Ureteral Obstruction and Malformations
Hydronephrosis and ureteral malformations are among the most common anomalies in the urinary tract in children. Most are now detected prenatally. Urinary tract dilation is present in 1 in 100 fetuses, but significant uropathy is found in only 1 in 5001,2 (Fig. 54-1).
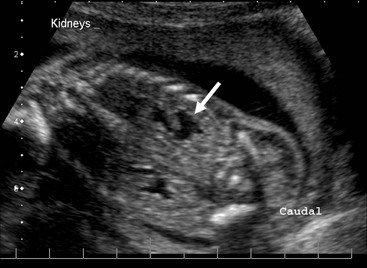
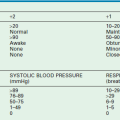
FIGURE 54-1 Normal kidneys are typically identifiable by 18 weeks in all fetuses. Dilated kidneys can be seen as early as 12–14 weeks of gestation. The arrow demonstrates left-sided caliectasis on a coronal fetal image.
Ureteropelvic Junction Obstruction in Children
Historically, the incidence of UPJ obstruction has been estimated at 1 in 5,000 live births. However, with the advent of antenatal ultrasonography (US), the prevalence of dilation has been found to be much higher. Retrospective reviews show that although the incidence of detected dilation has increased, the actual number of operations for UPJ obstruction has been relatively constant at 1 : 1,250 births.3,4 UPJ obstruction is more common in boys (2 : 1), and two-thirds occur on the left side. Bilateral dilation occurs in 5–10% of patients, and is much more frequently seen in younger children. Bilateral obstruction is much less common.5
Etiology
During development of the upper ureter, the lumen of the ureteral bud solidifies with ureteral lengthening and later recanalization.6 Failure to recanalize adequately is thought to be the cause of most intrinsic UPJ obstructions. Other causes of intrinsic UPJ obstruction include ureteral valves, polyps, and leiomyomas.7
The most common observation is ureteral narrowing of a variable length that joins the renal pelvis above the expected dependent position.8 At low volume, peristaltic waves of urine cross the UPJ. However, as the flow increases beyond a threshold, the renal pelvis dilates.9 The dilated pelvis may functionally kink the ureter further, increasing the pelvic pressure. In 20–30% of patients, the ureter is draped over a lower-pole vessel, producing an extrinsic UPJ obstruction. In most situations, there is also a coexisting luminal narrowing of the ureter.10
Histologic evaluation reveals a decrease or complete absence of smooth muscle fibers at the UPJ.11 Electron microscopy may show an increase in collagen deposition between the muscle fibers that is most likely a response to the obstruction as opposed to the cause.12 Fibrosis and interruption of the smooth muscle continuity block transmission of the peristaltic wave, while defective innervation also may play a role.13 UPJ obstruction also can be secondary, i.e., related to other ureteral pathology. It can be found in conjunction with high-grade vesicoureteral reflux (VUR), after cutaneous ureterostomy, and after decompression of the dilated urinary tract. VUR is present in 14% of patients with UPJ obstruction (Fig. 54-2).14,15
Clinical Presentation
Most renal dilation and obstruction are detected prenatally. Less frequently, it is detected because of an abdominal mass, urinary tract infection (UTI), or associated with other congenital anomalies (i.e., VACTERL syndrome). In older children, vague, poorly localized, cyclic or acute abdominal pain associated with nausea is common (Fig. 54-3). Some of these children are initially seen by gastroenterologists. The cause for the intermittent obstruction is unclear, but renal function is almost always preserved. Hematuria after minor trauma or vigorous exercise may be a presenting feature, most likely secondary to rupture of mucosal vessels in the dilated collecting system.5
Diagnosis
When the antenatal diagnosis of UPJ obstruction is made, the initial postpartum evaluation should be performed at 10 to 14 days of life to avoid false-negative studies resulting from the transitional nephrology of the newborn. Bilateral dilation is rarely associated with significant enough obstruction to cause oligohydramnios and warrant antenatal intervention. Ultrasound confirms the presence of pelvic and calyceal dilation, with variable thinning of the renal parenchyma. The Society for Fetal Urology (SFU) classification is typically used to describe the degree of dilation (Fig. 54-4).16 The presence of corticomedullary junctions is indicative of preserved function.15 Ultrasound is used to evaluate the contralateral kidney, the bladder, and the distal ipsilateral ureter to avoid confusion with a ureterovesical junction (UVJ) obstruction, but it does not provide functional information.
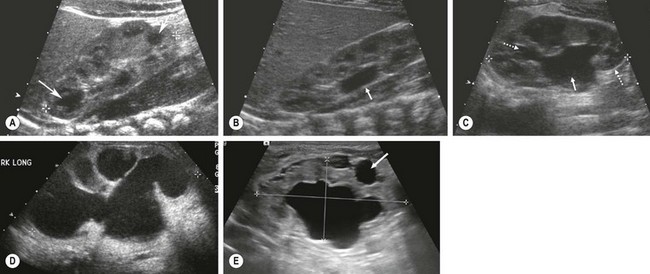
FIGURE 54-4 These neonatal ultrasound images come from infants with a history of prenatally detected renal dilation. (A) This ultrasound is normal for comparison purposes. There are dark renal pyramids (arrow) and no renal pelvic dilation. (B) This image shows isolated renal pelvic dilation (arrow) (SFU grade I). (C) This image shows dilation of the renal pelvis (solid arrow) and upper and lower-pole calyces (dotted arrows) (SFU grade II). (D) Calyceal dilation and cortical thinning are seen (SFU grade IV). (E) Hydronephrosis with peripheral cysts (arrow) indicating dysplasia is seen. This kidney had no function on renal scan.
In the past, routine antibiotic prophylaxis was utilized in all infants with prenatal dilation but the risk of UTI is very small in the absence of reflux.17 A voiding cystourethrogram (VCUG) was previously recommended in all patients being evaluated for UPJ obstruction. VUR increases the chance that infection will occur, even in a partially obstructed system. Between 5–30% of infants with prenatally detected dilation will have reflux, and the majority will spontaneously resolve without an infection.15 Children with isolated pyelectasis and no ureteral dilation have a very low incidence of reflux and do not need a screening VCUG.
The diuretic isotopic renogram is very useful for evaluating hydronephrosis, differential renal function, and renal drainage (Fig. 54-5). In this study, the transit of an injected radioisotope through the urinary tract is monitored by a gamma camera. The early uptake (first 1 to 2 minutes) of the tracer indicates the split renal function, while the washout, augmented by the administration of a diuretic, is evaluated and plotted by a computer to demonstrate drainage.18–20 The study is obtained with either 99mTc-mercaptoacetyltriglycine (99mTc-MAG3), whose clearance is predominantly via proximal tubular secretion, or with technetium-99m-labeled diethylenetriamine pentaacetic acid (99mTc-DTPA), whose renal clearance is by glomerular filtration. 99mTc-MAG3 is more efficiently excreted than 99mTc-DTPA and gives better images, particularly in patients with impaired renal function.19,20
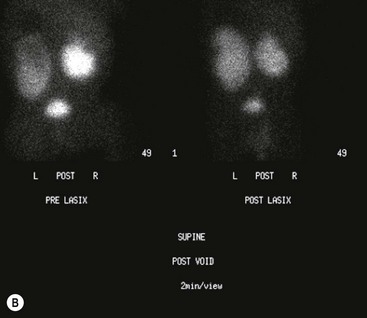
FIGURE 54-5 Renal scan in the evaluation of prenatally identified hydronephrosis. The ultrasound study showed diffuse caliectesis and pelvic dilation consistent with UPJ obstruction. (A) The dilated left kidney has reduced function. (B) Lasix should be administered once the renal pelvis is completely distended. In this case, it appears there is further accumulation (increased counts) after Lasix administration. Observation was chosen because the dilation was predominantly extrarenal, the kidneys were not palpable even after Lasix administration, and concern that the drainage time was perhaps falsely elevated. One year later, the renal dilation was markedly improved.
