Advances in Anesthesia, Vol. 28, No. 1, 2010
ISSN: 0737-6146
doi: 10.1016/j.aan.2010.08.001
Biomarkers: Understanding, Progress, and Implications in the Perioperative Period
Patients with coronary artery disease undergoing major noncardiac surgery have a considerable risk of perioperative cardiac morbidity and mortality and compromised long-term outcome [1,2]. Several preoperative risk stratification scores, as well as strategies based on physical examination and history, have been developed to predict the risk of cardiac complications following various surgical procedures performed in different patient populations [3,4].
Some of these scores have limitations, and generating them may include cardiac stress testing, with its associated risks and costs [4]. Moreover, although there are many risk stratification tools concerning cardiac outcomes, few exist for important noncardiac complications, such as infectious, neurologic, and renal complications, or for noncardiac causes of mortality.
Biomarkers have attracted the attention of clinicians and investigators as a means of stratifying risk in different patient populations, including surgical patients. They generally entail simple, minimally invasive tests (blood draws) that have potentially high yield in assessing risk stratification [5,6]. Therefore, it is important to understand the biology, pathophysiology, and usefulness of biomarkers during the perioperative period, because these indicators may have predictive value for postoperative outcomes, especially when these biomarkers are added to existing risk stratification techniques. For instance, C-reactive protein (CRP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) improved the average sensitivity of predicting perioperative major cardiovascular outcomes from 59% to 77% when added to a clinical risk prediction system [7]. The ultimate goal is to use these biomarkers to develop and evaluate therapies intended to improve surgical outcomes.
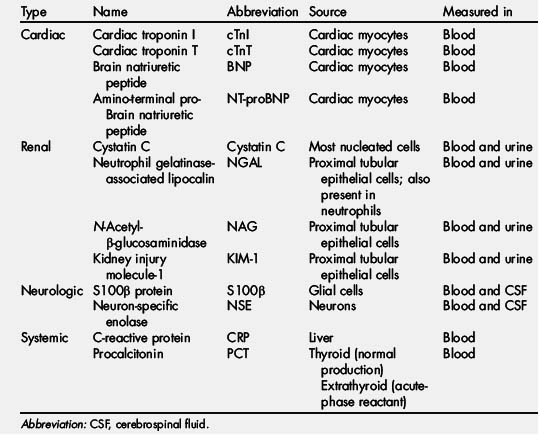
This article reviews and evaluates current knowledge and available evidence regarding the usefulness of some of the commonly studied infectious, inflammatory, neurologic, cardiac, and renal biomarkers in the perioperative period (Table 1).
CRP
Surgery induces an intense inflammatory response [8]. The release of inflammatory markers has been documented following cardiac surgery [9], and major noncardiac surgeries such as joint replacement, major vascular surgery, and colorectal surgery [10]. The perioperative inflammatory response is believed to contribute to poor perioperative outcomes. For example, 17% of postoperative deaths are attributed to cardiovascular causes [11]. Inflammation has been implicated in the pathophysiology of myocardial infarction; it is associated with the development of arterial plaque, starting with lipid deposition to plaque rupture and the resulting complications. If the underlying mechanism of perioperative myocardial infarction (PMI) is plaque rupture, then a negative stress test (which indicates that there is no flow-limiting stenosis) may fail to detect a nonobstructing plaque that may potentially rupture and cause cardiac ischemia or infarction [12]. In such circumstances, inflammatory markers such as CRP may more accurately predict perioperative cardiac morbidity and mortality [13]. There are many available markers of inflammation such as tumor necrosis factor (TNF) and the interleukins (IL) IL-2, IL-4. IL-6, IL-8, IL-10, and IL-14; however, this article focuses on CRP.
CRP, an acute-phase reactant produced in the liver, is a well-known marker of systemic inflammation; it greatly increases in response to acute injury. Its stable concentration over a long period of time is governed chiefly by the rate of hepatic production rather than by factors connected with its clearance [14]. CRP assays, being cost-effective, reliable, and fairly sensitive [15,17], seem to meet all criteria for the ideal biomarker. Indeed, the sensitivity of the high sensitivity CRP (hsCRP) assay seems to exceed even that of the original CRP assay.
