CHAPTER 22 Upper gastrointestinal and colorectal stenting
PART 1 UPPER GI TRACT STENTING
Background
Less than 10% of patients with gastro-esophageal cancer survive 5 years (Lee, 2001). Oral intake of nutrition is vital in maintaining quality of life for these patients, particularly when considered by the multidisciplinary team (MDT) to be suitable for palliative treatment only. The development of significant dysphagia to solids and, more particularly, liquids as a result of occlusive neoplastic stricturing requires palliation that is effective and minimally invasive (Box 22.1). In this section, it is intended to review upper gastrointestinal (GI) tract stenting.
History
Historically, palliation of dysphagia resulting from occlusive malignancy has required surgical intervention. Celestin (1959) described a traction technique that required endoscopic guidance of an introducer and a mini laparotomy to gain entry high in the stomach; this enabled the tube (Figure 22.1) to be pulled through. Subsequently, the Nottingham introducer precluded the requirement for a laparotomy.
These intubation procedures took less than an hour to perform but required a general anesthetic and hospitalization of between three and ten days (Celestin, 1959; Davis, 1972; Bancewicz, 1979; Unruh and Pagliero, 1985; Kratz et al., 1989; Dunn and Rawlinson, 1992).
Self-expanding metal stent (SEMS) structure
The vast majority of upper GI SEMS insertions are associated with neoplastic occlusion or tracheo-esophageal fistula. SEMS have also been used for benign conditions, such as achalasia, where other management options, such as myotomy, balloon dilatation or the injection of botulinum toxin, have failed. In a number of papers, the use of stents is not recommended for benign strictures (De Palma et al., 2001). Ackroyd (2001) suggests that SEMS should be used for benign strictures with caution as they can make matters worse, potentially making a stricture inoperable. The indications for upper GI SEMS insertion are given in Box 22.2.
Removable SEMS
If a temporary stent is being considered, subsequent removal is undertaken endoscopically (Nam et al., 2006; Shin et al., 2008). Removable stents have a lasso woven into their proximal end. Under endoscopic control, forceps are used to grasp the lasso, tension will draw the proximal end of the stent closed and away from the esophageal wall. Maintaining hold of the lasso, the scope and forceps are then drawn out together with the stent.
Approaches to esophageal and gastro-esophageal SEMS insertion
SEMS provide a significant benefit in palliating malignant dysphagia, but there is broad discussion as to the best method to employ for their insertion. The literature suggests a bias towards a joint endoscopic/fluoroscopic approach to SEMS insertion. Ramirez et al. (1997) reported that 83% of those members of the American Society of Gastrointestinal Endoscopy (ASGE) who responded to a survey used a combined endoscopic/fluoroscopic approach for the deployment of SEMS. Described by Sarper et al. (2003) and others, the joint approach uses endoscopy to pass the guide wire across the stricture, the subsequent stent deployment being undertaken using fluoroscopic guidance. Singhvi et al. (2000) suggest fluoroscopy is not necessary, advocating the use of a thin gastroscope, possibly in conjunction with a dilator such as the Savrey buginage, to assist with tight strictures. The routine use of general anesthetic for endoscopic SEMS insertion has also been reported (Singhvi et al., 2000; Sarper et al., 2003; Sabharwal et al., 2003; Soussan et al., 2005a, 2005b). The literature, however, also supports the use of fluoroscopy alone for deployment (Laasch et al., 2002; Saranovic et al., 2005; Law, 2008). The technical and clinical success rates for the deployment of esophageal SEMS are reported as similar whether using an endoscopic/fluoroscopic technique or fluoroscopic alone (Petruzziello and Costamagna, 2002).
Some units use endoscopy alone for the insertion of SEMS, but the advantages of this approach are limited. It can be used where there is limited access to fluoroscopy (Rathore et al., 2006). Singhvi et al. (2000) report using endoscopy alone as being a quick procedure (15 minutes) and resorting to the use of fluoroscopy is not routinely needed.
