Chapter 76 Use of Ablation to Treat Arrhythmias in Children and Patients with Congenital Heart Disease
Transcatheter Ablation of Arrhythmias in Children with Structurally Normal Hearts
Supraventricular tachycardia (SVT) accounts for the majority of childhood arrhythmias. Re-entrant tachycardia secondary to accessory pathways (APs) is responsible for 75% of SVTs in children with AV nodal re-entrant tachycardia and primary atrial tachycardia accounting for the rest.1 Ventricular tachycardia is rare (only 5% of all tachycardias).2
The overall acute success for AP ablation is 91%, with higher success for left-sided pathways (96%) versus septal pathways (87%) and right free wall pathways (86%).3,4 These results may be even better with the lower risk of complications such as heart block in the present era since the advent of three-dimensional mapping and cryo ablation. The risk of major complications in the Pediatric Electrophysiology Registry comprising 4651 procedures was 3.2% and included AV block, perforation, pericardial effusion, brachial plexus injury because of arm position, emboli, and pnemothorax.4,5–7 Body weight of 15 kg or less correlated with a higher complication rate in one study.4 The overall death rate was 0.15%, which was similar to that reported for adult ablations.3
Indications for radiofrequency (RF) ablation for cardiac arrhythmias in children are as follows:5
Procedural Implications
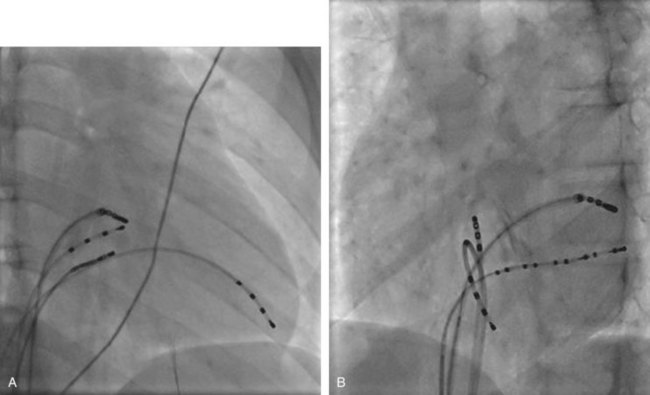
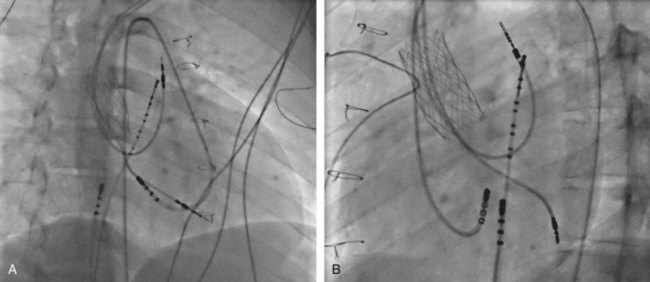
The ablation procedure, including catheter placement, programmed electrical stimulation, and recording and analysis of electrograms, is similar to that in adults. Standard CA includes placement of a multiple-polar (octapolar or decapolar) catheter in the coronary sinus from either the right internal jugular vein or the femoral vein and quadripolar catheters in the His bundle and right ventricular apex positions (Figure 76-1). Programmed electrical stimulation (PES) and pacing maneuvers are performed from the atrium and the ventricle to identify the location and characteristics of the AP (such as effective refractory period [ERP]) and inducibility of different arrhythmias. The procedure can be modified by using smaller caliber and fewer catheters in younger patients without compromising the procedure. This can be accomplished by the occasional use of an esophageal electrode for atrial stimulation and recording to replace an intracardiac catheter or by the use of His–right ventricular apex (RVA) multiple-polar catheter to obtain both RVA and His-bundle signals with a single catheter. Attention must be paid to the procedure, anesthesia duration, and fluoroscopy times, as younger patients are more susceptible to radiation effects. Ideally, pediatric interventional electrophysiology laboratories need to be specifically equipped with bi-plane, low-dose-rate pulsed fluoroscopy, and special shielding techniques. Availability of pediatric cardiac anesthesiologists, pediatric interventional cardiologists, and specially trained pediatric nursing personnel are essential to the safety and success of electrophysiology procedures in children.
Sedation and Anesthesia
In children older than 10 years of age who are not overly anxious or resistant to intravenous (IV) sedatives, conscious sedation and careful monitoring may be used. General anesthesia, with or without endotracheal intubation, may be necessary in children younger than 10 years or when comorbid pulmonary, neurologic, or hemodynamically significant structural heart disease coexist.5 Deeper sedation with general anesthesia as well as “breath holding” apnea techniques may be preferred if a para-Hisian septal pathway is suspected to minimize catheter instability related to respiratory motion.5 When no anesthesiologist is present, a nurse with sedation experience is crucial for close monitoring of the airway and vital signs and to avoid sedation overdose, especially during long procedures.
Ablation Energy Source
In the current era, RF energy or cryothermal (cryo) energy is used to interrupt abnormal pathways and ablate ectopic foci. The developing myocardium has the potential for the growth of lesions over time after application of RF energy, but no long-term follow-up studies are available to understand the implications. This potential should be considered when RF ablation is used in young children. Cryo energy application is preferred if the target is near the sinoatrial (SA) node, AV node, or His bundle to prevent damage to these areas of specialized conduction tissue that could result in SA node dysfunction or permanent heart block. Cryo ablation is preferred for ablation inside the coronary sinus as RF energy delivery in this region can result in blood coagulation, inadequate energy delivery, coronary artery injury, or perforation. Long-term recurrence risk of arrhythmia after cryo ablation, which may be as high as 10% to 20%, can be diminished by accurate mapping and requiring stringent time to success of less than 15 seconds after the onset of a cryo lesion before proceeding with a complete lesion.7
Approach to Ablation of Accessory Pathways
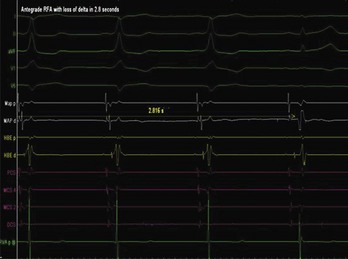
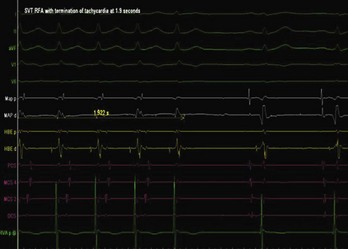
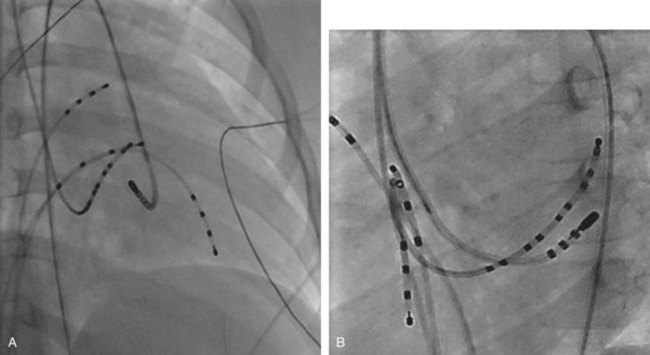
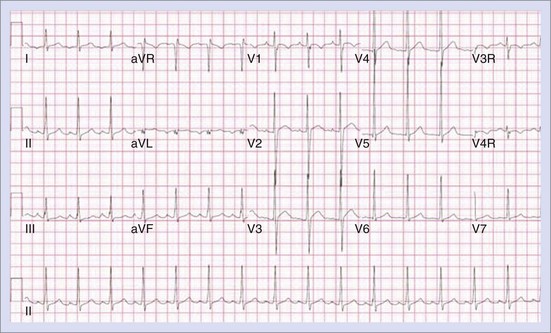
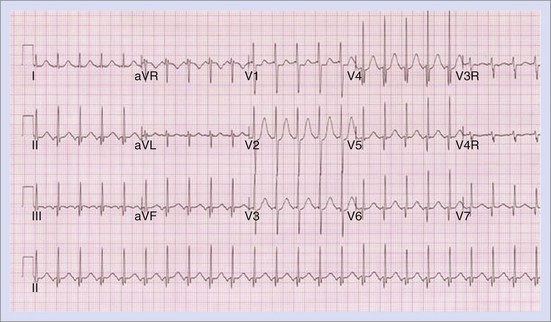
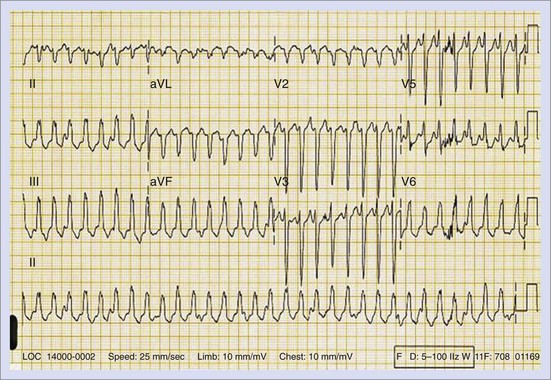
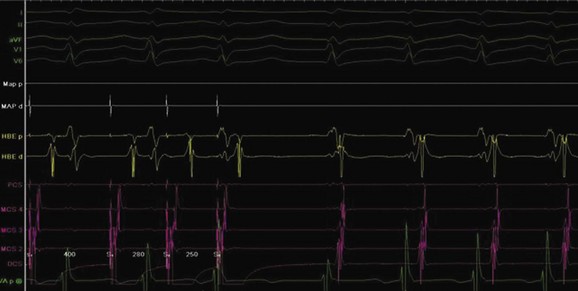
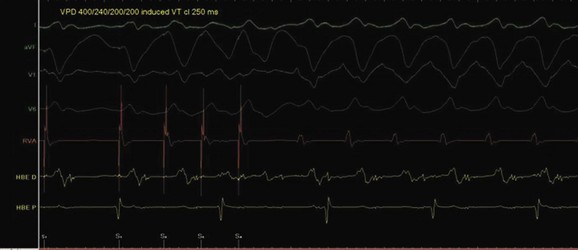
The approach to the ablation of APs depends on whether the pathway is concealed or manifest (in WPW syndrome) (Figure 76-2). Subjects with WPW syndrome should have the antegrade ERP and the shortest pre-excited R-R interval in AF determined. Indicators of high-risk AP include an ERP of less than 250 ms (Figure 76-3, A), the shortest pre-excited R-R interval of less than 220 ms, or the presence of multiple pathways.8–10 More recent studies have reported on a follow-up of 184 asymptomatic patients with WPW syndrome over 57 months (mean age, 10 years); 19 subjects (almost 10%) developed life-threatening arrhythmias, which makes a case for risk stratifying all patients with WPW syndrome at some point during the clinical course of their disease (Figure 76-3, B).11 Mapping of APs can be performed either during sinus rhythm with overt pre-excitation (Figure 76-4), during ventricular pacing by observing retrograde atrial conduction (Figure 76-5), or by observing retrograde atrial activation during SVT (Figure 76-6). If retrograde AP conduction is mapped during ventricular pacing, it must be ensured that fusion with retrograde AV nodal–His-Purkinje conduction, which is quite robust in children, does not occur.12 This can be confirmed with the administration of a bolus of IV adenosine during ventricular pacing, which blocks the retrograde AV nodal conduction but not the retrograde AP conduction. Additionally, other pacing maneuvers such as ventricular PES with single extrastimuli will eliminate retrograde AV nodal conduction. Rarely, children can have adenosine-sensitive antegrade or retrograde conducting APs.