In nonsurgical settings, CRP functions as a marker of atherosclerosis; when increased, it is considered a biochemical cardiovascular risk factor [18,20].
Cardiac Surgery
In cardiac surgery, postoperative serum concentrations of CRP are associated with the incidence of postoperative arrhythmias [9], and are predictive of septic complications, the need for catecholamine therapy, prolonged respiratory support, and prolonged intensive care unit (ICU) stay [21]. Moreover, Milazzo and colleagues [22] identified preoperative elevated concentrations of CRP as a predictor of recurrent ischemia up to six years postoperatively.
Noncardiac Surgery
The predictive power of CRP seems to be stronger than that of the clinical risk index [7]. Preoperative high levels are associated with short- and long-term morbidity and mortality after noncardiac [7]. Postoperative high levels of this marker are associated with complications after colorectal and bariatric surgeries [23,25].
So far, evidence provides no consensus on what value constitutes an abnormally increased hsCRP or even the range of detectable hsCRP in the general population. Because different studies have not used identical assays, a wide range exists in the values that are believed to constitute an increased level. Assays for hsCRP are new, only appearing commercially in the last decade. Most of the available clinical studies have accepted as normal or increased the values that have been designated as normal or increased by various laboratories, depending on the assay each laboratory used. However, more recent studies with the newer hsCRP assays have redefined a normal CRP level in a healthy individual as approximately ≤3 mg/L. Other clinicians might recognize an even lower cutoff (≤1 mg/L) as normal. Although the present assays are highly sensitive, these current values may change with future advances in technology and in the criteria governing specific assays [13,26–29].
Interventions that moderate the inflammatory response may reduce adverse outcomes [30]. Clinical studies show that, in some circumstances, a simple antiinflammatory intervention such as corticosteroid administration is associated not only with lower perioperative CRP levels but also with improved clinical outcomes [31,32]. In a randomized trial of 88 patients undergoing laparoscopic cholecystectomy, 8 mg of dexamethasone given 90 minutes before incision correlated with significantly lower CRP levels, significantly reduced postoperative fatigue, less nausea and vomiting, and a faster return to recreational activities [33]. Therefore, interventions targeted to decrease CRP levels, or to prevent their perioperative increase, may be warranted [34].
Brain or B-type natriuretic peptide and NT-proBNP
Congestive heart failure (CHF) has been shown to be a predictor of PMI following vascular surgery [3,35–37]. It increases the odds of dying from a PMI 12-fold if diagnosed within 12 months of a vascular surgery [1]. Diagnosis of CHF is a clinical challenge. Traditionally, the diagnostic approach begins with a thorough history and physical examination, complemented by chest radiographic examination and echocardiography. However, the history and physical examination can be misleading [38], and chest radiographic examination has its own limitations [39]. Echocardiography can also be misleading, in that 30% to 70% of patients with CHF present with a normal (>50%) or only mildly depressed left ventricular ejection fraction. Diastolic dysfunction may underlie CHF in many of these patients [40]. Most patients with asymptomatic left ventricular dysfunction remain undiagnosed [38]. Brain natriuretic peptide (BNP) and NT-proBNP have emerged as promising biomarkers, as simple and reliable tests that may be useful in establishing the diagnosis of CHF and correlating with its severity [38,41].
BNP is a 32-amino acid polypeptide secreted by cardiomyocytes in response to excessive ventricular or atrial stretching, or during ischemia. NT-proBNP is a 76-amino acid N-terminal fragment that is biologically inactive and is coreleased in the same conditions as BNP [42,43]. Biologically, they are distinguished by a significantly different half-life: NT-ProBNP has a longer half-life, 1 to 2 hours, in contrast with 20 minutes for BNP.
In nonsurgical settings, clinical studies have shown that plasma BNP is a powerful predictor of adverse cardiovascular events in patients with heart failure, acute coronary syndromes, primary pulmonary hypertension, and valvular disease [44,46].
