CHAPTER 6 Use of Biologics for Degenerative Joint Disease of the Knee
 Current cartilage-based techniques provide improved clinical outcomes when addressing full-thickness cartilage lesions of the knee.
Current cartilage-based techniques provide improved clinical outcomes when addressing full-thickness cartilage lesions of the knee.Introduction
Natural History
Approximately 60% of knees undergoing surgical arthroscopy for knee pain have articular cartilage changes.1 Many of these injuries are only partial thickness and are of indeterminate significance in terms of their association with symptoms and their long-term progression to full-thickness chondral defects. Those with full-thickness lesions undergo approximately 400,000 cartilage procedures per year in the United States.2 It is these cases that form the basis for the treatment options reviewed in this chapter.
Treatment Options
Enhanced Intrinsic Repair: Microfracture
Current microfracture techniques are based on this endogenous potential for regeneration, first proposed by Purdie,3 and first described by Steadman and colleagues.4
Postoperative Management
Classical training dictates a strict protocol of touch-down weight bearing and continuous passive motion (CPM) for 6 weeks.5 Animal models demonstrate an extended healing period up to 12 weeks, thus suggesting a potential benefit to a longer period of restricted load bearing. In contrast, a clinical study by Marder et al. compared this protocol to one of unrestricted weight bearing and no CPM for femoral chondral lesions less than 2 cm2. At a mean 4.2-year follow-up (2–9 years) there was no difference in Lysholm and Tegner scores between groups.6
Outcomes
At early,7 midterm,8 and long-term9 follow-up, good results have been reported in clinical function and pain relief with microfracture. Good fill grades, low body mass index, younger age, lack of associated meniscal and ligamentous injuries, and shorter duration of preoperative symptoms are predictive of improved outcomes.
Traditional Cell-Based Technique: Autologous Chondrocyte Implantation
Technique
Originally described by Brittberg et al.10 in 1994, autologous chondrocyte implantation (ACI) is a two-stage technique that requires harvesting, culturing, and implantation of autologous chondrocytes. In the initial stage, surgical arthroscopy is used to assess whether or not the patient is a candidate for ACI; to address any associated meniscal pathology; to template the size, location, and dimensions of the defect; to locate any other cartilage lesions; and to obtain cartilage for culture. Typically the lateral edge of the notch, at the site of an anterior cruciate ligament notchplasty, is a good location for cartilage harvesting. A small curette or gouge can be used to harvest two to three small samples of cartilage, each about 3–5 mm in size. Samples are sent to a central lab. There these autologous cells are cultured to expand the cell count by up to 50 times. Chondrocytes are stored until appropriate time for implantation.
Outcomes
Long-term outcomes demonstrate durable results with ACI at 10–20 years postprocedure.11 At a mean 12.8-year follow-up, 92% of patients were satisfied with results and would have the procedure again. Vasiliadis et al. examined the long-term magnetic resonance imaging appearance of lesions at 9–18 years. Though some degeneration was noted, the quality of the repair tissue was similar to that of surrounding normal cartilage.12 In a double-blind trial, Knutsen et al. did not note any difference between short-term outcomes with ACI and microfracture, with both techniques demonstrating good results.13
Emerging and Developing Technologies
Despite clinical success with current conventional techniques, limitations exist. Inferior biomechanical properties of the reparative tissue,14 and progressive degeneration of the mixed reparative tissue created,15 limit success with a microfracture technique. Osteochondral autografts are limited by the availability of transplantable tissue. ACI is costly and time consuming and requires two surgeries, harvesting of a periosteal flap and an open arthrotomy. New technologies are currently in varied stages of development, and will seek to expand the options available for addressing cartilage lesions. These techniques are broadly categorized as cell based, and non–cell based. It is important to note that the Food and Drug Administration (FDA) has yet to approve any of these new techniques. Carticel (Genzyme, Cambridge, MA), approved by the FDA in 1995 for ACI, remains the only approved implant system in this field. As with any novel technology, long-term data do not exist to support their use. A few selected devices and techniques are reviewed.
