1 Understanding lasers, lights, and tissue interactions
Summary and Key Features
• Lasers and flashlamps can destroy histological targets using the concept of selective photothermolysis (SP)
• Ablative lasers vaporize tissue; non-ablative lasers heat tissue without vaporization
• Selective histological damage requires heat confinement to desirable target structures. Selective photothermolysis combines appropriate wavelength (‘color’ of light), fluence (‘dose’ of light), pulse duration, and protective skin cooling for the treatment of a variety of diseases
• Understanding the optical and thermal properties of skin and its histological targets allows safe and optimal treatments using light sources
Light
Lasers that vaporize a thin layer or column of tissue have also been developed. The concept of fractional photothermolysis (FP), reported by Manstein and colleagues in 2004, recently launched another era of lasers in dermatology, in which patterns of very small non-selective thermal damage zones are used to stimulate skin remodeling without scarring. Laser-stimulated remodeling is a complex process that mimics large wound healing in some aspects, with epidermal regeneration, induction of metalloproteinases, and formation of new dermal matrix including elastin fibrils and collagen types I and III. Compared with gross wound healing, there is minimal inflammation and no scarring. A ‘cookbook’ approach should be avoided when choosing among these devices for various applications. When treating a particular patient with a particular device, a combination of fundamental understanding, careful observation of the appropriate clinical end points, dexterity, and clinical experience is far better than a set of instructions (Box 1.1).
Box 1.1
How to choose a light source?
2. Choose correct light wavelength (nm) based on histological target chromophore
3. Observe whether continuous wave (CW) or pulsed source is required
4. Choose right pulse width if necessary (seconds)
5. Choose pulse frequency (Hz), if necessary
6. Set skin cooling parameters
7. Choose appropriate light dose
8. Test laser to check whether it is working properly
9. Trigger single pulse on target skin and observe clinical end point
10. Adjust dosimetry if necessary
11. If no unwanted sign is observed, continue with treatment
a. Anesthetize area if necessary (e.g. tattoos, ablative lasers)
b. Wear appropriate personal protection (e.g. wavelength specific eye goggles / glasses, fume-resistant mask, gloves)
c. Turn smoke evacuator on if performing ablative procedures
d. Dress the area with petrolatum ointment and protect from sun exposure until treated area is healed
Light interactions with skin
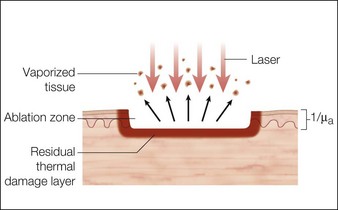
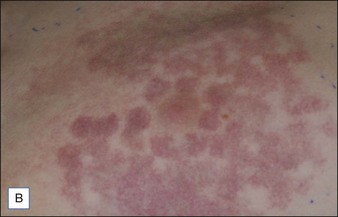
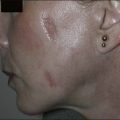
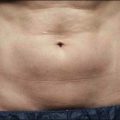
Laser dosimetry is extremely important for safe and effective results. In order to remove tissue, ablative lasers must raise local tissue temperature beyond the boiling point of 100oC, plus add much more energy needed for changing water into steam. The fundamental unit of energy is a joule (J). It takes 4.2 J to heat 1 cm3 of water by 1oC. In order to vaporize the same 1 cm3 of water, more than 2000 J are required. An ablative laser must deliver about 2500 J of energy per cm3 of vaporized tissue. Not only is a lot of energy required to ablate skin tissue – the energy must be delivered quickly to remove the hot tissue before heat is conducted deeply into the skin, causing a burn. The standard ablative lasers in dermatology are erbium (2940 nm) and CO2 (10 600 nm). The desired interaction of these ablative lasers is to precisely remove a thin layer for resurfacing or narrow column for fractional treatment of skin, leaving behind minimal residual thermal damage. A thin residual thermal damage layer, typically about 0.1 mm, is useful in practice for hemostasis. Minimum residual thermal injury is achieved with ablative lasers by a combination of wavelength, pulse duration, and power density (W/cm2) at the skin surface. A common mistake made by beginning laser users is to ‘turn down’ the power of a surgical CO2 laser in a misguided attempt to exercise caution. Unfortunately, turning down the power can cause burns because the process turns from rapid, precise vaporization with minimal thermal damage to bulk heating of the skin from unwanted residual heat. Fortunately, many of the ablative lasers made specifically for dermatology are designed to stay within a range of dosimetry for rapid tissue ablation, making this scenario less likely. The safest erbium and CO2 lasers are those emitting high power, high energy, and short (less than a few ms) pulses, designed specifically for dermatologic use with minimal residual thermal damage. Despite whatever safeguards an ablative laser may offer, the most reliable safeguard is an ability to recognize the desired and undesired immediate response end points. For example, immediate contraction of the skin is always a sign that substantial thermal injury of the dermis has occurred (Fig. 1.1).
