Compare and contrast rubeola and rubella infections
• Discuss congenital rubella syndrome
• Identify the five complications of mumps infections
• Compare and contrast the pathophysiology of varicella infection and varicella zoster infection
• Identify the four sequelae of chickenpox infections
• Explain the mechanism involved in post-herpetic neuralgia
• Design a therapeutic regimen to treat herpes zoster
• Compare and contrast the varicella vaccine and the varicella zoster vaccine
• Compare the transmission routes of hepatitis A and B
• Design a therapeutic regimen to treat hepatitis
• Compare and contrast the vaccines for hepatitis A and B
• Identify the host range for influenza types A, B, and C
• Explain the roles of hemagglutinins and neuraminidase in influenza infectivity
• Compare and contrast antigenic drift and antigenic shift
• Compare and contrast the pathophysiology of seasonal influenza and pandemic influenza
• Design a therapeutic regimen to treat influenza
• Identify the two types of influenza vaccines
• Compare and contrast inactivated and attenuated influenza vaccines
• Identify the disease caused by rotavirus
• Identify the rotavirus strains endemic in the United States
• Explain the pathophysiology of rotavirus infections
• Compare and contrast the three rotavirus vaccines
• Identify the diseases and syndromes caused by papilloma virus
• Identify the papilloma serotypes that cause skin and genital warts
• Identify the papilloma serotypes that cause 70% of cervical cancers
• Identify the therapeutic agents used to treat papilloma virus infections
• Identify the two papilloma vaccines
• Identify the advantages and limitations of papilloma vaccines
Key Terms
Antigenic drift
Antigenic shift
Bacteremia
Bacteriophage
Congenital rubella syndrome
Cytokine storm
Disseminated intravascular coagulopathy (DIC)
Elongation factor 2
Fimbriae
Gamma amino butyric acid
Hemagglutinin
Herd immunity
Intussusceptions
Koplik spots
Lipooligosaccharide
Neuraminidase
Nosocomial infections
Opisthotonos
Orchitis
Osteomyelitis
Otitis media
Pertactin
Post-herpetic neuralgia
Ramsay Hunt syndrome
Reye syndrome
Sabin vaccine
Salk vaccine
Tetanospasmin
Trismus
Vaccine-associated paralytic poliomyelitis (VAPP)
Introduction
Mass vaccination programs have been highly successful in reducing the 57 million deaths caused by infectious diseases in the world each year. The World Health Organization’s (WHO) global initiatives have eradicated smallpox and reduced the incidence of measles and polio deaths. In the last 7 years, vaccination reduced the incidence of measles-related deaths by 74%. Polio is now endemic only in four countries, as opposed to 185 countries in 1988.
In the United States, the success of mass vaccination programs may be one of the greatest public health achievements of the twentieth century. Prior to the institution of mass vaccination programs, 100,000 cases of diphtheria, whooping cough, and tetanus occurred each year. After the institution of vaccination programs, the incidence of these diseases is between 1 and 5000 cases per year. Vaccines against measles, mumps, rubella, and Haemophilus have also reduced annual disease incidence to less than 500 cases per year.
Mass vaccination programs are based on the concept of herd immunity. When a high proportion of a population is vaccinated, person-to-person disease transmission is interrupted by surrounding the infected person with vaccinated individuals. The percentage of vaccinated individuals necessary to disrupt transmission depends on the infectivity of the microbe and the number of secondary infections that are transmitted by a single index case in a susceptible population. Using these data, crude herd immunity thresholds for potentially vaccine-preventable diseases can be calculated mathematically. The threshold of herd immunity is the minimum percentage of the population that must be immunized to prevent person-to-person transmission. When the minimum threshold is reached, disease transmission is stopped, and the epidemic or pandemic is halted. Herd immunity thresholds for childhood diseases are provided in Table 25-1.
Table 25-1
Herd Immunity Thresholds for Common Childhood Diseases
| Infection | No. of Individuals Infected from an Index Case | Herd Immunity Threshold (%) |
| Diphtheria | 6–7 | 85 |
| Measles | 12–18 | 83–94 |
| Mumps | 4–7 | 75–86 |
| Pertussis | 12–17 | 92–94 |
| Polio | 5–7 | 80–86 |
| Rubella | 6–7 | 83–85 |
Modified from Fine P: Herd immunity: History, theory, practice, Epidemiol Rev 15(2):265, 1993.
The previous chapter discussed the general nature of vaccines, additives, and adjuvants. This chapter explores the natures of vaccine-preventable diseases and vaccines licensed for use in the United States.
Diphtheria
Diphtheria is a multiple-organ disease caused by gram-positive Corynebacterium diphtheriae. The three strains of corynebacteria are gravis, intermedius, and mitis. The gravis strain is the most virulent because it divides every 60 minutes and produces large amounts of exotoxin.
Diphtheria Exotoxin
The exotoxin-producing diphtheria bacteria carries a bacteriophage (bacterial virus) gene (Dtox) that codes for the exotoxin. Expression of the Dtox gene is controlled by a diphtheria iron-dependent repressor gene (DtoxR). If sufficient iron is available, the DtoxR gene synthesizes a repressor protein that inhibits the transcription and translation of the Dtox gene. When iron stores are exhausted, the repressor protein is not synthesized and the toxin gene is transcribed and translated.
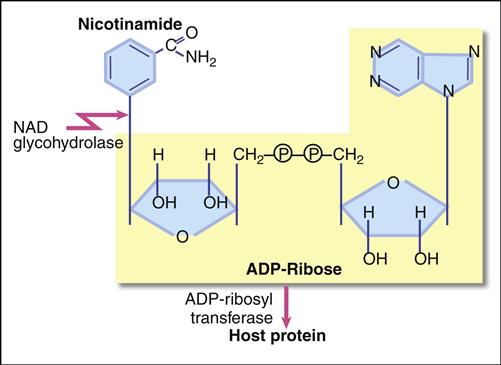
The diphtheria exotoxin is a classic two component A+B protein subunit toxin. Using the B subunit, the intact toxin binds to epidermal growth factor (EGF) receptors on epithelial cells. Protease activity associated with the EGF receptor cleaves the toxin into A and B fragments. The A fragments undergo endocytosis and target nicotinamide adenine dinucleotide (NAD), a coenzyme involved in redox reactions in the cytochrome system. The A subunit has both glycohydrolase and ribosyl transferase activities. Glycohydrolase splits the NAD molecule into nicotinamide and adenosine diphosphate (ADP) ribose. Using the ribosyl transferase function, the A subunit attaches ADP-ribose to histidine (diphthamide) found in the elongation factor 2 (EF-2) protein, which assists in joining of amino acids during protein synthesis (Figure 25-1). Exotoxin inactivation of EF-2 prevents peptide elongation. The reaction is as follows:
< ?xml:namespace prefix = "mml" ns = "http://www.w3.org/1998/Math/MathML" />

Diphtheria Pathophysiology
Diphtheria organisms colonize the tonsils and the pharynx. The secreted exotoxin destroys surrounding tissue, and leakage of blood and plasma in the necrotic tissue activates fibrin, which creates a pseudomembrane at the back of the throat. If untreated, the membrane ultimately obstructs airflow and causes death.
The exotoxin is also transported to the heart, muscle, adrenal glands, kidneys, liver, and spleen. The most frequent diphtheria complications are myocarditis and neuritis. Abnormal heart rhythms occur early in the infection, and necrosis of heart muscle often leads to heart failure.
Treatment
Treatment for active disease usually entails a combination of antitoxin and antibiotics. Equine diphtheria antitoxin is available from the Centers for Disease Control and Prevention (CDC). Antitoxin is most effective in the early stages of the infection because it neutralizes free diphtheria. A 14-day regimen of erythromycin or intramuscular procaine penicillin G also is recommended to treat active cases. Since the bacterium is transmitted by respiratory aerosols, persons coming in contact with the infected patient are given a course of benzathine penicillin G or oral erythromycin. Therapeutics used to treat diphtheria are listed in Table 25-2.
Table 25-2
Therapeutic Agents Used to Treat Diphtheria
| Penicillin G | Arrests bacterial cell wall synthesis by inhibiting cross-linking cell wall polymers |
| Erythromycin | Binds to 50S ribosomal subunits and prevents ribosomal translocation |
| Diphtheria antitoxin | Provides passive protection against circulating toxin. It has no effect on bound toxin |
Diphtheria Vaccine
Diphtheria toxoid is usually included with other antigens in combination vaccines of varying strengths. In the description of diphtheria vaccines, uppercase letters (D, P, or T) denote full strength diphtheria, pertussis, and tetanus. Lowercase letters (d and p) indicate reduced concentrations of diphtheria and pertussis in the vaccine. Acellular components of a vaccine are represented by the lowercase “a” (DTaP).
DTaP and DT vaccines are used through 6 years of age. The DTaP also is formulated with inactivated polio–hepatitis B (Pediarix). Adult Td can be used to vaccinate individuals 7 years and older. Tdap vaccines are available for children between 10 and 18 years of age (Boostrix), and Adacel is approved for individuals 11 to 64 years of age.
Following a four-vaccination series over 8 months, the clinical efficacy is 97%. However, diphtheria immunity wanes with age, and children and adults are at risk for contracting the disease. To maintain protective immunity, children vaccinated with DTaP should receive booster doses at 4 to 6 years of age and every 10 years thereafter. Older children vaccinated with adult Td or Tap should receive a booster shot every 10 years.