Fluoroscopically guided SEMS insertion: technique
Fine bore intubation is a natural skill progression for GI radiographers or radiologic technologists experienced in demonstrating esophageal pathology. (The techniques and benefits of technician performed fine bore intubation are described as a service development in Chapter 11.) In performing complicated fluoroscopically guided naso/orogastric intubation, GI radiographers and radiologic technologists are ideally placed to be involved in a joint approach with a clinician (gastroenterologist, surgeon or radiologist) in performing the first part of SEMS deployment by transgressing the esophageal lumen strictured by tumor (Law, 2008). Suggested protocol inclusions prior to upper GI SEMS insertion are offered in Box 22.3.
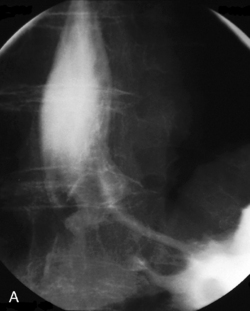
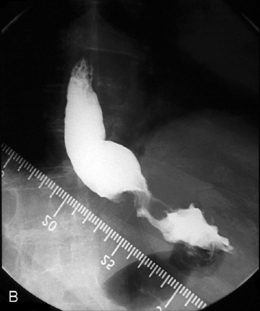
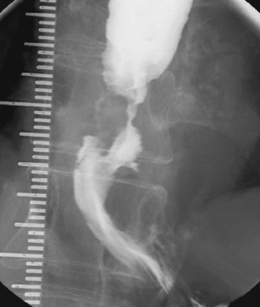
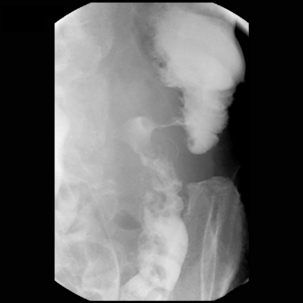
The barium swallow
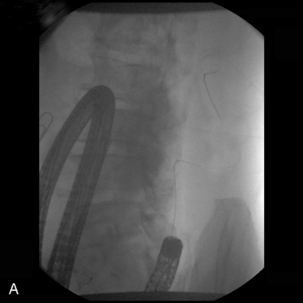
Intubation across the stricture
High esophageal stricture
Technically, there is nothing to preclude fluoroscopically guided stenting of malignant strictures close to the upper esophageal sphincter. Verschuur et al. (2007), in reporting the safety and effectiveness of stenting high in the esophagus, note that its limitations can include patient intolerance due to pain or globus sensation. Sihoe et al. (2004) reported that, due to the close proximity of the trachea and the upper esophagus, consideration should be given to pre-empt airway stenosis or fistulation by inserting a tracheal stent prior to the insertion of a high esophageal stent. Wyn et al. (2006), although supporting this premise, suggest that airway compression is rare.
Gastric and duodenal SEMS
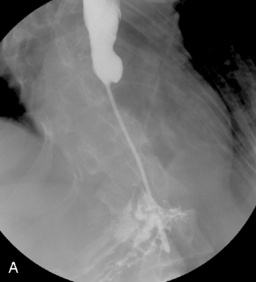
As a precursor to SEMS insertion, location and length of the stricture can be demonstrated fluoroscopically using a water-soluble contrast medium such as Ultravist 240 (Schering Health Care Ltd).
With the patient lying on their right side, the contrast pooling (whether in the stomach or duodenum) has the optimum chance of outlining the strictured lumen (Aviv et al., 2002; Lowe et al., 2007; Van Hooft et al., 2007; Hosono et al., 2007).