Cardiac Surgery
In cardiothoracic surgery patients, 2 recent clinical studies showed the importance of BNP and NT-proBNP. High levels BNP and NT-proBNP have been associated with the development of atrial fibrillation in patients undergoing cardiac or thoracic oncology surgery [47,48]. Moreover, NT-proBNP may be useful in the assessment of potential donor hearts and in the follow-up of patients undergoing lung transplantation [49,50]. A recent study indicates that plasma BNP levels do not correlate with left ventricular function after cardiac surgery but do correlate with the E/E′ ratio, which is an echocardiographic indicator of filling pressures and diastolic function [51].
Noncardiac Surgery
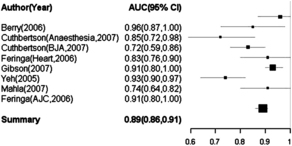
In noncardiac surgery, many investigators have confirmed that either preoperative BNP [52–56] or NT-proBNP [57–60] are helpful in predicting outcomes (Fig. 1). A clear example comes from Breidhardt and colleagues [61], who showed that preoperative increase of BNP predicts major perioperative cardiovascular complications in patients undergoing orthopedic surgery, and that the combination of BNP and the American Society of Anesthesiologists score is superior to BNP alone in predicting in-hospital cardiac events.
In light of these studies, both BNP and NT-proBNP are promising markers for postoperative cardiac outcomes. The current evidence does not support a universal cutoff point for BNP or NT-proBNP levels beyond which poor outcomes are anticipated, because existing studies have used different assays and various statistical methods to obtain their cutoffs; moreover, some did not indicate how they obtained their cutoff [62]. The specified cutoff points have varied between 40 and 189 pg/mL for BNP [52–56] and between 280 and 533 pg/mL for NT-proBNP [57–60]. Additional investigations are needed in order for consensus to be reached on what constitutes increased and predictive levels of both markers. Interventions should be sought that reduce concentrations of these 2 markers, and trials designed to assess the effects of these interventions on surgical outcomes.
Cardiac troponins
Troponins are proteins of the contractile system of the cardiac cells that act together through the tropomyosin complex to regulate muscle contraction [63]. Cardiac troponins have become the biomarker of choice for the diagnosis of acute myocardial ischemia and infarction [64]. Cardiac troponin T (cTnT) levels are detectable 3 to 12 hours after myocardial injury, and the concentration is in direct proportion to the extent of myocardial injury [65,67].
Cardiac troponins have also been used in the perioperative period to evaluate myocardial ischemia and infarction. Early studies considered them the best tool for diagnosing PMI in patients having noncardiac and cardiac surgery. Several investigators have shown that increased preoperative and postoperative levels of cTnI are associated with poor postoperative clinical outcomes [68]. However, at present, no single cutoff value has been recommended for use in the diagnosis of PMI. Moreover, cTnI may increase even before aortic cross-clamping and cardiac manipulation [69]. Martin and colleagues [70] suggested that cTnI levels lower than 0.15 μg/L were not associated with myocardial ischemia in the perioperative period of cardiac surgery. In contrast, cTnI levels higher than 0.15 μg/L were a strong indicator of ischemic damage.
Thielmann and colleagues [71] showed that preoperative cTnI levels higher than 1.15 ng/mL were associated with longer ICU stay and with higher in-hospital mortality. Moreover, levels of 0.15 ng/mL or higher were associated with higher morbidity and mortality 6 months after surgery [72]. In a study conducted by Lehrke and colleagues [73], cTnT measurement 48 hours after surgery was a strong predictor of severe cardiac failure and high postoperative mortality. A cTnT value exceeding 0.46 μg/L 48 hours after surgery carried a 6.7-fold risk of cardiac death.