Skin optics
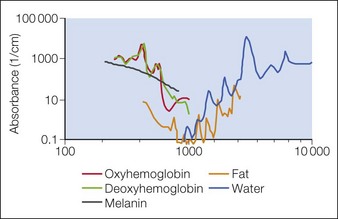
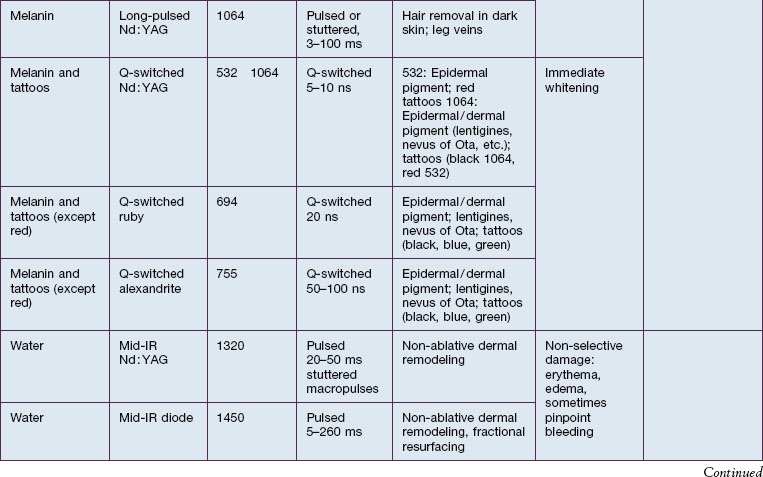
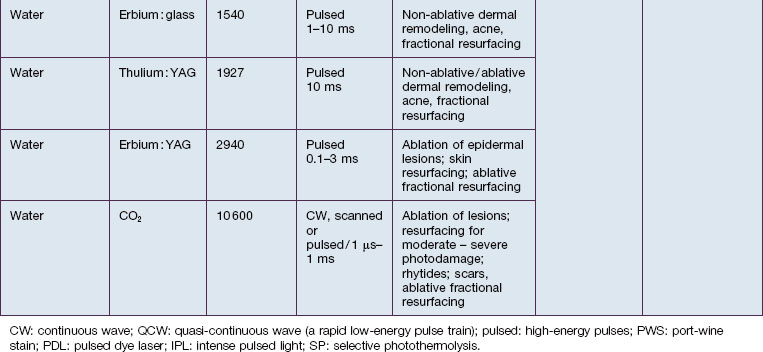
In skin, the most important chromophores are hemoglobin, melanin, exogenous pigments (e.g. tattoo ink, some drugs), water, and lipids. The intended target chromophore, depth of the target structures, and absorption of light by adjacent tissue influence the appropriate wavelength selection. Absorption spectra of various chromophores across the electromagnetic spectrum are summarized in Figure 1.2.
Selective photothermolysis
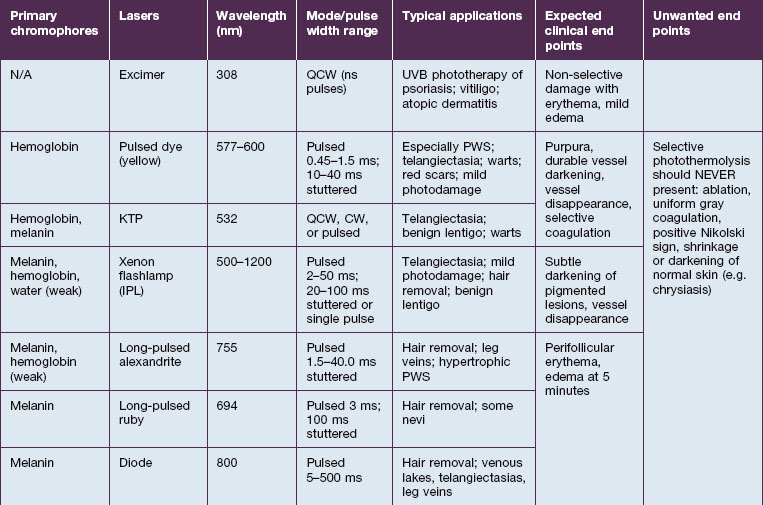
SP relies on fundamental choices being made correctly – wavelength, pulse duration, fluence, exposure spot size, and use of skin cooling. First, a wavelength (or, with IPLs, a range of wavelengths) must be used that is preferentially absorbed by the intended ‘target’ structures such as hair follicles, microvessels, tattoo inks, or melanocytes. Thus far, all lasers utilizing SP operate in the visible and near-infrared (NIR) spectrum. Generally, in the visible light spectrum, a target chromophore is treated using wavelengths of light of a complimentary color. For example, red tattoo ink absorbs green light and can be effectively treated with a frequency doubled Q-switched Nd : YAG laser operating at the green wavelength of 532 nm. Similarly, green tattoo ink is best removed with a red Q-switched laser, such as the ruby laser at 694 nm. Preferential absorption implies the avoidance of competing chromophores, not simply strong absorption in the intended target. For example, when treating dermal targets such as blood vessels it is important to minimize unwanted damage to the epidermis. Since every photon that reaches a blood vessel must first travel through the overlying epidermis, the best wavelengths for port-wine stain treatment are not simply those with strong absorption by blood. The proper wavelength(s) must also penetrate deeply enough to reach the intended targets. Across the visible and near-infrared spectrum from 400 to 1200 nm, longer wavelengths penetrate deeper into tissue. These reasons account for the use of yellow light pulsed dye lasers rather than the very strongly absorbed blue wavelengths for treating superficial vascular lesions. Long-pulsed dye lasers are the first example of a laser designed specifically for a medical application: treatment of port-wine stains in children (see Case study 2). On the microscopic scale, microvessels are selectively heated and damaged, with minimal injury to the rest of the skin structures. However, for a hypertrophic or deep vascular lesion, such as many adult port-wine stains and venous malformations, much better efficacy is often obtained using the deeply penetrating 755 nm near-infrared alexandrite laser, as detailed by Izikson et al in 2009. (Looking at Fig. 1.2, it is easy to observe that hemoglobin absorbs yellow light much more strongly than at 755 nm, a wavelength that is also well absorbed by melanin.) When alexandrite lasers are used for vascular lesion treatment, it is therefore imperative to use excellent skin cooling for epidermal protection; see Chang & Nelson 1999 and Altschuler et al 2000.