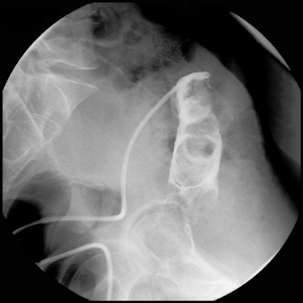
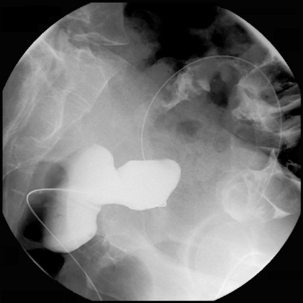
Technical considerations
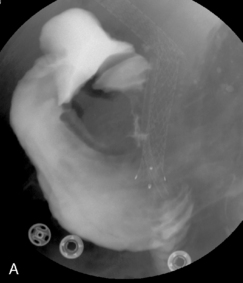
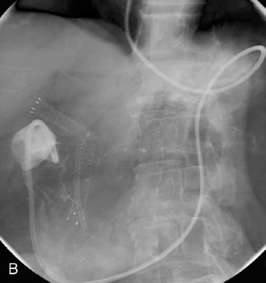
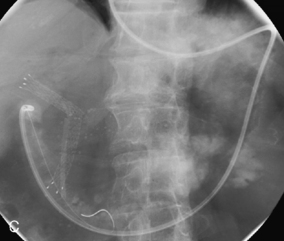
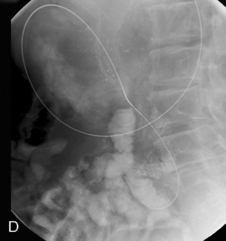
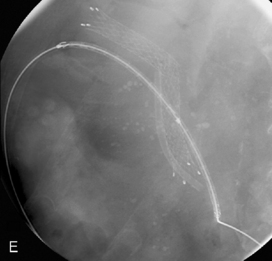
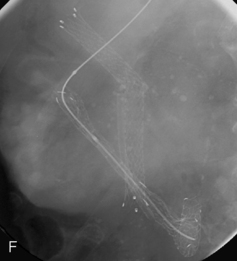
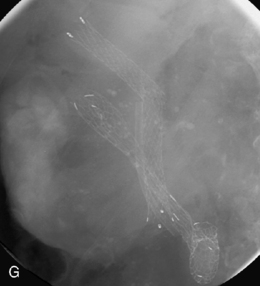
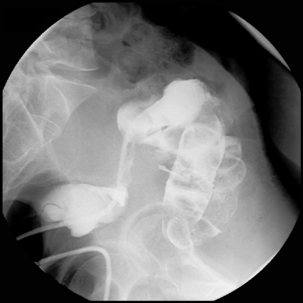
Figure 22.7 (A) Water-soluble contrast outlining stricture in 2nd part of the duodenum. Metal and plastic stent in the common bile duct; (B) 8fg fine bore tube placed at the proximal origin of stricture; (C) guide wire passed across stricture; (D) guide wire passed into jejunum; (E) stent delivery system passed over guide wire; (F) stent deployed; (G) delivery system withdrawn.
Patient after-care post upper GI SEMS insertion
The patient experience
The most common experience the patient can expect, particularly in the immediate post SEMS insertion period, is focal chest pain. Pain is caused by the radial force of the stent as it opens. Staying in hospital overnight will allow for monitoring and appropriate analgesia to provide pain relief. Complications are not infrequent. Although not related to large patient groups, literature has reported early complication rates of 13% and late complication rate of 20% (Aviv et al., 2002). A complication rate of 44% has been reported for SEMS placement for distal esophageal tumor stenoses (Sarper et al., 2003). Major complication rates of 37% have also been reported (Ross et al., 2007). Complications are given in Table 22.1.
| During procedure | Post procedure |
| Cardiorespiratory distress | Misplacement |
| Reflux | Perforation |
| Aspiration | Reflux |
| Failed deployment | Aspiration |
| Failed expansion | |
| Migration | |
| Delayed complications | Iatrogenic complications |
| Migration | Aspiration |
| Tumor overgrowth | Fistula formation |
| Food impaction | Sepsis |
| Bleeding | Respiratory failure |
PART 2 COLORECTAL SELF-EXPANDING METAL STENTS (SEMS)
Introduction
About 20 000 people die in the UK each year from colorectal malignancy (Starkey, 2002; Steele, 2006). Currently, most incidents of colorectal cancer occur in patients in their seventh and eighth decades of life when other comorbidities, such as vascular disease, may limit treatment options. Most patients with colorectal cancer present with colonic symptoms (see Chapter 10), while others may present with disease as a consequence of metastatic spread. A significant number of patients with colorectal cancer present with acute large bowel obstruction (Stipa et al., 2008) and it is in this group of patients where colonic stenting is particularly appropriate (Alcantara et al., 2007; Farrell, 2007; Dionigi et al., 2007).