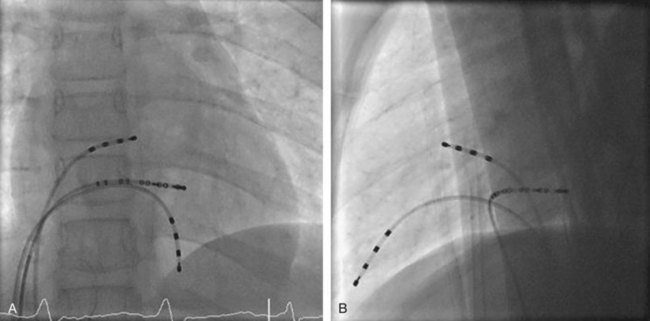
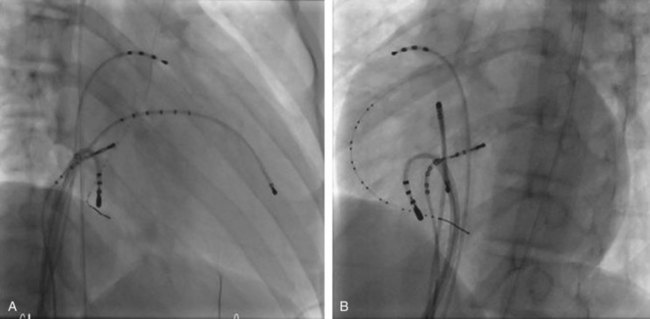
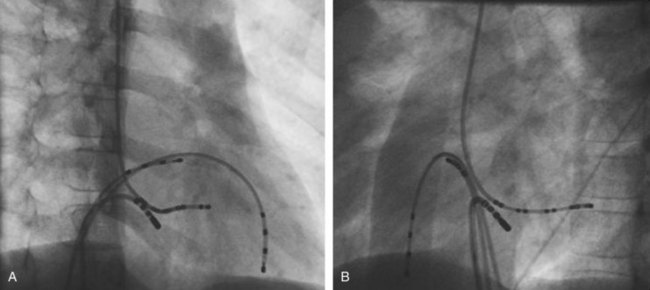
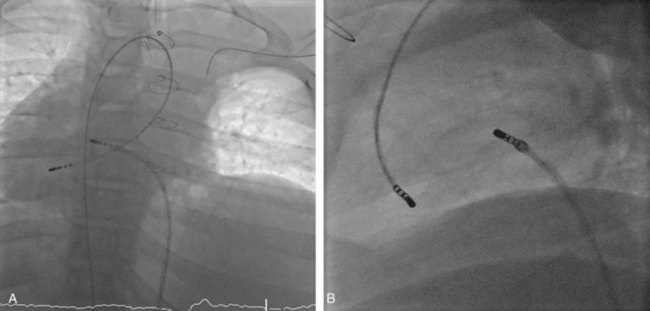
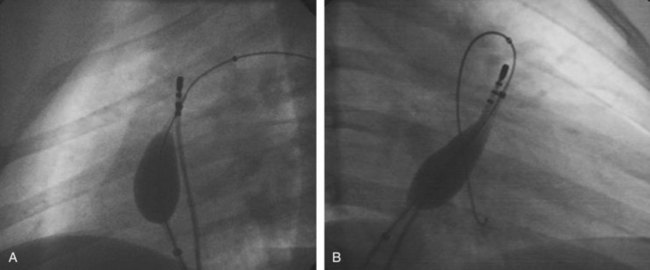
Approximately 60% of all APs are located on the left AV groove.12 Access to the left atrium is achieved by the trans-septal technique using a trans-septal puncture kit (which includes the sheath, the dilator, and the needle) with the assistance of bi-plane fluoroscopy, pressure monitoring, and contrast injection (Figure 76-7). Rarely, transesophageal echocardiogram (TEE) or intracardiac echocardiogram (ICE) may be helpful in the presence of complex heart disease. Heparinization is essential for ablation of left-sided pathways once trans-septal access is obtained; activated clotting time should be maintained above 250 seconds with heparin infusion or bolus doses for the duration of the procedure. In some pediatric laboratories, a retrograde trans-aortic technique is preferred for left-sided pathways (Figure 76-8) with established safety13; the presence of aortic valve disease or younger age (<5 years) may be relative contraindications to this approach because of the risk of damage to the aortic valve from the maneuvering of the ablation catheter.
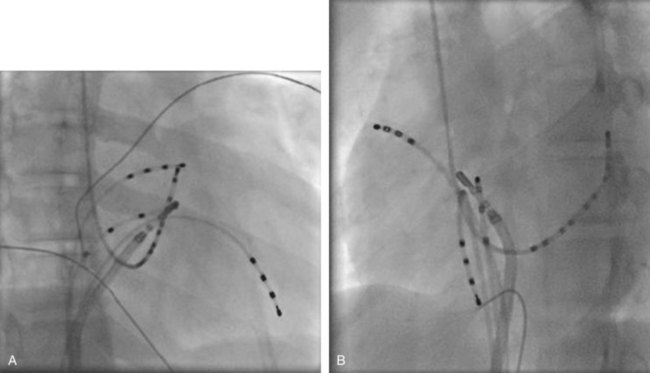
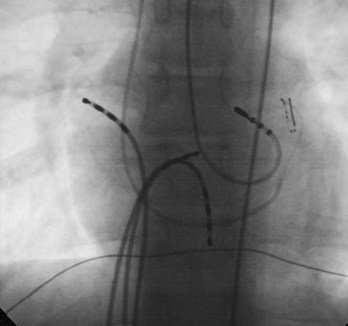
In cases with a left-sided AP, mapping is improved by placing a multiple-electrode catheter in the coronary sinus for initial pathway localization. Access to right-sided pathways, although straightforward from an access standpoint, may be technically challenging. The lower success rate of right AP ablation is related to mapping error and poor catheter stabilization. The tricuspid valve (TV) has a larger circumference than the mitral valve and differs with respect to the angle at which the valve attaches to the annulus. In addition, the absence of a reference catheter in an annular venous structure (such as the coronary sinus) increases mapping errors. The right coronary artery (RCA) runs along the ventricular aspect of the right epicardial AV groove and has a constant anatomic relation to the right AV annulus, regardless of the location of valve attachment. RCA mapping with various catheters has been used for pathway localization in selected patients in some EP laboratories (Figure 76-9). The efficacy and safety of this technique using a multiple-polar 2F microcatheter in a select group of children has been reported.14 Despite accurate mapping of the right AV annulus, the procedure is often confounded by catheter instability. It is often necessary to use a long sheath with specially directed curves that orient the catheter tip toward the right AV groove. Additionally, cryo ablation, by virtue of catheter adherence to the AV groove during a lesion, may be advantageous. This may be specifically helpful in right-sided anteroseptal and midseptal APs. In these locations, accurate mapping with minimal catheter movement is important to avoid any injury to collateral structures such as the His bundle (Figures 76-10 and 76-11). Catheter mapping from the right internal jugular approach is sometimes more helpful than the traditional femoral approach because of the angle of approach to the AV groove.