Pertussis (Whooping Cough)
The etiologic agent of whooping cough is Bordetella pertussis, an aerobic, gram-negative rod. The term whooping cough is derived from the unique paroxysmal coughing pattern associated with the disease. As a consequence of prolonged coughing, the individual is unable take air into the lungs. When the coughing stops, a protracted inspiratory effort with an associated high-pitched “whoop” occurs. Coughing begins 1 to 2 weeks after infection and lasts for 6 to 10 weeks.
B. pertussis produces a number of virulence factors that include filamentous hemagglutinin (FHA), fimbriae, adenylate cyclase, pertactin, and toxin production. B. pertussis attaches itself to cells in the respiratory tract using the FHA. Secondary attachment points are facilitated by fimbriae and pertactins. The pathology of the disease is caused by a pertussis toxin, a tracheal cytotoxin, and a dermonecrotic toxin.
Pertussis Toxin
The B. pertussis toxin (PTx) is an A+B subunit toxin. Attachment to the cell is facilitated by the B units, and the A unit is responsible for the toxicity. PTx is a ribosyl transferase, which binds ADP-ribose to a membrane-bound regulatory G1 molecule. Inactivation of the G1 protein allows uncontrolled conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Accumulating cAMP disrupts normal cellular function and alters hormone activity. For example, increased cAMP levels increase sensitivity to histamine, which results in increased capillary permeability, hypotension, and shock. PTx also downregulates both cellular and antibody-mediated immune responses, increasing the risk for secondary infections.
Tracheal Cytotoxin
The tracheal cytotoxin is a cell wall peptidoglycan fragment and cannot be classified as an exotoxin or an endotoxin. The toxin kills ciliated epithelial cells in the respiratory tract and stimulates the release of interleukin 1 (IL-1), which acts as an endogenous pyrogen.
Dermonecrotic Lethal Toxin
Dermonecrotic lethal toxin is an exotoxin that causes local necrosis of tissue adjacent to areas colonized by pertussis. The role of the lethal toxin in the pathophysiology of whooping cough is unknown.
Treatment
The treatment of choice is erythromycin, which reduces transmission and the number of organisms in the respiratory tract. Other drugs such as clarithromycin or trimethoprim-sulfamethoxazole can also be used in treatment (Table 25-3).
Table 25-3
Agents Used to Treat Whooping Cough
| Erythromycin | Binds to 50S ribosomal subunits and prevents ribosomal translocation |
| Azithromycin | Same as above |
| Clarithromycin | Same as above |
| Trimethoprim/Sulfamethoxazole | Inhibits bacterial synthesis of folic acid |
Vaccine
The original whole-cell, inactivated pertussis (wBP) vaccine was developed in the 1930s. Although the vaccine was effective, it contained small amounts of the lipopolysaccharide endotoxin. The endotoxin caused significant adverse health effects, including acute encephalopathy, febrile seizures, and hypotonic or hypertonic episodes. The vaccine has been discontinued in the United States.
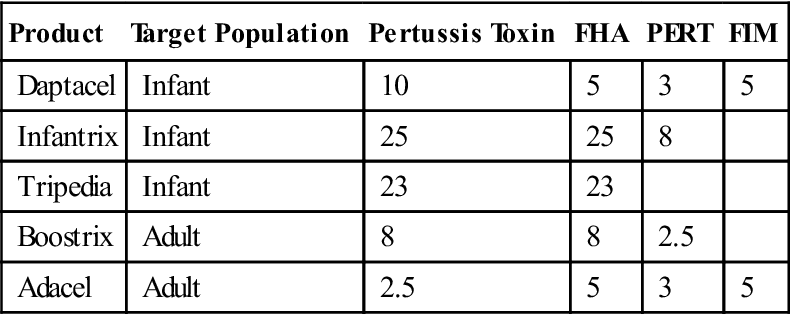
Acellular subunit pertussis (aBP) vaccines were introduced in 1996 for pediatric use and for adolescent and adult use in 2005. All aBP vaccines contain a pertussis toxoid. However, the concentrations of the filamentous hemagglutinin, fimbriae, and pertactin in vaccines vary considerably (Table 25-4). None of the vaccines contain the pertussis endotoxin.
Table 25-4
Composition of Acellular Pertussis Vaccines
< ?comst?>
| Product | Target Population | Pertussis Toxin | FHA | PERT | FIM |
| Daptacel | Infant | 10 | 5 | 3 | 5 |
| Infantrix | Infant | 25 | 25 | 8 | |
| Tripedia | Infant | 23 | 23 | ||
| Boostrix | Adult | 8 | 8 | 2.5 | |
| Adacel | Adult | 2.5 | 5 | 3 | 5 |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
FHA, Filamentous hemagglutinin; FIM, fimbriae; PERT, pertactin.
Modified from Centers for Disease Control and Prevention. Atkinson W, Hamborsky J, McIntyre S, editors: Epidemiology and prevention of vaccine preventable diseases, ed 10, Washington, DC, 2007, Public Health Foundation.
Following the standard four-dose immunization regimen, between 70% and 90% of the patients are protected from pertussis infection for 5 to 10 years.
Tetanus
Clostridium tetani is a gram-positive, anaerobic organism that is a common inhabitant in the intestines of horses, sheep, cattle, dogs, cats, rats, and guinea pigs. In soils contaminated with animal manure, the vegetative microbe derives nutrients from dead or decaying organic material. When nutrients become limited, the bacteria form spores, which are impervious to heat, chemicals, and other environmental toxins. Viable spores can persist in soil for decades.
Tetanus Toxin
C. tetani spores infect humans by accident, through a deep puncture wound. A deep wound ensures an oxygen-depleted (anaerobic) environment, which allows spores to germinate into vegetative bacteria. Vegetative bacteria release two protein exotoxins—tetanolysin and tetanospasmin. Tetanolysin lyses red blood cells but has no other physiologic function. Tetanospasmin is responsible for the recognized pathophysiology of tetanus.
Tetanospasmin is a 150-kiloDalton (kDal) A+B toxin. The B subunit binds to membrane gangliosides on neural pathways, and the complete toxin is transported to peripheral nerve end plates, the sympathetic nervous system, the spinal cord, and the brain. In the nerve endings, the A subunit inactivates synaptobrevin and prevents the release of gamma amino butyric acid (GABA) and glycine, which normally control the release of acetylcholine. Uncontrolled acetylcholine synthesis and increased nerve firing cause unpredictable muscle contractions, spasms, and seizures characteristic of tetanus.
Clostridium tetani and Disease
Four clinical forms of tetanus have been described: (1) generalized (most common), (2) local (common), (3) cephalic (rare), and (4) neonatal (common in the developing countries).
Generalized Tetanus
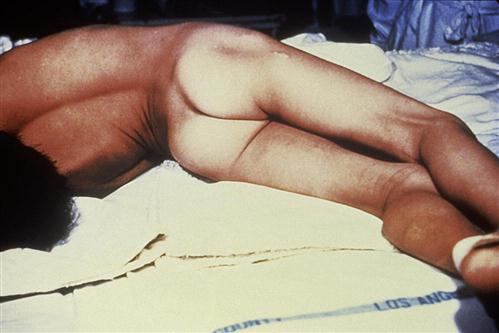
Trismus, or lockjaw, is the first sign of generalized tetanus. This is usually followed by a stiff neck, difficulty in swallowing or breathing, and abdominal muscle rigidity. Severe muscular contractions are frequent and last 5 to 10 minutes. Contractions in the long muscles of the back often arch the back in a characteristic form called opisthotonos, which often results in fractures of the spine (Figure 25-2). If the toxin reaches the lung, paralysis of respiratory muscles causes death.
Under the best of circumstances, the mortality rate from tetanus in unvaccinated individuals ranges between 40% and 78%.
Localized Tetanus
Localized tetanus is characterized by muscle contraction near the injury site. The contractions are self-limiting and seldom result in long-term complications.
Cephalic Tetanus
Cephalic tetanus occurs when the organism is present in the flora of the middle ear. Paralysis of facial muscles occurs when nerve transmission is inhibited in the branches of the fifth cranial nerve.
Neonatal Tetanus
Neonatal tetanus is a form of generalized tetanus that occurs in infants when spores infect the unhealed umbilical cord stump. Neonatal tetanus is rare in the United States but common in other countries when contaminated instruments are used to cut the umbilical cord. Globally, an estimated 257,000 deaths occur from neonatal tetanus each year.
Treatment
Tetanus immune globulin (TIG) or equine tetanus antitoxin is usually administered to persons with active tetanus. If TIG is unavailable, intravenous immunoglobulin (IVIG) containing anti-tetanus toxin antibodies is used. Both preparations neutralize and remove free toxins but have no effect on neuronal-bound toxin. To provide active immunity, immunization with the tetanus toxoid should begin as soon as the patient’s condition permits. Common therapeutic agents used to treat tetanus are listed in Table 25-5.