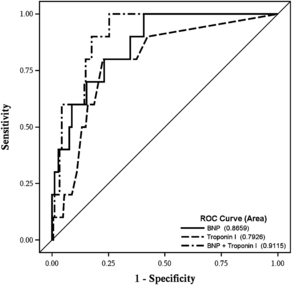
Cardiac troponins have also been used to identify clinical outcomes in patients undergoing major vascular surgery [74]. Early studies by Lee and colleagues [75] and Meltzer and colleagues [76] suggested an association between increased levels of cTn and postoperative outcomes. More recently, Landesberg and colleagues [77] showed that levels of cTnI greater than 0.6 ng/mL and cTnT greater than 0.03 ng/mL were independently associated with a respectively 2.15-fold and 1.89-fold increase in mortality. The investigators of the Coronary Artery Revascularization Prophylaxis (CARP) trial used cTnI to define PMI. They found that cTnI levels of 0.1 μg/L or higher occurred in 27% of patients undergoing elective vascular surgery and that these levels were a strong predictor of long-term risk of death among patients with diabetes [78]. Preoperative concentrations of cTnI were predictive of postoperative cardiac outcomes in major noncardiac surgery patients and their predictive power was mildly improved by adding preoperative BNP concentration into the model (Fig. 2).
In agreement with these last 2 studies, Filipovic and colleagues [79] found an association between cTnI levels and all-cause mortality 1 year after major noncardiac surgery, but a subsequent study by the same group of investigators could not show this association [80]. Controversies surround the validity of cTnT in patients with impaired renal function. However, Feringa and colleagues [81] showed that, after adjustment for estimate of the glomerular filtration rate, minor increases in troponin T from 0.03 to 0.09 ng/mL were strongly associated with late mortality and major adverse cardiac events after major vascular surgery.
Procalcitonin
Procalcitonin (PCT) is a 116-amino acid peptide produced by the c-cells in the thyroid. Its concentration in the serum of healthy individuals is low (<0.1 ng/mL); however, it may be considered an acute-phase reactant because of its production and release during inflammatory stress [63,82]. Traditionally identified as a marker for infectious conditions, PCT at increased levels has been shown more recently to be associated with noninfectious processes such as trauma, surgery, cardiogenic shock, burns, heat stroke, acute respiratory distress syndrome, and rejection after transplantation [83–87]. In these conditions it is produced outside the thyroid by the liver and circulating mononuclear cells.
Cardiac Surgery
Procalcitonin has been investigated in the context of cardiac surgery. It is not clear whether the type of cardiac surgery influences the surge of PCT after surgery. Prat and colleagues [88] could not identify differences in PCT levels between patients undergoing coronary artery bypass graft (CABG) and open heart valvular surgery. In contrast, Sponholz and colleagues [89] and Franke and colleagues [90] reported that valvular surgery induces larger increments of PCT levels than on-pump CABG, and this in turn induces larger increases than off-pump CABG.
Procalcitonin seems to be predictive of poor outcomes after cardiac surgery. An early study by Loebe and colleagues [91] showed that an increased PCT level, higher than 1 ng/mL, after cardiac surgery was predictive of poor postoperative outcomes. Two recent studies showed that procalcitonin significantly increased in patients who underwent cardiac surgery and subsequently developed infectious and noninfectious complications, compared with patients who did not experience complications [88,92]. Procalcitonin proved more accurate than CRP in predicting those complications [92]. In heart transplantation, PCT is also a reliable marker in the diagnosis and monitoring of postoperative infections [93,94]. Moreover, PCT levels were not affected by the use of immunosuppressants [94,95].
Noncardiac Surgery
Schneider and colleagues [96] retrospectively analyzed the association of PCT concentration with postoperative mortality, morbidity, and length of stay in 220 patients who were admitted after surgery to an ICU. The researchers found a significant and logarithmic association between PCT concentration and outcome. Moreover, PCT was an independent predictor of mortality and duration of hospital stay after surgery in the survivors. In patients undergoing oncologic surgery, PCT seems to be a more useful marker than CRP for monitoring the postoperative course and diagnosing severe bacterial infections [97,98]. Procalcitonin is also superior to high sensitivity CRP (hsCRP) as an independent predictor of graft failure in renal transplantation [99]. However, Mommertz and colleagues [100] found no association between perioperative neurologic deficit and PCT in patients who had carotid endarterectomy.
