Chapter 29 Urinary Porphyrins for the Detection of Heavy Metal and Toxic Chemical Exposure
 Introduction
Introduction
Toxic chemicals affect human biochemistry at any level of long-term exposure. Fortunately, the body has mechanisms for transforming, eliminating, or compartmentalizing the many toxic chemicals encountered over a lifetime. Nonetheless, these “safety” mechanisms may be inadequate or even inappropriate in modern industrialized society, especially for susceptible people such as the elderly, individuals with poor nutritional habits, and others who are physiologically stressed.1,2
Porphyrins measured in urine serve as such a biomarker. Porphyrins are particularly well suited as biomarkers for two reasons. First, they are produced in the highly active heme pathway, so any disturbance tends to cause rapid and relatively large accumulations of intermediates. Second, some of the enzymes of the porphyrin-producing pathway are highly sensitive to the presence of various toxins, and they are rapidly turned over. Their short half-lives of minutes or hours cause a demand for rapid synthesis in response to changing environmental signals. The enzymes normally associate with nutrient metal ions to produce heme and cobalamin (and, in plants, chlorophyll), sometimes called the pigments of life.3
The presence or elevation of various urinary porphyrin species can flag a potentially toxic condition. Metals and other toxic chemicals with prooxidant reactivity can inactivate porphyrinogenic enzymes, deplete glutathione and other antioxidants, and increase oxidant stress, all of which lead to damage to membranes, enzymes, and other proteins in cells.4,5 In addition, porphyrinogens (precursors to porphyrins in the reduced state) themselves are easily nonenzymatically oxidized to porphyrins by toxic metals such as mercury. Thus, the distribution pattern of porphyrins in the urine serves as a functional fingerprint of toxicity.6
 Definitions
Definitions
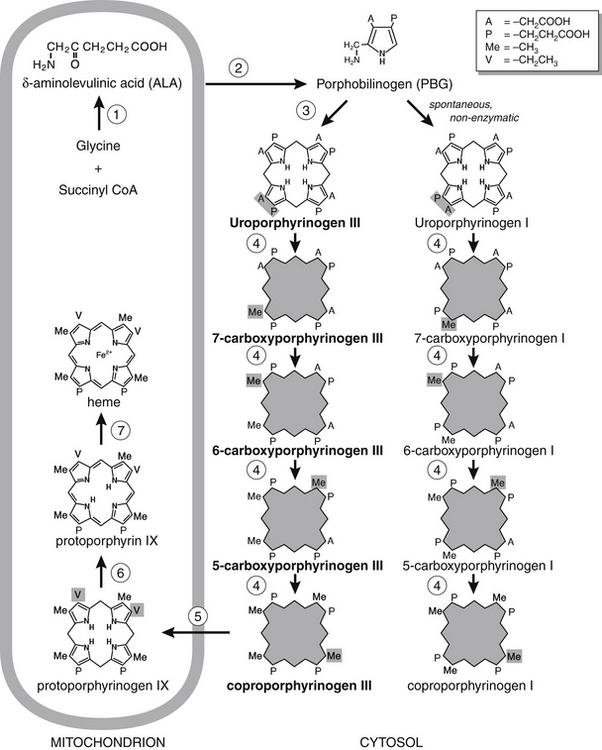
Porphyrins are oxidized by-products that have escaped from the heme biosynthetic pathway, an essential pathway occurring in all nucleated mammalian cells. Heme is the all-important iron-binding molecule essential for the proper function of many proteins, including hemoglobin (oxygen transport), cytochrome c (energy production), and cytochrome P-450 (detoxification). Biosynthesis of heme involves eight enzymes (Figure 29-1), five of which produce intermediate molecules that are collectively called porphyrinogens. Some porphyrinogens escape the intracellular pathway to become oxidized by other cellular processes to porphyrins. In turn, some porphyrins are excreted in urine and feces.
