Chapter 37 Upper Airway Disease
Rhinitis and Rhinosinusitis
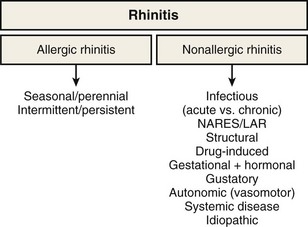
Rhinitis
Rhinitis is defined as the presence of two or more symptoms of nasal discharge (anterior or posterior), blockage with sneeze, or itch for more than 1 hour on most days. It is an umbrella term that encompasses multiple diseases with distinct immunopathogenic mechanisms and correspondingly specific diagnostic and treatment strategies (Figure 37-1). Although rhinitis is subdivided into two broad categories of allergen-induced rhinitis and nonallergic rhinitis, disease overlap is common. Thus, a careful history and directed investigations are required to establish the exact diagnosis. In practice, inflammatory changes usually are continuous from nasal to sinus mucosa (see Figure 37-1); therefore, the designation rhinosinusitis is more accurate, although its use may lead to clinical confusion with the separate group of diseases that are historically classified under sinusitis. Apart from viral colds, allergic rhinitis (AR) is the most common cause of nasal symptoms.
Epidemiology, Risk Factors, and Pathophysiology
Allergic Rhinitis
Up to 30% of adults and 40% of children are affected, and worldwide the prevalence of AR continues to increase (Figure 37-2). The condition has marked effects on quality of life and is responsible for reduced school and workplace attendance (by 3% to 4%) and performance (by 30% to 40%). The resulting economic burden is high, and rhinitis and related AR are common. It is estimated that nearly 500 million people worldwide have AR, and it is one of the most common reasons for attendance with a primary care practitioner.
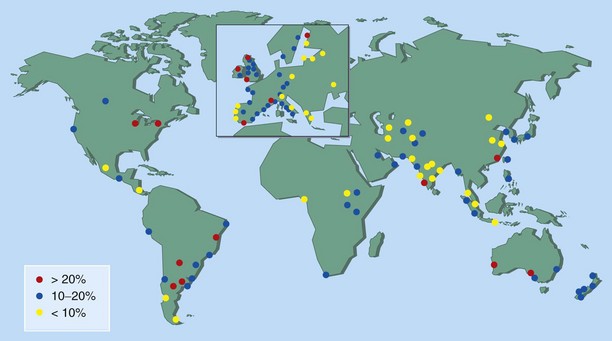
Figure 37-2 Global prevalence of hay fever in 13- to 14-year-olds.
(From Strachan D, Sibbald B, Weiland S, et al: Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood [ISAAC], Pediatr Allergy Immunol 8:161–176, 1997.)
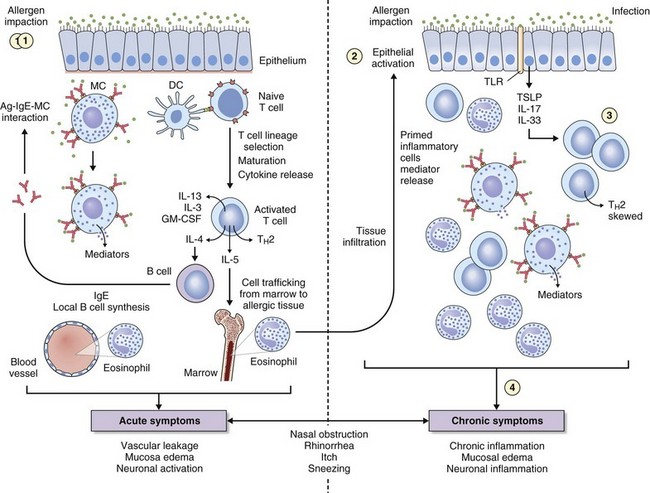
The key immunologic event that initiates AR is binding of allergen to specific IgE on mast cells found in the nasal mucosa. Cross-linking of two or more high-affinity IgE molecules in response to allergen binding leads to mast cell activation and degranulation with release of mediators, initiating an immune cascade (Figure 37-3). This is termed the immediate response. With the release of histamine, leukotrienes, prostaglandins, bradykinin, and other mediators (platelet-activating factor, substance P, tachykinins) comes the immediate onset of symptoms of sneezing, itching, and “running,” typically seen in instances of intermittent allergen contact—for example, with hay fever. An additional immunologic event in up to 70% of affected persons is a further influx of inflammatory cells consisting predominantly of eosinophils, basophils, and T cells expressing TH2 cytokines such as interleukin (IL)-4 (B cell IgE class switching) and IL-13 (mucus hypersecretion). Clinically this process is characterized by further obstruction, decreased olfaction, and mucosal irritability with immunopathologic changes similar to those seen in chronic asthma (see Figure 37-3). Local mucosal allergen–specific IgE production by nasal B cells is now confirmed and leads to local (skin prick test–negative) rhinitis. Emerging evidence also indicates that activated nasal epithelium–derived cytokines such as thymic stromal lymphopoietin (TSLP), IL-25 (i.e., IL-17E), and IL-33 can further promote disease through initiation, enhancement, and maintenance of TH2 inflammation at the mucosal surface where allergen deposition and sampling occur. In addition, the potential for innate mucosal immune mechanisms such as Toll-like receptor (TLR) signaling system to drive or skew TH2 responses is increasingly recognized.