Modifed ACI Techniques: Matrix-Associated Chondrocyte Implantation (MACI) and Hyalograft
Postoperative Management
The rehabilitation protocol is similar to those of the traditional ACI technique. Midterm experience with a more intensive protocol in competitive athletes has demonstrated an earlier return to sport, and improved 5-year follow-up.16
Outcomes
In an early randomized trial comparing traditional ACI with a periosteal flap to MACI, Zeifang et al. found no difference in efficacy between the two groups.17 In a 2- to 7-year follow-up with Hyalograft, good results were achieved with isolated lesions. However, less successful outcomes were noted with complex and salvage cases, highlighting the limitations of these techniques.18
Scaffold-Based Techniques
TruFit Osteobiologic Plugs
Technique
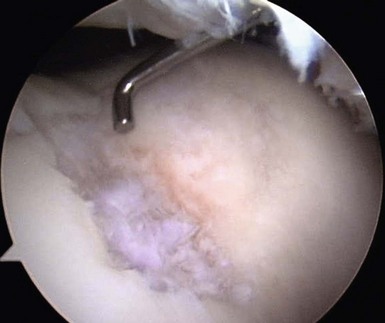
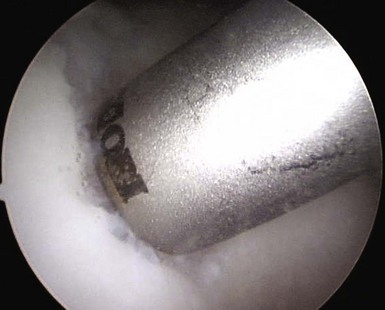
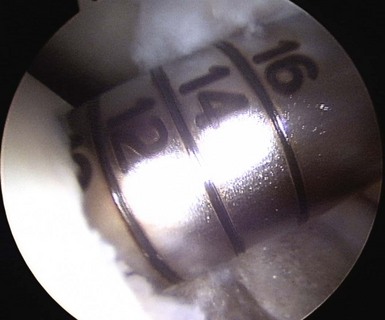
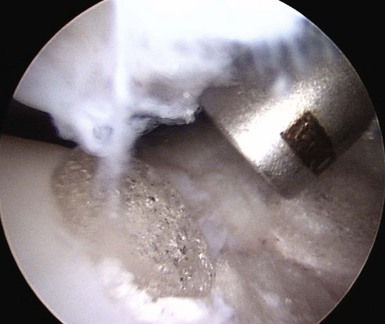
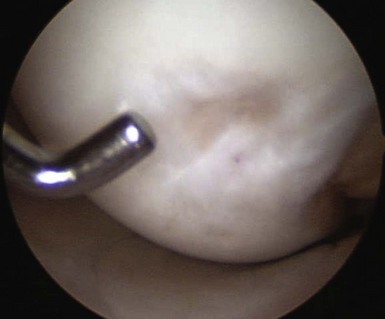
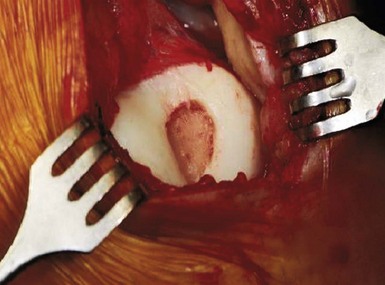
When addressing a primary cartilage lesion of the femoral condyle during arthroscopy, the size and location of the lesion are defined (Fig. 6–1). A perpendicular approach must be obtained to seat the plug flush with surrounding cartilage. Tamps of increasing diameter are inserted to determine the appropriate-diameter plug (Fig. 6–2). An obturator is secured in the cutting tube and it is placed into the lesion. The obturator is removed, and the cutting tube is firmly held in place while it is malleted to a depth of 10–12 mm (Fig. 6–3). Care must be taken to ensure that the cutting tube remains perpendicular to the lesion. Failure to do so can damage surrounding cartilage, create a nontubular defect, and break the cutting tube. Hand reaming is performed until the reamer is flush with the cutting tube. The cutting tube is removed and the defect created is examined.