The different molecular species of porphyrins that occur in the urine of healthy individuals form a predictable, characteristic pattern. An elevation of one or more porphyrins is designated porphyrinuria. The term porphyria is reserved for primary conditions exhibiting specific clinical symptoms caused by an inherited defect in one or more of the heme biosynthetic enzymes. Porphyrinopathy is an umbrella term for any disorder in porphyrin metabolism. Enzymatic defects associated with common porphyrias are listed in Table 29-1.
| ENZYMATIC DEFECT | PORPHYRIA |
|---|---|
| δ-Aminolevulinic acid (ALA) synthetase | Aminolevulinic aciduria (porphyrinogen precursor) |
| ALA dehydratase | Plumboporphyria |
| Porphobilinogen deaminase | Acute intermittent porphyria |
| Uroporphyrinogen cosynthetase | Congenital erythropoietic porphyria |
| Uroporphyrinogen decarboxylase | Porphyria cutanea tarda and hepatoerythropoietic porphyria |
| Coproporphyrinogen oxidase | Hereditary coproporphyria |
| Protoporphyrinogen oxidase | Variegate porphyria |
| Ferrochelatase | Protoporphyria |
 Porphyrinopathies
Porphyrinopathies
One class of toxic chemicals capable of subtle yet insidious health effects that may mimic other disorders, especially in children, is the heavy metals. Lead, mercury, arsenic, aluminum, and cadmium are well-documented examples. Long-term exposure to these metals often results in organ-specific accumulation that compromises target organ physiology. Similarly, long-term exposure to organic chemicals such as herbicides, pesticides, and industrial and manufacturing by-products can have a deleterious effect on the body’s biochemistry that results in the decline of cellular function.4 Whatever the cause, porphyrins induce oxidative cellular damage when they accumulate.
Clinical Applications
The effect of chemicals on the porphyrin pathway has been the subject of many scientific reports. Lead, mercury, or arsenic toxicity induces porphyrinuria,7 as do polychlorinated phenyls (e.g., dioxin, polychlorinated biphenyls)8 and many drugs9 (Table 29-2). In long-term cultures of adult rat hepatocytes, exposure to submicromolar concentrations of lead increased porphyrin excretion after a delay of 15 to 22 days.10 A study of practicing dentists reported correlations between elevations of urinary 5-carboxyporphyrin, precoproporphyrin, and coproporphyrin, and behavioral changes that were related to urinary excretion of mercury.11,12 Together, elevations of these porphyrins served as biomarkers of mercury toxicity.
TABLE 29-2 Interpretation of Abnormal Urinary Porphyrin Test Results: Relationship to Heme Pathway Defects and Possible Causes (with Emphasis on Toxic Metals)
| ABNORMAL TEST RESULT* | HEME PATHWAY ENZYME AFFECTED | POSSIBLE ENVIRONMENTAL CAUSE† |
|---|---|---|
| Uroporphyrin and 7-carboxyporphyrin (sometimes) | Uroporphyrinogen decarboxylase | Arsenic2 or certain organic chemicals |
| 5-Carboxyporphyrin and coproporphyrin | Uroporphyrinogen decarboxylase | Mercury6 |
| 6-Carboxyporphyrin (sometimes) | Coproporphyrinogen oxidase | Certain organic chemicals |
| Precoproporphyrin‡ (almost always accompanied by elevated coproporphyin III) | Uroporphyrinogen decarboxylase (possibly) | Mercury6 |
| Coproporphyrin III, coproporphyrin I (sometimes) | Coproporphyrinogen oxidase | Lead or mercury2 Certain organic chemicals |
| Coproporphyrin I/III ratio >1 | Hepatobiliary dysfunction4 Porphobilinogen deaminase |
Arsenic |
* Reference ranges vary depending on the laboratory doing the analysis. The reference range for the particular laboratory conducting the analysis should be used. Inherited disorders in the enzymes of heme biosynthesis are relatively rare, but such a possibility should be considered if urinary porphyrins are greatly elevated. A specialist in inherited disorders should be consulted if such a disorder is suspected.
† When urinary porphyrin results are being evaluated to arrive at a diagnosis of metal or chemical toxicity, the following should be ruled out: use of ethanol, estrogens, oral contraceptives, antibiotics, sedatives, analgesics, dietary brewer’s yeast; also pregnancy, liver disease, malignancies, and hematologic diseases such as pernicious or iron deficiency anemias.