The technique for diuretic renography is standardized.20 Patients should be hydrated intravenously (15 mL/kg) 15 minutes before injection of the radionuclide. An indwelling catheter maintains an empty bladder and monitors urine output. The diuretic (1 mg/kg furosemide, up to 40 mg) is not administered until the activity peaks in the hydronephrotic kidney and renal pelvis. The tracer activity is then monitored for an additional 30 minutes, and a quantitative analysis is performed. Historically, persistence of more than 50% of the tracer in the renal pelvis 20 minutes after diuretic administration (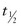 >20) is diagnostic of obstruction, although the applicability of this threshold in pediatric patients is debatable. False-positive results may occur when the immature neonatal kidney fails to respond to diuretic, when the diuretic is administered prior to maximal renal pelvic distension, when the patient is dehydrated, when the bladder is distended, or when the pelvis is significantly dilated.
>20) is diagnostic of obstruction, although the applicability of this threshold in pediatric patients is debatable. False-positive results may occur when the immature neonatal kidney fails to respond to diuretic, when the diuretic is administered prior to maximal renal pelvic distension, when the patient is dehydrated, when the bladder is distended, or when the pelvis is significantly dilated.
Magnetic resonance urography (MRU) can be used at any age. T2-weighted images are independent of renal function, and hydronephrosis is readily detected. The anatomic images are excellent (Fig. 54-6). Enhanced MR images with gadolinium can give information regarding differential function if one kidney is anatomically and functionally normal.21
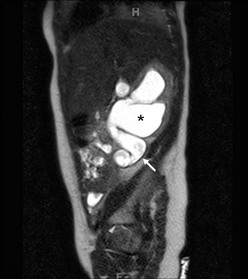
FIGURE 54-6 In this 6-month-old infant, an ultrasound showed a dilated ureter below the kidney, but not at the level of the bladder. This coronal image shows marked dilation of the proximal ureter (arrow) and pelvis (asterisk) due to a proximal ureteral valve.
Rarely, when imaging is equivocal, invasive pressure flow studies may be indicated.22 These tests assume that obstruction produces a constant restriction to outflow that necessitates elevated pressure to transport urine at high flow rates. However, not all obstructions are constant. If the obstruction is intrinsic, a linear relationship exists between pressure and flow. However, in some cases, the results reflect only the response of the renal pelvis to distention and may be positive in the absence of obstruction. These studies require general anesthesia in children and have limited applicability.
Retrograde urography at the time of operative correction is helpful if uncertainty exists regarding the site of obstruction. This is rarely required because a well-performed ultrasound evaluation and radionuclide study will exclude distal obstruction.23 As there are risks with using instruments in the infant male urethra and the ureteral orifice, these retrograde studies are not usually performed.
Management
Indications for Intervention
Intermittent obstruction and pain are probably the most reliable indication for operation. Diminished function, delayed drainage, progression of pelvic and calyceal dilation on ultrasound, and loss of renal function are all potential indicators of obstruction. Randomization to operative and observational arms is complicated by a difficult decision that a parent has to make for the asymptomatic child.24 The morphologic appearance of a dilated renal pelvis on excretory urography or ultrasound is not a good indication for operation because many of these findings will resolve without the need for operation (see Fig. 54-5).25 Neonatal hydronephrosis can often be explained by physiologic polyuria and natural kinks and folds in the ureter.
The ongoing debate in the management of neonatal UPJ obstruction centers on the definition of significant obstruction. Diuretic renography has limitations in the neonate, although using the ‘well-tempered’ approach increases its value.20 The standard half-time of 20 minutes for obstruction in the neonate is misleading in many cases.
Differential renal function or individual kidney uptake is the most useful information obtained during renography.24–26 An indication for operation is diminished renal function in the presence of an obstructive pattern on renography. The threshold is arbitrary, but most surgeons believe that less than 35–40% function in the hydronephrotic kidney warrants correction. However, one series of patients with dilated kidneys and no more than 25% total renal function were found to improve to more than 40% of total function in all cases without operative correction.25 Long-term studies of kidneys with greater than 40% function have shown that fewer than 15–20% will require operation for diminishing function, UTIs, or unexplained abdominal pain.26,27 Some of these kidneys will regain some of the lost function.
If the child is initially seen with acute pain or infection, it is advisable to wait one to two weeks to allow the inflammation to resolve. Percutaneous drainage or stent placement for sepsis is rarely required preoperatively. It should be avoided in the absence of infection because of the inflammation that develops from a tube in the renal pelvis. Exploration of a poorly functioning kidney requires assessment of the renal parenchyma. If the parenchyma is grossly dysplastic or frozen-section analysis shows only dysplasia, then nephrectomy should be performed. No test accurately predicts recovery of function. Thus, nephrectomy is rarely performed in the infant with UPJ obstruction.
Operative Techniques
A dismembered pyeloplasty is the preferred technique to correct UPJ obstruction (Fig. 54-7). A successful outcome is achieved with construction of a funnel-shaped, dependent UPJ complex. The renal pelvis and upper ureter are mobilized and the ureter is divided just below the obstructed segment. It is spatulated on its lateral border through the aperistaltic segment. Usually, it is necessary to resect some of the renal pelvis to avoid postoperative obstruction. If this segment is particularly long, a flap of renal pelvis can be created. Foley YV-plasty and the Culp spiral flap were designed to maintain the continuity of the ureter and the pelvis.28,29 These techniques are used in unusual cases of malrotation, fusion anomalies, or long, stenotic segments.
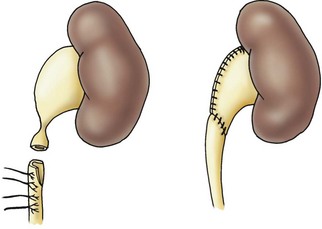
FIGURE 54-7 Dismembered pyeloplasty showing reduction of the renal pelvis and spatulation of the ureter (see the text).
Pyeloplasties are frequently performed without diversion so it is important to be as gentle as possible.30 Excessive handling of the pelvis and ureter increases edema. A stent is typically left in place after a laparoscopic repair. Even if leakage from the anastomosis occurs, a satisfactory outcome can usually be expected. A Penrose drain is left near the anastomosis and can usually be removed within 48 hours. If drainage is prolonged, the child can be discharged with the drain in place. Renal drainage is indicated in solitary kidneys or when simultaneous bilateral pyeloplasties are performed. In reoperation, it is technically more difficult to achieve a watertight anastomosis and internal drainage (stent, nephrostomy or nephrostent) is indicated.
Extrinsic UPJ obstruction associated with an aberrant lower-pole vessel requires division of the ureter at the UPJ and performance of a standard dismembered pyeloplasty after transposing the ureter to a nonobstructed position. This is preferable to laparoscopic transposition of the crossing vessel. In the case of an intrarenal pelvis or when significant scarring is found at reoperation, a ureterocalicostomy is a useful technique.31 A portion of the lower pole should be resected to prevent a postoperative stricture. The ureter is spatulated and then anastomosed to the exposed calyx in the lower pole.
The open approach still has a role in infants and young children. Laparoscopic pyeloplasty has been performed in all ages and the age of the patient is inversely related to benefits of decreased pain and convalescence.32 Open pyeloplasty can be performed through a flank, anterior extraperitoneal approach, or posterior lumbotomy approach. The anterior approach involves a transverse incision from the edge of the rectus to the tip of the 12th rib.33 The retroperitoneum is entered and the UPJ is exposed, with the kidney left in situ. In infants, this is a muscle-splitting incision with low morbidity. The posterior lumbotomy also can be easily performed in infancy and provides direct access to the UPJ.34 The kidney does not require mobilization, and the ureter and renal pelvis can usually be delivered into the incision. In bilateral cases, the child does not need to be repositioned. The lumbotomy approach should not be used with a malrotated kidney or a kidney that has an intrarenal pelvis. An anterior or flank approach is always preferred for reoperation although laparoscopy and endopyelotomy have largely replaced open re-operative pyeloplasty.35
Endoscopic approaches (endopyelotomy) for UPJ obstruction were popularized in the 1980s and 1990s, but have been replaced by laparoscopic approaches.36,37 Endopyelotomy successfully relieves primary UPJ obstruction in 70% of children.38 As the success pales in comparison to pyeloplasty, it is not routinely utilized for primary repair. Endopyelotomy clearly has a role in recurrent UPJ obstruction, in which the success rate is >95%.38 Depending on the age of the patient and the size of the ureter, this can be performed in either an antegrade or retrograde fashion (Fig. 54-8).