Biomarkers of kidney dysfunction
Acute kidney injury (AKI) remains a common postoperative complication. The incidence of dialysis-dependent acute renal failure after cardiac surgery is approximately 1%, and approximately half of these patients die from this complication [101,103]. Early detection of AKI in the perioperative period may permit early renoprotective interventions, which may in turn translate into more favorable postoperative outcomes; thus, simple and specific biomarkers would be useful to monitor AKI.
Cystatin C
Cystatin C is a 13-kDa endogenous cysteine proteinase inhibitor that is synthesized at a constant rate and released into the plasma by all nucleated cells in the body. It is freely filtered at the glomerulus, not secreted or reabsorbed, and nearly completely catabolized by proximal renal tubular cells. Cystatin C can be measured in plasma and urine [104]. Cystatin C has a lower variability of measurements, a shorter half-life, and a lower distribution volume than creatinine [105]. Because of these properties, it has been suggested that it may be more sensitive than creatinine to early and mild changes in kidney function [106,108]. However, factors such as age, gender, and body mass index may still affect the serum levels of this protein [104,109,110]. A recent study by Villa and colleagues [111] showed that cystatin C correlated better with glomerular filtration rate than creatinine in patients in ICUs with mild kidney dysfunction. Moreover, a meta-analysis conducted by Dharnidharka and colleagues [106] indicated that the serum cystatin C concentration was a more sensitive indicator of renal dysfunction than serum creatinine.
Cardiac surgery
Several investigators have studied the clinical value of cystatin C in identifying patients with, or at risk for, renal dysfunction after cardiac surgery [112,113]. In a cohort study of 110 elderly patients having cardiac surgery requiring cardiopulmonary bypass, serum cystatin C could not detect mild renal injury earlier than plasma creatinine [114]. Similar results were reported by Liangos and colleagues [115], who found that cystatin C levels 2 hours after cardiopulmonary bypass could not predict AKI. However, these results are disputed by a recent study demonstrating that serum cystatin C predicted AKI about 2 days earlier than clinically significant increases in creatinine [116]. Increased levels of urinary cystatin C have also been associated with early detection of kidney dysfunction after cardiac surgery [117]. In a prospective study of 376 patients, Ledoux and colleagues [113] found that preoperative estimation of renal function from serum cystatin C was strongly associated with hospital morbidity and mortality.
Noncardiac surgery
In contrast with cardiac surgery, cystatin C seems to correlate better with kidney dysfunction in the setting of noncardiac surgery. Lebkowska and colleagues [118] showed that patients with delayed graft function after cadaveric renal transplantation showed significantly higher levels of cystatin C than those with normal graft function. Cystatin C correlated with perioperative levels of neutrophil gelatinase–associated lipocalin (NGAL) and creatinine. Moreover, cystatin C has also been used to detect early kidney dysfunction in living-related donor kidney transplantation. Gourishankar and colleagues [119] found that cystatin C correlated with all other markers of kidney function and detected acute changes in kidney function immediately after donor nephrectomy.
Cystatin C has been used clinically to monitor the perioperative effect of nephrotoxic agents such as cyclooxygenase inhibitors or the effect of a variety of perioperative interventions on kidney function. In a randomized, controlled, double-blinded trial, Puolakka and colleagues [120] found that cystatin C levels were not affected by the administration of parecoxib in patients undergoing laparoscopic hysterectomy, and accordingly the researchers suggested that 80 mg of this analgesic was not associated with perioperative kidney dysfunction. Cystatin C has also been used to evaluate the effects of perioperative fluid optimization in patients undergoing surgery [121]. Boldt and colleagues [122] assessed the effect of different kinds of colloid therapy (albumin vs hydroxyethylstarch) on patients undergoing cardiac surgery and found that differences in the increase of cystatin C after surgery did not reach statistical significance between the 2 groups being studied.
Cystatin C has also been used to investigate the effects of sevoflurane on kidney function. Laisalmi and colleagues [123] showed in a double-blinded controlled study that cystatin C levels did not differ in healthy women undergoing breast surgery who received ketorolac during high fresh gas flow of sevoflurane anesthesia. They concluded that, in healthy women, the use of ketorolac is not associated with kidney injury in patients having general anesthesia with sevoflurane.