‡ The detection of precoproporphyrin is specifically diagnostic for mercury toxicity.6
Upregulation of the heme biosynthetic pathway, with the concomitant increase in ALA, is another mechanism by which porphyria can be precipitated. Increased ALA production is usually a normal physiologic response to provide enough of this preporphyrin precursor to meet the body’s demand for heme. However, overproduction of ALA can overwhelm even a normally functioning heme biosynthetic pathway, resulting in the inappropriate accumulation of ALA or the porphyrins.6 Active porphyria commonly occurs when overproduction of ALA coincides with inhibition of one or more of the porphyrinogenic enzymes. Often porphyria is the result of a chemical insult to a porphyrinogenic enzyme combined with an external stressor that provokes dysregulation of the heme biosynthetic pathway. It is estimated that among patients with a porphyrinogenic enzyme deficiency, as many as 90% are healthy throughout adulthood, until porphyria is triggered midlife by toxic chemicals or drugs, an acute illness or worsening chronic condition, or a major dietary change (Table 29-2).11
 Conclusion
Conclusion
Use of porphyrin measurements as biomarkers of chemical toxicity is reasonable in combination with other laboratory tests of toxicant concentrations in body fluids or hair. The clinician should realize that many conditions unrelated to primary or toxicant-induced porphyria can cause porphyrinuria.13 When considering a urinary porphyrin result, the clinician should be mindful that the distribution of normal urinary porphyrin values, representing healthy individuals, overlaps significantly with values representing those who have had porphyria at one time or another.
1. Rowland I., ed. Nutrition, toxicity, and cancer. Boca Raton, FL: CRC Press, 1991.
2. Baker S. Detoxification and healing. New Canaan, CT: Keats Publishing; 1997.
3. Thunell S. Porphyrins, porphyrin metabolism and porphyrias. I. Update. Scand J Clin Lab Invest. 2000;60:509–540.
4. Chang L., Magos L., Suzuki T. Toxicology of metals. Boca Raton, FL: CRC Press, 1996.
5. Fowler B.A., Oskarsson A., Woods J.S. Metal- and metalloid-induced porphyrinurias: relationships to cell injury. Ann N Y Acad Sci. 1987;514:172–182.
6. Woods J.S., Bowers M.A., Davis H.A. Urinary porphyrin profiles as biomarkers of trace metal exposure and toxicity: studies on urinary porphyrin excretion patterns in rats during prolonged exposure to methyl mercury. Toxicol Appl Pharmacol. 1991;110:464–476.
7. Woods J.S. Porphyrin metabolism as indicator of metal exposure and toxicity. In: Goyer R.A., Cherian M.G. Handbook of experimental pharmacology. Berlin: Springer-Verlag; 1995:19–52.
8. Doss M.O. Porphyrinurias and occupational disease. In: Silbergeld E., Fowler B. Mechanisms of chemical-induced porphyrinopathies. New York: New York Academy of Sciences; 1987:204–218.
9. Moore M.R., Disler P.B. Drug-induction of the acute porphyrias (review). Adverse Drug React Acute Poisoning Rev. 1983;2:149–189.
10. Quintanilla-Vega B., Hernandez A., Lopez M.L., et al. Porphyrin production and excretion by long-term cultures of adult rat hepatocytes and effect of lead exposure. Toxicology. 1995;102:275–283.
11. Woods J.S., Martin M.D., Naleway C.A., et al. Urinary porphyrin profiles as a biomarker of mercury exposure: studies on dentists with occupational exposure to mercury vapor. J Toxicol Environ Health. 1993;40:235–246.
12. Woods J.S. Altered porphyrin metabolism as a biomarker of mercury exposure and toxicity. Can J Physiol Pharmacol. 1996;74:210–215.
13. Donnay A., Ziem G. Porphyria protocol packet (on evaluating disorders of porphyrin metabolism in chemically sensitive patients). Baltimore: MCS Referral and Resources; 1995.