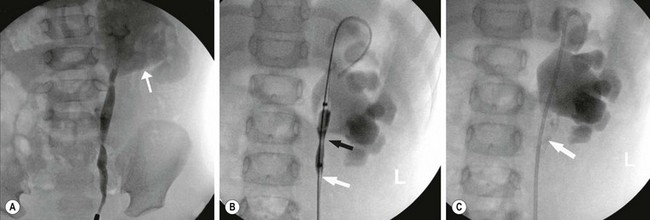
FIGURE 54-8 Retrograde endopyelotomy. Sixteen-month-old infant with persistent hydronephrosis and preserved function, but no washout on renal scan 6 months after undergoing a dismembered pyeloplasty. (A) The retrograde pyelogram shows a UPJ configuration with dilute contrast in a dilated renal pelvis (arrow). (B) A balloon catheter is passed retrograde and inflated. The black arrow shows the narrowing (waist) and the white arrow demonstrates the cutting wire that is positioned laterally. (C) An indwelling stent is positioned after the endopyelotomy is performed. The white arrow points out the extravasation that is indicative of an appropriate depth of incision.
The first laparoscopic pyeloplasty in a child was reported in 1995 by Peters.39 The first series was published by Tan in 1999.40 Laparoscopic pyeloplasty has been reported in children as young as 2 months.41 The introduction of robotic surgery with articulating instruments and three-dimensional visualization has made intracorporeal suturing easier and more precise. The success rate with minimally invasive techniques is equivalent to open pyeloplasty.42,43 The benefits of laparoscopic and robotic surgery over an open approach may include a decreased length of hospitalization, decreased analgesic requirements, improved cosmesis, and quicker return to normal activity.42
Laparoscopic pyeloplasties are mostly performed using the Anderson–Hynes dismembered technique (Fig. 54-9). This can be performed through either a transperitoneal or retroperitoneal approach using a similar technique once access and exposure are obtained. With both transabdominal and retroperitoneal approaches, the child is placed in a flank or modified flank position.
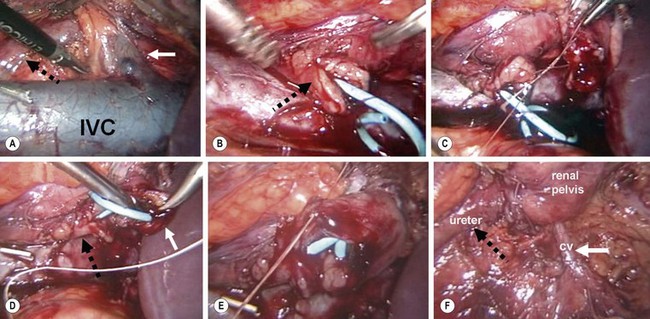
FIGURE 54-9 Laparoscopic pyeloplasty. (A) Transperitoneal view of crossing vessel (solid white arrow) that is causing intermittent obstruction of the ureter (dotted black arrow). IVC, inferior vena cava. (B) The ureteropelvic junction has been transected and the ureter spatulated (arrow), and a double J stent has been positioned in the bladder and out the proximal ureter. This can be accomplished via a retrograde or antegrade approach. (C) The posterior anastomosis between the renal pelvis and ureter is being performed. (D) The proximal end of the stent is inserted into the renal pelvis (solid white arrow). The ureter is marked with the dotted black arrow. (E) The anterior anastomosis is being performed. (F) The completed anastomosis is seen with the crossing vessel (white arrow, CV) now located posterior to the pyleloplasty. Again, the ureter is marked with the dotted black arrow. (Images courtesy of R. Sherburne Figenshau, MD.)
UPJ Obstruction in a Duplex Kidney
In a duplex kidney, the lower pole is most commonly affected because the upper pole lacks a true pelvis.44 Ultrasound may not be reliable for diagnosis because the duplex nature of the kidney may not be identified. A pyelogram or renogram will show a small nonobstructed upper segment.
Surgical Results and Complications
The results of operative correction have been uniformly successful when performed at children’s hospitals.27,30,35 The rate of recurrent UPJ obstruction is less than 1%, and the nephrectomy rate is less than 2%. The most common early complications are prolonged urinary extravasation and delayed drainage through the anastomosis. If a significant leak develops, either a stent or a percutaneous nephrostomy tube can be inserted. Once diversion is instituted, the leak will usually cease within 48 hours. Late scarring at the anastomotic site is common, but rarely occurs due to a leak.
Ureteral Abnormalities
Ureteral development begins during the fourth week of gestation when the ureteral bud arises from the mesonephric duct.45 The bud elongates cephalad, and forms the ureter, renal pelvis, calyces, and collecting tubules. The distal end of the mesonephric duct from the ureteral bud to the vesicourethral tract is called the common excretory duct and expands in trumpet fashion into the bladder and urethra to form half of the trigone. The attachment of the ureter to the mesonephric duct switches from a posterior to an anterolateral location. With expansion and absorption of the common excretory duct into the urinary tract, the orifices of the ureteral bud and mesonephric duct become independent and move away and settle in the bladder and urethra, respectively.
Alterations in bud number, position, and time of development result in anomalies. VUR results from caudal displacement of the ureteral bud, whereas ureteral ectopia and obstruction result from cranial displacement. Renal development and dysplasia are related to the ureteral orifice location.46
Ureteral Duplication
Duplication is the most common ureteral anomaly. Both sides are equally affected, and girls are affected twice as often as boys. The autopsy incidence is approximately 1%, but the incidence was 2–4% in clinical series in which pyelograms were obtained for urinary symptoms.45,46 Many of the duplicated units show congenital dysplasia (scarring) and hydronephrosis. There is an increased incidence of infection because both VUR and obstruction are much more common in duplicated systems.45
A partial or complete duplication of the ureter occurs when a single bud branches prematurely or when two ureteral buds arise from the mesonephric duct. A bifid renal pelvis is the highest level of bifurcation and occurs in 10% of the population. Other incomplete duplications occur throughout the ureter (Fig. 54-10). An inverted-Y ureter is the rarest of all branching anomalies.47 This is presumably the result of separate ureteral buds that fuse before entering the metanephros.
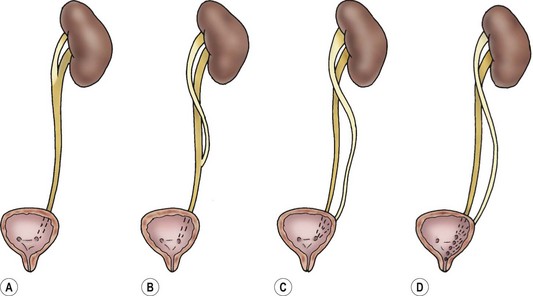
FIGURE 54-10 Types of duplication. (A) Bifid pelvis. (B) ‘Y’ ureter. (C) ‘V’ ureter. (D) Complete duplication with various ectopic orifices.
In complete duplications, reflux into the lower renal moiety is the most common cause of renal disease. The more caudal ureteral bud ends up laterally and cranially deviated in the bladder and has a shorter intramural tunnel. The upper-pole ureter enters the bladder adjacent or distal to the lower ureter, as defined by the Weigert–Meyer law.48 Reflux is identified in up to two-thirds of children with duplicated systems that develop infection.49 Reflux may occur into the upper-pole ureter if the ureteral orifices are immediately adjacent or if the upper ureter is distally located at the level of the bladder neck without any submucosal support (Fig. 54-11).
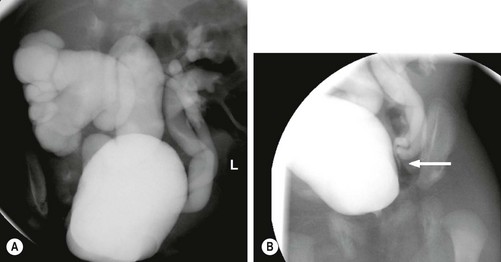
FIGURE 54-11 Ureteral duplication and reflux. (A) This VCUG shows massive reflux into the lower pole of a duplex right kidney. There is also reflux into both moieties on the left. (B) Lateral oblique views show that the two left ureters join (arrow) just outside the bladder
The treatment of VUR in duplicated ureters follows the same principles as that in a single system. Initial treatment includes preventive antibiotics and radiographic monitoring. Low grades of VUR are associated with the same rate of spontaneous resolution as a single system. The distal ureters share a common vascular supply so reimplantation involves mobilization and reimplantation of the common sheath.50 If an associated lower-pole UPJ obstruction is noted, ipsilateral end-to-side pyeloureterostomy is an effective management for both obstruction and reflux.51 Even if significant scarring is present in the lower pole, reimplantation is usually effective unless major ureteral dilation is present. In the latter case, lower-pole nephroureterectomy may be needed.