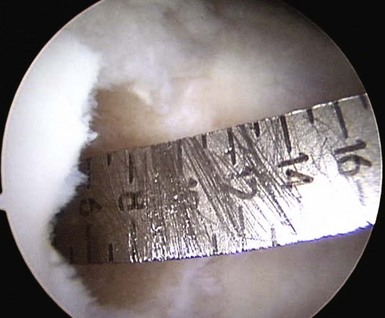
Measurements are taken from the base of the lesion to the chondral surface, in four quadrants (Fig. 6–4). A plug is cut to match this depth, attempting to achieve a flush position with surrounding cartilage. The plug is inserted and adjustments can be made with the appropriately sized tamp. Care must also be taken to ensure a perpendicular insertion of the plug, as it can be crushed or fractured if it is not in line with the hole. A similar open technique can be used to address patellar lesions. For larger or oblong-shaped lesions, more than one plug can be used. A narrow bony bridge (2–3 mm) should be maintained between plugs, to prevent collapse (Fig. 6–5).
Outcomes
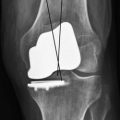
Limited successful early follow-up exists with TruFit plugs19 (Fig. 6–6). Some caution must be used, as case reports have noted failure of the plugs to incorporate and giant cell reactions requiring revision surgery.20 A prolonged course of physical therapy and rehabilitation may be necessary when addressing large lesions.21
Cartilage Repair Device Plugs
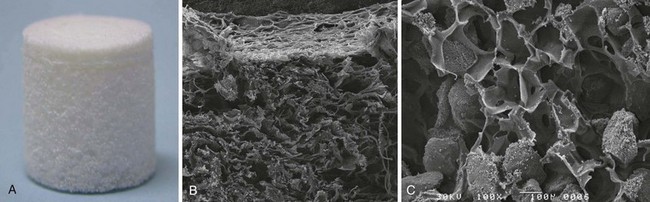
The Cartilage Repair Device (CRD) (Kensey Nash, Exton, PA) is a biphasic scaffold comprised of type I collagen in the chondral region, and β-tricalium phosphate and polylactic acid, with interconnected pores, in the subchondral bone region (Fig. 6–7).
Indications
These plugs are intended for the treatment of primary full-thickness cartilage lesions of the knee.
Cell-Based Techniques
DeNovo Natural Tissue (NT) Grafting
The DeNovo NT (Zimmer, Warsaw, IN; and ISTO Technologies, St. Louis, MO) graft is derived from juvenile allograft cartilage. Fragments are minced and then placed in suspension (Fig. 6–9). Its use is not subject to FDA approval as it is considered an allograft tissue product similar to osteochondral allografts and ligament allografts used in knee reconstructions.
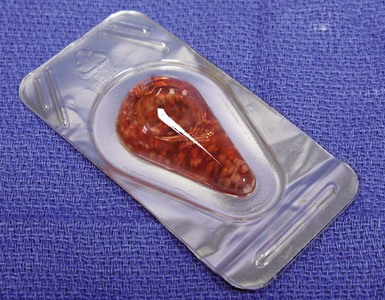
Figure 6–9 DeNovo NT in suspension.
(Courtesy of Zimmer, Warsaw, IN; and ISTO Technologies, St. Louis, MO.)
Indications
DeNovo NT grafts are indicated for symptomatic full-thickness cartilage defects of the knee.