Neutrophil Gelatinase-associated Lipocalin
NGAL, a 25-kDa protein that belongs to the superfamily of lipocalins, has been measured in plasma and urine. NGAL binds to 2 receptors, the megalin multiscavenger complex and the 24p3 receptors. It has been suggested that NGAL also acts as an acute-phase reactant that is increased not only during the infective process but also during noninfective systemic diseases. The kidney can release NGAL in response to damage or stress, and thus serum or urinary NGAL can be used to detect AKI [124,125]. It has also been suggested that serum and urinary NGAL may be useful in distinguishing between septic and nonseptic AKI. Bagshaw and colleagues [126] found that septic kidney injury was associated with higher initial and peak values of urinary and serum NGAL than nonseptic kidney dysfunction. Several investigators have also studied the predictive value of NGAL in the perioperative setting, as discussed later [127–130].
Cardiac surgery
Urinary and serum levels of NGAL were increased in patients with postoperative kidney injury after cardiac surgery [131,132]. In addition, a study by Che and colleagues [133] found that urinary NGAL was highly predictive of kidney dysfunction. Its predictive value was higher than that of other markers such as cystatin C, urinary interleukin 18, serum creatinine and N-acetyl-β-d-glucosaminidase. These results were partially replicated by McIlroy and colleagues [134], who observed that urinary NGAL detected AKI only in patients with a preoperative estimated glomerular filtration rates of 90 to 120 mL/min.
Noncardiac surgery
Serum NGAL has been tested in liver transplantation. Intraoperative increases of NGAL were significantly associated with postoperative acute kidney dysfunction. Moreover, the difference between intraoperative and preoperative levels was also predictive of renal dysfunction. This delta value is particularly important because proper intraoperative interventions targeted to reduce or prevent increases of NGAL may mitigate or prevent postoperative kidney injury [135].
Several researchers have investigated the predictive value of NGAL in patients undergoing renal transplantation. They found that NGAL performs better than serum creatinine in detecting graft dysfunction [136,138].
N-Acetyl-β-Glucosaminidase
N-Acetyl-β-glucosaminidase (NAG), a proximal tubular epithelial cell glycosidase of 130 kDa, is normally excreted in low amounts in urine [139]. Early clinical studies showed that NAG is increased after kidney injury and can predict acute renal dysfunction in critically ill patients [140,142].
Cardiac surgery
In a small pilot study, Backlund and colleagues [143] showed that the urinary NAG/creatinine ratio increased during aortic operations. The researchers observed that the NAG/creatinine ratio increased soon after the induction of anesthesia and before the aortic cross-clamp. It was argued that early intraoperative increases in NAG might be related to hypotension or administration of nephrotoxic drugs such as antibiotics [144]. Also, increased NAG levels were found in patients with low intraoperative hematocrit who received blood transfusion during cardiac surgery [145]. In an early clinical study of 36 patients, a positive correlation was found between serum creatinine levels and urinary excretion of NAG after cardiac surgery [146]. However, Gormley and colleagues [147] found that, although NAG concentration increased in patients undergoing cardiac surgery, it did not adequately predict postoperative AKI [115,148].
Noncardiac surgery
NAG has been extensively used to assess volatile anesthesia-induced nephropathy. For instance, an early study investigated the comparative effects of 2 hours of hypotensive anesthesia with sevoflurane or isoflurane under 5 L/min total gas flow in patients having no preoperative renal dysfunction. Patients who received sevoflurane showed a transient increase of the NAG index; the researchers therefore concluded that sevoflurane in hypotensive patients caused a temporary, reversible disturbance of renal tubular function [149]. Kanbak and colleagues [150] investigated the effect of sevoflurane on kidney dysfunction in patients undergoing liver transplantation and showed that NAG increased during the intraoperative period, with a peak level occurring during the anhepatic phase. The researchers concluded that increased NAG concentrations did not correlate with blood urea nitrogen and creatinine levels. In a double-blinded, placebo-controlled study, NAG was used as a biomarker to detect renal injury in patients who received ketorolac for mastectomy surgery performed under sevoflurane-based anesthesia. The investigators could not show significant differences in NAG levels in patients who received or did not receive ketorolac [151].