Ureteral Triplication
This is one of the rarest anomalies of the upper urinary tract and results from either several ureteral buds or early branching. In most cases, all three ureters drain into a single orifice.52 Triplication presents with incontinence, infection, and symptoms of obstruction, and is associated with both ectopia and ureteroceles.53,54 Ureteral quadruplication has also been described.55
Retrocaval Ureter
The retrocaval or circumcaval ureter is a right ureter that passes behind the vena cava (Fig. 54-12).56 This is the result of a developmental error in the formation of the vena cava. The supracardinal vein (vena cava) lies dorsal to the developing ureter, whereas subcardinal veins lie ventral to the ureter. If the subcardinal vein persists as the vena cava, the ureter passes behind the vena cava and anterior to the iliac vein. If both veins persist, the ureter passes between the duplicated vena cava.57
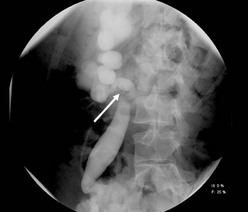
FIGURE 54-12 Retrocaval ureter. A retrograde pyelogram shows reverse J-hooking of the ureter (arrow) as it passes behind the inferior vena cava. Note that the ureteral caliber is the same proximally and distally which indicates no obstruction.
Symptoms are related to chronic ureteral obstruction and infection, and rarely occur in children.58 The radiographic appearance depends on the level of obstruction. The more common distal obstruction appears as a ‘reversed J’ on intravenous pyelogram. Less commonly, the ureter crosses at the level of the UPJ. Both of these can be confused with UPJ obstruction and should be suspected when pyelectasis and dilation of the upper third of the ureter are seen.
Megaureter
Megaureter is not a diagnosis, but a descriptive term for a dilated ureter. Normal ureteral diameter in children is rarely greater than 5 mm. Ureters greater than 7 mm are considered megaureters.59 The ultrasound appearance of the dilated and tortuous ureter is usually striking (Fig. 54-13). Pelvicalyceal dilation and parenchymal scarring or thinning depend on the primary disease process. Megaureter is the second most common urinary tract abnormality detected prenatally.2 Infection and intermittent abdominal pain can also be presenting symptoms.60
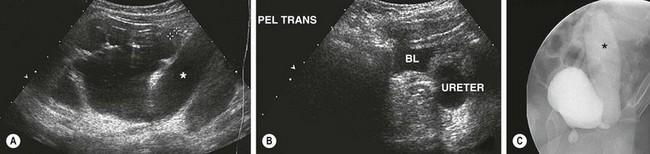
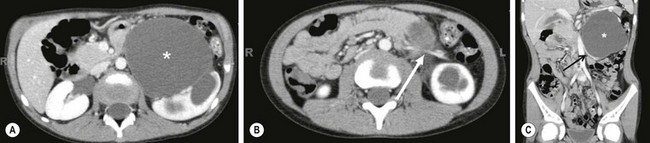
FIGURE 54-13 This patient has congenital megaureter. (A) Renal ultrasound image shows diffuse caliectasis and cortical thinning with a markedly dilated left ureter (asterisk). (B) Ultrasound image of the bladder confirms the markedly dilated ureter adjacent to the bladder (BL). A MAG-3 scan was performed and showed preserved renal function on the left side. (C) The voiding cystourethrogram shows reflux into the markedly dilated left ureter (asterisk). Note that the contrast agent in the ureter is diluted (compared with the bladder), which is indicative of partial obstruction on that side.
Primary obstructive megaureter is most commonly caused by a distal adynamic ureteral segment, but ureteral valves60 and ectopic ureteral insertion can also cause obstruction. Proximal smooth muscle hypertrophy and hyperplasia are seen. A normal caliber catheter will usually pass through the distal 3–4 mm segment, but the peristaltic wave does not propel urine across this area. This absent peristalsis is not a result of a ganglionic abnormality as seen in megacolon.61 The distal ureter may exhibit a variety of histologic abnormalities, but the common finding is a disruption of muscular continuity that prevents propulsion of urine.11,62
These anomalies can be classified as nonobstructed, refluxing, and obstructed.60 Some ureters also have reflux and simultaneous obstruction. Standard imaging allows classification and appropriate management. The diagnosis of a nonobstructed, nonrefluxing megaureter is one of exclusion and is made only when the secondary causes of megaureter have been excluded and diagnostic tests do not show obstruction. Box 54-1 gives clinical examples of each classification. Any normal ureter will dilate if the volume of urine exceeds emptying capacity. Moreover, bacterial endotoxins and infection alone can cause dilation that will resolve after treatment of the infection.63,64
Ultrasound almost always distinguishes megaureters from UPJ obstruction. The degree of distal ureteral dilation is often much more pronounced than the degree of renal pelvic dilation or caliectasis. A VCUG should be obtained in all patients. If significant reflux is found, delayed drainage films must be obtained to exclude simultaneous obstruction with a normal caliber distal ureteral segment. In a partially obstructed system, the contrast density in the ureter is decreased because of dilution related to stasis in the ureter (see Fig. 54-13).
Treatment
Nonoperative management is based on clearance half-time and relative renal function of the hydronephrotic and contralateral kidneys. If observation is chosen, suppressive antibiotics are recommended and the child is followed with serial ultrasound and/or renal scans. Neonatal megaureter with obstruction found by renography, but with preserved function can be safely observed. Most ureters will become radiographically normal over time.65–68 Operative correction for decreasing function or recurring infections will be needed in only 10–25% of patients by age 7 years. Evidence of delayed obstruction after normalization of radiographs has not been seen in these children.
Ureteral tapering with preservation of the ureteral blood supply was popularized in the early 1970s.69 A longitudinal segment of ureter is excised and then closed over a 10–12 French catheter. When the ureter is tunneled submucosally, the suture line is positioned against the detrusor to decrease the chance of fistula. Initial repairs involved tailoring the entire ureter, but this was found to be unnecessary because the upper ureteral tortuosity and dilatation often disappears after tapering the distal ureter.70 Ureteral folding techniques have been popularized because they theoretically decrease the risk of ischemic injury while achieving the decreased intraluminal diameter necessary for a successful reimplant.71,72 However, the increased bulk is technically a problem in infant bladders. Although dissection is usually both intravesical and extravesical, extravesical reimplants alone have been described and may have lower morbidity.73 A vesicopsoas hitch is a useful adjunct that helps achieve a longer submucosal tunnel length without risking ureteral kinking, although excisional tailoring usually eliminates this need. A nonrefluxing, nonobstructed reimplantation can be achieved in most patients with megaureters.72,74 Recognized complications include persistent obstruction, reflux, and urinary extravasation. Most of these can be managed nonoperatively with drainage. Lower grades of postoperative VUR will often resolve.
Balloon dilation and stenting have been described in older children (mean 3 years) with a success rate greater than 95%.75,76 This approach should definitely be considered in older children because of the marked decrease in morbidity associated with a completely endoscopic approach.
Ectopic Ureter
An ectopic ureter is defined as one that does not drain on the trigone, but at the bladder neck or more caudally. Embryologically, this results from a cranial insertion of the ureteral bud on the mesonephric duct that allows distal migration with the mesonephric duct as it is absorbed into the urogenital sinus.77
The incidence of ureteral ectopia is approximately 1 in 2000.46 Eighty per cent of ectopic ureters are reported in association with a duplicated renal system. As clinical problems are more common in girls with ectopia, only 15% of ectopic ureters have been reported in boys.46,77 Ectopia is bilateral 20% of the time.46 Single ectopic ureters are rare but are more common in boys.78
Ectopic Ureter in Girls
The fundamental difference between ureteral ectopia in boys and girls arises from ureteral insertion distal to the continence mechanism in girls (Fig. 54-14). Approximately one-third of ureters open at the level of the bladder neck, one third are in the vestibule around the urethral opening, and the remainder empty into the vagina, uterus, or cervix. All of these insertions are along the course of the mesonephric duct remnant (Gartner’s duct).