Table 25-5
Agents Used in the Treatment of Tetanus
| Tetanus immunoglobulin | Provides passive immunity by neutralizing free toxin |
| Metronidazole | Is considered a prodrug, which results in the production of cytotoxic radicals |
| Penicillin G | Arrests bacterial cell wall synthesis by inhibiting cross-linking cell wall polymers |
| Erythromycin | Binds to 50S ribosomal subunits and prevents ribosomal translocation |
| Clindamycin | Bacteriostatic agent that binds to 50S ribosomal subunit |
| Tetracycline | Blocks binding of animoacyl transfer ribonucleic acid (tRNA) to the ribosomal A site |
Tetanus Vaccine
Like the diphtheria toxin, tetanus toxin is treated with formalin to create a toxoid. It is offered as a fluid toxoid or absorbed to aluminum hydroxide. Although both formulations induce antibodies, a higher antibody response and duration of protection is induced by the adsorbed toxoid. Tetanus toxoid is available as a stand-alone vaccine or combined with diphtheria toxoid and acellular pertussis to form the DTaP vaccine. Three doses of tetanus toxoid in children 7 years of age and older or four doses in infants confer immunity in 100% of individuals. Without booster doses, immunity wanes, and antibody levels fall below the protective levels after 10 years.
Haemophilus Influenzae Type B
Haemophilus influenzae is an encapsulated gram-negative coccobacillus. It is the etiologic agent of meningitis, otitis media, epiglottitis, pneumonia, arthritis, occult febrile bacteremia, septic arthritis, cellulitis, and purulent pericarditis.
A polyribosyl phosphate (PRP) capsule appears to be the major Haemophilus virulence factor. It protects the organism from phagocytosis and the effects of complement. There are different capsular serotypes (A–F), however, H. influenzae type b (Hib) serotype accounts for 95% of all infections. Haemophilus is endemic in the United States, with epidemics occurring every 3 to 5 years. Most epidemics originate in nurseries or daycare centers, and a single index case can infect 90% of contacts with 48 hours.
Treatment
Children with Hib respiratory infections usually require hospitalization and treatment with cephalosporin or chloramphenicol and ampicillin. Since many Hib strains produce a β-lactamase and are resistant to ampicillin, these medications are not used as monotherapy for severe disease. Third-generation cephalosporins are the drugs of choice to treat invasive Haemophilus infections and meningitis. Glucocorticoids are also useful for reducing the risk of deafness associated with meningitis (Table 25-6).
Table 25-6
Agents Used to Treat Haemophilus Infections
| Ceftriaxone | Arrests bacterial cell wall synthesis by cross-linking cell wall polymers |
| Cefotaxime | Same as above |
| Meropenem | Arrests bacterial cell wall synthesis in a manner similar to penicillin |
| Chloramphenicol | Inhibits the ribosomal peptidyl transferase which elongates polypeptides |
| Ampicillin | Interferes with transpeptidation step of peptidoglycan biosynthesis |
| Dexamethasone | Suppresses migration of polymorphonuclear leukocytes into inflamed areas |
| Rifampin | Binds to β-prime subunit of deoxyribonucleic acid (DNA)-dependent ribonucleic acid (RNA) polymerase and inhibits the transcription of messenger RNA (mRNA) |
Vaccines
To convert the capsules to a T cell–dependent antigen, Haemophilus capsular polysaccharides are conjugated to proteins such as the diphtheria toxoid, the tetanus toxoid, or meningococcal outer membrane proteins (Table 25-7). Three conjugate vaccines are licensed in the United States for the vaccination of infants as young as 6 weeks of age. Each conjugate vaccine uses a different protein carrier.
Table 25-7
Conjugate Vaccines Licensed by the U.S. Food and Drug Administration
| Vaccine | Carrier Protein |
| HbOC (Hib TITER) | Diphtheria protein |
| PRT-T ActHIB | Tetanus toxoid |
| PRP-OMP (Pedvax HIB) | Meningococcal protein |
From Centers for Disease Control and Prevention. Atkinson W, Hamborsky J, McIntyre S, editors: Epidemiology and prevention of vaccine preventable diseases, ed 10, Washington, DC, 2007, Public Health Foundation.
Pneumococcus
Streptococcus pneumoniae is a gram-positive bacterium that is most commonly found in the nasopharynx in children and adults. In high density populations such as military installations or prisons, 50% to 60% of the population may be asymptomatic carriers. Pneumococci are the most common cause of community-acquired pneumonia (CAP), meningitis, and bacteremia.
A polysaccharide capsule surrounding the bacterium is the major virulence factor. It prevents phagocytosis and inhibits the deposition of the complement fragment C3b.
Capsules form the basis for pneumococcal serotyping. More than 90 different serotypes have been reported, and 23 to 25 pneumococcal serotypes infect humans. Two different numbering systems are used to identify pneumococcal serotypes. In the American system, serotypes are numbered in the order in which they were discovered and associated with disease. A Danish numbering system, which is more accepted by clinicians and microbiologists, groups serotypes with similar antigenicity. Predominant serotypes vary by geographic area, socioeconomic status, and age of the patient. Ten common serotypes account for 65% of invasive diseases in adults. Other serotypes (4 and 14) and serogroups (6, 7, 8, 9, 18, 19, and 23) are associated with pneumococcal infections in children younger than 6 years of age.
Pneumococcal Diseases
From the initial colonization in the respiratory tract, the organism spreads into the paranasal sinuses, the middle ear, the lung, and the brain. In children and older adults, pneumococci also cause osteomyelitis, endocarditis, cellulitis and brain abscesses.
Pneumonia
S. pneumoniae is the major cause of community-acquired lobar pneumonia. In lobar pneumonia, an entire lung lobe is infected and filled with serous fluid. A subsequent inflammatory response creates a purulent exudate in the infected lobe. A lobe containing fluid or exudates is commonly termed a consolidated lung. Pneumonia is a leading cause of death in children. Each year 1.8 million children under the age of 6 years die from pneumonia worldwide.
Otitis Media
In some individuals, the organism migrates up the eustachian tube to the middle ear. Inflammatory cells occlude the eustachian tubes and create negative pressure in the middle ear. If the pressure is not equalized by normal ventilation, serous fluid accumulates in the ear. Increased fluid pressure on the tympanic membrane causes pain and difficulty hearing. Severe infections may result in damage to the auditory nerve or perforation of the eardrum.
Bacteremia
In 25% to 30% of pneumonia cases, bacteria enter the blood. More than 50,000 pneumococcal bacteremia cases are reported each year, with the highest incidences in older adults and in infants. Most adults have evidence of a focal infection and frank bacteremia. In children 2 years of age or younger, occult bacteremia may occur in a healthy, febrile child without focal lung lesions. The fatality rate is 20% to 60% in patients with pneumonia and bacteremia.
Pneumococcal Meningitis
Streptococcus pneumoniae is the leading cause of bacterial meningitis among children under 5 years of age. In the United States, an estimated 3000 to 6000 cases are reported each year. The symptoms and sequelae are similar to those described for Haemophilus infection. The mortality rate is approximately 30% in younger individuals and 80% in older adults.
Treatment
Third-generation cephalosporins (ceftriaxone or cefotaxime) and vancomycin comprise the antibiotic regimen of choice. Evidence suggests that administration of corticosteroids such as dexamethasone also reduces the mortality rate and neurologic sequelae.
Vaccines
Pneumococcal vaccines are unique in that they do not prevent infection. However, they are highly effective in preventing invasive or systemic diseases in patients who develop pneumonia.
Capsular Polysaccharide Vaccines
The polyvalent vaccine (Pneumovax), or PPV23, contains capsular antigens from 23 different pneumococcal serotypes. These serotypes cause 96% of pneumonias and invasive disease in the developed countries. The vaccine also includes six serotypes that most frequently cause invasive drug-resistant pneumococcal infections in children. Over 80% of adults vaccinated form antibodies within 2 to 3 weeks, and antibodies may persist for 5 to 10 years. The vaccine is recommended for individuals over 50 years of age and for individuals aged 2 to 64 years who have a high risk of pneumococcal disease. Individuals at risk for developing severe disease include those with chronic conditions such as chronic cardiovascular disease, chronic pulmonary disease, diabetes, alcoholism, or liver cirrhosis.
The Pneumovax vaccine has several limitations. Capsular polysaccharides are T cell–independent type II antigens, which produce only transient immunoglobulin M (IgM) antibodies. Some children under 2 years of age may not respond to the PPV23 vaccine because of a developmental block in the production of IgG2 protective antibodies.
Pneumococcal Conjugate Vaccines
In 2000, the U.S. Food and Drug Administration (FDA) licensed the first conjugate pneumococcal vaccine (PCV7). Prevnar comprises capsular polysaccharides from seven pneumococcal serotypes (4, 9V, 14, 19F, 23F, 18C, and 6B). These serotypes account for 86% of bacteremia, 83% of meningitis, and 65% of otitis media cases in the United States. The conjugate vaccine acts as a T cell–dependent antigen that generates a primary and anamnestic response in both adults and young children.
Prevnar
Prevnar is recommended for children younger than 12 months and for children between the ages of 24 and 59 months who have underlying medical conditions. Over 90% of vaccinated individuals develop antibodies after completion of the four-dose immunization series.
Prevnar 13
Prevnar 13 is indicated for active immunization for the prevention of invasive disease caused by S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F and otitis media caused by serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. It is approved for use in children aged 6 weeks to 5 years.
Neisseria Meningitidis
N. meningitidis is a gram-negative organism with a specialized endotoxin referred to as a lipooligosaccharide (LOS). An anti-phagocytic capsule and the LOS are the major neisserial virulence factors. Thirteen different capsular serotypes have been reported. However, only five serogroups (A, B, C, Y, and W135) infect humans. As with other pathogenic bacteria, the major serotype causing disease differs by geographic region. For example, serotype A is a common cause of disease in Africa, but it is rarely found in the United States.