Nonallergic Rhinitis
Nonallergic, Noninfectious Rhinitis
By definition, patients with nonallergic rhinitis (NAR) are skin prick–negative for common aeroallergens. NAR occurs in around 25% of persons with rhinitis symptoms. However, mixed rhinitis (a combination of allergic and nonallergic forms) can occur in up to 44% to 87% of patients. NAR incidence is higher in women. The nonallergic form of rhinitis incorporates a very heterogeneous group of conditions, and it is possible to further subclassify NAR into etiologic subtypes: a group for which the etiology is known and one for which it cannot be established, termed idiopathic rhinitis. The latter type is a diagnosis of exclusion, and approximately 60% of NAR cases will fall into this category. A summary of considerations in the differential diagnosis for NAR is presented in Box 37-1. An essential division is between NAR with eosinophilic inflammation in the upper airway and that without.
Nonallergic Rhinitis Without Eosinophilia
Autonomic Rhinitis
The nasal mucosa receives a rich efferent innervation from both the parasympathetic and sympathetic nervous system. Nasal glandular secretion is largely mediated by the parasympathetic fibers, the main postganglionic neurotransmitter being acetylcholine (ACh) acting through muscarinic receptors (predominantly the M3 subtype). The sympathetic fibers mediate vascular tone and can regulate nasal airflow by potent effects on venous erectile tissue. The primary neurotransmitter is norepinephrine (noradrenaline). In autonomic rhinitis, there is no evidence of nasal inflammation, but of autonomic dysfunction or imbalance. Nasal and, in some patients, cardiovascular reflexes are abnormal, and there may be association with the chronic fatigue syndrome. Topical ipratropium is useful in decreasing watery rhinorrhea; capsaicin applications also may relieve symptoms for several months after a few weeks of treatment. Epinephrine (adrenaline) and other sympathomimetics lead to vasoconstriction of the nasal mucosa, with increased nasal patency. Both α- and β-adrenergic blockers increase nasal resistance and can produce symptoms of nasal stuffiness (Box 37-2). Stimulation of the parasympathetic system leads to an increase in nasal secretions. However, patients who have this condition also have increased responsiveness to both histamine and methacholine, which results in nasal blockage and rhinorrhea. It also is associated with hypertrophy of the inferior turbinates, and nasal polyps are sometimes present. Certain stimuli such as cold air, exercise, mechanical or thermal factors, and humidity changes result in rhinorrhea and other symptoms of rhinitis, and a period of nasal hyperresponsiveness often follows viral infection. This observation is consistent with general neuronal dysregulation leading to excessive and troublesome neural hyperreactivity and imbalance with certain environmental exposures.