NAG has also been used to assess the effect of perioperative renoprotective interventions. For instance, a prospective randomized, double-blind study investigated the effects of N-acetylcysteine infusion in patients undergoing abdominal aortic repair. The researchers found that N-acetylcysteine does not decrease renal injury as assessed by an increase in the urinary NAG/creatinine ratio [144]. NAG was also measured in a randomized controlled trial that ascertained whether the administration of N-acetylcysteine prevented AKI in 30 patients undergoing knee arthroplasty. Similar to the study discussed earlier, NAG increased in the perioperative period and the infusion of N-acetylcysteine did not prevent AKI [152]. NAG was one of the biomarkers used by Izumi and colleagues [153] to assess the efficacy of human atrial natriuretic peptide in patients with renal dysfunction undergoing cardiac surgery. The researchers found that the administration of human atrial natriuretic peptide prevented the increase of NAG associated with surgery and improved creatinine clearance.
Kidney Injury Molecule-1
During kidney injury, the kidney injury molecule-1 (KIM-1) glycoprotein is shed from cells into the urine. Ninety percent of KIM-1 expression in the human kidney occurs in the proximal tubule system, which might explain why KIM-1 seems to be specific to ischemic acute tubular necrosis (ATN) [154]. In patients with established acute renal failure, urinary KIM-1 levels are useful in assessing its severity, and have a prognostic value [155]. Han and colleagues [156] showed that patients with acute ischemic ATN had significantly higher levels of KIM-1 than patients with other forms of kidney dysfunction.
Cardiac surgery
In patients undergoing cardiac surgery, KIM-1 was increased before an increase in serum creatinine was detected [130]. Moreover, KIM-1 was a better biomarker than urinary NAG, NGAL, and IL-18 for predicting AKI in a cohort of 103 patients who underwent cardiac surgery [115].
Noncardiac surgery
The expression of KIM-1 has been assessed in cadaveric-donor and living-donor kidneys. The levels of expression of KIM-1 were significantly higher in cadaveric-donor kidneys than in living-donor kidneys [157]. In 2007, van Timmeren and colleagues [158] reported that high KIM-1 excretion was a predictor of graft loss. This predictive value was independent of donor age, creatinine clearance, and proteinuria, and was associated with hazard ratios of a magnitude similar to those of proteinuria. However, a later study by Hall and colleagues [136] found that KIM-1 was not a predictor of graft dysfunction in 98 patients who underwent cadaveric-donor kidney transplant. In summary, KIM-1 is an attractive biomarker of AKI but, because of the small number of clinical trials, its real predictive value in the perioperative context is unclear.
Biomarkers for neurologic injury
S100b Protein
The S100b protein is a low molecular weight glial protein of approximately 10 kDa that belongs to a multigenic family of calcium-mediated proteins (S100 proteins) [159]. S100b is currently labeled as a marker of generalized blood-brain barrier dysfunction that is released into the cerebrospinal fluid (CSF) after neuronal injury [160]. S100b has a half-life of 25 minutes [161]. Increased S100b is observed after infarction and after traumatic and toxic injury of the central nervous system [162–165]. However, S100b measurements differ depending on the assay used for its detection. Thus, researchers have suggested the need for international standardization among methods to measure S100b, to facilitate effective assessment of the predictive validity of this biomarker in clinical practice [166].
Cardiac surgery
Several clinical studies have shown that the S100b protein increased in patients undergoing cardiac surgery using cardiopulmonary bypass [167,169]. Serum S100b levels seem to be influenced by the age and gender of patients, by the use of cardiotomy suction or cell saver, and on-pump versus off-pump cardiac surgery, all of which might explain the varying and, in some instances, contradictory results of different trials [170,171]. Also, genetic factors may influence serum concentrations of S100b. Kofke and colleagues [172] showed that S100b levels were higher in ApoE4 carriers than in ApoE2 and ApoE3. Kilminster and colleagues [173] showed that S100b protein release during and after cardiopulmonary bypass correlated positively with both age of patient and duration of extracorporeal perfusion. However, the relationship between S100b levels and neurologic/neuropsychological outcomes remains controversial [174]. In a small cohort study, serum levels of S100b protein higher than 0.3 μg/L were more commonly observed in patients who had postoperative sleep disturbances after cardiac surgery [175]. The timing of S100b sampling may be important if this biomarker is used to predict outcomes. Georgiadis and colleagues [176] showed that S100b levels 24 hours after the operation had a sensitivity and specificity of approximately 90% in identifying patients with postoperative cerebral lesions.