Approximately 5% to 10% of the patients may have multiple pathways or multiple SVT mechanisms.9 Post-ablation EP testing includes repeat programmed electrical stimulation to exclude the presence of multiple pathways or confirm the recovery of the recently ablated AP. After a presumed successful ablation, adenosine confirms the absence of antegrade or retrograde AP conduction. Isoproterenol is used to provide adrenergic stimulation during programmed electrical stimulation to indicate lack of inducibility of SVT. This protocol is used during the “waiting period” after ablation to confirm a successful ablation with noninduction of SVT and absence of residual or additional APs. The “waiting period” after the successful lesion may vary from 30 to 60 minutes.
Ablation for Atrioventricular Nodal Re-entry Tachycardia
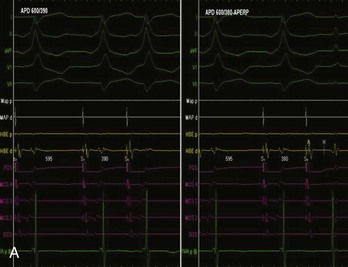
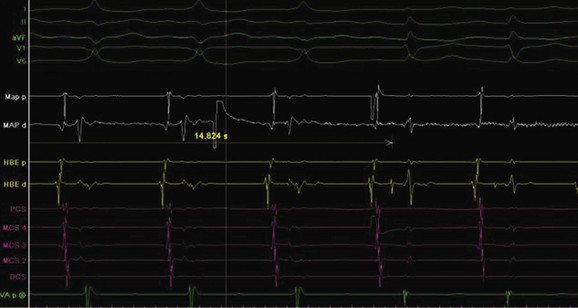
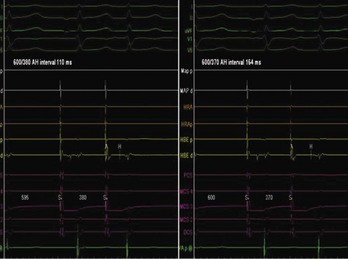
AV nodal re-entry tachycardia (AVNRT) is infrequent in infants and young children, but its incidence increases with age.1 Although the mechanism of AVNRT induction is similar in children and adults, the same definition of dual AV node physiology—that is, 50-ms increase in the atrium to His (A-H) with a 10-ms decrease in atrium-to-atrium (A-A) extrastimulus (Figure 76-12)—may not be applicable to the pediatric population; typical and atypical AVNRT may be induced without clear-cut evidence of dual AV node physiology. Slow pathway elimination or modification is performed by RF or cryo energy delivery to the area of slow conduction in the triangle of Koch. Although the dimensions of the triangle of Koch are relatively constant in adults of different sizes, the area increases with growth in children. In children with a body weight less than 20 kg, the area is 50 mm2 or less.15 This small dimension, along with the proximity of the AV node and His bundle region, should be considered carefully when applying RF energy in small children with AVNRT. With RF application in the slow pathway region, accelerated junctional rhythm is observed. Care needs to be taken to preserve fast pathway conduction (by either atrial pacing or by watching the ventriculoatrial [VA] conduction in junctional rhythm) (Figure 76-13). Successful elimination of AVNRT can be achieved with a 95% success rate and a low recurrence risk by using RF energy. Optimal temperatures during RF ablation should not exceed 55° C and often 45° to 48° C is sufficient.16
Radiofrequency Ablation Versus Cryothermal Ablation
With the advent of cryo technology, more operators are choosing cryo ablation over RF ablation as the first line of approach to avoid AV block. Success and recurrence risks have been reported to be similar in both procedures, but procedure time may be longer in cryo ablation.17 More recent studies have shown overall lower success rate of 83% with cryo ablation with a 6-mm catheter tip in comparison with the standard RF ablation success rate of 93%.7 In addition, this study compared the use of 6-mm cryo catheters for cryo ablation with RF ablation and found a 0.7% incidence of heart block with RF ablation, no heart block with cryo ablation, and no difference in procedure or fluoroscopy times. The use of 8-mm tip cryo catheters has also been shown to be effective, with 91% acute success with a 2.8% long-term recurrence risk18; a 6.5% incidence of transient AV block with complete recovery was seen in all subjects, and pacemaker implantation was not needed in any. Cryo lesions are usually placed in the slow pathway location in a sinus or paced rhythm while monitoring the antegrade fast pathway conduction; junctional rhythm is usually not seen, in contrast to slow pathway modifications with RF energy. Because the cryo ablation catheter is stable, an EPS (using PES) for a slow pathway can be performed during placement of the lesion to assess the efficacy of the cryo ablation.
Ablation for Ectopic Atrial Tachycardia
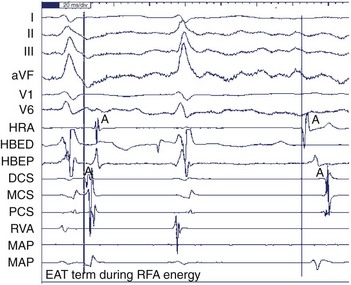
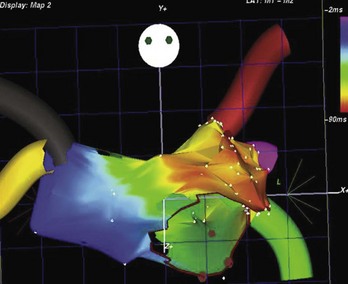
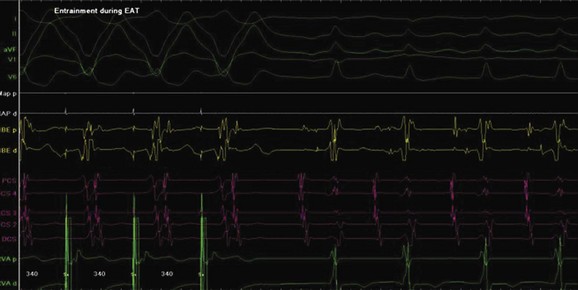
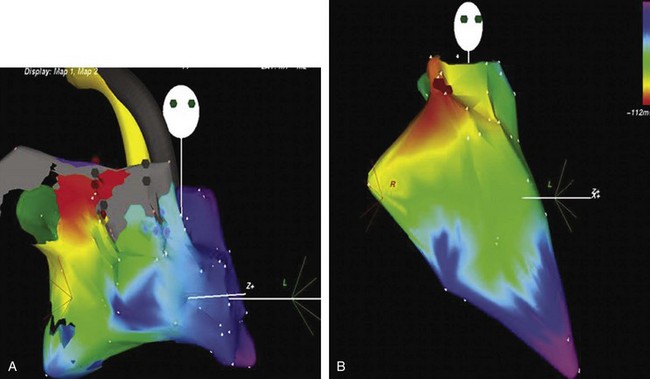
Ectopic atrial tachycardia (EAT) is an uncommon arrhythmia accounting for 5% to 20% of all SVTs.19 The tachycardia may be incessant and result in tachycardia-induced cardiomyopathy. In younger patients (<1 year), conventional drug therapy is attempted, but in older patients, catheter ablation is often preferred, especially if the tachycardia is incessant. A small percentage of patients may have spontaneous resolution of their tachycardia over time.12 PES is often of little help, as the SVT is often focal and automatic; however, it may respond to catecholamines. EATs can be left sided or right sided in origin (Figures 76-14 and 76-15) and sometimes can have a triggered mechanism. Use of conventional mapping with complementary three-dimensional systems, such as contact and noncontact mapping, is often helpful in decreasing fluoroscopy and procedure times and in increasing chances of ablation success.20 Depending on the cycle length and location of the EAT, mapping (Figures 76-16 and 76-17) and entrainment maneuvers (Figure 76-18) are performed before using RF ablation or cryo ablation. In the pediatric population, some of these ectopic tachycardias may be sensitive to sedation and anesthesia and may be noninducible because of suppression of the automatic focus by the sedation or the anesthetic. This may make mapping difficult in the very young, in whom conscious sedation may not be an option. Multiple P-wave morphologies and multiple foci may be present, which necessitates careful mapping in both atria before ablation is performed. Some of the secondary ectopic foci will become apparent after ablation of the initial clinical tachycardia.