Technique
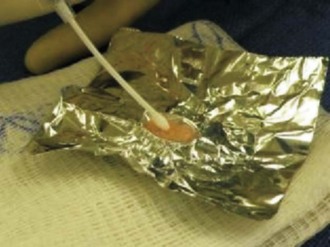
Surgical arthroscopy is used to identify the lesion, and to address any associated meniscal pathology. A mini-arthrotomy is then performed, exposing the lesion. Débridement of overlying fibrous and calcified tissue is performed to isolate a healthy bed with a stable shoulder of surrounding cartilage. The surgeon should avoid violating the subchondral bone layer. The defect is irrigated and a thin layer of fibrin glue is applied to stop bleeding. The cross-sectional area of the defect is measured with a ruler. One pack of graft is required per square inch (2.5 cm2) of defect. Simultaneously, the supplied suspension of cartilage fragments is opened and storage fluid is removed with a large angiocatheter. Foil is pressed into the lesion to create a matching mold (Fig. 6–10). This mold is then used to create a fibrin gel suspension of the cartilage fragments. Fragments are evenly spread in the foil defect. A layer of fibrin glue is then carefully applied to fill the defect to three fourths of the depth of the mold (Fig. 6–11). The construct is then left for up to 10 minutes to allow the fibrin to fully set. The defect is once again irrigated and dried. A further thin fresh layer of fibrin glue is then applied to the base of the defect, followed by the DeNovo construct (Fig. 6–12). It should be even to slightly countersunk. Time must be allowed for the fibrin to once again set. The knee is then cycled to ensure that the implant is stable.
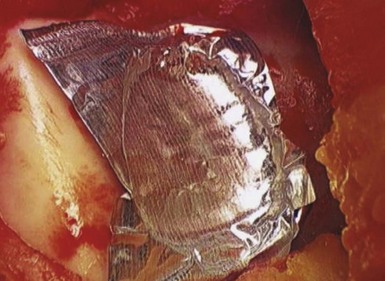
Figure 6–10 A foil template in a trochlear defect.
(Coutesy of Zimmer, Warsaw, IN; and ISTO Technologies, St. Louis, MO.)
DeNovo Engineered Tissue (ET) Grafting
DeNovo ET (Zimmer, Warsaw, IN; and ISTO Technologies, St. Louis, MO) also employs juvenile allograft cartilage. Chondrocytes are isolated from the allograft cartilage and expanded in a cell bank. A hyaline-like cartilage graft is created from these cells by placing them in a matrix that is both chondro-inductive and chondro-conductive (Fig. 6–13).
Combined Procedures
Osteochondral transplantation is reviewed in Chapter 7 in this text. A study by Lonner et al. demonstrated good early results with the use of autologous osteochondral allografts to the femoral condyles at the time of patellofemoral arthroplasty.23 In a similar fashion, the option exists to combine partial knee arthroplasty with one of the cartilage procedures reviewed in this chapter, to address less advanced lesions in the remaining compartments. Long-term outcomes do not exist with these developing techniques.
Associated Procedures
Osteotomies about the knee are performed in conjunction with cartilage restorative procedures to address associated malalignment. Failure to correct a structural deformity will result in overload to the affected compartment, and early failure. Distal femoral, proximal tibial, and tibial tubercle osteotomies are all used to unload a specific compartment, and improve weight-bearing kinematics across the knee.24 Ligament reconstructions are necessary to provide a stable articulation following injury, and reduce the incidence of recurrent cartilage injury.25 Other soft tissue reconstructions, including a proximal realignment, can also improve patellar kinematics, thus reducing stress on patellofemoral surfaces.
Patient Education
All patients should be educated regarding appropriate knee health. Knees bear significant multiples of body weight in day-to-day activities. Thus appropriate weight management and nonimpact conditioning are important in reducing pain and improving function.26 Muscle strengthening, focusing on the entire lower extremity,27 can significantly decrease pain scores in patients with arthritic knees. We encourage all patients with symptomatic cartilage lesions and arthritis of the knee toward a self-directed nonimpact conditioning program focused on a stationary bike and/or an elliptical trainer for a minimum 30 minutes, 5 days per week.
