Chapter 38 VACCINATIONS DURING PREGNANCY
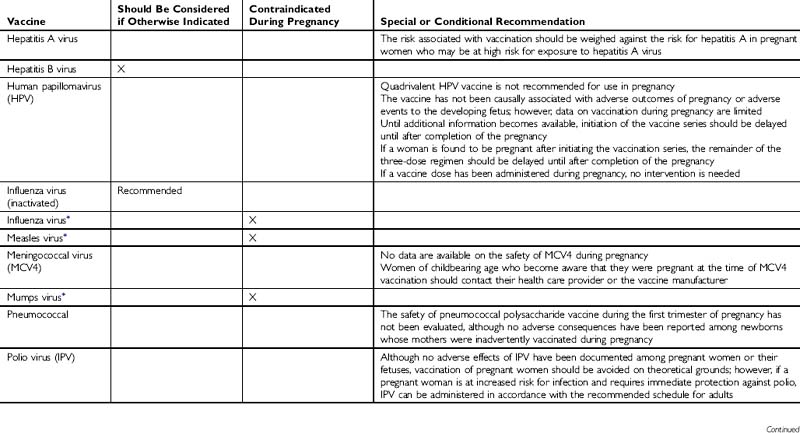
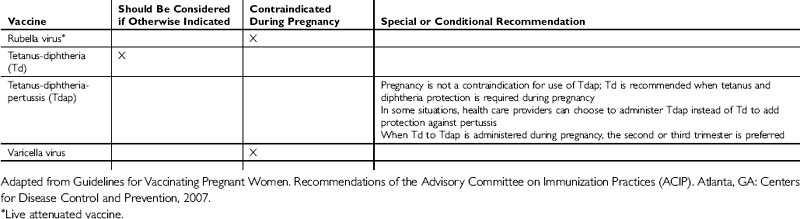
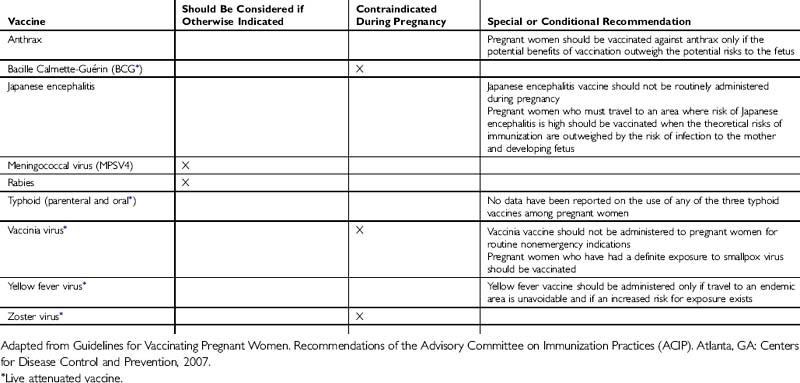
The administration of vaccines during pregnancy often poses concerns to physicians and patients about the risk of transmitting a virus to a developing fetus (Tables 38-1 and 38-2). However, for a developing fetus, this risk from vaccination of the mother during pregnancy is theoretical. There is no evidence of risk from vaccinating pregnant women with inactivated viral or bacterial vaccines or toxoids.
Live-virus vaccines, however, do pose a theoretical risk to the fetus. In general, live-virus vaccines are contraindicated in pregnant women because of the theoretical risk of transmission of the vaccine virus to the fetus. If a live-virus vaccine is inadvertently given to a pregnant woman, or if a woman becomes pregnant within 4 weeks after vaccination, she should be counseled about the potential effects on the fetus. Such vaccination is not ordinarily an indication to terminate the pregnancy.
Advisory Committee on Immunization Practices. Use of anthrax vaccine in the United States. MMWR Recomm Rep. 2000;49(No. RR-15):1-20.
Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54(No. RR-7):1-21.
Centers for Disease Control and Prevention: Prevention of Tetanus, Diphtheria and Pertussis Among Pregnant Women: Provisional ACIP Recommendations for the Use of Tdap Vaccine, 2006. Available at http://www.cdc.gov/nip/recs/provisional_recs/tdap-preg.pdf; accessed February 24, 2008.)
Cetron MS, Marfin AA, Julian KG, et al. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR. 2002;51(No. RR-17):1-11.
Guidelines for Vaccinating Pregnant Women. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: Centers for Disease Control and Prevention, 2007.
Inactivated Japanese encephalitis virus vaccine. recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1993;42(No. RR-1):1-15.
Kroger AT, Atkinson WL, Marcuse EK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-15):1-48.
Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(No. RR-2):1-24.
Prevention of pneumococcal disease. recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46(No. RR-8):1-24.
Prevots DR, Burr RK, Sutter RW, et al. Poliomyelitis prevention in the United States: updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(No. RR-5):1-22.
Rotz LD, Dotson DA, Damon IK, et al. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2001;50(No. RR-10):1-25.
Sur DK, Wallis DH, O’Connell TX. Vaccinations in pregnancy. Am Fam Physician. 2003;68:E299-E309.
Typhoid immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1990;39(No. RR-10):1-5.