Junctional Ectopic Tachycardia
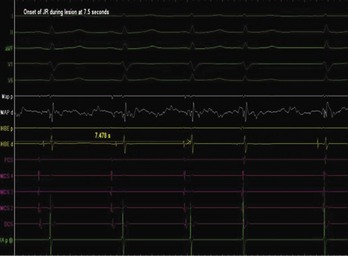
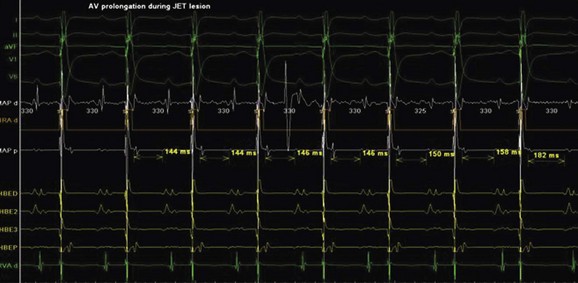
Junctional ectopic tachycardia (JET) is an automatic tachycardia arising from the AV node or the His bundle, which can be seen either as a congenital (Figure 76-19) or as a postoperative form. Congenital JET may present early in life (in the first year of life, shortly after birth) and is often familial in 50% of the cases, and the etiology is unclear.21 Some pathology reports have shown inflammatory changes at the crest of the ventricular septum, and others have linked it to anti-SSA and anti-SSB antibodies.22,23 In contrast, the postoperative form is seen in the immediate postoperative period after repair of ventricular septal defect (VSD) or of complex CHD such as palliation of tetralogy of Fallot, AV canal, and single ventricle and transposition of the great artery.19 Treatment of congenital JET initially usually consists of medical therapy with amiodarone in combination with digoxin, β-blockers, or flecanide. If the tachycardia is incessant with associated hemodynamic compromise, cryo or RF ablation is an option. Because RF has a high risk of causing AV block, cryo ablation is the preferred choice. Mapping is performed during tachycardia, and the region of interest is the anterior septum near the bundle of His. Either RF or cryo energy may be used to successfully eliminate the automatic focus.24,25 The earliest His signal during tachycardia is targeted (Figure 76-20), and care is taken to monitor antegrade conduction and AV or P-R interval during lesion application. Postoperative JET almost never needs invasive intervention and can be managed with medications such as IV amiodarone.19 It is self-limiting a few days after surgery once the inotropic medications are weaned and surgical stresses have abated.
Permanent Junctional Reciprocating Tachycardia
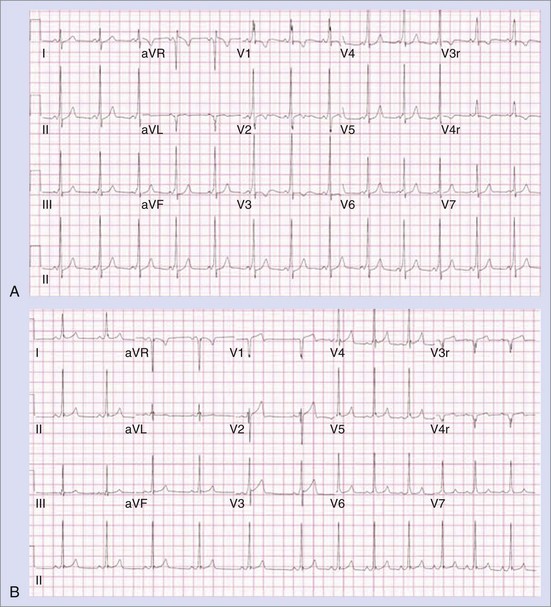
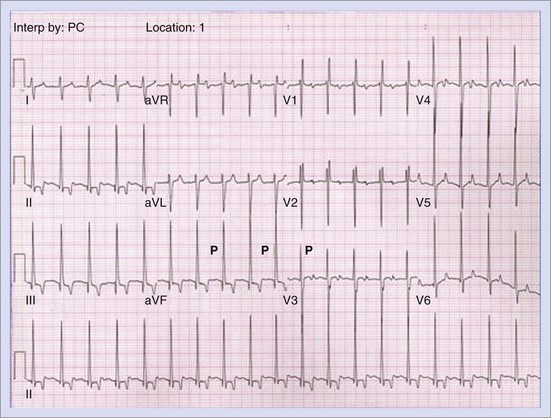
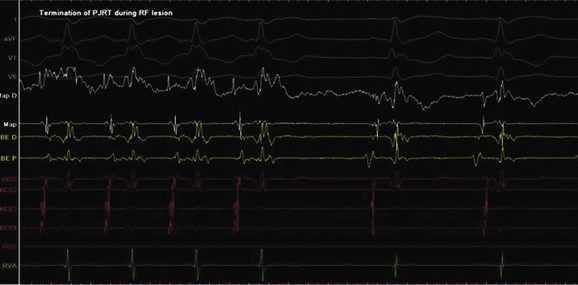
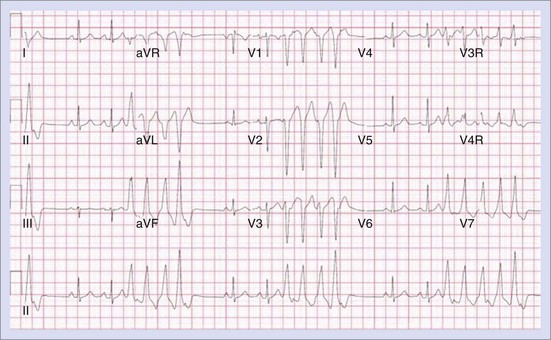
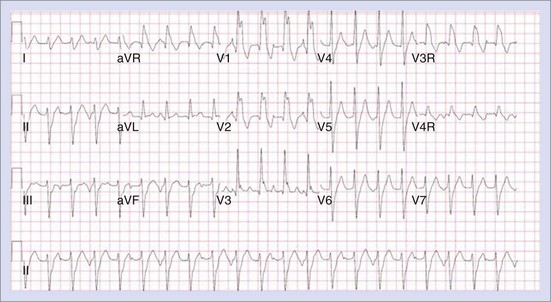
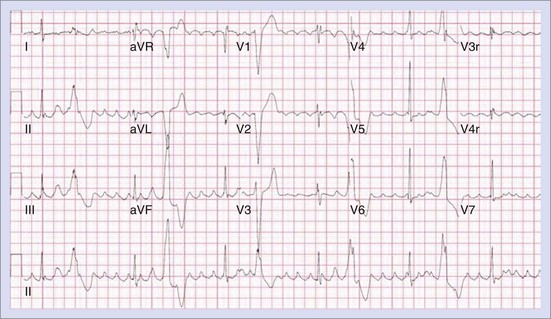
Permanent junctional reciprocating tachycardia (PJRT) is, in fact, a misnomer, as this disease is characterized by an orthodromic reciprocating tachycardia mediated by a slowly conducting concealed accessory pathway.26 Because of the retrograde decremental pathway properties, the tachycardia may be incessant and result in tachycardia-induced cardiomyopathy. The tachycardia is characterized by narrow QRS morphology with a long R-P interval and negative P waves in leads 2 and 3 and aVF with earliest retrograde atrial activation in the posteroseptal right atrium (Figures 76-21 and 76-22). Medical management can be frustrating because of the incessant nature of the tachycardia, so catheter ablation is often the treatment of choice. Differential diagnosis includes atypical AV node re-entry tachycardia and ectopic atrial tachycardia, both of which can be associated with long R-P interval tachycardias. Diagnosis is confirmed by an EPS and maneuvers that include demonstrating atrial pre-excitation with a ventricular premature beat during His refractoriness, response to adenosine, and entrainment with ventricular pacing during tachycardia. Most of these pathways are located in the right posteroseptal region (Figure 76-23), but some have been reported in other locations such as right-lateral and left-posteroseptal AV groove.27 Success rates of RF and cryo ablation may be as high as 95%, with a recurrence risk greater than those with other APs.28 Complications include risk of injury to the AV node and the RCA, especially when ablating in the mouth of the coronary sinus.6
Ventricular Arrhythmia
Outflow Tract Ventricular Tachycardia
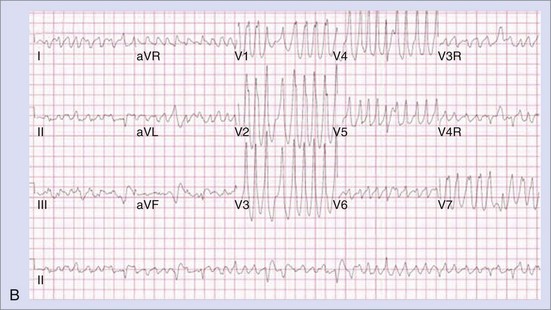
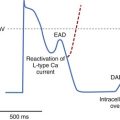
Outflow tract ventricular tachycardias present fairly early in life, with equal distribution between the two genders.29 Most patients are asymptomatic and are often diagnosed at routine physical examinations as having “an irregular heart beat.” Other patients may experience palpitations, lightheadedness, or presyncope. Most ventricular tachycardias in the young arise from the right ventricular outflow tract (RVOT). This predisposition to a specific region of the heart (perivalvar area) raises the possibility of “fiber disruption during development” as an etiology.30 Other etiologies such as subclinical myocarditis or pericarditis may enhance fiber disruption or cause local dysautonomic changes predisposing to triggered activity and arrhythmogenesis. These tachycardias are triggered by delayed after-depolarizations (DADs) that are dependent on calcium release, which is inhibited by adenosine, and hence they are described as “adenosine sensitive.”30 The electrocardiogram (ECG) typically shows a left bundle branch block (LBBB) with inferior axis morphology, and the tachycardia may be sustained or nonsustained (Figures 76-24 and 76-25). The tachycardia, which is usually suppressed by increasing heart rates, can be observed by 24-hour Holter monitoring or exercise stress tests. Clinical investigations, including echocardiography and magnetic resonance imaging (MRI), reveal structurally normal hearts. Since the arrhythmia is usually non–life threatening, indications for treatment include the presence of symptoms and ventricular dysfunction. Medical therapy may include β-blockers or calcium channel blockers, which have shown variable success rates.31 Spontaneous resolution can occur in some in adolescence. Ablation success rates have been greater than 90%, but the availability of bi-plane fluoroscopy and three-dimensional mapping are essential.31,32 The arrhythmia is often suppressed by sedation and anesthesia, necessitating isoproterenol, aminophylline, and epinephrine to enhance triggered activity and tachycardia induction. Complications to avoid include complete or right bundle branch block (RBBB) and damage to the coronary arteries and the aortic valve when ablation is performed in the left ventricular outflow tract (LVOT).31
Fascicular Ventricular Tachycardia
Fascicular ventricular tachycardia is the most common form of idiopathic left-sided VT seen in the pediatric population. It is characterized by an RBBB and left-axis deviation configuration (Figure 76-26). This VT is seen in structurally normal hearts and can be induced by atrial or ventricular stimulation, which suggests a re-entry mechanism.33 Belhassen described verapamil sensitivity as a characteristic of this arrhythmia, so it is also called Belhassen tachycardia.34 More recently three different types have been described: (1) the more common left posterior fascicular VT with a QRS morphology of RBBB and superior axis; (2) the uncommon left anterior fascicular VT with RBBB and right-axis deviation; and (3) the rare upper septal fascicular VT with a narrow QRS and normal or right-axis deviation. Different etiologies for this abnormal re-entry have been suggested, including false tendons extending to the basal LV septum and abnormalities in the Purkinje network that facilitate the arrhythmia.35 Clinical presentation is often similar to that of SVT and includes paroxysmal episodes of palpitations, fatigue, and dizziness. The ECG may have typical findings of RBBB with superior axis. Twenty-four-hour Holter monitoring is helpful if the arrhythmia is nonsustained. The arrhythmia is sensitive to calcium channel blockers (such as verapamil).34 Curative therapy using RF or cryo ablation involves mapping of the LV during tachycardia or sinus rhythm. LV access is obtained by the antegrade trans-septal approach or the retrograde aortic approach (Figure 76-27). Different strategies include targeting the diastolic potential (which represents the antegrade limb of the VT circuit) in the LV midseptum, pace mapping in the LV midseptum for a perfect QRS match, and targeting of the VT exit site at the apical septum using presystolic fused Purkinje potentials.36 Success rates for ablation vary between 80% and 85%, with a 5% risk of recurrence.37 Complications to avoid include damage to the femoral artery and the aortic valve and formation of a left-sided thrombus.