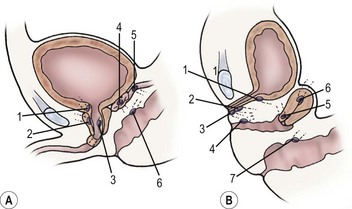
FIGURE 54-14 (A) Ureteral ectopia in a boy. Possible sites are above the external sphincter (1–3), or into the seminal vesicle (4,5), or anorectal (6). (B) Ureteral ectopia in a girl may be located at the bladder neck (1), or beyond the continence mechanism in the urethra (2), or on the perineum (3). Uterine or vaginal insertion (4–6) may also cause incontinence. Anorectal insertion (7) can also occur.
Fifty per cent of affected girls initially have continuous urinary incontinence despite what appears to be a normal voiding pattern.46,77 If the system is markedly hydronephrotic and functions poorly, urine leakage may occur only in the upright position and may be confused with stress incontinence. Persistent foul-smelling vaginal discharge can suggest an ectopic ureter. When the ectopic ureter is present in the urethra or the bladder neck, both obstruction and reflux are frequently found.
The diagnosis of an ectopic ureter may be obvious or can be difficult. When the distal end exits in the vagina, cervix or uterus, the kidney may not visualize on ultrasound if the renal moiety is small and atrophic, and not associated with hydronephrosis. Often significant hydronephrosis is found in the upper pole of a duplicated system and the ultrasound image may show a dilated ectopic ureter behind the bladder. However, the upper pole may also be a small remnant (Fig. 54-15). A scan using dimercaptosuccinic acid (DMSA) is a good test for localizing a small ectopic kidney when an orthotopic kidney is not identified on standard imaging and there is a high suspicion of an ectopic ureter in the vagina, cervix or uterus. As long as there is some dilation, MRU may be the most precise method for making this diagnosis.79 A VCUG should be obtained to exclude occult reflux.80
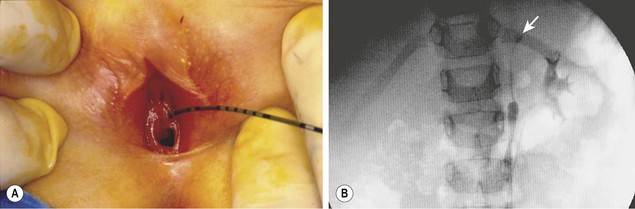
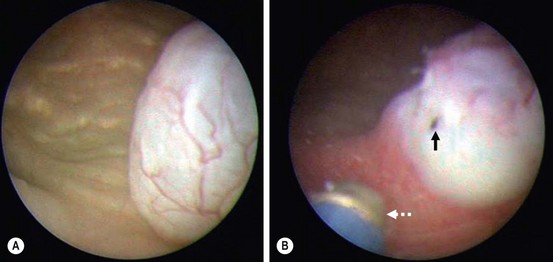
FIGURE 54-15 This 3-year-old girl had continuous urinary incontinence despite a normal voiding pattern. The renal ultrasound image was essentially normal on both sides with no evidence of hydronephrosis or an echogenic upper pole. (A) A ureteral catheter has been inserted into an ectopic left ureter. (B) The retrograde ureterogram shows a very small upper left ureter and a small cystic calyx (arrow) in the left upper pole medial to the lower-pole collecting system that was opacified through an orifice on the trigone. The patient was continent after a laparoscopic left upper pole partial nephroureterectomy.
The diagnosis is confirmed with physical examination, panendoscopy, and retrograde pyelography. Dyes that stain urine may have a role. Urine in the bladder changes color, whereas the poorly concentrated urine is evident as persistent clear leakage. Meticulous examination of the area around the urethral meatus and vagina will often reveal an asymmetry or bead of fluid coming from an opening that can be probed and injected in retrograde fashion (see Fig. 54-15). Vaginoscopy with attention to the superior lateral aspect of the vagina may reveal a large ectopic orifice.
Ectopic Ureter in Boys
The most common sites of ectopic ureteral insertion in boys are the posterior urethra (40–50%) and the seminal vesicle (20–60%), depending on the age at presentation.81 Symptoms in boys may not occur until after the onset of sexual activity and include prostatitis, seminal vesiculitis, or an infected seminal vesical cyst causing painful bowel movements. The genital insertion accounts for the common presentation with epididymitis. He may have postvoid dribbling secondary to pooling of urine in the prostatic urethra, but incontinence is never as pronounced as in a female.
Management of Ectopic Ureters
Operative treatment is dependent on the associated renal parenchyma.82,83 Single-system ectopic ureters that enter the genital system usually have poor function, and nephroureterectomy is appropriate. When the ectopic ureter is associated with duplication, the function in the upper pole is usually poor, and an open or laparoscopic partial nephroureterectomy has historically been the most common approach. The distal ureter can be left open. A ureteroureterostomy can be performed proximally or distally as well. The dilated upper pole is diverted into the normal caliber lower pole system. There are potential concerns regarding the size discrepancy of the ureters and injury to the recipient ureter, but large series show excellent success with a very low complication rate.84 This approach avoids potential injury to the lower pole of the kidney and can be performed through a small inguinal incision85 or laparascopically.86 The obstruction, dilation, and incontinence usually resolve following ureteroureterostomy. Even if the upper pole is poorly functioning, it should not cause significant long-term problems. A common sheath ureteral reimplantation can be performed with tailoring of the ureter in the upper pole, but the increased morbidity and complication rate associated with ureteral tapering limit the utility of this approach.
The distal ureteral stump rarely causes a problem in genital ectopia. However, if urethral or bladder neck insertion of the ectopic ureter and reflux into the ureter is identified preoperatively, excision of the distal stump is important, but can be tedious.83 If the plane of dissection is kept immediately adjacent to the ureter behind the bladder, the bladder neck and sphincter should not be damaged. A transvesical approach can also be useful and aids in exposure of the urethral insertion. In a postpubertal girl, access to the urethra can be performed transvaginally as well.
Bilateral Single Ectopic Ureters
This is a rare finding in which the altered ureteral embryologic development is associated with failure of normal bladder neck development.87 Genital and anal anomalies are commonly present. In girls, the ureter inserts into the distal urethra. They are usually initially seen with infection or are noted to have continuous urinary leakage. The bladder is usually poorly developed because it has never stored urine. Boys have somewhat larger bladders because some urine will have entered it. However, because the bladder neck is not formed normally, they also have some degree of urinary incontinence.
Ureteroceles
Ureteroceles are cystic dilatations of the terminal, intravesical ureter that usually have a stenotic orifice.46 In children, ureteroceles are most commonly associated with the upper pole of a duplex system (80%) and an ectopic orifice (60%) in the urethra. In adults, they are usually part of a completely intravesical single system. Ureteroceles occur four to seven times more frequently in girls and are more common in whites. Bilateral ureteroceles are found 10% of the time.
A single embryologic theory does not explain all ureteroceles. Historically, a persistent membrane (Chwalla’s) at the junction of the Wolffian duct and urogenital sinus was theorized to cause a ureterocele.88 It is more likely that a ureterocele is the result of an abnormal induction of the trigone and distal ureter by many of the genes and growth factors that are important in renal and ureteral growth and development. Gross inspection of the intravesical portion of ureteroceles shows deficiencies in the trigonal musculature of patients with ureteroceles that are not present in in ectopic ureters without ureterocele formation. This results in a pseudodiverticulum (ureterocele eversion) and reflux into laterally displaced, poorly supported ureters. This is further supported by the clinical observation of multicystic dysplasia and the absence of hydronephrosis in association with a ureterocele.89
The classification of ureteroceles can be confusing. The current recommended nomenclature classifies ureteroceles as either intravesical (entirely within the bladder) or ectopic (some portion is situated permanently at the bladder neck or in the urethra).90
Presentation and Diagnosis
Most ureteroceles are identified prenatally although 10–15% still present postnatally due to infection.91–93 The obstructed renal unit may be palpable, but most have no clinically apparent abnormality. Bladder outlet obstruction is rare because most ureteroceles decompress during micturition, but the most common cause of urethral obstruction in girls is urethral prolapse of a ureterocele (Fig. 54-16).