Drug-Induced Rhinitis
The main drugs implicated in pharmacologic rhinitis are listed in Box 37-2. This entity is mostly noninflammatory—for example, antihypertensives, particularly beta blockers, can cause nasal obstruction by abrogation of the normal sympathetic tone, which maintains nasal patency. Exogenous estrogens in oral contraceptives or hormone replacement therapy also evoke rhinitis in some patients. Overuse of α-agonists results in rhinitis medicamentosa: a tachyphylaxis of α-receptors to extrinsic and intrinsic stimuli. The mucosa becomes swollen and reddened. Aspirin hypersensitivity is an inflammatory form of drug-induced rhinitis (see earlier).
Differential Diagnosis for Rhinitis
A summary of considerations in the differential diagnosis for rhinitis is provided in Box 37-1. Neoplasms, foreign bodies, and trauma all can produce obstruction, pain, purulent discharge, and epistaxis. In adults, the possibility of neoplasm should always be considered in patients who have persistent symptoms, particularly if these are unilateral. In children, the presence of a foreign body should be considered if the nasal discharge is unilateral and foul-smelling. Local disease in the pharynx and larynx also may involve the nose and paranasal sinuses (e.g., enlarged adenoids), as may dental disease (e.g., maxillary dental root infection), which may spread to the maxillary sinus.
Rhinosinusitis
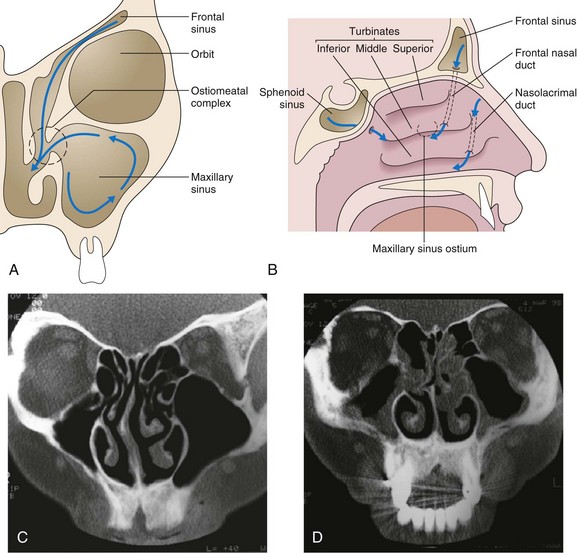
Evolutionary developments in humans have meant that the head is now held upright, so that sinuses, which reach down to the level of the top jaw, drain at the level of the nasal bridge, against gravity. This process is dependent on efficient mucociliary clearance and a patent ostiomeatal (sinus drainage point) complex and is easily compromised by mucosal swelling or mucociliary failure from any cause. The sinus drainage pathways are illustrated in Figure 37-4.
Chronic Rhinosinusitis
Mucus Clearance Defects
The nose and paranasal sinuses are lined with ciliated epithelium, which in a coordinated fashion moves a mucus blanket toward the nasopharynx. This mucus is important for the entrapment and removal of particulate material and toxic substances, which include bacteria and allergens. The integrity of the mucociliary clearance pathway is vital to the appropriate drainage and ventilation of the paranasal sinuses and the nose (see Figure 37-4). Primary ciliary dyskinesia is inherited as an autosomal recessive trait and is characterized by the presence of sinusitis, bronchiectasis, situs inversus (Kartagener syndrome, present in 50% of these patients), and male infertility that results from dyskinetic sperm. Various ciliary structural defects have been described (e.g., absence of inner or outer dynein arms or both), but some cilia appear normal (Young syndrome). Recent work suggests that deficiency of inducible nitric oxide synthase (iNOS) may be the common underlying abnormality. Presentation is with chronic sinusitis, bronchiectasis or bronchitis, and obstructive azoospermia.