Most colorectal cancers which cause intestinal obstruction are on the left side of the colon, where the diameter of the colon is less and the consistency of feces is harder. It has been hypothesized that the relatively high mortality of urgent surgery can be avoided by placing a stent across the obstructing tumor and allowing the colon to decompress and the patient to return to a normal physiological state before elective surgery is undertaken. This, it is believed, returns the patient to the risk level for morbidity and mortality associated with elective surgery. Additionally, the use of colorectal stenting in the emergency situation might simplify the type of surgery. In an acutely obstructed patient, it may be necessary to perform colostomy to decompress the colon, closing the distal colonic stump. In some circumstances, it may not even be possible to remove the primary tumor at this time. The patient is then left with a colostomy and probably the need for further surgery at some time in the future when the urgent situation has passed. If a stent is placed and elective surgery is undertaken, then a primary colocolic anastomosis may be undertaken and the tumor removed, thereby improving the quality of the patient’s existence and reducing the need for second surgery. Under some circumstances, for example, where the patient is not fit even for elective surgery or has extensive metastatic disease, colonic stenting may be definitive therapy in the acutely obstructed patient, thereby avoiding the need for potentially futile surgery (Athreya et al., 2006; Soto et al., 2006; Small et al., 2008).
Other indications
While the majority of colorectal stenting is undertaken for the obstructed patient with colonic cancer, other indications exist. Colonic strictures from diverticular disease or from Crohn’s disease may be treated with stenting when the patient is too unfit for the surgical option. The presence of a fistula between the colon and other structures, such as the bladder, small bowel and vagina, can lead to extremely debilitating symptoms for the patient and may be effectively treated by stenting (Laasch et al., 2003; Small et al., 2008).
Patient preparation
For the patient who presents with acute colonic obstruction, full clinical biochemical, hematological and radiological evaluation is important to reduce risk. Clinical assessment is important to determine the presence of comorbidities, particularly cardiac and respiratory disease. Hematological assessment is necessary because the patient may be anemic as a consequence of bleeding from the tumor and this may need correcting. Biochemical abnormality may be present because of dehydration, vomiting and renal failure. These deficiencies need to be corrected. It is rare that colorectal stenting is required so urgently that the patient cannot be adequately resuscitated from a hematological and biochemical standpoint prior to stenting. While radiological evaluation is most commonly undertaken using a plain film of the abdomen to determine the presence of obstruction, CT of the abdomen at least is essential. It may be valuable to add a CT of the chest in order to determine the presence of metastatic disease.
Stent placement technique
Colonoscopy or not?
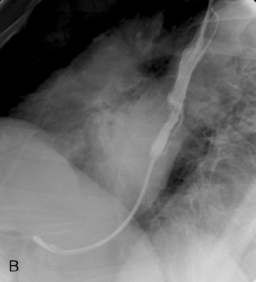
It is generally true to say, with upper GI or lower GI enteral stenting, that the further the lesion from the point of access, the more appropriate it is to use endoscopy. Obstructing tumors in the rectosigmoid can usually be traversed and stented using fluoroscopic guidance alone, without colonoscopy (Athreya et al., 2006). However, with more proximal tumors, in the descending colon up to the transverse colon, colonoscopy has a definite advantage in that it allows more rapid access to the tumor and therefore reduces radiation dose to the patient, but most importantly to staff (Figures 22.8,22.9A,22.9B).
Fluoroscopic stent placement technique
It is appropriate to have a variety of hydrophilic catheters and guide wires to hand. With the patient in the left lateral position, a catheter (such as the Headhunter 1, Bernstein or Law 2) loaded with a guide wire is inserted into the rectum and negotiated slowly up to the level of the tumor. This is generally not difficult but can be time consuming. Once arrival at the distal margin of the tumor is achieved, it may be appropriate to inject water-soluble contrast in order to outline the tumor (Figure 22.10).