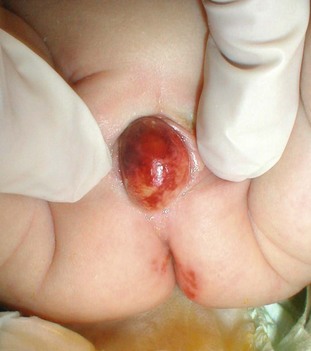
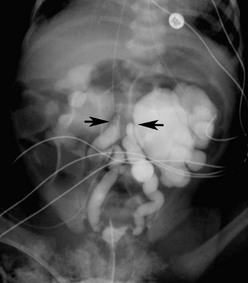
FIGURE 54-16 This 2-week-old baby presented with sepsis and was found to have this prolapsing ectopic ureterocele. The ureterocele was aspirated with return of purulent debris and underwent prompt decompression. Recovery was uneventful.
Abdominal ultrasound reveals a well-defined cystic intravesical mass that is associated within the posterior bladder wall (Fig. 54-17). This can be followed to a dilated ureter in the pelvis and to upper-pole hydroureteronephrosis in a duplicated system. The thickness and echogenicity of the renal parenchyma are often consistent with dysplasia and poor function. A VCUG typically shows ipsilateral lower pole or contralateral reflux.94
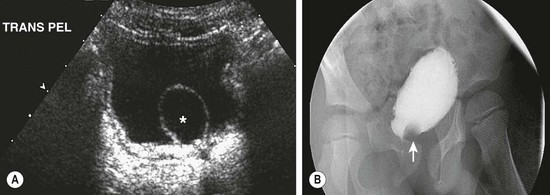
FIGURE 54-17 (A) Ultrasound image of the bladder demonstrates a ureterocele (asterisk). (B) The ureterocele appears as a nonopacified filling defect (arrow) at the base of the bladder on the cystogram.
During cystoscopy, the bladder should be examined when it is full and also completely empty because compressible ureteroceles may not be evident in a full bladder or may appear as a bladder diverticulum. The dilated lower end of an ectopic ureter or megaureter may elevate the trigone, creating the cystoscopic, radiographic, and ultrasound appearance of a ureterocele, a so-called pseudoureterocele.95
Treatment
The goals for ureterocele management include control of infection, preservation of renal function, protection of normal ipsilateral and contralateral units, and continence. There is a subset of ureteroceles associated with multicystic dysplasia, no hydroureter, and no reflux. The multicystic moiety usually involutes and the ureterocele rarely causes symptoms and can be observed.89 Up to 10–15% of prenatally identified ureteroceles have these clinical findings. Neonates given suppressive antibiotics rarely develop a febrile UTI.92,93 If significant hydroureteronephrosis is found, it is assumed that there is significant urinary tract obstruction and antibiotics should be initiated.
The usual treatment of duplex ectopic ureteroceles has been upper-pole heminephrectomy through a separate flank incision, ureterocele excision, and ipsilateral lower-pole ureteral reimplant via a lower incision. The bladder-level operation may require repair of a sizable defect in the bladder base and tapering or plication of the lower ureter. The distal extent of the ureterocele can often be dissected through the bladder neck. Incomplete excision may result in an obstructing urethral flap. Also, resection of the entire ureterocele risks damaging the continence mechanisms of the bladder neck. Experienced surgeons report excellent results with low rates of reoperation (<10%) and low complication rates (<10%).96–98 These approaches assume that ureterocele excision is an essential component of management. However, because the distal ureter and bladder defect may not cause symptoms after decompression, an absolute indication to proceed with a simultaneous bladder operation is rarely present. In older children, when absence of function is noted on the affected side (upper and lower pole), nephroureterectomy is the preferred option.
Another option is upper-pole partial nephroureterectomy that avoids bladder-level reconstruction and its potential risks.91,99 Nearly all of the ureter can be removed either through a flank incision or laparoscopically. The need for subsequent bladder-level excision and reconstruction varies between 10–62%, and is largely dependent on the presence of reflux.91,93,99
Most partial nephrectomy specimens show dysplasia, but some show only inflammatory and obstructive changes.99,100 In patients with preserved function, a pyeloureterostomy or ureteroureterostomy (high or low) may be performed, along with distal ureterectomy and ureterocele decompression.82
Ureterocele incision is the least invasive technique for upper pole preservation (Fig. 54-18). Using a 3 French Bugbee electrode to incise the ureterocele just above the bladder neck is the recommended approach because reflux does not always develop (see Fig. 54-18).92 Endoscopic incision successfully decompresses the ureterocele most of the time.92,100–103 It is the definitive procedure in more than 90% of infants with intravesical ureteroceles. However, subsequent reconstructive surgery may be needed in 50–90% of patients with ectopic ureteroceles. Reflux into the ureterocele moiety is the most common indication for reconstruction in these infants. Previous decompression of the system makes this reconstruction easier.93 For an infected system, ‘unroofing’ of the ureterocele is advocated only as an initial drainage procedure prior to the definitive operation because it invariably results in reflux.103,104
References
1. Dicke, JM, Blanco, VM, Yan, Y, et al. The type and frequency of fetal renal disorders and management of renal pelvis dilation. J Ultrasound Med. 2006; 25:973–977.
2. Lee, RS, Cendron, M, Kinnamon, DD, et al. Antenatal hydronephrosis as a predictor of postnatal outcome: A meta-analysis. Pediatrics. 2006; 118:586–593.
3. Hubert, KC, Palmer, JS. Current diagnosis and management of fetal genitourinary abnormalities. Urol Clin North Am. 2007; 34:89–101.
4. Capello, SA, Kogan, BA, Giorgi, LJ, et al. Prenatal ultrasound has led to earlier detection and repair of ureteropelvic junction obstruction. J Urol. 2005; 174:1425–1428.
5. Carr, MC, El-Ghoneimi, A. Anomalies and surgery of the ureteropelvic junction in children. In: Wein AJ, Kavoussi LR, Novick AC, eds. Campbell-Walsh Urology. 9th ed. Philadelphia: WB Saunders; 2007:3359–3382.
6. Ruano-Gil, D, Coca-Payeras, A, Tejedo-Mateu, A. Obstruction and normal recanalization of the ureter in the human embryo: Its relation to congenital ureteric obstruction. Eur Urol. 1975; 1:287–293.
7. Arams, HJ, Buchbinder, ME, Sutton, AP. Benign ureteral lesions: Rare causes of hydronephrosis in children. Urology. 1977; 9:517–520.
8. Stephens, FD. Ureterovascular hydronephrosis and the ‘aberrant’ renal vessels. J Urol. 1982; 128:984–987.
9. Koff, SA, Hayden, LJ, Cirulli, C, et al. Pathophysiology of UPJ obstruction: Experimental and clinical observations. J Urol. 1986; 136:336–338.
10. Starr, NT, Maizels, M, Chou, P, et al. Microanatomy and morphometry of the hydronephrotic ‘obstructed’ renal pelvis in asymptomatic infants. J Urol. 1992; 148:519–524.
11. Hanna, MK, Jeffs, RD, Sturgess, JM, et al. Ureteral structure and ultrastructure: II. Congenital UPJ obstruction and primary obstructive megaureter. J Urol. 1976; 116:725–730.
12. Kjurhuus, JC, Nerstrom, B, Gyrd-Hansen, N, et al. Experimental hydronephrosis: An electrophysiologic investigation before and after release of obstruction. Acta Chir Scand Suppl. 1976; 472:1728.
13. Wang, Y, Puri, P, Hassan, J, et al. Abnormal innervation and altered nerve growth factor messenger ribonucleic acid expression in ureteropelvic junction obstruction. J Urol. 1995; 154:679–683.
14. Hollowell, JG, Altman, HG, Snyder, HM, III., et al. Coexisting UPJ obstruction and vesicoureteral reflux: Diagnostic and therapeutic implications. J Urol. 1989; 142:490–493.