Humans are the only reservoir, and the organism is commonly found in the posterior nasal pharynx. At any point in time, 10% of the adolescent population are asymptomatic carriers. During an epidemic, the carrier rate may exceed 80%. Infection is transmitted from carriers to nonimmune individuals through respiratory droplets.
Meningococcal Diseases
N. meningitidis is the etiologic agent of meningitis and pneumonia. When disseminated by blood (bacteremia), bacteria can infect multiple organs. The onset of disease is rapid, and patients become symptomatic within 24 hours of exposure. Under the best of circumstances and appropriate antimicrobial therapy, the case fatality rate is 10% to 15%. In cases of fulminating bacteremia, the case fatality rate is 40%. Approximately 20% of patients surviving meningitis have sequelae such as neurologic deficits or loss of hearing, skin, digits, or limbs because of gangrene.
Neisserial Meningitis
Meningitis is an inflammation of the meninges and infection of the subarachnoid cerebrospinal fluid (CSF). Rapid bacterial growth, an inflammatory response, and release of LOS are responsible for both the pathophysiology and disease sequelae such as deafness and gangrene in hands and feet.
Bacterial growth causes obstruction of normal CSF flow, increased cranial pressure, and destruction of brain tissue. The inflammatory response to the organism often damages cranial nerve VIII, which controls hearing.
LOS enters the bloodstream and binds to a lipid-binding protein (LBP). In turn, the complex is transferred to a CD14 and Toll-like receptor 4 (TLR4) expressed by monocyte and endothelial cell membranes. CD14 intracellular signaling triggers three major physiologic events that include activation of the coagulation complement pathways and septic shock.
Activation of the Coagulation Pathway
Disseminated intravascular coagulopathy (DIC) and gangrene are caused by activation of the coagulation pathway. LOS stimulates endothelial cells to express tissue factor (TF). Interactions between clotting factor VII (FVIIa) and TF activate the coagulation cascade and the formation of microthrombi. These small blood clots circulate in peripheral blood until they are trapped in the small capillaries of the hands and feet. Occlusion of capillary blood flow results in tissue necrosis and gangrene. Depletion of platelets and clotting factors ultimately results in plasmin activation and seepage of blood into skin. Minor hemorrhages in skin cause small (petechiae) or large red or purple (purpura) spots on the lower extremities.
Activation of the Complement Cascade
LOS also activates the alternative complement pathway. Complement fragment C5a is an anaphylatoxin, which releases histamine and preformed mediators from mast cells and basophils. Other complement fragments attract neutrophils in the infection nidus. Vasodilatation and neutrophil chemotaxis are major contributors to septic shock induced by N. meningitidis.
Meningococcal Septic Shock
Meningococcal septic shock is a consequence of a fulminating meningococcal bacteremia. Systemic manifestations of hypotension, shock, adrenal hemorrhage, and multiple-organ failure are also common. Meningococcal bacteremia carries a mortality rate of 40%.
Incidence and Frequency
The incidence of meningococcal disease is highest in the late winter and early spring. Infants under 12 months of age, adolescents, and young adults between the ages of 18 and 23 years are highly susceptible to meningococcal infections. Populations with the highest risk for developing the disease are college freshman living in dormitories and military recruits.
Infection rates for each serotype are age dependent. In infants under 1 year of age, 65% of the cases are caused by serotype B, for which no licensed vaccine is available. In older individuals, 75% of meningococcal disease is caused by serogroups C, Y, or W-135.
Treatment
Bacterial meningitis runs a rapid clinical course and has a high mortality rate. Treatment is predicated on bacterial morphology and gram stain characteristics. Therapy regimens for gram-positive and gram-negative bacteria are provided in Table 25-8.
Table 25-8
Therapeutic Agents Used to Treat Meningitis Caused by Gram-Positive and Gram-Negative Bacteria
| Gram-positive cocci | Vancomycin plus ceftriaxone or cefotaxime |
| Gram-negative cocci | Penicillin G |
| Gram-positive bacilli | Ampicillin plus an aminoglycoside |
| Gram-negative bacilli | Broad-spectrum cephalosporin plus an aminoglycoside |
Modified from Razonable RR, Keating MR: Meningitis: Treatment and medication, Emedicine. emedicine.medscape.com/article/232915-overview. Accessed 11/01/10.
Vaccines
Three meningitis vaccines are licensed in the United States: Menomune, which is also called the Meningococcal Polysaccharide Vaccine 4 (MPSV4), Menactra (MCV), and Menveo.
Menomune
Menomune is a quadrivalent vaccine, which contains (A, C, Y, and W-135) capsular polysaccharides. Because Menomune contains T cell–independent antigens, it has poor immunogenicity in children under 5 years of age and does not generate memory cells. The vaccine is recommended for individuals 2 to 55 years of age and for unvaccinated individuals in high-risk groups. High-risk groups include children entering high school, college freshman living in dormitories, military recruits, and individuals with complement deficiencies or a dysfunctional spleen.
Menactra
Menactra is a quadrivalent vaccine consisting of (A, C, Y, and W-135) capsular polysaccharides linked to the diphtheria toxoid. The toxoid evokes a T cell–dependent response to both the polysaccharides and the toxoid. Because of its capability to evoke a memory response, the vaccine has a long duration of protection and reduces the carrier state. Menactra is licensed for use among persons 11 to 55 years of age. Young adolescents 11 to 12 years of age are usually vaccinated during a preadolescent health care visit.
Menveo
Menveo is composed of meningococcal (A, C, Y, and W-135) oligosaccharides conjugated to the diphtheria CRM197 protein. It is indicated for immunization to prevent invasive meningococcal disease and approved for use in individuals 11 to 55 years of age. It does not protect against infection with serotype B bacteria.
Polio
Poliovirus is an enterovirus, which is a genus within the picornavirus family. By definition, picornaviruses are small, icosahedral, non-enveloped, double-stranded ribonucleic acid (RNA) viruses. The three polio serotypes (P1, P2, and P3) have little antigenic cross-reactivity and immunity to one serotype does not confer immunity to other serotypes.
Humans are the only natural hosts for polioviruses. The virus is transmitted via the fecal–oral route and quickly infects the lymph tissue in the gastrointestinal tract. Following rapid replication, the virus enters the bloodstream and produces several different clinical outcomes.
Subclinical Infections
The majority of polio infections are asymptomatic. These individuals have no clinical symptoms but continue to shed the virus in saliva and feces for extended periods.
Abortive Infections
Some individuals infected with poliovirus develop a minor, nonspecific illness with symptoms similar to influenza infections. Most people recover within 1 week.
Nonparalytic Aseptic Meningitis
The virus infects the meninges, or tissue layers, covering the brain. Meningitis is characterized by stiffness of the neck, back, and legs. Usually, a complete recovery occurs within 10 days, often without supportive therapy.
Paralytic Polio
Over 85% of paralytic infections are caused by the P1 virus strain, which unilaterally infects motor neurons in the anterior horn of the spinal cord and the brain stem. Destruction of infected neurons disrupts the transmission of nerve impulses to select muscle groups, causing a flaccid paralysis.
Types of Paralytic Polio
Three types of paralytic polio exist: (1) The most common form is spinal polio (79%), with asymmetrical paralysis of the lower limbs. (2) Bulbar polio is associated with weakness in muscles innervated by the trigeminal (V), glosso-pharyngeal (IV), and accessory nerves (VI). These nerves control the muscles in the neck and tongue and the muscles used for swallowing. (3) Bulbospinal polio affects the spinal cord at the C3-5 level, which inhibits the function of the diaphragm and results in death.
Treatment
Currently, no antiviral treatment for polio is available. Most therapies are only supportive.
Post-Polio Syndrome
Between 20% and 40% of persons with a history of paralytic polio completely recover from the initial infection but experience new muscle weakness and paralysis 30 to 40 years after the initial event. The etiology of the post-polio syndrome (PPS) is unclear. One possible cause of PPS is reactivation of a latent polio virus. A second possible cause of PPS is the loss of slow-firing motor neurons in the anterior horn. To replace the rapid-firing neurons destroyed in the initial infection, a compensatory hypertrophy of small, slow twitch neurons occurs, restoring motor function. Over time, these neurons become fatigued and dysfunctional, and motor function is lost.
Vaccines
Two polio vaccines are licensed for use in the United States. The original inactivated poliovirus (IPV) vaccine developed by Jonas Salk consists of the three (P1–P3) formalin-inactivated polio strains. Over 95% of vaccinated individuals develop high levels of antibodies, which are protective against paralytic disease. The duration of immunity is unknown. A major disadvantage of the vaccine is that it does not stimulate the mucosal immune system, and individuals can be infected and transmit wild-type viruses. The vaccine was used extensively from 1955 to 1963 to eradicate polio in the United States.
The Sabin oral poliovirus vaccine (OPV) contains attenuated P1–P3 polio strains, which can replicate in the intestine. The OPV induces mucosal immunity and the synthesis of surface immunoglobulin A (sIgA), which prevents infection by the wild-type virus. Continued shedding of the virus also contributes to the natural vaccination of unprotected individuals.
Until 2000, a person was considered protected against polio following an IVP and OPV vaccination series. However, the risk of vaccine-associated paralytic poliomyelitis (VAPP) now outweighs the benefit of OPV. OPV is no longer recommended for routine vaccination in the United States. In other parts of the world, OPV is widely used in polio eradication programs.