The second essential factor for SP is to use a pulse duration that allows heat to be confined during the laser pulse in or near the target structures. The moment that heat is formed in a target by preferential absorption of photons, the target begins to cool by conduction. Therefore, heating of the target is a balance between the rate of photon absorption and the rate of cooling. The concept of a particular target’s thermal relaxation time (TRT) is useful in clinical practice to pick the correct pulse duration. TRT is simply defined as the time required for substantial cooling of the target structure. TRT is strongly related to target size, and this variation accounts for the wide range of laser pulse durations needed for optimal dermatological lasers. A simple and useful approximation is that TRT ≈ d2, when TRT is in units of seconds, and d is the target size in millimeters. For example, a 1 mm leg vein cools in about 1 second, while a 0.2 mm telangiectasia, typical for rosacea, cools in about 0.04 seconds (40 ms), and a 0.03 mm venule in a child’s port-wine stain cools in about 0.001 seconds (1 ms). The optimal laser or IPL pulse duration is typically about equal to the TRT. In this example, a very long exposure from a low-power KTP (532 nm) laser would be appropriate for treating the leg vein. A higher power KTP or pulsed dye (595 nm) laser operated at about 20–40 ms would be appropriate for the rosacea-associated telangiectasia, and a pulsed dye laser operated at about 1 ms would be appropriate for the pediatric port-wine stain. This extreme dependence of TRT on target size applies all the way down to the nano-scale of subcellular targets. Q-switched lasers are used in dermatology because their 10–100 nanosecond pulse durations are shorter than the TRT for targets such as tattoo ink particles, melanosomes, and drug pigmentation deposits (see Case study 1).
Pearl 5
Pulse duration in seconds is directly proportional to the square of the target size in millimeters.
The third factor for optimal SP is sufficient fluence to affect the targets. In general, the fluence necessary is inversely related to absorption by the target structures – stronger absorption requires lower fluence, and vice versa. This is the reason, for example, that a typical alexandrite laser fluence for treatment of a port-wine stain (see Case study 2) is 40 J/cm2, while that for pulsed dye laser treatment of the same lesion may be only 8 J/cm2.