Use of Ablation in Patients with Congenital Heart Disease
Accessory Pathways and Dual Atrioventricular Nodal Pathways in Congenital Heart Disease
The overall incidence of CHD is approximately 1%, with almost half these patients requiring cardiac surgery.12 The prevalence of accessory pathways in CHD varies between 1 and 3 per 1000, including manifest WPW and concealed APs.38 Although these pathways have been associated with all types of anatomic CHD lesions, WPW syndrome is especially common in Ebstein anomaly and varies between 9% and 20%.39 These pathways are often multiple and right sided. APs are seen in L-looped ventricles with associated Ebstein anomaly.40 WPW patterns have been reported in hypertrophic cardiomyopathy.41 AV reciprocating tachycardia has been reported with twin AV nodes and dual AV node pathways in complex congenital heart diseases such as heterotaxy syndromes with L-looped hearts.42 These tachycardias are often poorly tolerated in the presence of residual hemodynamic issues. Although medical therapy is an option, invasive ablation therapy is preferred for a variety of reasons, including patient choice, side effects, adverse effects of medication in the presence of the congenital heart lesion, poor compliance to medical therapy, and the need for risk stratification.
Challenges in Mapping of Accessory Pathways
The different challenges in attempting an invasive EPS in CHD patients include the following:
Mapping and Ablation of Accessory Pathways in D-Transposition of the Great Arteries with Mustard or Senning Procedures
The concepts of mapping and ablation are similar to those used in structurally normal hearts. In D-transposition of the great arteries after the Mustard or Senning baffle procedure, both APs and AVNRTs have been described.48 Three-dimensional mapping, with or without contrast angiography in the baffle, helps delineate the systemic side of the baffle. For understanding the pulmonary venous atrial anatomy, the use of pulmonary artery contrast injection with delayed acquisition of angiogram pictures in the cardiac levophase is often helpful. The His bundle electrogram may not be easily recordable from the systemic venous side and may need retrograde aortic catheter positioning. Targeting the slow pathway from the right side may be challenging, as the baffle suture lines often traverse the inferior portion of Koch’s triangle. Hence, a transbaffle puncture or a retrograde aortic approach may be necessary to map the slow pathway, which is usually on the pulmonary venous atrial side.49 Cryo ablation may need to be considered to reduce the risk of heart block, as the exact position of the compact AV node and slow pathway inputs may not be clear. Similarly, mapping of the AV grove for concealed and manifest APs can be performed with either the antegrade or the retrograde approach on the ventricular side.
Mapping and Ablation of Accessory Pathways in Hearts with a Single Ventricle
In patients with a complex single ventricle, mapping for slow pathways and APs after the Fontan procedure can be challenging. The decision to ablate is often made on the basis of symptoms and impending surgery that might block future catheter access into the atrial chambers (such as the Fontan procedure). After a Fontan procedure, transconduit or transbaffle puncture (both intracardiac and extracardiac) can be performed safely to achieve access into the pulmonary venous atrium, if needed.50 In this patient group, careful attention needs to be paid to certain issues, including (1) arterial line placement and close monitoring of blood pressure, as tachycardia or pacing maneuvers may be poorly tolerated because of ventricular dysfunction; (2) aggressive anticoagulation protocol to avoid thrombus formation in the pulmonary venous atrium by monitoring activated clotting time (ACT) after heparin administration and maintaining it above 250 seconds. The anatomic displacement of the compact AV node and penetrating His bundle in complex CHD can result in difficulty in finding a good His bundle signal, which makes mapping of the para-Hisian and slow pathways challenging.47,51,52 In such situations, cryo ablation may have to be considered in AVNRT and septal APs associated with complex heart disease.53 The success rate of ablation for SVT (other than postoperative atrial flutter and atrial tachycardia) in CHD was reported to be 86.7% in the pediatric registry data.4 The recurrence rate has been reported to be higher (15% to 20%) than in structurally normal hearts.19
Mapping and Ablation in Ebstein Anomaly
An increased prevalence of pre-excitation is seen in patients with the diagnosis of Ebstein anomaly. The challenges include multiple pathways, the ambiguous location of the right AV annulus, and the presence of tricuspid insufficiency. To better locate the right AV grove and the right-sided pathways, a 2F multiple-polar recording catheter is often placed from the retrograde approach in the RCA (see Figure 76-9) before mapping is started.14 Placement of such a catheter helps identify the proximity of the right coronary artery to the AV groove and ablation site to avoid coronary artery injury. The electrograms at the successful site are often complex and fractionated.54,55 In a series of 21 patients with 34 right-sided pathways, the acute success rate was 76% with a recurrence of 25%.54 An analysis of the pediatric registry data showed that 16% of patients with CHD and SVT had Ebstein anomaly and that 29% of these patients had multiple pathways.55 The acute success rate in this multiple-center trial was 83%.55
Adult-Type Typical Atrial Flutter or Reverse Typical Atrial Flutter
These types of atrial flutter may be seen in patients with CHD in their 20s and 30s, who typically present with sawtooth P waves with a positive or negative morphology in the inferior leads. Impulse propagation in a typical, or counterclockwise, flutter involves the isthmus of atrial tissue between the IVC and the tricuspid annulus, which is called the cavo-tricuspid isthmus (CTI), and travels up the septal aspect along the AV groove near the His bundle and then back down the lateral wall of the atrium to re-enter the CTI.56 The reverse typical, or clockwise, flutter has a reverse activation compared with typical flutter. These arrhythmias have been seen in all CHD lesions, including postoperative ASD, VSD, tetralogy of Fallot (TOF), D-transposition of the great arteries (D-TGA), and single-ventricle Fontan repair of hypoplastic left and right heart variants.19,57 Attention must be paid to the anatomic barriers in the older forms of D-TGA repairs (intra-atrial baffle operations such as the Mustard and Senning procedures) and single-ventricle Fontan repairs, where the CS and part of the CTI may be on the “other side” (pulmonary venous portion) of the baffle, and catheter access to part of the CTI is by transbaffle puncture or retrograde A-A access.58
Mapping and Ablation of Typical Flutter
It is essential to define the anatomy using echocardiography, computed tomography (CT), MRI, angiography, or all of these before ablation. Noninvasive body-surface mapping of intra-atrial re-entry tachycardia (IART) has been shown to help analyze flutter wave isopotential maps and predict the activation sequence inside the atrium; this may assist in intracardiac mapping and ablation.59 However, these techniques are more experimental and not proven to be practical for universal use.