Oral Polio Vaccine-Associated Paralytic Poliomyelitis
Since the OPV attenuated virus is able to replicate in the body, it has the ability to mutate or revert to a neurotropic form. From 1980 to 1995, VAPP accounted for 95% of all polio cases in the United States. The rate of VAPP is 1 case per 2.4 million vaccinations. A majority of these cases occurred in healthy vaccine recipients or household contacts of vaccine recipients.
Measles (Rubeola)
Rubeola is an enveloped, single-stranded RNA paramyxovirus that is closely related to the animal distemper, mumps, and respiratory syncytial viruses. It causes an acute viral disease that infects children and young adults. Classic rubeola begins with infection of the nasopharynx. Macrophages also transport the virus to the local lymph nodes. Replication of the virus in the lymph nodes allows the virus to enter the bloodstream and spreads the virus to multiple organs and to the skin.
The rubeola rash begins at the hairline, face, and upper neck. Over several days, the rash moves downward to the hands and feet. Early in the disease course, characteristic white spots, called Koplik spots, are found on the cheeks inside the mouth.
Complications
Because the rubeola virus localizes in multiple organs, major and minor sequelae are not unexpected. Minor complications include diarrhea and otitis media. More serious sequelae are immune suppression, encephalitis, and subacute sclerosing panencephalitis (SSPE).
Immune Suppression
Rubeola infections cause a prolonged immunosuppression in infected individuals. Measles-infected lymphocytes undergo apoptosis. As a consequence, increased susceptibility to secondary intestinal and respiratory infections occurs.
Encephalitis
Encephalitis occurs in 0.1% of measles infections and is fatal in 25% of cases. Survivors often have moderate to severe brain damage that results in intellectual disabilities.
Subacute Sclerosing Panencephalitis
SSPE is a rare, progressive deterioration of the central nervous system associated with a slow or persistent measles infection. Progressive deterioration of brain function results in ataxia, myoclonic seizures, dementia, and death. Onset is generally 7 to 10 years after the initial measles infection. It is considered rare in the developed countries. Fewer than 10 cases per year are reported in the United States.
Atypical Rubeola
Atypical measles is a hypersensitivity reaction in individuals who received the killed measles vaccine (KMV) between 1963 and 1967. This vaccine sensitized an estimated 600,000 to 900,000 persons to measles antigens. A second exposure to measles vaccines elicits delayed hypersensitivity reactions in the skin and inflammation of the serous membranes lining the pericardial, pleural, and peritoneal cavities.
Modified Measles
Modified measles is a mild illness that occurs in infants who have maternal antibodies to measles and in adults who have received IgG prophylaxis for measles infections. It is characterized by small, discrete measles rashes of short duration.
Treatment
Treatment for measles is supportive. Antibiotics may be indicated when the individual is diagnosed with secondary bacterial infections.
Vaccines
In 1963, the Edmonstron-Enders B strain measles vaccine, in both killed and attenuated forms, was licensed in the United States. Further attenuation of the Edmonstron-Enders measles strain produced the virus used in the current vaccine, which was licensed in 1968. The attenuated vaccine is available as a stand-alone product or in combination with rubella or mumps and rubella (MMR). Vaccination produces a mild, noncommunicable infection. Ninety-five percent of children vaccinated at 12 to 15 months of age produce antibodies and are protected from subsequent infection. If a second dose of vaccine is given at 4 to 6 years of age, 99% of children develop high titers of antibody, an anamnestic response, and lifelong immunity. Side effects of the vaccine range from fever to encephalopathy (Table 25-9).
Table 25-9
Adverse Reactions of the Measles–Mumps–Pertussis Vaccine
| Symptom | Percentage or Frequency |
| Fever | 5–15 |
| Rash | 5 |
| Joint symptoms | 25 |
| Thrombocytopenia | ≤1/30,000 |
| Encephalopathy | ≤1/1,000,000 |
Modified from Centers for Disease Control and Prevention. Atkinson W, Hamborsky J, McIntyre S, editors: Epidemiology and prevention of vaccine preventable diseases, ed 10, Washington, DC, 2007, Public Health Foundation.
Mumps
Mumps is an acute viral illness caused by a single-stranded RNA paramyxovirus. Person-to-person transmission occurs by the respiratory route. The virus initially localizes in the nasopharynx and regional lymph nodes. Following several replication cycles in the lymph node, the virus enters the bloodstream and infects multiple organs, including the meninges, pancreas, testes, ovaries, and salivary glands. The most common symptom is unilateral or bilateral parotitis, which is an inflammation of the parotid salivary glands located on either side of the face.
Complications of Mumps
Complications of mumps infections include encephalitis, aseptic meningitis, and inflammation of the ovaries (oophoritis), breasts (mastitis), or testes. Inflammation of the testes (orchitis) is common in postpubescent males. The inflammation is bilateral in 30% of males and is characterized by testicular swelling and tenderness. Orchitis results in testicular atrophy in 30% of affected males, but sterility is rare. The mumps virus also causes unilateral deafness in approximately 1 per 20,000 infected individuals.
Vaccine
The current vaccine, licensed in 1967, is a live attenuated strain of mumps grown in chick embryo fibroblasts. Efficacy is estimated to be 75% to 91% after a single dose and 85% after two doses; immunity is believed to last 25 years or more.
The vaccine is available as a single-antigen preparation or in combination with measles and rubella (MMR), and additionally in combination with varicella (MMRV). Following vaccine licensure, the incidence of mumps decreased rapidly in the United States. Only 258 cases of mumps were reported in 2004.
Recent data suggest that the mumps virus may be evolving or a high rate of vaccine failure is present. In 2006, a multiple-state outbreak of mumps with 6500 cases was documented. The majority of infections occurred in college students (median age 22 years) who had been vaccinated with one or two doses of the MMR vaccine.
Rubella (German Measles)
The rubella virus is an enveloped, single-stranded RNA virus closely related to the eastern and western encephalitis viruses. German physicians were the first to describe the illness in the scientific literature, and thus the common name for the infection came to be German measles. The scientific name rubella is derived from the Latin word, meaning “little red,” to denote that the lesions were smaller than those of rubeola.
The virus is transmitted by the respiratory route, and initial replication occurs in the nasopharnyx and lymph nodes in the head and neck. After 5 to 7 days, the virus enters the bloodstream and infects the distal lymph nodes. Viral replication in tissue and nodes produces a second viremia. Infection of skin cells causes a maculopapular rash that appears 14 to 17 days after the initial infection. The rash begins on the face and progresses downward to the hands and feet.
Complications
Congenital Rubella Syndrome
Congenital rubella syndrome (CRS) is the major complication associated with rubella infections. Measles infection of the mother during the first trimester of pregnancy is associated with encephalitis, hearing loss, and blindness in the newborn infant, and, later, intellectual disabilities. Between 1964 and 1965, a rubella epidemic resulted in 12.5 million cases of rubella infection. CRS occurred in 20,000 newborns and resulted in 15,000 neonates being either deaf or blind, 2000 with encephalitis, and 1800 children with intellectual disabilities.
Rubella Infections in the Second and Third Trimesters
If the mother is infected with rubella during the fourth and fifth months of pregnancy, deafness is the most common complication in the offspring. Ocular, cardiac, and neurologic abnormalities have also been reported.
Arthritis
In 70% of women, rubella infection causes arthritis and arthralgia in the fingers, wrists, or knees. Symptoms may persist for 2 to 6 weeks.
Vaccine
The current licensed rubella vaccine is the RA27/3 live attenuated virus grown in human diploid fibroblasts. Individuals 1 year and older develop antibodies after a single dose. Antibodies protect against infection and viremia for 15 years or more.
The vaccine also is available as a single-antigen preparation, as MMR, or as MMR combined with varicella as MMRV. A mass vaccination program undertaken in 1969 eradicated rubella in the United States.
ChickenPox (Varicella Zoster Virus)
Varicella zoster virus (VZV), which causes chickenpox, is an enveloped deoxyribonucleic acid (DNA) virus and a member of the herpes virus family. It causes two diseases: (1) chickenpox (varicella) and (2) shingles (zoster), which is a reactivation of a latent varicella virus.
The virus enters the host through the respiratory route and the conjunctiva. Like other viruses, the initial replication occurs in the nasopharynx and in the lymph nodes. A second viremia infects multiple organs and skin cells. Some viruses infect sensory nerves and take up lifelong residence in the sensory dorsal root ganglia near the spinal cord.
In contrast to measles and rubella infections, the skin rash begins on the torso (centripetal distribution) and progresses outward to the arms, legs, and head. Lesions progress from red papules to generalized blisters (vesicular lesion) containing a clear fluid and infective virus. Successive crops of lesions may occur over several days. When all lesions have scabbed over, the individual is no longer infectious.
Treatment of Varicella Infections
Treatment is designed to reduce or manage symptoms and prevent secondary bacterial infections. Therapeutic agents used to treat chickenpox are listed in Table 25-10.
Table 25-10
Agents Used to Treat Chickenpox
| Diphenhydramine | First-generation antihistamine |
| Hydroxyzine | H1 receptors agonist |
| Acyclovir | Synthetic purine nucleoside that inhibits the replication of viral deoxyribonucleic acid (DNA) |
| Varicella zoster immunoglobulin, human | Provides passive immunity and binds free virus |
| Acetaminophen | Reduces fever by acting directly on hypothalamic heat-regulating centers |
Complications of Varicella Infection
Disseminated Varicella Neonatorum
Disseminated varicella neonatorum is observed in infants born to women who develop active varicella infections 5 days before or after delivery. Because the neonate is often without the benefit of maternal antibodies and cannot generate an immune response, infants have overwhelming hemorrhagic infections of the lungs and liver. Mortality rate in these infants is over 30%.