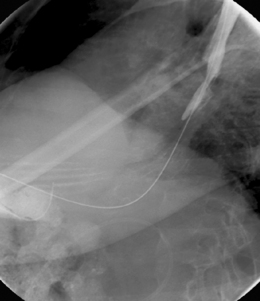
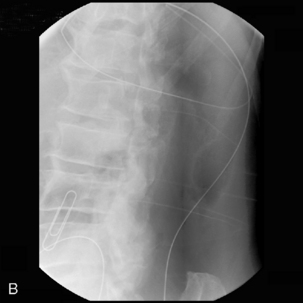
The catheter and guide wire can then be negotiated across the tumor into the dilated colon above (Figures 22.11 and 22.12). This can occasionally be very difficult or impossible and there is a variety of tricks to improve success. Access across the tumor is most easily achieved using a standard non-stiff, straight tipped, hydrophilic guide wire. Occasionally, stiff or angled wires may be helpful. A change of catheter should be considered if difficulty is encountered. The more curved (cobra) or even straight catheters may be successful when the primary choice of catheter fails. Intravenous Buscopan to reduce peristalsis may help, as might insufflation of the rectum and distal colon with gas. The placement of a stiffening sheath into the rectum and sigmoid can help to prevent tube looping, but this also removes the effectiveness of the anal sphincter in maintaining continence.
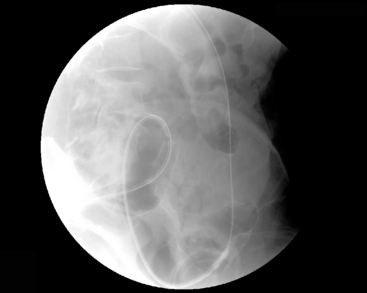
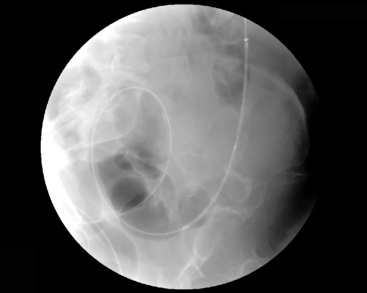
If the proximal margin of the tumor has not been outlined, with the catheter passed through the tumor lumen into dilated colon, the guide wire can be removed and a water-soluble contrast infused. A relatively long, relatively stiff, nitinol plastic coated guide wire should then be passed into the proximal segments of colon. As much wire as possible should be inserted beyond the stricture (Figure 22.13). It may be necessary to allow a soft guide wire to loop its passage through the stricture (Figure 22.14). However, depending on the configuration of the colon, the length of wire passed across the stricture may, by force of circumstances, be short (Figure 22.15).
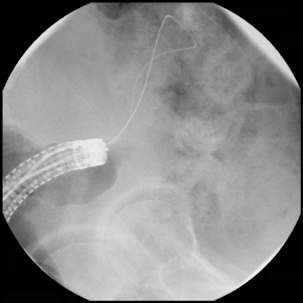
Figure 22.14 Using joint colonoscopic/fluoroscopic guidance a soft wire is allowed to loop to pass across the strictured lumen.
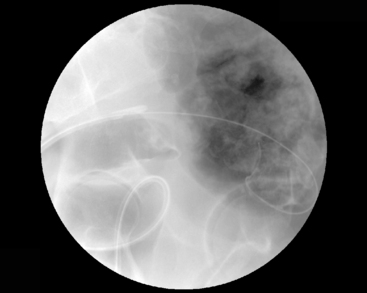
Figure 22.15 Maximum position of guide wire due to a tight loop of bowel immediately proximal to the tumor site.
If the delivery system does not easily pass through the stricture due to the tightness or rigidity of the lumen, pulling the wire to place it under tension can assist in railroading the delivery system into place. If the wire is to be placed under tension, the amount of free wire across the stricture must be very closely monitored as the tension will draw the wire out. Should the amount of wire extracted give concern, stop the advance of the delivery system and reinsert the wire.
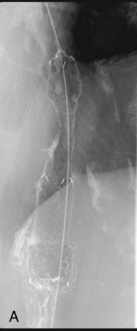
With tumors above the rectosigmoid junction, it is probably best to place the middle of the stent in the middle of the tumor, so that there are equal lengths of stent above and below the tumor (Figure 22.16). It is appropriate to use a length of stent so that at least 2, preferably 3 or 4 cm, of stent lie above and below the margins of the tumor. This allows for any slippage or movement during placement (Figure 22.17).