• Mucous membranes become swollen and inflamed, which may result in blockage of the sinus ostia, thus preventing clearance (this is particularly critical at the ostiomeatal complex).
• If viruses or bacteria damage the epithelial cell layer, the integrity of cilial clearance is destroyed.
• Some bacteria produce toxins that inhibit cilial clearance mechanisms.
• Mucus during infection becomes thick and difficult to clear.
Chronic Rhinosinusitis With Nasal Polyps
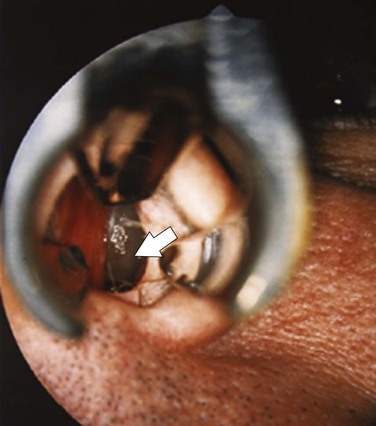
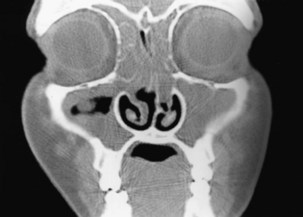
Chronic rhinosinusitis with nasal polyps (CRSwNP) represents a more distinct immune phenotype with better characterization. Nasal polyps (Figure 37-5) result from prolapse of the mucous membranes lining the nose and on examination are seen as pale, grapelike swellings arising predominantly from the middle meatus. These lesions are insensitive to pain but cause blockage and hyposmia and often are associated with asthma and aspirin hypersensitivity. They also may be infection-related, being common in persons with cystic fibrosis. Classification of polyps is similar to that of rhinitis (Box 37-3).
Nasal polyposis demonstrates a strong heritable component, with a relative risk of 18 times the normal rate of 4% in the population and 6 times the normal rate with an affected father and mother, respectively. A strong association of AERD with CRSwNP is recognized. A number of genes have been found to be associated with AERD (e.g., leukotriene C4 synthase promoter region); these vary among different populations. HLA-DQB1 is associated with allergic fungal sinusitis. The genetics of cystic fibrosis are discussed in Chapter 46; heterozygotes for cystic fibrosis are overrepresented in the chronic rhinosinusitis population. Primary ciliary dyskinesia is also genetic, with an incidence of approximately 1 in 20,000. Various structural ciliary defects have been described, but one common defect—a lack of iNOS in nasal mucosa—has recently been found.
Clinical Features
Diagnosis, Evaluation, and Tests
Imaging Techniques
Since the advent of CT, plain sinus radiographs now have only a very limited role in the diagnosis of acute rather than chronic sinusitis, because opacification or a fluid level may be seen in a sinus or gross soft tissue swelling may be evident. The imaging investigation of choice is CT, which is the best technique to demonstrate mucosal disease and underlying anatomic abnormalities (see Figure 37-4). The detailed anatomy of both bone and soft tissue is well delineated, and axial and coronal sections can be obtained. The coronal cuts provide views of the ostiomeatal complex, important for planning surgery for acute and chronic sinusitis (Figure 37-6). Preoperatively, coronal sections at 3 to 4 mm give maximal anatomic detail, whereas axial views provide vital information regarding the relation of the optic nerve to the posterior ethmoidal and sphenoid sinuses. Magnetic resonance imaging (MRI) is of very limited value, because bone is not well imaged. MRI is useful, however, in distinguishing one type of soft tissue from another and has the advantage of avoiding irradiation.