Reye Syndrome
Reye syndrome is associated with aspirin use during an active varicella zoster virus (VZV) infection. In the infected cells in the brain, liver, and kidney, aspirin inhibits mitochondrial function and the generation of ATP. Without ATP, cells cannot synthesize the proteins necessary for survival. As a consequence, a loss of neurons in the brain, degeneration of the proximal tubules in the kidney, and hepatocyte destruction occur.
Congenital Infections
Infection of the fetus during the first 20 weeks of gestation is associated with congenital varicella syndrome, which includes low birth weight, skin scarring, muscular atrophy in the arms and legs, encephalitis, and microencephaly. After 20 weeks of gestation, VZV infection causes a latent infection that is associated with reactivation varicella, or shingles.
Varicella Zoster (Shingles)
In young individuals, varicella reactivation in nerve tracts is prevented by a vigorous cellular immune response. As the immune system wanes with age, the risk of varicella reactivation increases. Shingles or varicella zoster results from a reactivation and eruption of the latent varicella virus in the dorsal root ganglia and supporting cells. The virus moves down the nerve tact and localizes in the skin, where it causes a unilateral, painful skin rash. Eruptions usually occur in the sensory ganglia of the face, head, or torso.
Complications of Herpes Zoster
Post-Herpetic Neuralgia
After resolution of the zoster lesions, persistent pain, or post-herpetic neuralgia (PHN), may persist for a year. Although the pathology is unclear, PHN is believed to involve neuronal atrophy, scarring of the dorsal root ganglia, and loss of epidermal nerve innervations. Sensory loss is often surrounded by areas where normal stimuli (e.g., light touching) cause intense pain.
Ramsay Hunt Syndrome
In Ramsay Hunt syndrome, varicella infection of the seventh cranial nerve (CNVII) gives rise to ulceration of the ears, tongue, and soft palate. It begins with lesions on the external ear canal, with or without facial paralysis.
Treatment of Herpes Zoster
Treatment is directed at inhibiting viral replication and prevention of PHN. Nucleoside analogs are often effective in reducing the duration of zoster infections and the severity of PHN. Therapeutic agents used to treat zoster are listed in Table 25-11.
Table 25-11
Agents Used to Treat Herpes Zoster
| Acyclovir | Synthetic purine nucleoside analog that inhibits the replication of viral deoxyribonucleic acid (DNA) |
| Famciclovir | Active metabolite, penciclovir, inhibits viral DNA synthesis/replication |
| Valacyclovir | L-valyl ester of acyclovir, which is converted to acyclovir |
| Varicella zoster vaccine | Boosts immunity against herpes zoster virus (shingles) in older patients |
Vaccines
Varicella Vaccine
The varicella vaccine (Varivax) was licensed in 1995 and is indicated for the vaccination of individuals 1 year of age and older. The vaccine comprises an attenuated strain of VZV that has been passed sequentially through human embryonic lung cells, embryonic guinea pig fibroblasts, WI-38, and MRC-5 human diploid cells. After two doses of the vaccine, over 99% of individuals develop protective antibodies, and the risk of varicella infection is reduced by 80% to 90%. Efficacy studies have shown that protection lasts 10 years or longer. Breakthrough infections in vaccinated individuals are rare and usually milder than the infections in unvaccinated individuals.
Varicella Zoster Vaccine
In 2006, a varicella zoster vaccine (Zostavax) was approved for use in persons 60 years of age and older. Zostavax contains a 14-fold higher concentration of the attenuated strain used in the Viravax vaccine. The vaccine reduces the incidence of shingles by 51.3% and PHN by 61.3%. The vaccine efficacy, however, declines with age. In persons 80 years of age and older, the efficacy is only 18%. With the scheduled use of Viravax, this vaccine will become unnecessary.
Hepatitis
Viral hepatitis is a generic term for liver inflammation caused by six hepatitis viruses (A, B, C, D, E, and G). Types A, B, and E are more prevalent and cause acute hepatitis, chronic hepatitis, or both, in humans. Because of the occult and chronic nature of hepatitis C virus infection, it is viewed as the most serious form of hepatitis. The pathophysiology of hepatitis has not been fully delineated. However, cellular injury is not apparent until cytotoxic T cells accumulate in the portal and periportal areas of the liver. This suggests that the immune response to the virus-infected cells causes tissue destruction and hepatitis.
Hepatitis A
The hepatitis A virus (HAV) is a non-enveloped, single-stranded RNA enterovirus that is a member of the picornavirus family. The virus is transmitted via the fecal–oral route and occasionally by the parenteral route. The incidence of symptomatic illness varies with age. Children younger than 6 years of age usually have asymptomatic infections. In adolescents and adults, infection is symptomatic, with nausea, abdominal discomfort, dark urine, and jaundice (yellowing of the skin and eyes). Symptoms usually last less than 2 months but may persist for as long as 6 months.
Hepatitis B
The hepatitis B virus (HBV) is a member of the Hepadnaviridae family and is closely related to animal hepatitis viruses. Unlike other viruses, HBV can persist outside the body for extended periods and is highly resistant to extreme temperatures. The viral DNA is circular but only partially double-stranded.
The presence of hepatitis B surface antigen (HBsAg), which is an envelope protein, is considered evidence for acute HBV infections. Another antigen called HBeAg is only found when free virus is present in blood. It is used as a marker for infectivity and the ability to spread the virus to others.
HBV is transmitted through mucosal exposure to the blood or other bodily fluids of the infected person. Although rare, it is also possible to transmit HBV through percutaneous exposure, such as with tattooing, ear piercing, or needle sticks. HBV can also be transmitted perinatally from mother to infant.
Several populations are at high risk for developing hepatitis B. Intravenous drug users are a high-risk group because of needle sharing. Other high-risk populations are household contacts of individuals with hepatitis, residents and staff of facilities for developmentally disabled persons, and health care workers who have possibly been exposed to the blood, blood products, or bodily fluids of patients.
Acute Hepatitis B
After a nonspecific stage, which is characterized by malaise, nausea, and vomiting that last for 3 to 10 days, jaundice emerges and lasts for 1 to 3 weeks. In addition to jaundice, hepatic tenderness and hepatomegaly are often present.
Chronic Hepatitis
Chronic active infection is responsible for most HBV morbidity and mortality. Fifteen to twenty percent of infected individuals develop cirrhosis of the liver within 5 years. When compared with the general population, persons with chronic HBV disease also have a 12- to 300-fold higher risk of developing liver cancer. If cirrhosis is present, the risk of hepatocellular carcinoma is further increased.
Treatment
Therapeutic agents are available for treatment of HBV with the goals of reducing inflammation, fibrosis, and progression to cirrhosis. Therapeutic agents are listed in Table 25-12.
Table 25-12
Agents Used to Treat Hepatitis Infections
| Interferon alpha 2b (IFN-α2b) | Prevents the spread of virus to uninfected cells |
| Amantadine | Prevents penetration of virus into host by inhibiting uncoating of virus |
| Famciclovir | Active metabolite, penciclovir, inhibits viral deoxyribonucleic acid (DNA) synthesis |
| Entecavir | Guanosine nucleoside analogue which inhibits hepatitis B virus (HBV) polymerase |
| Prednisone | Anti-inflammatory agent |
Vaccines
Hepatitis A Vaccine
Havrix and VAQTA are two killed or inactivated virus vaccines licensed in the United States for use in children and adults. The vaccine is prepared from tissue culture–adapted virus is grown in diploid human fibroblasts, purified, and formalin treated. Viral fragments are adsorbed to aluminum adjuvants.
Both vaccines are efficacious. After a single dose of either vaccine, protective antibody develops in 97% of children and adults within 4 weeks. The incidence of clinical hepatitis is reduced by 94% in children receiving two doses of the vaccine. Data from computer-based kinetic models suggest that antibodies may persist for 20 years or longer. The duration of cell-mediated immunity to the virus is unclear.
Hepatitis B Vaccines
Recombivax and Engerix–B are licensed for both adult and pediatric populations. Both are recombinant subunit vaccines containing HBsAg protein. The vaccines differ only in the concentration of the HBsAg antigen and the nature of aluminum adjuvants. Recombivax contains aluminum hydrophosphate sulphate. Engerix-B uses aluminum hydroxide as an adjuvant and trace amounts of thimerosal from the manufacturing process.
In addition to single-antigen vaccines, two combination vaccines have been licensed in the United States. Comvax is HBV combined with Haemophilus influenzae type B vaccine. It can be administered to infants aged 12 to 15 months when the mother’s HBV status is unknown. Pediatrix is the first licensed multiple-component vaccine. It contains diphtheria–tetanus–acellular pertussis (DTaP Infantrix), hepatitis B virus (Engerix), and the three-strain inactivated polio virus (IPV). This vaccine can be used interchangeably with other HBV vaccines.