Stent types
As described for esophageal stents (see Part 1) nitinol or stainless steel stents are available. The most appropriate stent in current use has smooth wire ends and no sharp edges. Stents range from 22 to 30 mm in diameter and up to 14 cm in length. Colorectal stents are most commonly uncovered because of the better bowel wall grip achieved. Tumor in-growth is of less concern in the colon due to the bowel lumen diameter that can be achieved post stenting. However, covered stents may be appropriate in patients with fistula (Laasch et al., 2003; Shin et al., 2008; Small et al., 2008).
Outcomes
Stent placement and technical success is expected in about 90% of cases (Sebastian et al., 2004; Baraza et al., 2008). Failure occurs most commonly because of inability to negotiate either the catheter or colonoscope through the colon up to the level of the tumor. This may be because of tortuosity of the bowel or the presence of diverticular disease or stricture. Failure to negotiate a catheter or guide wire across the stricture may occur because the stricture is very tight (uncommon) or because of an adverse relationship of the lumen of the colon and the lumen of the tumor. Once the guide wire has been placed across the tumor into the proximal colon, failure to place a stent is extremely rare.
Complications
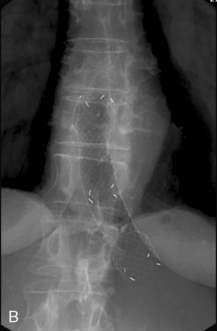
Apart from failure to complete the procedure, complications are few. Perforation of the distal colon or perforation of the tumor can occur and, in some series, appear quite common. If the position markers are not held rigidly in place during SEMS deployment, as the stent opens the radial force can migrate the stent into the more proximal colon (Figure 22.18). Stent migration appears to be less common in the colon than at the gastro-esophageal junction, perhaps a function of the use of uncovered stents more frequently in the colon. In experienced hands, perforation is uncommon. Failure of a technically well placed stent to function adequately is most likely because of impaction of solid feces into the stent and enemas, together with oral stool softeners, may resolve the situation (Athreya et al., 2006; Pericoli Ridolfini et al., 2007; Shin et al., 2008).
Late complications
Stent migration can occur late, but is less common than immediate migration. Stent occlusion is the most common later sequel and is usually due to tumor through-growth, although fecal impaction may occur. Late perforation is reported (Salvi et al., 2004; Pericoli Ridolfini et al., 2007).
Post-stent course
In the majority of patients who undergo colorectal stenting for acute colonic obstruction as a bridge to surgery, further clinical and radiological assessment for the detection of metastatic disease or preliminary treatment with chemotherapy or radiotherapy may be appropriate under certain circumstances. Elective surgery can then be undertaken, the stent being removed with the surgical specimen. There does not appear to be any disadvantage to the surgeon caused by the presence of the stent. Some anxieties have been raised about the possibility that the presence of the stent causes dissemination of tumor cells with a possible increased risk of metastatic liver disease (Maruthachalam et al., 2007), but these concerns have yet to be confirmed.
Summary
Ackroyd R., Watson D.I., Devitt P.G., et al. Expandable metallic stents should not be used in the treatment of benign oesophageal strictures. J. Gastroenterol. Hepatol.. 2001;16(4):484-487.
Adam A., Ellul J., Watkinson A., et al. Palliation of inoperable esophageal carcinoma: a prospective randomized trial of laser therapy and stent placement. Radiology. 1997;202:344-348.
Alcantara M., Serra X., Bombardó J., et al. Colorectal stenting as an effective therapy for preoperative and palliative treatment of large bowel obstruction: 9 years’ experience diseases of the colon and rectum. Tech. Coloproctol.. 2007;11(4):316-322.
Athreya S., Moss J., Urquhart G. Colorectal stenting for colonic obstruction: the indications, complications, effectiveness and outcome – 5 year review. Eur. J Radiol.. 2006;60(1):91-94.
Aviv R.I., Shyamalan G., Khan F.H., et al. Use of stents in the palliative treatment of malignant gastric outlet and duodenal obstruction. Clin. Radiol.. 2002;57(7):587-592.