Skin cooling: limiting thermal damage to the intended targets
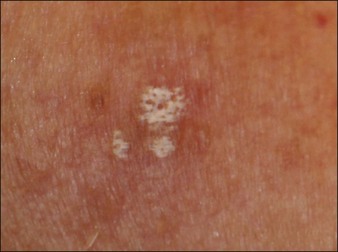
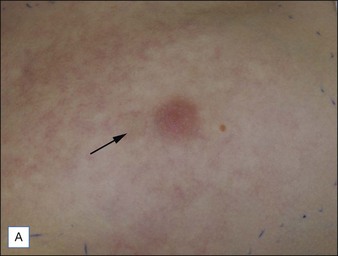
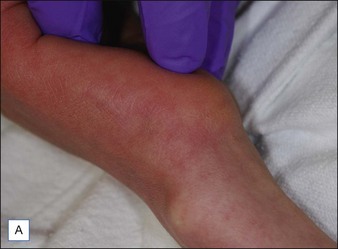
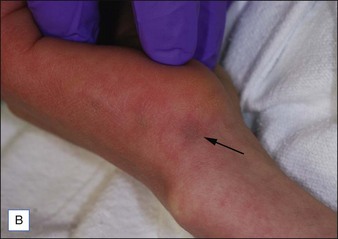
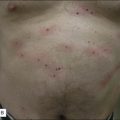
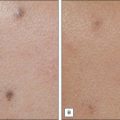
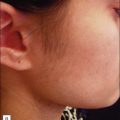
For the choice of proper dosimetry, it is crucial to be familiar with the particular device being used, and to carefully observe skin response to treatment. The combination of laser wavelength, pulse duration, spot size, skin cooling, and dosimetry can suggest initial treatment parameters, but only careful observation of immediate clinical end points will ensure efficacy (Figs 1.3–1.5), helping to avoid side effects. Common clinical end points are summarized in Table 1.1.
Alster TS, Tanzi EL, Lazarus M, et al. The use of fractional laser photothermolysis for the treatment of atrophic scars. Dermatologic Surgery. 2007;33(3):295–299.
Anderson RR, Farinelli W, Laubach H, et al. Selective photothermolysis of lipid-rich tissues: a free electron laser study. Lasers in Surgery and Medicine. 2006;38(10):913–919.
Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524–527.
Chang CJ, Nelson JS. Cryogen spray cooling and higher fluence pulsed dye laser treatment improve port-wine stain clearance while minimizing epidermal damage. Dermatologic Surgery. 1999;25(10):767–772.
Grossman MC, Dierickx C, Farinelli W, et al. Damage to hair follicles by normal-mode ruby laser pulses. Journal of the American Academy of Dermatology. 1996;35(6):889–894.
Haedersdal M, Katsnelson J, Sakamoto FH, et al. Enhanced uptake and photoactivation of topical methyl aminolevulinate after fractional CO2 laser pretreatment. Lasers in Surgery and Medicine. 2011;43(8):804–813.
Haedersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers in Surgery and Medicine. 2010;42(2):113–122.
Ibrahimi OA, Syed Z, Sakamoto FH, et al. Treatment of tattoo allergy with ablative fractional resurfacing: a novel paradigm for tattoo removal. Journal of the American Academy of Dermatology. 2011;64(6):1111–1114.
Izikson L, Nelson JS, Anderson RR, et al. Treatment of hypertrophic and resistant port wine stains with a 755 nm laser: a case series of 20 patients. Lasers in Surgery and Medicine. 2009;41(6):427–432.
Katz TM, Goldberg LH, Friedman PM, et al. Fractional photothermolysis: a new therapeutic modality for xanthelasma. Archives of Dermatology. 2009;145(10):1091–1094.
Kim BJ, Lee DH, Kim MN, et al. Fractional photothermolysis for the treatment of striae distensae in Asian skin. American Journal of Clinical Dermatology. 2008;9(1):33–37.
Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers in Surgery and Medicine. 2004;34(5):426–438.
Sakamoto FH, Doukas AG, Farinelli WA, et al. Selective photothermolysis to target sebaceous glands: Theoretical estimation of parameters and preliminary results using a free electron laser. Lasers in Surgery and Medicine. 2012;44(2):175–183.
Sakamoto FH, Wall T, et al. Lasers and flashlamps in dermatology. In: Wolff K, Goldsmith LA, Katzet SI, et al, eds. Fitzpatrick’s dermatology in general medicine, vol II. Columbus: The McGraw-Hill Companies, Inc.; 2007:2263–2279.
Tannous ZS, Astner S. Utilizing fractional resurfacing in the treatment of therapy-resistant melasma. Journal of Cosmetic Laser Therapy. 2005;7(1):39–43.
Wenzel S, Landthaler M, Baumler W, et al. Recurring mistakes in tattoo removal. A case series. Dermatology. 2009;218(2):164–167.
Zenzie HH, Altshuler GB, Smirnov MZ, et al. Evaluation of cooling methods for laser dermatology. Lasers in Surgery and Medicine. 2000;26(2):130–144.