Influenza
The influenza virus is a helical-shaped, single-stranded RNA virus within the Orthomyxoviridae family. The viral RNA is unique in that it is composed of eight separate genomic segments. Extending from the protein capsid are hemagglutinins and neuraminidase molecules. Hemagglutinins are spikes that facilitate viral binding to sialic acid residues in mammalian cell membranes. Neuraminidase is a mushroom-shaped glycoprotein enzyme, which contributes to the infectivity of the virus. As the virus exits the cell, it coats itself with host cell membrane containing sialic acid residues. Sialic acids are the target molecules for hemagglutinins. As a consequence, the hemagglutinins on one virion bind to sialic residues on another virion, creating large, noninfectious aggregates. Neuraminidase destroys sialic acid residues and frees the individual viruses to infect other cells. On the basis of the types of nuclear material, three basic influenza types exist: A, B, and C.
Type A Influenza
The natural hosts for type A viruses are wild aquatic birds. Influenza type A viruses from wild birds can infect multiple species, including humans, birds, and swine. When transmitted to humans, type A influenza causes moderate to severe disease. Type A influenza is usually associated with worldwide epidemics known as pandemics.
Type B Influenza
Influenza type B has no animal reservoir and causes only mild disease in humans. However, it is responsible for geographically localized epidemics that appear every 3 to 5 years.
Type C Influenza
Type C influenza can infect humans and pigs. In humans, it causes a mild respiratory disease or an asymptomatic infection. It does not have an impact on public health, as do types A and B, because it rarely causes epidemics.
Influenza Strains or Subtypes
Hemagglutinins and neuraminidase are the major influenza virulence factors. Sixteen hemagglutinins and 9 different neuraminidase molecules exist. An influenza strain expresses only one hemagglutinin and one neuraminidase serotype. Both molecules are antigenic and are not cross-reactive. Current serotypes of influenza A viruses are the H1N1 and H3N2 viruses.
Antigenic Drift
From year to year, genetic and epidemiologic pressures on influenza types A and B cause point mutations in genes coding for the antigenic epitopes in hemagglutinins (H) and often neuraminidase (N). Less than a 1% change in the hemagglutinin or neuraminidase structure creates novel epitopes that are not recognized by pre-existing antibodies or memory cells. The phenomenon is known as antigenic drift. An example of antigenic drift occurred in 1997–1998. Early in 1997, the type A/Wuhan/H3N2 was the dominant influenza strain included in the 1997 vaccine. Late in the same year, a drift variant called type A/Sydney/H3N2 became the predominant virus. Although only a small difference in hemagglutinin structure was present, the two strains were antigenically dissimilar. The 1997 vaccine failed to protect against infection with the type A/Sydney/H3N2 strain, which caused epidemics in late 1997 and early 1998.
Antigenic Shift
Antigenic shift occurs when a radical and abrupt change in influenza type A virus hemagglutinins occurs. In some cases, a 50% change occurs in the hemagglutinin structure. Antigenic shift can be the result of a direct jump from an unknown animal strain to humans or a reassortment of two or more influenza viruses within the same cell.
Evidence suggests that the 1918 influenza pandemic was the result of a direct jump from pigs to humans. The type A H1N1 pandemic flu killed 500,000 people in the United States. Worldwide, the death toll was estimated at 50 million.
Viral reassortment is a more complex form of antigenic shift. It occurs when two viruses simultaneously infect the same animal. For example, pigs carry an endemic strain of influenza and can be infected with both human and avian influenza strains. Within an infected porcine cell, reassortment of genetic material from both viruses creates a new, hybrid virus. The virus that caused the 2009 pandemic influenza (type A H1N1) is a quadruple reassortment virus. It contains genes from pigs normally found in Europe and Asia, avian–swine influenza genes, and human influenza genes.
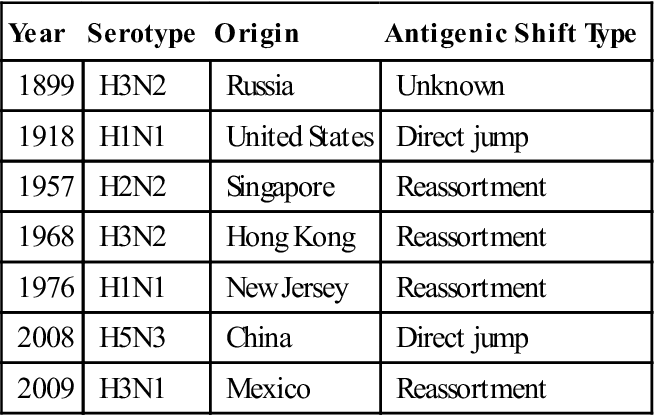
Seven reassortments or antigenic shifts in type A influenza have been documented in the last 115 years. Influenza pandemics are listed in Table 25-13.
Table 25-13
Influenza Pandemics of the Twentieth Century
< ?comst?>
| Year | Serotype | Origin | Antigenic Shift Type |
| 1899 | H3N2 | Russia | Unknown |
| 1918 | H1N1 | United States | Direct jump |
| 1957 | H2N2 | Singapore | Reassortment |
| 1968 | H3N2 | Hong Kong | Reassortment |
| 1976 | H1N1 | New Jersey | Reassortment |
| 2008 | H5N3 | China | Direct jump |
| 2009 | H3N1 | Mexico | Reassortment |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Transmission and Temporal Patterns
Person-to-person transmission of influenza virus is facilitated via large (5-micron diameter) droplets generated by sneezing or coughing. In adults, transmission can occur 1 day before symptoms occur to 5 days after symptoms begin. Transmission also occurs by touching virus-contaminated inanimate objects. Influenza virus remains viable for 24 to 28 hours on hard, nonporous materials.
Types of Influenza
Seasonal Influenza
In seasonal or yearly influenza, infections are localized in the lung because hemagglutinins can only bind to the epithelial cells in the respiratory tract. The incubation period is 2 to 4 days followed by fever, myalgia, sore throat, nonproductive cough, and headache. Influenza complications include secondary bacterial pneumonia, Reye syndrome, and myocarditis. During an average influenza season, 36,000 persons die from influenza infections in the United States. If the predominate type A strain expresses the genetically unstable H3 hemagglutinin, mortality increases significantly. Over 90% of deaths occur in persons 65 years of age and older.
Pandemic Influenza Mortality
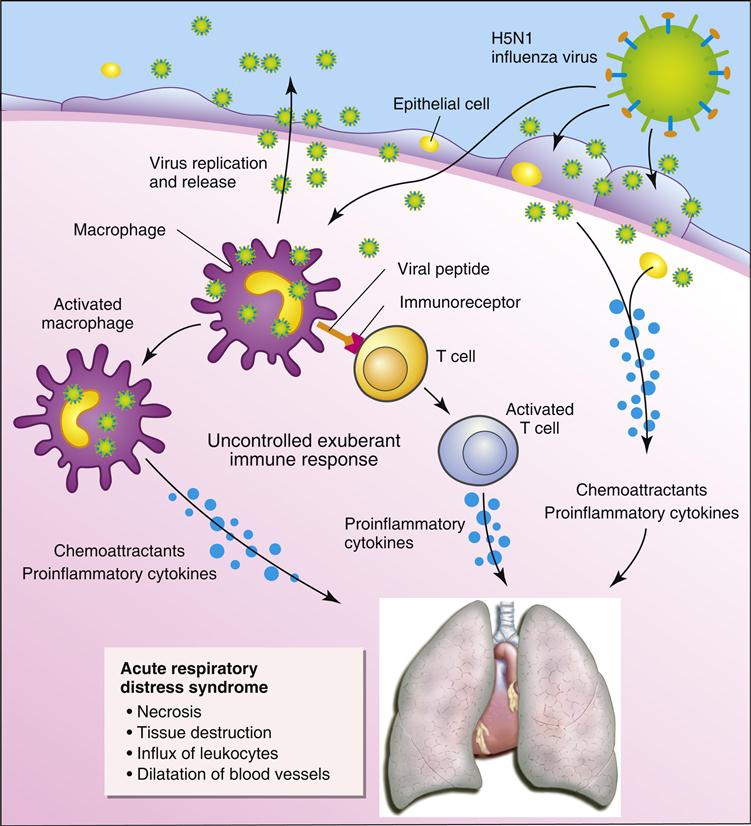
Pandemic influenza strains usually cause a severe infection that is often fatal. Unlike the seasonal flu viruses, pandemic strains can infect multiple organs. An aberrant immune response known as a cytokine storm is responsible the pathophysiology of pandemic influenza.
Infection of macrophages and monocytes by a pandemic avian influenza strain (H5N1) causes an exaggerated and prolonged production of proinflammatory cytokines and chemokines. T cells activated following interactions with macrophages also produce proinflammatory cytokines. The cytokine storm causes pulmonary edema, alveolar hemorrhage, and acute respiratory distress syndrome (Figure 25-3). The major cytokine contributors to the pathophysiology are tumor necrosis factor alpha (TNF-α), IL-6, and interferon gamma (IFN-γ).
Treatment
Treatment for influenza is designed to prevent the spread of the virus and boost immunity with the administration of influenza vaccines. Therapeutic agents are listed in Table 25-14.
Table 25-14
Agents Used to Treat or Prevent Influenza Infections
| Amantadine | Inhibits fusion between viral and target cell membrane |
| Remantadine | Same as above |
| Zanamivir | Neuraminidase inhibitor |
| Oseltamivir | Same as above |
| Peramivir | Same as above |
Vaccines
Two vaccines are licensed by the FDA. A trivalent inactivated vaccine (TIV) was licensed in 1940, and the live attenuated influenza vaccine (LAIV) became commercially available in 2003. The composition of these vaccines changes every year but usually contains both type A and B strains.