Treatment
Allergic Rhinitis
Treatment for AR has five major components:
Medical Suppressive Therapy
The classification system and treatment plan for AR according to ARIA are shown in Figures 37-7 and 37-8, respectively. Intermittent rhinitis is defined as that accompanied by symptoms for fewer than 4 days per week or fewer than 4 weeks in total. Persistent symptoms are defined as those present for more than 4 days per week and lasting more than 4 weeks in total duration. Classification of disease severity as mild, moderate, or severe is based on effects on day-to-day well-being and ability to sleep or work. Thus, the diagnosis takes into account severity of disease in terms of effects on quality of life, as well as disease duration, which in turn guides pharmacotherapy and immunotherapy intervention. However, in view of the spectrum of respiratory diseases (i.e., NAR) that can manifest with rhinitis symptoms and the possible need for use of allergen-specific immunotherapy, the key concept of seasonality is very useful in the diagnostic algorithm and has been retained by several guidelines and standards of care documents such as that from the British Society for Allergy Clinical Immunology (BSACI).
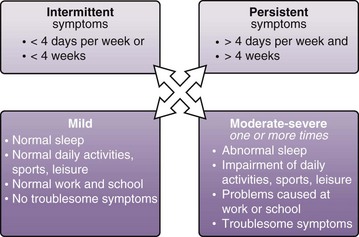
Figure 37-7 Classification of rhinitis by frequency and severity.
(From Bousquet J, Van Cauwenberge P, Khaltaev N, et al: Allergic rhinitis and its impact on asthma, J Allergy Clin Immunol 108[suppl]:S147–S334, 2001.)
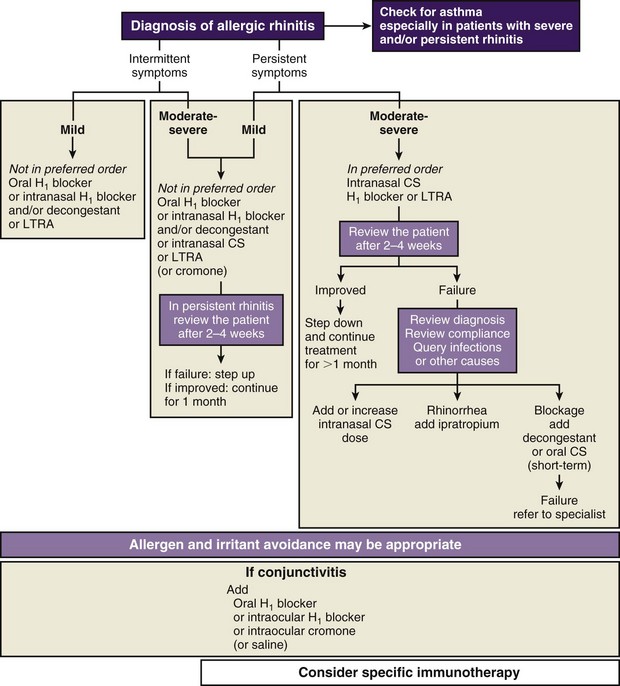
Figure 37-8 Approach to treatment of rhinitis based on the classification shown in Figure 37-7. CS, corticosteroids; H1, histamine receptor type H1; LTRA, leukotriene receptor antagonist.
(From Bousquet J, Khaltaev N, Cruz AA, et al: Allergic Rhinitis and Its Impact on Asthma [ARIA] 2008 update [in collaboration with the World Health Organization, GA[2]LEN and AllerGen], Allergy 63[Suppl 86]:8–160, 2008.)