Noncardiac surgery
Serum levels of S100b have also been studied in the perioperative period of noncardiac surgery [177,178]. In patients with chronic cervical myelopathy who underwent uncomplicated spine surgery, S100b did not predict functional outcomes; however, in patients with a complicated postoperative course, S100b had prognostic significance [179]. The same group of researchers investigated the predictive value of S100b in patients with intradural spine lesions. The postoperative time course of serum S100b did not correlate with functional outcomes [180]. Cerebrospinal levels of S100b were measured by Winnerkvist and colleagues [178] to identify patients at risk of neurologic complications in the perioperative period of surgery for thoracoabdominal aneurysm. The researchers found that patients who developed neurologic complications had significantly higher levels of S100b; however, the concentrations of this biomarker began to increase after the onset of clinical symptoms. In a small study in patients undergoing major vascular surgery, the serum concentration of S100b increased during the perioperative period, but the cerebrospinal levels remained low in some patients. The researchers argued that, in the absence of central nervous injury, surgical trauma itself may be responsible for the observed serum S100b increments [181,182].
Several studies have investigated the predictive value of S100b in the context of carotid endarterectomy (CEA), but the results remain controversial. In a cohort of 43 patients, intraoperative serum concentrations of S100b increased during catorid endarterectomy; however, these increased concentrations failed to predict cognitive outcome [183]. S100b did not significantly increase in patients undergoing CEA under local anesthesia [184]. Wijeyaratne and colleagues [185] also found no significant differences in S100b levels between patients who underwent CEA under either general or local anesthesia.
Neuron-specific Enolase
Neuron-specific enolase (NSE), located in neurons and neuroendocrine cells, is a neuronal form of the cytoplasmic glycolytic enzyme enolase. It has a molecular weight of 78 kDa and a dimeric structure composed of two γ subunits [186,187].
Cardiac surgery
In patients undergoing cardiac surgery, Ramlawi and colleagues [170] were able to show a significant association between postoperative neurocognitive dysfunction and NSE. However, no relationship between NSE concentrations and perioperative diffusion-weighted imaging lesions on magnetic resonance scanning was found in patients who had aortic valve replacement [169]. In a small cohort of patients, those who had the ApoE4 allele showed significantly higher postoperative levels of NSE than those with ApoE2 and ApoE3 [172]. Carriers of ApoE4 had an increased likelihood of developing Alzheimer disease [188]. Therefore, it is possible that genetic susceptibility explains increased levels of some biomarkers and postoperative cognitive dysfunction after cardiac surgery.
Noncardiac surgery
Lases and colleagues [182] investigated the predictive value of NSE in patients undergoing aortic aneurysm repair. The researchers concluded that neither NSE nor S100b added useful clinical information to that gleaned from the motor-evoked potential monitoring during surgery. In patients undergoing orthotopic liver transplantation, postreperfusion levels of NSE correlated highly with decreased regional cerebral oxygen saturation levels; however, the investigators did not assess neurologic outcomes [189].
NSE has been the focus of research in patients undergoing carotid endarterectomy (CEA). It increases during and after CEA, but its serum levels seem to depend on the anesthetic technique used during surgery [185]. In a study by Sahlein and colleagues [183], NSE failed to predict cognitive dysfunction in patients undergoing CEA. NSE was also used to assess neurologic damage in a randomized controlled trial that compared CEA with carotid artery stenting (CAS). The researchers found that NSE levels were significantly higher in the CAS group of patients than in those who underwent CEA [190].
Support: Supported by internal funds. None of the authors has a personal financial interest in the topics discussed in this article.