Preparation of Trivalent Inactivated Vaccine (TVI)
The epidemic strains of the vaccine are propagated in the embryonic fluid of fertilized chicken eggs, harvested by centrifugation, purified, inactivated by betapropiolactone, and disrupted by detergent treatment. Subunit hemagglutinin and neuraminidase proteins are purified and prepared for inclusion into the vaccine. Only subunit vaccines are licensed in the United States. TIV is 70% to 90% effective in adults under 65 years of age. In older adults, the vaccine is only 30% to 40% effective in preventing clinical illness. It does, however, reduce hospitalization and prevent influenza-induced deaths by 50% to 80%.
Live Attenuated Influenza Vaccine (LAIV)
LAIV uses influenza strains adapted to replicate at 25°C by the passage of the virus through primary chicken kidney cells at progressively lower temperatures. When administered by the intranasal route, the vaccine strains infect the nasal pharynx and the throat, which are usually at 25°C, but cannot infect the lung that has a temperature of 37°C. In the nasal pharynx and the throat, the virus produces hemagglutinins and neuraminidase, which stimulate mucosal immunity. It is 87% effective against infection with the vaccine strains. In vaccinated adults, 20% to 27% fewer febrile and upper respiratory illnesses occur.
Rotavirus
Rotavirus is a double-stranded RNA virus that consists of three concentric protein shells. This virus is found in every part of the world and is the major cause of severe diarrhea in children. Groups at risk for rotavirus infection are children in daycare centers, hospitalized children (nosocomial infection), and parents of infected children. Each year in the United States, 55,000 children between 4 and 36 months require hospitalization for rotavirus infections.
The virus enters the body by the oral route and infects the villous epithelium in the upper two thirds of the small intestine. Viral replication causes damage to the intestinal mucosa which results in the malabsorption of sodium and glucose and decreased levels of lactase, phosphatase, and sucrase. This leads to isotonic diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis.
The major groups, subgroups, and serotypes are determined by the differences in viral capsid proteins. Seven major groups of rotavirus (A–G) have been described, but most human infections are caused by group A. Two structural viral proteins (VP4 and VP7) are the major virulence factors and determine the serotype. Eight different VP4 protein isoforms (P1–P8) have been described. These isoforms act as viral enterotoxins that cause diarrhea. The VP7 structural protein is a cased glycoprotein, also known as the G protein. Nine different allelic forms of the G protein (G1–G9) are known to exist. A rotavirus strain expresses only one P and one G isoform. Over 80% of diarrheal disease in the United States is caused by the P8G1 serotype.
Treatment
In most cases, the individual recovers without medications. Medications such as antidiarrheals and antiemetics should be used with extreme caution.
Vaccine
In 1998, the FDA licensed Rotashield (RRV-TV), a rhesus-based reassortment vaccine for administration to infants. The vaccine was withdrawn within a year because it caused bowel obstructions as a consequence of the intestine wrapping around itself (intussusceptions).
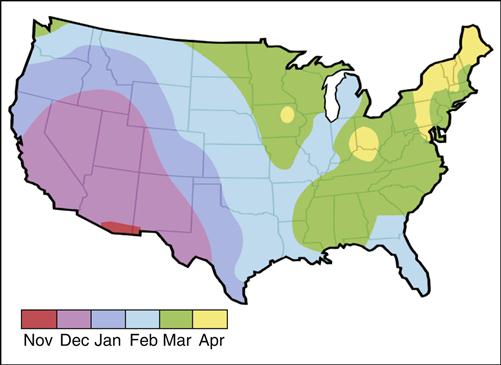
The FDA subsequently licensed Rotarix and RotaTeq for the prevention of rotavirus infections. RotaTeq is a reassortment vaccine developed from human and bovine rotavirus strains. The vaccine is composed of four reassortment human viruses (G1–G4), a bovine G6 virus, and attachment proteins P1A (human) and P7 (bovine). The duration of RotaTeq protection lasts, at least, throughout the rotavirus season. In the United States, the rotavirus season begins on the west coast between November and January. It moves quickly to the southern states, and then to the northern and the midwestern states (Figure 25-4).
Rotarix is indicated for the prevention of rotavirus gastroenteritis caused by G1 and non-G1 types (G3, G4, and G9), which are the four predominant strains in the United States. The vaccine contains a live attenuated virus associated with the P8G1 serotype. After two doses of the vaccine, 77% to 86% of the recipients develop high antibody titers. The vaccine is efficient in reducing both gastroenteritis and hospitalizations for rotavirus infections over 2 years.
Papilloma Virus
Papilloma virus is a small DNA virus. On the basis of antigenic differences in viral capsid L1 proteins, 100 different human papilloma virus serotypes have been classified. Sixty serotypes cause warts on hands and feet. The remaining 40 serotypes infect the mucosa or the genital area. Two serotypes (6 and 11) cause 99% of genital warts and 80% of laryngeal papillomas. Twenty-three other serotypes are oncogenic and cause cervical changes that can transition into cervical intraepithelial neoplasia (CIN) and cancers of the anus, penis, vulva, and vagina. Serotypes 16 and 18 cause 70% of cervical cancers in women and 90% of anal cancers in men.
Papilloma virus infection is the most common sexually transmitted disease in the United States and, perhaps, the world. New infections are estimated at 6.2 million annually. Over 70% of new infections are being reported in the 15- to 24-year age group, and the infection rate among adolescent girls may be as high as 64% and among boys may be greater than 20%. Recent computer-based models also estimate that 80% of sexually active women will be infected with the papilloma virus by age 50.
Infection estimates are both alarming and deceiving. Estimates are based on infection with any one of the 23 genital strains. Only four of these strains pose a high risk of adverse health effects, and 91% of infections resolve within 2 years and cause no further health effects. The incidence of infections with vaccine strains causing genital warts or cervical cancer may be relatively low in the United States.
Human papilloma virus (HPV) has a two-stage reproductive cycle. Basal cells are infected with the virus when the epithelial layer is disturbed during sexual intercourse. The virus targets keratinocyte stem cells and remains dormant in these cells until HPV genes are activated as the keratinocytes leave the basal layer. Replication of the virus occurs in highly differentiated keratinocytes at the epithelial surface. Desquamation of epithelial cells releases the virus into the environment.
Treatment
Biologic modifiers are used to reduce symptoms and external lesions. Infected cells can also be destroyed by cytotoxic agents. Therapeutic agents used to treat HPV infections are listed in Table 25-15.
Table 25-15
Agents Used to Treat Human Papilloma Virus Infections
| Imiquimod | Induces secretion of interferon alpha (IFN-α), tumor necrosis factor (TNF), interleukin 1 (IL-1), IL-6, and IL-8 |
| IFN-α n3 and IFN-α2b | Prevents the spread of virus to uninfected cells |
| Podofilox | Topical anti-mitotic |
| Podophyllin | Cytotoxic agent that arrests mitosis in metaphase |
| Fluorouracil | Interferes with the synthesis of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), which creates thymine deficiency |
| Trichloroacetic acid and bichloroacetic acid | Keratolytic agents that chemically cauterize skin and keratins |
| Salicylic acid | Desquamation of the horny layer of skin |
| Papillomavirus vaccine, quadrivalent (Gardasil) | Vaccine containing serotypes 9, 11, 16, and 18 |
| Papillomavirus vaccine, bivalent (Cervarix) | Vaccine containing serotypes 16 and 18 |
Vaccines
Gardasil and Cervarix are FDA-licensed HPV vaccines. The quadrivalent HPV recombinant vaccine (Gardasil) contains L proteins that reassemble to form an empty viral shell called a viral-like protein (VLP). Gardasil contains VLPs from serotypes 6, 11, 16, and 18. Cervarix is a bivalent vaccine that contains only serotypes 16 and 18. To sustain the immune response, VLPs are adsorbed to an aluminum adjuvant and stabilized with sodium chloride, L-histidine, polysorbate 30, and sodium borate.
Both vaccines have advantages and disadvantages. Since VLPs do not contain DNA or other genetic material, they cannot infect cells, reproduce, or induce neoplastic changes. A major disadvantage of both vaccines is that they are only effective against papilloma strains used in the vaccine. They do not protect against 21 other strains that may cause cervical cancers in women and penile cancers in men.
Gardasil
Within 1 month of completing the three-dose series, 99.5% of subjects develop high levels of IgG antibody to all four L1 serotypes. This suggests that the vaccine is highly immunogenic and that memory cells have been created. The vaccine is also highly efficacious. Double-blind studies showed that the vaccine reduced the incidence of advanced stage CIN2/3 and carcinoma in situ (CIS). CIS is synonymous with high-grade dysplasia in the cervix. The vaccine is also 99% effective against genital warts. The duration of protection is believed to be 3 to 5 years.
Cervarix
Cervarix is a bivalent (types 16 and 18) HPV vaccine prepared from L1 proteins produced in bioreactors by using a baculovirus expression system. The vaccine is indicated in girls and women (aged 10–25 years) for the prevention of diseases caused by oncogenic HPV types 16 and 18. These diseases include cervical cancer, cervical intraepithelial neoplasia grades 1 and 2, and adenocarcinoma in situ. The vaccine is 93% effective in preventing diseases caused by serotypes 16 and 18.
Summary