Topical Corticosteroids
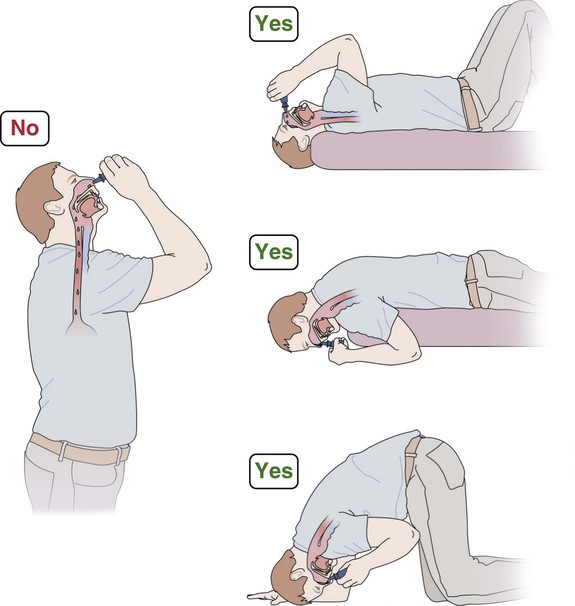
Metaanalysis has shown that topical corticosteroids constitute the most effective treatment for AR. Regular use is needed, and preseasonal dosing reduces development of seasonal rhinitis symptoms. For polyps or marked nasal blockage, betamethasone drops or oral corticosteroids for the first 2 weeks of therapy may be needed, followed by a nonabsorbable drop formulation of fluticasone propionate (Flixonase Nasule), particularly in the long term for polyp management (Figure 37-9). Side effects of topical corticosteroids include local irritation and minor epistaxis. Systemic steroid absorption is low except with betamethasone and dexamethasone, which should be reserved for only short-term use.
Noninfectious Causes
Intrinsic Rhinitis
Anticholinergics (e.g., ipratropium bromide) are useful for troublesome rhinorrhea (i.e., intrinsic rhinitis) (Figure 37-10), particularly when eosinophils are absent from nasal secretions. When eosinophilia is present, a response to topical corticosteroid therapy is usual. α-Agonist decongestants, such as pseudoephedrine and xylometazoline, should be used sparingly. Surgical procedures may help if nasal obstruction is predominant.
Immune Defects
Therapy of immune-related rhinitis is directed toward correction of the underlying defect.
Acute Rhinosinusitis
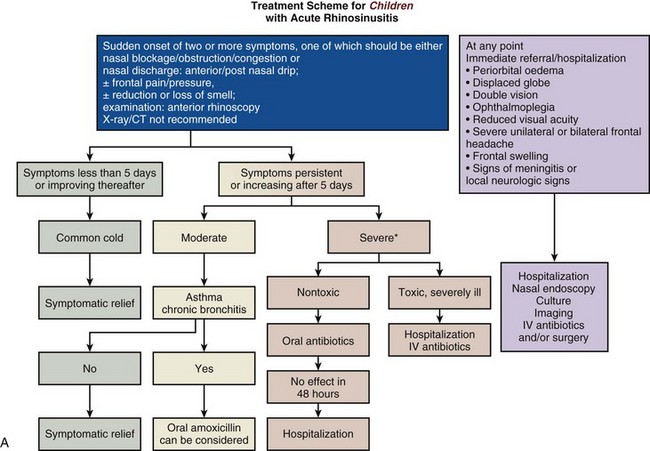
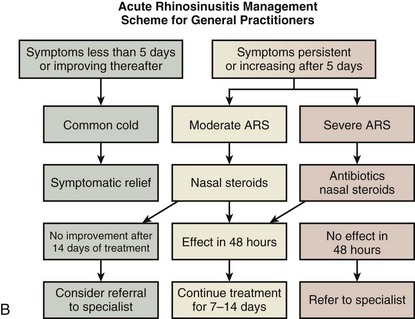
Recent evidence suggests that topical nasal corticosteroids help, either in conjunction with antibiotics or alone, to reduce symptom severity and hasten recovery. There is no suggestion that they lead to more recurrences or to more adverse events. In a number of rhinosinusitis scenarios, acute intervention will be required, as shown in the EPOS guidelines (Figure 37-11, A and B).