Bancewicz J. Celestin tubes (letter). Br. Med. J.. 1979;2(6200):1292.
Baraza W., Lee F., Brown S., et al. Combination endo-radiological colorectal stenting: a prospective 5-year clinical evaluation. Colorectal Dis.. 2008. [Epub ahead of print]
Celestin L.R. Permanent intubation in inoperable cancer of the oesophagus and cardia: a new tube. Ann. R. Coll. Surg. Engl.. 1959;25:165-170.
Davis R.S. Insertion of Celestin tubes (letter). Br. Med. J.. 1972;4(5834):235.
De Palma G.D., Iovino P., Masone S., Persico M., et al. Self-expanding metal stents for endoscopic treatment of esophageal achalasia unresponsive to conventional treatments. Long-term results in eight patients. Endoscopy. 2001;33(12):1027-1030.
Dionigi G., Villa F., Rovera F. Colonic stenting for malignant disease: review of literature. Surg. Oncol.. 2007;16(Suppl. 1):S153-S155.
Dunn D.C., Rawlinson N. Surgical diagnosis and management, second ed. Blackwell Scientific Publications, 1992.
Farrell J.J. Preoperative colonic stenting: how when why? Curr. Opin. Gastroenterol.. 2007;23(5):544-549.
Hosono S., Ohtani H., Arimoto Y., et al. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant obstruction: a meta-analysis. J. Gastroenterol.. 2007;42(4):283-290.
Kratz J.M., Reed C.E., Crawford F.A., et al. A comparison of endoesophageal tubes: improved results with Atkinson tube. J. Thorac. Cardiovas. Surg.. 1989;97(1):19-23.
Laasch H., Marriott A., Wilbraham L., et al. Effectiveness of open versus antireflux stents for palliation of distal esophageal carcinoma and prevention of symptomatic gastrointestinal reflux. Radiology. 2002;225(2):359-365.
Laasch H.U., Wilbraham L., Marriott A., Martin D.F. Treatment of colovaginal fistula with coaxial placement of covered and uncovered stents. Endoscopy. 2003;35(12):1081.
Law R.L. Radiographer involvement with stent insertion to palliate symptomatic dysphagia resulting from neoplastic obstruction. Radiography. 2008;14:39-44.
Lee S.H. The role of oesophageal stenting in the non surgical management of oesophageal strictures. Br. J. Radiol.. 2001;74(886):891-900.
Lowe A.S., Bechett C.G., Jowett S., et al. Self-expandable metal stent placement for the palliation of malignant gastroduodenal obstruction: experience in a large UK centre. Clin. Radiol.. 2007;62(8):738-744.
Maruthachalam K., Lash G.E., Shenton B.K., et al. Tumour cell dissemination following endoscopic stent insertion. Br. J. Surg.. 2007;94(9):1151-1154.
Nam D.H., Shin J.H., Song H.Y., et al. Malignant esophageal-tracheobronchial strictures: parallel placement of covered retrievable expandable nitinol stents. Acta Radiologica. 2006;47(1):3-9.
Pericoli Ridolfini M., Sofo L., Di Giorgio A., et al. Late complication after colon self-expandable metal stent placement: a case report. Minerva Chirurgie. 2007;62(1):69-72.
Petruzziello L., Costamagna G. Stenting in oesophageal strictures. Dig. Dis.. 2002;20:154-166.
Quine M.A., Bell G.D. Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut. 1995;36:462-467.
Ramirez F.C., Dennert B., Zierer S.T., et al. Esophageal self expanding metal stents – indications, practice, technique and complications; results of a national survey. Gastrointest. Endosc.. 1997;45(5):360-364.
Rathore O.I., Cross A., Patchett S.E., et al. Direct-vision stenting: the way forward for malignant oesophageal obstruction. Endoscopy. 2006;38(4):382-384.
Ross W.A., Alkassab F., Lynch P.M., et al. Evolving role of self-expanding metal stents in the treatment of malignant dysphagia and fistulas. Gastrointest. Endosc.. 2007;65(1):70-76.
