Chapter 71 Use of Paralytic Agents in The Intensive Care Unit
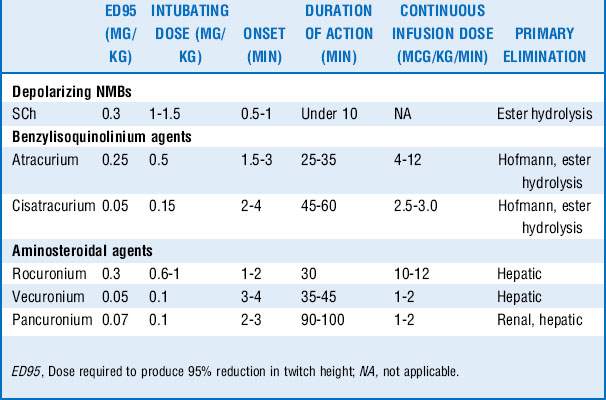
11 How do nondepolarizing agents differ in their dosing and duration of action?
A summary of the pharmacology of the commonly used NMBs is given in Table 71-1.
22 How do muscle relaxants interact with other commonly used drugs in the ICU?
A variety of drugs either augments or inhibits the actions of nondepolarizing muscle relaxants. A summary is given in Table 71-2.
Table 71-2 Drug-drug interaction of neuromuscular blocking agents
| Drugs that potentiate the action of nondepolarizing NMBs | Drugs that antagonize the actions of nondepolarizing NMBs |
|---|---|
| Local anesthetics | Phenytoin |
| Lidocaine | Carbamazepine |
| Antimicrobials (aminoglycosides, polymyxin B, clindamycin, tetracycline) | Sodium valproate |
| Antiarrhythmics (procainamide, quinidine) | Ranitidine |
| Magnesium | Steroids |
| Calcium channel blockers | Azathioprine |
| β-Adrenergic blockers | |
| Immunosuppressive agents (cyclophosphamide, cyclosporine) | |
| Dantrolene | |
| Diuretics | |
| Lithium carbonate | |
| Inhaled anesthetics | |
| Tamoxifen |
23 Describe how serum electrolyte, acid–base status, and temperature alter the action of neuromuscular blockade
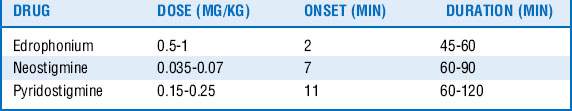
26 How can the effects of nondepolarizing muscle relaxants be reversed?
The easiest and most effective way for the effect of muscle relaxants to terminate is to wait for the drug to be metabolized and eliminated from the body. The effects of nondepolarizing muscle relaxants may be actively reversed in two ways, however. The first involves increasing the amount of ACh in the neuromuscular junction to overcome the muscle relaxant competing for the receptor. Drugs called cholinesterase inhibitors (anticholinesterases) inhibit the enzyme that hydrolyzes ACh, thus effectively overwhelming the muscle relaxant so that it cannot work. A list of these agents is in Table 71-3. Of note, physostigmine is another cholinesterase inhibitor that crosses the blood–brain barrier and is not used for the reversal of neuromuscular blockade. Pyridostigmine is primarily used for the treatment of myasthenia gravis and not for reversal of neuromuscular blockade. The second way muscle relaxants may be actively reversed involves the use of a novel drug that binds aminosteroid muscle relaxants. See question 28.
1 Akha A.S., Rosa J., Jahr J.S., et al. Sugammadex: cyclodextrins, development of selective binding agents, pharmacology, clinical development, and future directions. Anesthesiology. 2010;28:691–708.
2 De Jonghe B., Lacherade J.C., Sharshar T., et al. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37(Suppl):S309–S315.
3 Forel J., Roch A., Marin V., et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–2757.
4 Griffiths R.D., Hall J.B. Intensive care unit–acquired weakness. Crit Care Med. 2010;38:779–787.
5 Murray M.J., Cowen J., DeBlock H., et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002;30:142–156.
6 Naguib M., Lien C.A. Pharmacology of muscle relaxants and their antagonists. In: Miller R.D., ed. Miller’s Anesthesia. 7th ed. New York: Churchill Livingstone; 2009:859–911.
7 Papazian L., Forel J., Gacouin A., et al. Neuromuscular blocking agents in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116.
8 Perry J.J., Lee J.S., Sillberg V.A., et al. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev 2. 2008. CD002788, 2008
9 Routsi C., Gerovasili V., Vasileiadis I., et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14:R74.