Once the activation sequence is confirmed using intracardiac electrograms, and in some cases, advanced mapping techniques such as electroanatomic or noncontact mapping, the CTI is accessed and the flutter entrained to demonstrate the re-entry mechanism before ablation. Long sheaths may facilitate catheter stability and easy access to CTI. The use of larger-tip (8-mm and 10-mm) ablation catheters helps deliver higher power (50 to 100 W) and create larger lesions. Some operators have also used closed and open irrigated tip catheters for applying better lesions.60 These modalities must be used with caution because of the higher risk of myocardial perforation in the pediatric population. After successful ablation, the documentation of bi-directional CTI block may be challenging because of the anatomic barriers to catheter placement (such as a baffle), but making this determination has been to shown to reduce the risk of recurrence.61
Atypical Flutters
Intra-atrial Re-entry Tachycardia
The incidence of these arrhythmias may be as high as 30% among patients who had postoperative Mustard and Senning intra-atrial repairs (surgery that is rarely performed in the current era) and 50% among patients who had the Fontan procedure within a decade following surgery.61–63 IART can also be seen in four-chamber postoperative conditions such as TOF and ASD, along with the typical CTI-dependent flutter. Unlike some of the arrhythmias commonly seen in adults, such as AF, arrhythmia control is preferred over rate control. IART is a significant cause of morbidity and mortality in this group of patients. Even mildly elevated heart rates can cause hemodynamic alterations. Additionally, a heightened risk of atrial thrombosis is present. Even though medical therapy is often the first line of treatment, the lack of efficacy and the proarrhythmic side effects of different antiarrhythmic drugs have led to the use of ablation therapy as a common approach to the management of IART in patients with CHD.
Mapping and Ablation of Intra-atrial Re-entry Tachycardia
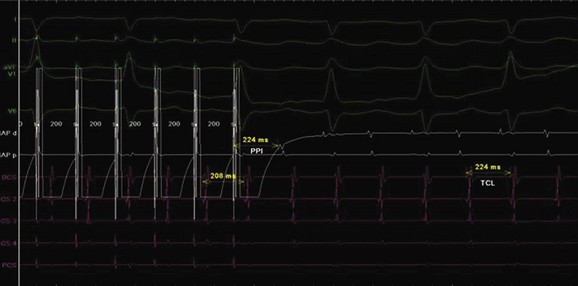
The P-wave morphology is often different from adult-type flutter (Figure 76-30), and the rates may be slower (because of associated scarring and fibrosis). This may lead to occasional one-on-one rapid ventricular conduction with resultant hemodynamic compromise.64 Sustained hemodynamic stable tachycardia is essential for mapping and entrainment and is aided by advanced electroanatomic and noncontact mapping systems, which allow the creation of activation and substrate maps. To help with better understanding of the complex anatomy, MRI and CT scan images can be merged with intra-procedure geometry obtained in the EP laboratory by using advanced mapping systems. Intracardiac echocardiography has been shown to help guide atrial baffle puncture, identify anatomic structures, and monitor tissue contact during ablation.63 Once the propagation of the tachycardia has been deciphered, entrainment mapping can be used to confirm the target ablation sites (Figure 76-31). The aim is to create a line of conduction block and to achieve transmural lesions; it may be necessary to resort to large-tip and irrigated-tip catheters to accomplish this.60 The CTI is accessed with a retrograde approach, especially in patients who have had a Senning or Mustard repair (Figure 76-32). The line of block can be created by either a series of ablation applications or “drag” lesions at the proposed site. These circuits, which can be seen along the lateral wall of the right atrium at the atriotomy site and involve the crista terminalis, can combine with the CTI and cause a “figure-of-8”–type flutter configuration. The other flutter focus site could be in the lateral superior right atrial wall, which is the bypass cannulation site. The combination of advanced mapping and enhanced lesion creation has led to over 90% acute success. Unfortunately, a 40% recurrence rate has been seen in the single-ventricle population within 3 years.65,66 This is significantly better than the 73% acute success rate observed in the multiple-institutional pediatric RF registry in 271 patients with CHD between 1989 and 1999.4
Nonfocal Atrial Tachycardia
NFAT emanates from a focal source and can be induced and terminated by pacing.67 The mechanism underlying NFAT may be triggered, re-entrant, or both and is indistinguishable from other forms of atrial tachycardia on ECGs. The incidence varies from 8% to 40%.68,69 These arrhythmias are more commonly seen in older patients who had surgery for CHD at a young age. The average age at clinical presentation was 27 years (range, 5 to 52 years) in one of the studies.67 NFAT is distinguished from macro–re-entrant IART by careful analysis of the three-dimensional propagation map, which shows a radial spread of activation from an atrial point source. Some of the triggered NFATs may respond to adenosine because of membrane hyperpolarization and shortening of refractory periods, which may be caused by the effect on potassium channels.70,71
Mapping and Ablation Nonfocal Atrial Tachycardia
Focal sites have been found in both atrial chambers with ablation success rate of approximately 75%.67–69 In a study of 62 atrial tachycardias in 43 patients following CHD surgery, the incidence of focal atrial tachycardia was approximately 15% with an acute RF ablation success of 100%.69
Surgical Ablation
Residual hemodynamic issues such as valvar insufficiency, severe atrial or Fontan baffle dilation, and high recurrence of IART after catheter ablation following a Fontan procedure has led to an increased use of surgical ablation as a potential treatment option with variable results. The recurrence risk of IART may be lower with surgical ablation using either RF or cryo ablation (such as surgical Fontan baffle revision with a modified right atrial Maze procedure), but the risks of such a procedure may be significantly higher than with catheter ablation.72
Complications
The overall risk of complications (786 with CHD of 7524 enrolled patients) was 7.8% for ablation of all SVTs in CHD.4 Serious complications in this group were recorded as 4.2% and included third-degree AV block, brachial plexus stretch injury, cardiac arrest, Horner’s syndrome, and SVC thrombus. The overall mortality risk was 0.3%. Both morbidity and mortality risks are lower in the current era.
Ventricular Arrhythmias
Ventricular Tachycardia
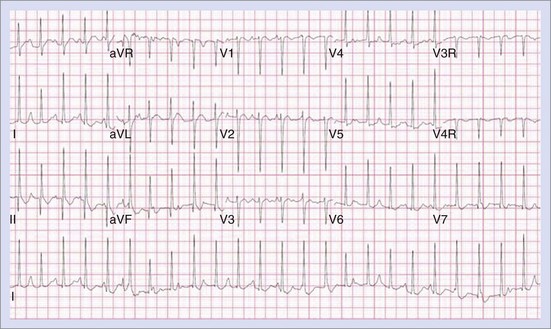
Ventricular tachycardia (VT) continues to be a challenge, especially in postoperative patients with TOF, and has been implicated in the etiology of sudden death in this patient population.73,74 The incidence of VT has been reported as 11.9% in patients with TOF with a sudden death risk of 8.3% after 35 years of follow-up.75 However, the risk of sudden death in younger patients after TOF surgery is low; the presence of inducible VT by PES (Figure 76-33) and increased QRS duration may be helpful in predicting the risk.74 Correction of TOF involves patch closure of the VSD with relief of right ventricular outflow obstruction, which may involve infundibulectomy and trans-annular patch. Scarring and fibrosis around these sites of incision and patch repairs, as well as associated residual hemodynamic issues (such as outflow obstruction and pressure overload, pulmonary insufficiency, and volume overload, or all of these) contribute to macro–re-entrant VT.76
Ventricular arrhythmias and sudden death have also been described in D-TGA after a Mustard procedure (incidence of 9%) with an inverse correlation with the RV ejection fraction and a positive correlation with age and QRS duration greater than 140 ms.77 Although atrial arrhythmias were present in 44% of their patients, the authors of this study did not predict the occurrence of VT or sudden death.78 The risk factors for development of VT in TOF include later age at repair, moderate or severe tricuspid regurgitation, increased QRS duration, and depressed ventricular function. Clinical presentation includes isolated premature ventricular contractions, which may be monomorphic or polymorphic in 18% to 40% of the postoperative patients with TOF.78 Other symptoms may include palpitations and syncope.
Mapping and Ablation
VT ablation in this group with CHD can be challenging because of complex anatomy, thickened hypertrophied myocardium, hemodynamic instability in the presence of ventricular dysfunction, noninducibility of clinical VTs, and broad scar-related isthmuses.79 Different strategies have been described, including activation sequence analysis, pace mapping, entrainment maneuvers, and, more recently, substrate mapping in sinus rhythm. The last is used if the tachycardia is not inducible or poorly tolerated. It is imperative to perform three-dimensional mapping such as electroanatomic contact mapping or noncontact mapping for substrate mapping (Figures 76-34 and 76-35). The success in a series of 30 subjects described in two studies was 89% with a 20% recurrence risk.80,81 In another series of 11 patients, where substrate mapping was used as the strategy, the acute success rate was 100% with a 9% recurrence rate after a mean follow-up of 30 months.82 The critical isthmus in this study was within the distinct anatomic isthmuses bordered by unexcitable tissue in the RVOT at the site of trans-annular repair and around the VSD patch. Since sudden death can still occur in patients who have no inducible VT adjunct therapy after a successful RF ablation may include an implantable cardioverter-defibrillator).83
Key References
Alexander ME, Cecchin F, Walsh EP, et al. Implications of implantable defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72-76.
Avari JN, Jay KN, Rhee EK. Experience and results during transition from radiofrequency ablation to cryoablation for treatment of pediatric atrioventricular nodal reentrant tachycardia. PACE. 2008;31:454-460.
Deal BJ, Mavroudis C, Backer C. Beyond Fontan conversion: Surgical therapies of arrhythmias including patients with associated complex congenital heart disease. Ann Thorac Surg. 2003;76:542-553.
Friedman RA, Walsh E, Silka M, et al. Radiofrequency catheter ablation in children with and without congenital heart disease. Report of the writing committee. PACE. 2002;25(6):1000-1017.
Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicenter study. Lancet. 2000;356:975-981.
Kanter RJ, Papagiannis J, Carboni MP, et al. Radiofrequency catheter ablation of supraventricular tachycardia substrates after Mustard and Senning operations for D-transposition of the great arteries. J Am Coll Cardiol. 2000;35:428-441.
Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair. Circulation. 2004;109:1994-2000.
Ko JK, Deal BJ, Strasburger JF, et al. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028-1032.
Kugler JD, Danford DA, Houston K, et al. Pediatric Radiofrequency Catheter Ablation Registry: Update of immediate results. In: Imai Y, Momma K, editors. Proceedings of the Second World Congress of Pediatric Cardiology and Cardiac Surgery. Armonk, NY: Futura, 1998.
Lermann BB. Response of nonreentrant catecholamine mediated VT to endogenous adenosine and acetylcholine: Evidence for myocardial receptor mediated effects. Circulation. 1993;87:382-390.
Lermann BB, Stein KM, Markowitz SM. Mechanism of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 1997;8:571-583.
Lesh MD, Van Hare GF, Scheinman MM, et al. Comparison of retrograde and transeptal methods for ablation of left free wall accessory pathways. J Am Coll Cardiol. 1993;22:542-549.
Nogami A, Naito S, Tada H, et al. Demonstration of diastolic and presystolic Purkinje potentials as critical potentials in a macroreentry circuit of verapamil-sensitive idiopathic left ventricular tachycardia. J Am Coll Cardiol. 2000;36:811-823.
Opel A, Murray S, Kamath N, et al. Cryoablation versus radiofrequency ablation for treatment of atrioventricular nodal reentrant tachycardia: Cryoablation with 6 mm tip catheters is still less effective than radiofrequency ablation. Heart Rhythm. 2010;7:340-343.
Reich JD, Auld D, Hulse E, et al. The Pediatric Radiofrequency Ablation Registry’s experience with Ebstein’s anomaly: Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol. 1998;9:1370-1377.
Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation: A long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280.
Schaffer MS, Silka MJ, Ross BA, et al. Inadvertent atrioventricular block during radiofrequency catheter ablation: Results of the Pediatric Radiofrequency Ablation Registry. Circulation. 1996;94:3214.
Teo WS, Klein GJ, Guiraudon GM, et al. Multiple accessory pathways in the Wolff-Parkinson-White syndrome as a risk factor for ventricular fibrillation. Am J Cardiol. 1991;67:889-891.
Ticho BS, Saul JP, Hulse JE, et al. Variable location of accessory pathways associated with the permanent form of junctional reciprocating tachycardia. Am J Cardiol. 1992;70:1559-1564.
Treidman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intaatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
Van Hare GF, Lesh MD, Ross BA, et al. Mapping and radiofrequency ablation of intraatrial reentrant tachycardia after the Senning and Mustard procedure for transposition of great arteries. Am J Cardiol. 1996;77:985-991.
Walsh EP. Interventional electrophysiology in patients with congenital heart disease. Circulation. 2007;115:3224-3234.
1 Ko JK, Deal BJ, Strasburger JF, et al. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028-1032.
2 Benson DW, Smith WM, Dunnigan AM. Mechanisms of regular wide QRS tachycardia in infants and children. Am J Cardiol. 1982;49:1776-1788.
3 Bromberg BI, Lindsay BD, Cain ME, et al. Impact of clinical history and electrophysiologic characterization of accessory pathways on management strategies to reduce sudden death among children with Wolff-Parkinson White syndrome. J Am Coll Cardiol. 1996;27:690-695.
4 Kugler JD, Danford DA, Houston K, et al. Pediatric Radiofrequency Catheter Ablation Registry: Update of immediate results. In: Imai Y, Momma K, editors. Proceedings of the Second World Congress of Pediatric Cardiology and Cardiac Surgery. Armonk, NY: Futura, 1998.
5 Friedman RA, Walsh E, Silka M, et al. Radiofrequency catheter ablation in children with and without congenital heart disease. Report of the writing committee. PACE. 2002;25(6):1000-1017.
6 Schaffer MS, Silka MJ, Ross BA, et al. Inadvertent atrioventricular block during radiofrequency catheter ablation: Results of the Pediatric Radiofrequency Ablation Registry. Circulation. 1996;94:3214.
7 Opel A, Murray S, Kamath N, et al. Cryoablation versus radiofrequency ablation for treatment of atrioventricular nodal reentrant tachycardia: Cryoablation with 6 mm tip catheters is still less effective than radiofrequency ablation. Heart Rhythm. 2010;7:340-343.
8 Klein GJ, Bashore TM, Sellers TD, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301:1080-1085.
9 Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12:144-150.
10 Teo WS, Klein GJ, Guiraudon GM, et al. Multiple accessory pathways in the Wolff-Parkinson-White syndrome as a risk factor for ventricular fibrillation. Am J Cardiol. 1991;67:889-891.
11 Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation: A long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280.
12 Hoffman JIE. SVT incidence, mortality and natural history. In Anderson RH, Baker EJ, McCarthy FJ, et al, editors: Paediatric cardiology, ed 2, London, UK: Churchill Livingston, 2002.
13 Frias PA, Taylor MB, Kavanaugh-McHugh A, et al. Low incidence of valve insufficiency following retrograde aortic radiofrequency catheter ablation in young patients. J Interven Cardiol Electrophysiol. 1999;3:181-185.
14 Shah MJ, Jones TK, Cecchin F. Improved localization of right sided accessory pathways with microcatheter assisted right coronary artery mapping in children. J Cardiovasc Electrophysiol. 2004;15(11):1238-1243.
15 McGuire MA, Johnson DC, Robotin M, et al. Dimensions of the triangle of Koch in humans. Am J Cardiol. 1992;70(7):829-830.
16 Rhodes LA, Wieand TS, Vetter VL. Low temperature and low energy radiofrequency modification of atrioventricular node slow pathway in pediatric patients. PACE. 1999;22:1071-1078.
17 Avari JN, Jay KN, Rhee EK. Experience and results during transition from radiofrequency ablation to cryoablation for treatment of pediatric atrioventricular nodal reentrant tachycardia. PACE. 2008;31:454-460.
18 Silver E, Silva JN, Ceresnak SR, et al. Cryoablation with an 8 mm tip catheter for pediatric atrioventricular nodal reentrant tachycardia is safe and efficacious with a low incidence of recurrence. PACE. 2010;33(6):681-686.
19 Walsh EP. Interventional electrophysiology in patients with congenital heart disease. Circulation. 2007;115:3224-3234.
20 Abrams DJ, Earley MJ, Sporton SC, et al. Comparison of contact and electroanatomic mapping to identify scar and arrhythmia late after the Fontan procedure. Circulation. 2007;115:1738-1746.
21 Villain E, Vetter VL, Garcia JM, et al. Evolving concepts in management of congenital junctional ectopic tachycardia: A multicenter study. Circulation. 1990;81:1544-1549.
22 Bharati S, Moskowitz WB, Scheinman M, et al. Junctional tachycardias: Anatomic substrate and its significance in ablative procedures. J Am Coll Cardiol. 1991;18(1):179-186.
23 Dubin AM, Cuneo BF, Strassburger JF, et al. Congenital junctional ectopic tachycardia and congenital complete AV block: A shared etiology. Heart Rhythm. 2005;3:313-315.
24 Van Hare GF, Velvis H, Langberg JJ, et al. Successful transcatheter ablation of congenital junctional ectopic tachycardia in a 10 month old infant using radiofrequency energy. PACE. 1990;13:730-735.
25 Shah MJ, Wieand T, Vetter VL. Cryoablation of congenital familial ectopic tachycardia with preservation of atrionodal function in an infant. J Cardiovasc Electrophysiol. 2007;18:773-776.
26 Gaita F, Montefusco A, Riccardi R, et al. Cryoenergy catheter ablation: A new technique for treatment of permanent junctional reciprocating tachycardia in children. J Cardiovasc Electrophysiol. 2004;3:263-268.
27 Ticho BS, Saul JP, Hulse JE, et al. Variable location of accessory pathways associated with the permanent form of junctional reciprocating tachycardia. Am J Cardiol. 1992;70:1559-1564.
28 Critelli G. Permanent junctional reciprocating tachycardia: An update. Card Electrophysiol Rev. 2001;5:307-314.
29 Lermann BB, Stein SM, Markowitz SM, et al. Idiopathic right ventricular outflow tract tachycardia: A clinical approach. PACE. 1996;19:2120-2137.
30 Lermann BB. Response of nonreentrant catecholamine mediated VT to endogenous adenosine and acetylcholine: Evidence for myocardial receptor mediated effects. Circulation. 1993;87:382-390.
31 Cole RC, Marrouche NF, Natale A. Evaluation and management of ventricular outflow tract tachycardia. Card Electrophysiol Rev. 2002;6:442-447.
32 Lerman BB, Stein KM, Markowitz SM. Mechanism of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 1997;8:571-583.
33 Zipes DP, Foster PR, Troup PJ, et al. Atrial induction of ventricular tachycardia: Reentry versus triggered automaticity. Am J Cardiol. 1979;44:1-8.