15. Coplen, DE, Austin, PF, Yan, Y, et al. Correlation of prenatal and postnatal ultrasound findings with the incidence of vesicoureteral reflux in children with fetal renal pelvic dilatation. J Urol. 2008; 180:1631–1634.
16. Nguyen, HT, Herndon, CD, Cooper, C, et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. Journal of Pediatric Urology. 2010; 6:212–231.
17. Islek, A, Güven, AG, Koyun, M, et al. Probability of urinary tract infection in infants with ureteropelvic junction obstruction: Is antibiotic prophylaxis really needed? Pediatric Nephrology. 2011; 26:1837–1841.
18. Heyman, S, Duckett, JW. Extraction factor: An estimate of single kidney function in children during routine radionucleotide renography with 99m technetium diethylenetriamine pentaacetic acid. J Urol. 1988; 140:780–783.
19. Chung, S, Majd, M, Rushton, HG, et al. Diuretic renography in the evaluation of neonatal hydronephrosis: Is it reliable? J Urol. 1993; 150:765–768.
20. Conway, JJ. ‘Well-tempered’ diuresis renography: Its historical development, physiological and technical pitfalls and standardized technique protocol. Semin Nucl Med. 1992; 22:74–84.
21. Grattan-Smith, JD, Little, SB, Jones, RA. MR urography evaluation of obstructive uropathy. Pediatric Radiology. 2008; 38(Suppl 1):S49–S69.
22. Veenboer, PW, de Jong, TP. Antegrade pressure measurement as a tool in modern pediatric urology. World J Urol. 2011; 29:737–741.
23. Rushton, HG. Pediatric pyeloplasty: Is routine retrograde pyelography necessary? J Urol. 1994; 152:604–606.
24. Palmer, LS, Maizels, M, Cartwright, PC, et al. Surgery versus observation for managing obstructive grade 3 to 4 unilateral hydronephrosis: A report from the Society for Fetal Urology. J Urol. 1988; 159:222–228.
25. Ulman, I, Jayanthi, VR, Koff, SA. The long-term follow-up of newborns with severe unilateral hydronephrosis initially treated nonoperatively. J Urol. 2000; 164:1101–1105.
26. Yiee, J, Wilcox, D. Management of fetal hydronephrosis. Pediatr Nephrol. 2008; 23:347–353.
27. Cartwright, PC, Duckett, JW, Keating, MA, et al. managing apparent ureteropelvic junction obstruction in the newborn. J Urol. 1992; 148:122–124.
28. Foley, FE. A new plastic operation for stricture at the UPJ: Report of 20 cases. J Urol. 1937; 38:643.
29. Culp, OS, DeWeerd, JH. Pelvic flap operation for certain types of ureteropelvic obstruction. Mayo Clin Proc. 1951; 26:483–488.
30. Austin, PF, Cain, MP, Rink, RC. Nephrostomy tube drainage with pyeloplasty: Is it necessarily a bad choice? J Urol. 2001; 57:338–341.
31. Casale, P, Mucksavage, P, Resnick, M, et al. Robotic ureterocalicostomy in the pediatric population. J Urol. 2008.
32. Tanaka, ST, Grantham, JA, Thomas, JC, et al. A comparison of open vs. laparoscopic pediatric pyeloplasty using the pediatric health information system database-do benefits of laparoscopic approach recede at younger ages. Journal of Urology. 2008; 180:1479–1485.
33. Duckett, JW, Gibbons, MD, Cromie, WJ. An anterior extraperitoneal muscle-splitting approach for pediatric renal surgery. J Urol. 1980; 123:79–80.
34. Orland, SM, Snyder, HM, Duckett, JW. The dorsal lumbotomy incision in pediatric urological surgery. J Urol. 1987; 138:963–966.
35. Lindgren, BW, Hagerty, J, Meyer, T, et al. Robot-Assisted laparoscopic reoperative repair for failed pyeloplasty in children: Safe and highly effective treatment option. J Urology. 2012; 188:932–938.
36. Nakada, SY, Johnson, M. Ureteropelvic junction obstruction: Retrograde endopyelotomy. Urol Clin North Am. 2000; 27:677–684.
37. Streem, SB. Percutaneous endopyelotomy. Urol Clin North Am. 2000; 27:685–693.
38. Kim, EH, Tanagho, YS, Traxel, EJ, et al. Endopyelotomy for pediatric ureteropelvic junction obstruction. A review of our 25-year experience. J Urol. 188, 2012.
39. Peters, CA, Schlussel, RN, Retik, AB. Pediatric laparoscopic dismembered pyeloplasty. J Urol. 1995; 153:1962–1965.
40. Tan, HL. Laparoscopic Anderson-Hynes dismembered pyeloplasty in children. J Urol. 1999; 162:1045–1048.
41. Cascio, S, Tien, A, Chee, W. Laparoscopic dismembered pyeloplasty in children younger than 2 years. J Urol. 2007; 177:335–338.
42. Minnillo, BJ, Cruz, JA, Sayao, RH, et al. Long-term experience and outcomes of robotic assisted laparoscopic pyeloplasty in children and young adults. J Urology. 2011; 185:1455–1460.
43. Lam, PN, Wong, C, Mulholland, TL, et al. Pediatric laparoscopic pyeloplasty: 4-year experience. J Endourol. 2007; 21:1467–1471.
44. Ossandon, F, Androulakakis, P, Ransley, PG. Surgical problems in pelviureteral junction obstruction of the lower pole moiety in incomplete duplex systems. J Urol. 1981; 125:871–872.
45. Mackie, GG, Awang, H, Stephens, FD. The ureteric orifice: The embryologic key to radiologic status of the kidneys. J Pediatr Surg. 1975; 10:473–481.
46. Schlussel, RN, Retik, AB. Ectopic ureter, ureterocele and other anomalies of the ureter. In: Wein AJ, Kavoussi LR, Novick AC, et al, eds. Campbell-Walsh Urology. 9th ed. Philadelphia: WB Saunders; 2007:3383–3387.
47. Klauber, GT, Reid, EC. Inverted Y reduplication of the ureter. J Urol. 1972; 107:362–364.
48. Meyer, R. Normal and abnormal development of the ureter in the human embryo: A mechanistic consideration. Anat Rec. 1946; 96:355.
49. Fehrenbaker, LG, Kelalis, PP, Stickler, GB. Vesicoureteral reflux and ureteral duplication in children. J Urol. 1972; 107:862–864.
50. Barrett, DM, Malek, RS, Kelalis, PP. Problems and solutions in surgical treatment of 100 consecutive ureteral duplications in children. J Urol. 1975; 114:126–130.
51. Shelfo, SW, Keller, MS, Weiss, RM. Ipsilateral pyeloureterostomy for managing lower-pole reflux with associated ureteropelvic junction obstruction in duplex systems. J Urol. 1997; 157:1420–1422.
52. Kohri, K, Nagai, N, Kaneko, S, et al. Bilateral trifid ureters associated with fused kidney, ureterovesical stenosis, left cryptorchidism and angioma of the bladder. J Urol. 1978; 120:249–250.
53. Zaontz, MR, Maizels, M. Type I ureteral triplication: An extension of the Weigert-Meyer law. J Urol. 1985; 134:949–950.
54. Finkel, LI, Watts, FB, Cobrett, DP. Ureteral triplication with a ureterocele. Pediatr Radiol. 1983; 13:346–348.
55. Soderdahl, DW, Shiraki, IW, Schamber, DT. Bilateral ureteral quadruplication. J Urol. 1976; 116:255–256.
56. Considine, J. Retrocaval ureter. Br J Urol. 1966; 38:412–423.
57. Hollinshead, WH. Anatomy for Surgeons, vol 2, 3rd ed. Philadelphia: Harper & Row; 1982.
58. Zhang, XD, Hou, SK, Zhu, JH, et al. Diagnosis and treatment of retrocaval ureter. Eur Urol. 1990; 18:207–210.
59. Hellstrom, M, Hjalmas, K, Jacobsson, B, et al. Normal ureteral diameter in infancy and childhood. Acta Radiol. 1985; 26:433–439.