Chronic Rhinosinusitis
With CRS, the aims of treatment are to remedy any underlying cause (e.g., immunologic defect, anatomic abnormality that prevents drainage) and to restore the integrity of the mucous membranes to allow normal ventilation of the sinuses and drainage. Nasal douching with saline relieves symptoms and improves the endoscopic appearance. Topical corticosteroids may help to reduce mucous membrane swelling and improve drainage. Initially, betamethasone drops taken in the head-down position (see Figure 37-9) are briefly used, but nonabsorbed fluticasone propionate (Flixonase Nasule) is safer for long-term use. Prolonged courses of macrolide antibiotics produced improvements equivalent to those achieved with FESS, possibly because of their antiinflammatory activity. Amphotericin douching is ineffective.
Surgical Interventions for Acute and Chronic Rhinosinusitis
Major changes have occurred in recent years as a result of the advent of high-resolution CT scans and FESS. Better demonstration of the nasal and sinus anatomy is achieved with CT scans, as well as of the important ostiomeatal complex, the vital region in which sinus drainage by mucociliary clearance occurs. Obstruction in this zone is very important in the generation of chronic sinus disease. The main aim of FESS is to restore adequate drainage for the frontal, maxillary, and ethmoidal sinuses (see Figure 37-4). When this fails, more radical sinus surgery may be needed, but complete investigation for underlying medical factors (e.g., immune deficiency) should be undertaken first.
Nasal Polyps
Unilateral nasal polyps (Figure 37-12) warrant appropriate investigation to exclude transitional cell papilloma, squamous cell carcinoma, encephalocele, or other pathologic conditions. In the absence of contraindications and clinical suspicion regarding the nature of the polyp, a medical polypectomy accomplished with use of prednisolone (0.5 mg/kg, enteric-coated) plus betamethasone drops (two in each nostril three times a day with the head upside down) for 5 days, up to 14 days as indicated by clinical need, can be as effective as surgery and is superior with respect to control of concomitant asthma. This should be followed by long-term corticosteroid drops—initially betamethasone for 2 weeks, then almost nonabsorbed fluticasone propionate. Subsequently, a trial of a leukotriene receptor antagonist should be undertaken for 2 to 4 weeks, with continuation if beneficial. Other measures being evaluated include regular saline douching and topical lysine aspirin in patients sensitive to this on nasal challenge. Failure of medical treatment is an indication for surgery.
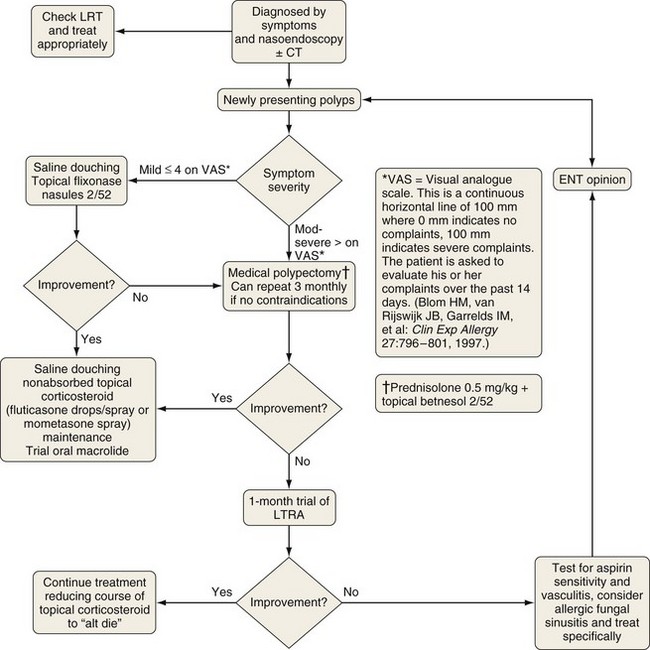