34 Belhassen B, Rotmensch HH, Laniado S. Response of recurrent sustained ventricular tachycardia to verapamil. Br Heart J. 1981;46:679-682.
35 Thakur RK, Klein GJ, Sivaram CA, et al. Anatomic substrate for idiopathic left ventricular tachycardia. Circulation. 1996;93:497-501.
36 Nogami A, Naito S, Tada H, et al. Demonstration of diastolic and presystolic Purkinje potentials as critical potentials in a macroreentry circuit of verapamil-sensitive idiopathic left ventricular tachycardia. J Am Coll Cardiol. 2000;36:811-823.
37 Chen M, Yang B, Zou J, et al. Non-contact mapping and linear ablation of the left posterior fascicle during sinus rhythm in the treatment of idiopathic left ventricular tachycardia. Europace. 2005;7:138-144.
38 Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation. 1993;87:866-873.
39 Lev M, Givson S, Miller RA. Ebstein’s disease with Wolff Parkinson White syndrome. Am Heart J. 1955;49:724-741.
40 Benson DJr, Gallagher JJ, Oldham HN, et al. Corrected transposition with severe intracardiac deformities with Wolff Parkinson White syndrome in a child: Electrophysiologic investigation and surgical correction. Circulation. 1980;61:1256-1261.
41 Perosio AM, Suarez LD, Bunster AM, et al. Pre-excitation syndrome and hypertrophic cardiomyopathy. J Electrocardiol. 1983;16:29-40.
42 Epstein ER, Saul JP, Weidling SN, et al. Atrioventricular reciprocating tachycardia involving twin atrioventricular nodes in patients with complex congenital heart disease. J Cardiovasc electrophysiol. 2001;12(6):671-679.
43 Shim D, Lloyd TR, Cho KJ, et al. Transhepatic cardiac catheterization in children: Evaluation of efficacy and safety. Circulation. 1995;92:1526-1530.
44 Neghme RA, Carboni MP, Care J, et al. Transthoracic percutaneous access for electroanatomic mapping and catheter ablation of atrial tachycardia in patients with a lateral tunnel Fontan. Heart Rhythm. 2006;3(1):37-43.
45 Lesh MD, Van Hare GF, Scheinman MM, et al. Comparison of retrograde and transseptal methods for ablation of left free wall accessory pathways. J Am Coll Cardiol. 1993;22:542-549.
46 Thiene G, Wenink AC, Frescura C, et al. Surgical anatomy and pathology of the conduction tissues in atrioventricular defects. J Thorac Cardiovasc Surg. 1981;82:928-937.
47 Anderson RH, Becker AE, Arnold R. The conduction tissues in congenitally corrected transposition. Circulation. 1974;50:911-923.
48 Kanter RJ, Papagiannis J, Carboni MP, et al. Radiofrequency catheter ablation of supraventricular tachycardia substrates after Mustard and Senning operations for D-transposition of the great arteries. J Am Coll Cardiol. 2000;35:428-441.
49 Perry JC, Boramanand NK, Ing FF. “Transseptal” technique through atrial baffles for 3-dimensional mapping and ablation of atrial tachycardia in patients with D-transposition of the great arteries. J Interven Cardiol Electrophysiol. 2003;9:365-369.
50 Dave AS, Aboulhosn J, Child JS, et al. Transconduit puncture for catheter ablation of atrial tachycardia in a patient with extracardiac Fontan palliation. Heart Rhythm. 2010;7(3):413-416.
51 Levine J, Walsh EP, Saul JP. Catheter ablation of accessory pathways in patients with congenital heart disease including heterotaxy syndrome. Am J Cardiol. 1993;72:689-694.
52 Chetaille P, Walsh EP, Triedman JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart Rhythm. 2004;1:168-173.
53 Bar-Cohen Y, Cecchin F, Alexander ME, et al. Cryoablation for accessory pathways located near normal conduction tissues or within the coronary venous system in children and young adults. Heart Rhythm. 2006;3:253-258.
54 Cappato R, Schlüter M, Weiss C, et al. Radiofrequency current catheter ablation of accessory atrioventricular pathways in Ebstein’s anomaly. Circulation. 1996;94(3):376-383.
55 Reich JD, Auld D, Hulse E, et al. The Pediatric Radiofrequency Ablation Registry’s experience with Ebstein’s anomaly: Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol. 1998;9:1370-1377.
56 Coscio FC. Endocardial mapping of atrial flutter. In: Taboul P, Waldo AL, editors. Atrial arrhythmias. St Louis: Mosby, 1990.
57 Roos-Hesselink J, Perlroth MG, McGhie J, et al. Atrial arrhythmias in adults after repair of tetralogy of Fallot: Correlations with clinical, exercise, and echocardiographic findings. Circulation. 1995;91:2214-2219.
58 Van Hare GF, Lesh MD, Ross BA, et al. Mapping and radiofrequency ablation of intraatrial reentrant tachycardia after the Senning and Mustard procedure for transposition of great arteries. Am J Cardiol. 1996;77:985-991.
59 Aiba T, Shimizu W, Noda T, et al. Non-invasive characterization of intra-atrial re-entrant tachyarrhythmias after surgical repair of congenital heart disease. Circ J. 2009;73:451-460.
60 Treidman JK, Delucca JM, Alexander ME, et al. Prospective trial of electroanatomically guided, irrigated catheter ablation of atrial tachycardia in patients with congenital heart disease. Heart Rhythm. 2005;2:700-705.
61 Chan DP, Van Hare GF, Mackall JA, et al. Importance of atrial flutter isthmus in postoperative intra-atrial re-entry tachycardia. Circulation. 2000;86:969-974.
62 Flinn CJ, Wolff GS, Dick M, et al. Cardiac rhythm after Mustard operation for complete transposition of great arteries. N Engl J Med. 1984;3310:1635-1638.
63 Cecchin F, Johnsrude CL, Perry JC, et al. Effect of age and surgical technique on symptomatic arrhythmias after Fontan procedure. Am J Cardiol. 1995;76:386-391.
64 Piechl P, Kautzner J, Gebauer R. Ablation of atrial tachycardias after correction of complex congenital heart diseases: Utility of intracardiac echocardiography. Europace. 2009;11:48-53.
65 Garson A, Bink-Bolkens MTE, Heisslin PS, et al. Atrial flutter in the young: A collaborative study of 380 cases. J Am Coll Cardiol. 1985;6:871-878.
66 Treidman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intaatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
67 Chen SA, Tai CT, Chiang CE, et al. Focal atrial tachycardia: Reanalysis of the clinical and electrophysiologic characteristics and prediction of successful radiofrequency ablation. J Cardiovasc Electrophysiol. 1998;9:355-365.
68 Sesslar SP, Alexander M, Berul C, et al. Ablation of nonautomatic focal atrial tachycardia in children and adults with congenital heart disease. J Cardiovasc Electrophysiol. 2006;17(4):359-365.
69 Kammeraad JA, Balaji S, Oliver RP, et al. Nonautomatic focal atrial tachycardia: Characterization and ablation of a poorly understood arrhythmia in 38 patients. PACE. 2003;26:736-742.
70 De Groot NM, Zeppenfeld K, Wijffels MC, et al. Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery: Role of circumscribed areas with heterogeneous conduction. Heart Rhythm. 2006;5:526-535.
71 Iwai S, Markowitz SM, Stein KM, et al. Response to adenosine differentiates focal from macroreentrant atrial tachycardia: Validation using three dimensional electroanatomic mapping. Circulation. 2002;106:2793-2799.
72 Deal BJ, Mavroudis C, Backer C. Beyond Fontan conversion: Surgical therapies of arrhythmias including patients with associated complex congenital heart disease. Ann Thorac Surg. 2003;76:542-553.
73 Marin-Garcia J, Moller JH. Sudden death after operative repair of tetralogy of Fallot. Br Heart J. 1977;39:1380-1385.
74 Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair. Circulation. 2004;109:1994-2000.
75 Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicenter study. Lancet. 2000;356:975-981.
76 Gatzoulis MA, Till JA, Reddington AN. Depolarization-repolarization inhomogeniety after repair of Tetralogy of Fallot: The substrate for malignant ventricular tachycardia? Circulation. 1997;95:401-404.
77 Schwerzmann M, Salehian O, Harris L, et al. Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J.. 2009;30:1873-1879.
78 Chandar JS, Wolff GS, Garson AJr, et al. Ventricular arrhythmias in postoperative tetralogy of Fallot. Am J Cardiol. 1990;65:655-661.
79 Furoshima H, Chinushi M, Sugiura H, et al. Ventricular tachycardia late after repair of congenital heart disease: Efficacy of combination therapy with radiofrequency catheter ablation and class III antiarrhythmic agents and long term outcome. J Electrocardiol. 2006;39:219-224.
80 Gonska BD, Cao K, Raab J, et al. Radiofrequency catheter ablation of right ventricular tachycardia late after repair of congenital heart defects. Circulation. 1996;94:1902-1908.
81 Morwood JG, Treidman JK, Berul CI, et al. Radiofrequency catheter ablation of ventricular tachycardia in children and young adults with congenital heart disease. Heart Rhythm. 2004;1:301-308.
82 Zeppenfeld K, Schalij MJ, Bartelings MM, et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease. Electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116:2241-2252.
83 Alexander ME, Cecchin F, Walsh EP, et al. Implications of implantable defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72-76.