Sabharwal T., Hamady M.S., Chui S., et al. A randomized prospective comparison of the Flamingo wall stent and Ultraflex stent for the palliation of dysphagia associated with lower third oesophageal carcinoma. Gut. 2003;52:922-926.
Salvi P.F., Leone G., Corona F., et al. Colonic perforations after self-expandable metallic stenting: two cases. G. Chir.. 2004;25(6–7):211-216.
Saranovic D.J., Djuric-Stefanovic A., Ivanovic A., et al. Fluoroscopically guided insertion of self expanding metal esophageal stents for palliative treatment of patients with malignant stenosis of esophagus and cardia: comparison of covered and uncovered stent types. Dis. Esoph.. 2005;18(4):230-238.
Sarper A., Oz N., Cihangir C., et al. The efficacy of self expanding metal stents for palliation of malignant esophageal strictures and fistulas. Eur. J. Cardio-thorac. Surg.. 2003;23:794-798.
Sebastian S., Johnston S., Geoghegan T., et al. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am. J. Gastroenterol.. 2004;99(10):2051-2057.
Shin S.J., Kim T.I., Kim B.C., et al. Clinical application of self-expandable metallic stent for treatment of colorectal obstruction caused by extrinsic invasive tumors. Dis. Colon. Rectum.. 2008;51(5):578-583.
Sihoe A.D.L., Wan I.Y.P., Yin A.P., et al. Airway stenting for irresectable esophageal cancer. Surg. Oncol.. 2004;13(1):17-25.
Singhvi R., Abbasakoor F., Manson J.M., et al. Insertion of self-expanding metal stents for malignant dysphagia: assessment of a simple endoscopic method. Ann. R. Coll. Surg. Engl.. 2000;82(4):243-248.
Small A.J., Young-Fadok T.M., Baron T.H. Expandable metal stent placement for benign colorectal obstruction: outcomes for 23 cases. Surg. Endosc.. 2008;22(2):454-462.
Soussan E.B., Antonietti M., Lecleire S., et al. Palliative esophageal stent placement using endoscopic guidance without fluoroscopy. Gastroenterol. Clin. Biol.. 29(8–9), 2005.
Soussan E.B., Antonietti M., LeCleire S. Palliative oesophageal stent. Gastroenterol. Clin. Biol.. 2005;29(8–9):785-788.
Soto S., López-Rosés L., González-Ramírez A. Endoscopic treatment of acute colorectal obstruction with self-expandable metallic stents: experience in a community hospital. Surg. Endosc.. 2006;20(7):1072-1076.
Starkey B.J. Screening for colorectal cancer. Ann. Clin. Biochem.. 2002;39(4):351-365.
Steele R.J. Modern challenges in colorectal cancer. Surgeon. 2006;4(5):285-291.
Stipa F., Pigazzi A., Bascone B., et al. Management of obstructive colorectal cancer with endoscopic stenting followed by single-stage surgery: open or laparoscopic resection? Surg. Endosc.. 2008;22(6):1477-1481.
Teague R.. Clinical practice guidelines update: safety and sedation during endoscopic procedures. British Society of Gastroenterology. 2003. Online http://www.bsg.org.uk
Unruh H.W., Pagliero K.M. Pulsion intubation versus traction intubation for obstructing carcinomas of the esophagus. Ann. Thorac. Surg.. 1985;40(4):337-342.
Van Hooft J., Mutignani M., Repici A. First data on the palliative treatment of patients with malignant gastric outlet obstruction using the Wall-flex enteral stent: a retrospective multicenter study. Endoscopy. 2007;39(5):434-439.
Verschuur S.M., Kuipers E.J., Siersema P.D. Esophageal stents for malignant strictures close to the upper esophageal sphincter. Gastrointest. Endosc.. 2007;66(6):1082-1090.
Wyn D., Shah R., Talwar A. Left main stem airway obstruction after esophageal stent placement for metastatic mediastinal mass. J. Bronchol.. 2006;13(2):72-73.
Yoon C.J., Shin J.H., Song H.Y., et al. Removal of retrievable esophageal and gastrointestinal stents: experience in 113 patients. Am. J. Roentgenol.. 2004;183(5):1437-1444.