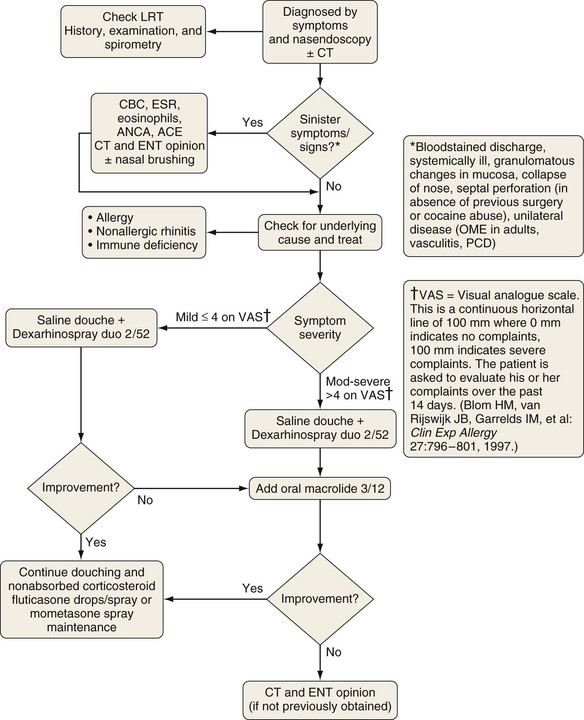
Figure 37-12 Treatment of chronic rhinosinusitis with nasal polyps (CRSwNP). Symptoms and signs are rated using a visual analogue scale. See Figure 37-10. CT, computed tomography; ENT, ear-nose-throat; LRT, lower respiratory tract; LTRA, leukotriene receptor antagonist.
(Modified from Scadding GK, Durham SR, Mirakian R, et al: BSACI guidelines for the management of rhinosinusitis and nasal polyposis, Clin Exp Allergy 38:260–275, 2008.)
Clinical Course and Prevention
Controversies and Pitfalls
• Not all patients who have nasal symptoms have AR—neoplasm or foreign body may be the cause.
• Unilateral discharge in children probably indicates the presence of a foreign body, but in adults it may be a sign of carcinoma.
• Unilateral lesions must be biopsied to exclude malignancy.
• Nasal decongestants should be used sparingly, if at all.
• Most medical treatment failures result from poor compliance; once-daily treatment is best, if possible.
• The common cold, a cause of widespread morbidity, remains a major research challenge.
• Intrinsic rhinitis can be troublesome to treat, and further research is required.
• Turbinates and polyps can be difficult to distinguish from one another; in general, however, turbinates are rigid and pain-sensitive, whereas polyps are mobile and insensitive to irritating or painful stimuli.
• Facial pain in the absence of nasal symptoms is rarely caused by sinus disease, so other causes such as migraine, dental problems, and temporomandibular joint syndrome should be considered.
• Chronic refractory sinusitis should stimulate investigation for underlying immune or other defects.
• CT scan is the investigation of choice—plain radiography or magnetic resonance imaging has a limited role.
Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and Its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160.
Bousquet J, van Cauwenberge P, Aït Khaled N, et al. Pharmacologic and anti-IgE treatment of allergic rhinitis ARIA update (in collaboration with GA2LEN). Allergy. 2006;61:1086–1096.
Ragab S, Scadding GK, Lund VJ, Saleh H. Treatment of chronic rhinosinusitis and its effects on asthma. Eur Respir J. 2006;28:68–74.
Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19–42.
Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of rhinosinusitis and nasal polyposis. Clin Exp Allergy. 2008;38:260–275.
Thomas M, Yawn BP, Price D, et al. European Position Paper on Rhinosinusitis and Nasal Polyps Group EPOS Primary Care Guidelines: European position paper on the primary care, diagnosis, and management of rhinosinusitis and nasal polyps 2007—a summary. Prim Care Respir J. 2008;17:79–89.