60. Khoury, AE, Bagli, DJ. Reflux and megaureter. In: Kavoussi LR, Novick AC, Partin AW, et al, eds. Campbell-Walsh Urology. 9th ed. Philadelphia: WB Saunders; 2007:3423–3481.
61. Leibowitz, S, Bodian, M. A study of the vesical ganglia in children and their relationship to the megaureter megacystis syndrome and Hirschsprung’s disease. J Clin Pathol. 1963; 16:342–350.
62. Tanagho, EA. Embryologic basis for lower ureteral anomalies: A hypothesis. Urology. 1976; 7:451–464.
63. Boyd, SD, Raz, S, Ehrlich, RM. Diabetes insipidus and nonobstructive dilation of urinary tract. Urology. 1980; 16:266–269.
64. Kass, EJ, Silver, TM, Konnak, JW, et al. The urographic findings in acute pyelonephritis: Non-obstructive hydronephrosis. J Urol. 1976; 116:544–546.
65. Mollard, P, Foray, P, De Godoy, JL, et al. Management of primary obstructive megaureter without reflux in neonates. Eur Urol. 1993; 24:505–510.
66. Cozzi, F, Madonna, L, Maggi, E, et al. Management of primary megaureter in infancy. J Pediatr Surg. 1993; 28:1031–1033.
67. Keating, MA, Escala, J, Snyder, HM, et al. Changing concepts in management of primary obstructive megaureter. J Urol. 1989; 142:636–640.
68. Baskin, LS, Zderic, SA, Snyder, HM, et al. Primary dilated megaureter: Long-term follow-up. J Urol. 1994; 152:618–621.
69. Hendren, WH. Operative repair of megaureter in children. J Urol. 1969; 101:491–507.
70. Hendren, WH. Commentary: Surgery of megaureter. In: Whitehead D, Leiter E, eds. Current Operative Urology. Philadelphia: Harper & Row; 1984:473–482.
71. Fretz, PC, Austin, JC, Cooper, CS. Long-term outcome analysis of Starr plication for primary obstructive megaureters. J Urol. 2004; 172:703–705.
72. Perdzynski, W, Kalicinski, ZH. Long-term results after megaureter folding in children. J Pediatr Surg. 1996; 31:1211–1217.
73. McLorie, GA, Jayanthi, VR, Kinahan, TJ, et al. A modified extravesical technique for megaureter repair. Br J Urol. 1994; 74:715–719.
74. Peters, CA, Mandell, J, Lebowitz, RL, et al. Congenital obstructed megaureters in early infancy: Diagnosis and treatment. J Urol. 1989; 142:641–645.
75. Christman, MS, Kasturi, S, Lambert, SM, et al. Endoscopic management and the role of double stenting for primary obstructive megaureters. J Urol. 2012; 187:1018–1022.
76. Garcia-Aparicio, L, Rodo, J, Krauel, L, et al. High pressure balloon dilation of the ureterovesical junction—first line approach to treat primary obstructive megaureter? J Urol. 2012; 187:1834–1838.
77. Schulman, CC. Les implantations ectopiques de l’uretère. Acta Urol Belg. 1972; 40:201–478.
78. Johnston, JH, Davenport, TJ. The single ectopic ureter. Br J Urol. 1969; 41:428–433.
79. Kreissl, MC, Lorenz, R, Ohnheiser, G, et al. Dystopic dysplastic kidney with ectopic ureter: Improved localization by fusion of MR urography and (99m)Tc-DMSA SPECT datasets. Pediatr Radiol. 2008; 38:241–244.
80. Wyly, JB, Lebowitz, RL. Refluxing urethral ectopic ureters: Diagnosis by the cyclic voiding cystourethrogram. AJR Am J Roentgenol. 1984; 142:1263–1267.
81. Berrocal, T, Lopez-Pereira, P, Arjonilla, A, et al. Anomalies of the distal ureter, bladder, and urethra in children: Embryologic, radiologic, and pathologic features. Radiographics. 2002; 22:1139–1164.
82. Lashley, DB, McAleer, IM, Kaplan, GW. Ipsilateral ureteroureterostomy for the treatment of vesicoureteral reflux or obstruction associated with complete duplication. J Urol. 2001; 165:552–554.
83. Plaire, JC, Pope, JC, Kropp, BP, et al. Management of ectopic ureters: Experience with upper tract approach. J Urol. 1997; 158:1245–1247.
84. Lashley, DB, McAleer, IM, Kaplan, GW. Ipsilateral ureteroureterostomy for the treatment of vesicoureteral reflux or obstruction associated with complete ureteral duplication. J Urol. 2001; 165:552–554.
85. Prieto, J, Ziada, A, Baker, L, Snodgrass, W. Ureteroureterostomy via inguinal incision for ectopic ureters and ureteroceles without ipsilateral lower pole reflux. J Urol. 2009; 181:1844–1848.
86. Storm, DW, Modi, A, Jayanthi, VR. Laparoscopic ipsilateral ureteroureterostomy in the management of ureteral ectopia in infants and children. J Pediatr Urol. 2011; 7:529–533.
87. Noseworthy, J, Persky, L. Spectrum of bilateral ureteral ectopia. Urology. 1982; 19:489–494.
88. Chwalla, R. The process of formation of cystic dilations of the vesical end of the ureter and of diverticula at the ureteral ostium. Urol Cutan Rev. 1927; 31:499.
89. Coplen, DE, Austin, PF. Outcome of prenatally detected ureteroceles associated with multicystic dysplasia. J Urol. 2004; 172:1662–1665.
90. Glassberg, KI, Braren, V, Duckett, JW, et al. Suggested terminology for duplex systems, ectopic ureters and ureteroceles. J Urol. 1984; 132:1153–1154.
91. Caldamone, AA, Snyder, HM, Duckett, JW. Ureteroceles in children: Follow-up of management with upper tract approach. J Urol. 1984; 131:1130–1132.
92. Cooper, CS, Passerini-Glazel, G, Hutcheson, JC, et al. Long-term follow-up of endoscopic incision of ureteroceles: Intravesical versus extravesical. J Urol. 2000; 164:1097–1100.
93. Husmann, DA, Ewalt, DH, Glenski, WJ, et al. Ureterocele associated with ureteral duplication and nonfunctioning upper pole segment: Management by partial nephroureterectomy alone. J Urol. 1995; 154:723–726.
94. Sen, S, Beasley, SW, Ahmed, S, et al. Renal function and vesicoureteric reflux in children with ureteroceles. Pediatr Surg Int. 1992; 7:192–194.
95. Sumfest, JM, Burns, MW, Mitchell, ME. Pseudoureterocele: Potential for misdiagnosis of an ectopic ureter as a ureterocele. Br J Urol. 1995; 75:401–405.
96. Coplen, DE, Barthold, JS. Controversies in the management of ectopic ureteroceles. Urology. 2000; 56:665–668.
97. Scherz, HC, Kaplan, GW, Packer, MG, et al. Ectopic ureteroceles: Surgical management with preservation of continence: Review of 60 cases. J Urol. 1989; 142:538–541.
98. Shekarriz, B, Upadhyay, J, Fleming, P, et al. Long-term outcome based on the initial surgical approach to ureterocele. J Urol. 1999; 162:1072–1076.
99. Rickwood, AM, Reiner, I, Jones, M, et al. Current management of duplex-system ureteroceles: Experience with 41 patients. Br J Urol. 1992; 70:196–200.
100. Tank, ES. Experience with endoscopic incision and open unroofing of ureteroceles. J Urol. 1986; 136:241–242.
101. Hagg, MJ, Mourachov, PV, Snyder, HM, et al. The modern endoscopic approach to ureterocele. J Urol. 2000; 163:940–943.
102. Husmann, D, Strand, B, Ewalt, D, et al. Management of ectopic ureterocele associated with renal duplication: A comparison of partial nephrectomy and endoscopic decompression. J Urol. 1999; 162:1406–1409.
103. Monfort, G, Guys, JM, Coquet, M, et al. Surgical management of duplex ureteroceles. J Pediatr Surg. 1992; 27:634–638.
104. Snyder, HM, Johnston, JH. Orthotopic ureteroceles in children. J Urol. 1978; 119:543–546.