1 Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177-182.
2 McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196-201.
3 Insall JN. Intra-articular surgery for degenerative arthritis of the knee: a report of the work of the late K. H. Pridie. J Bone Joint Surg [Br]. 1967;49:211-228.
4 Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21:761-767.
5 Mithoefer K, Williams RJ3rd, Warren RF, et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique: surgical technique. J Bone Joint Surg [Am]. 2006;88(Suppl 1 Pt 2):294-304.
6 Marder RA, Hopkins GJr, Timmerman LA. Arthroscopic microfracture of chondral defects of the knee: a comparison of two postoperative treatments. Arthroscopy. 2005;21:152-158.
7 Mithoefer K, Williams RJ3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg [Am]. 2005;87:1911-1920.
8 Asik M, Ciftci F, Sen C, et al. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy. 2008;24:1214-1220.
9 Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-484.
10 Brittberg M, Lindahl A, Nilsson A, et al, Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med, 331, 1994, 889-895.
11 Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-1124.
12 Vasiliadis HS, Danielson B, Ljungberg M, et al. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943-949.
13 Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg [Am]. 2004;86:455-464.
14 Buckwalter JA, Martin JA, Olmstead M, et al. Osteochondral repair of primate knee femoral and patellar articular surfaces: implications for preventing post-traumatic osteoarthritis. Iowa Orthop J. 2003;23:66-74.
15 Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119-1125.
16 Della Villa S, Kon E, Filardo G, et al. Does intensive rehabilitation permit early return to sport without compromising the clinical outcome after arthroscopic autologous chondrocyte implantation in highly competitive athletes? Am J Sports Med. 2010;38:68-77.
17 Zeifang F, Oberle D, Nierhoff C, et al. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924-933.
18 Nehrer S, Dorotka R, Domayer S, et al. Treatment of full-thickness chondral defects with Hyalograft C in the knee: a prospective clinical case series with 2 to 7 years’ follow-up. Am J Sports Med. 2009;37(Suppl 1):81S-87S.
19 Melton JT, Wilson AJ, Chapman-Sheath P, Cossey AJ. TruFit CB bone plug: chondral repair, scaffold design, surgical technique and early experiences. Expert Rev Med Devices. 2010;7:333-341.
20 Sgaglione NA, Florence AS. Bone graft substitute plug failure with giant cell reaction in the treatment of osteochondral lesions of the distal femur: a report of 2 cases with operative revision. Arthroscopy. 2009;25:815-819.
21 Carmont MR, Carey-Smith R, Saithna A, et al. Delayed incorporation of a TruFit plug: perseverance is recommended. Arthroscopy. 2009;25:810-814.
22 Lu Y, Adkisson HD, Bogdanske JP, et al. In vivo transplantation of neonatal ovine neocartilage allografts: determining the effectiveness of tissue transglutaminase. J Knee Surg. 2005;18:31-42.
23 Lonner JH, Mehta S, Booth REJr. Ipsilateral patellofemoral arthroplasty and autogenous osteochondral femoral condylar transplantation. J Arthroplasty. 2007;22:1130-1136.
24 Gardiner A, Gutiérrez Sevilla GR, Steiner ME, Richmond JC. Osteotomies about the knee for tibiofemoral malalignment in the athletic patient. Am J Sports Med. 2010;38:1038-1047.
25 Granan LP, Bahr R, Lie SA, Engebretsen L. Timing of anterior cruciate ligament reconstructive surgery and risk of cartilage lesions and meniscal tears: a cohort study based on the Norwegian National Knee Ligament Registry. Am J Sports Med. 2009;37:955-961.
26 Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36:1109-1117.
27 Thorp LE, Wimmer MA, Foucher KC, et al. The biomechanical effects of focused muscle training on medial knee loads in OA of the knee: a pilot, proof of concept study. J Musculoskelet Neuronal Interact. 2010;10:166-173.