Chapter 6 Ultrasound of the Brachial Plexus
The use of ultrasound for imaging the brachial plexus and its terminal branches has significantly increased since the first description of its use in anesthesiology for plexus blockade in 1989.1 With continuous improvement in imaging technology and evidence of the benefits of ultrasound for interventional approaches to, and diagnostic evaluation of, the brachial plexus, it is certain that the technology will increase its penetration into the routine clinical practice of anesthesiologists, neurologists, and musculoskeletal specialists in the coming years.
Equipment, Terminology, and Limitations of Ultrasound
The use of ultrasound for imaging of the brachial plexus has advantages over other imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI). Ultrasound equipment is relatively inexpensive and portable, and can be brought to the bedside for examination in patients who cannot readily be transported. The imaging of vascular and soft tissues around the plexus is excellent, and ultrasound allows for dynamic imaging with the upper extremity in various positions (as is done during imaging of the ulnar nerve at the elbow described in Chapter 5.) This dynamic imaging is ideal for interventional procedures with ultrasound guidance of needle placement, such as local anesthetic injection. Disadvantages of ultrasound imaging include the loss of resolution when imaging deeper structures requiring lower-frequency transducers, and the difficulty of imaging around bony structures, such as the intervertebral foramina, where bone will not allow penetration of ultrasound energy. An additional disadvantage is the requirements for knowledge, training, and perhaps certification in this new imaging modality for nonradiologists. The American and European Societies of Regional Anesthesia have made recommendations for ultrasound training curricula for residents and postgraduate practicing physicians.2
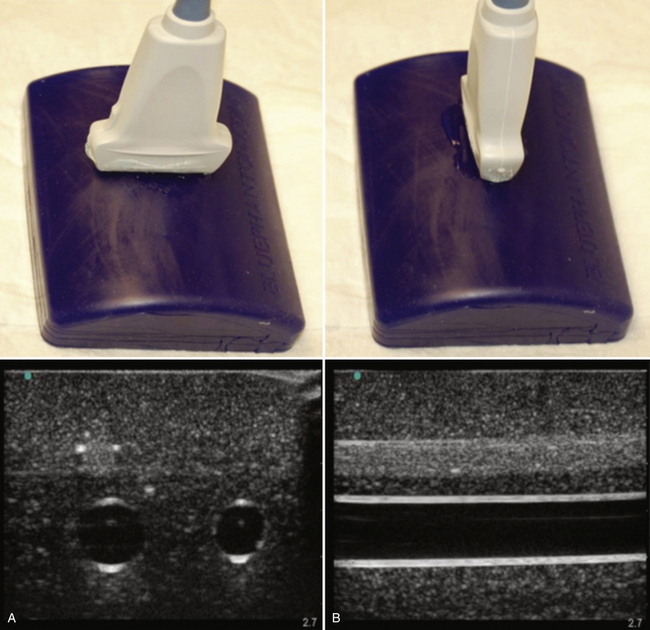
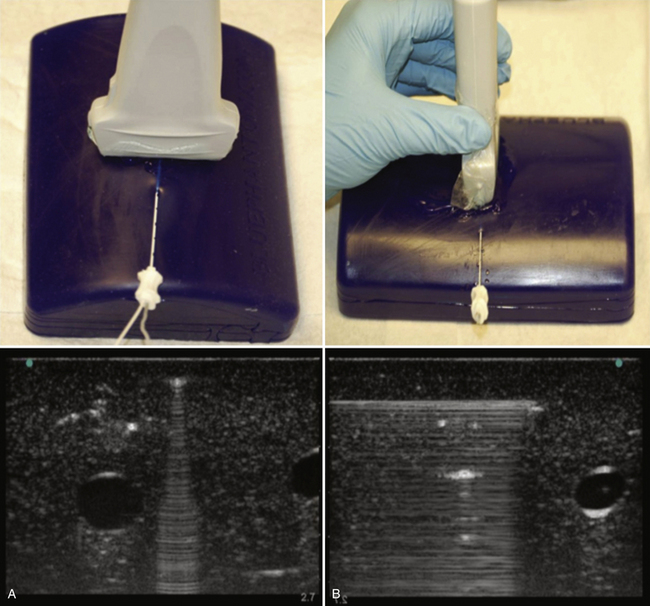
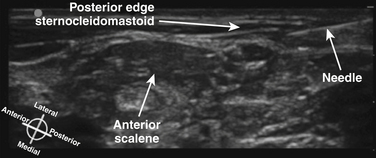
The terminology used for imaging and ultrasound-guided nerve block describes the probe-to-target orientation and the probe-to-needle orientation. Nerves and vascular structures are most easily located in a cross-sectional view, also known as short-axis. Because nerves display some anisotropy (see Chapter 1), a probe inclination of 90 degrees to the course of the target nerve produces the best image. After locating a nerve using the short-axis transducer position, rotation of the probe on its axis at 90 degrees produces a longitudinal or long-axis view (Fig. 6.1). Once a target nerve is identified in short-axis or long-axis view, a needle may be inserted using ultrasound guidance. If the needle is inserted perpendicular to the ultrasound plane such that only a bright spot is seen as the needle crosses the beam, this is known as out-of-plane. If the needle is inserted parallel to the ultrasound plane such that the tip and needle shaft are seen throughout the insertion, this is known as in-plane (Fig. 6.2). Both out-of-plane and in-plane techniques are used by anesthesiologists, each having advantages and disadvantages; neither has been proved to be superior.
Ultrasound Appearance of Tissues
The ultrasonographic appearance of normal nerves and other tissues has been well described.3 The epineurium and connective tissue within nerves appear hyperechoic, and nerve fascicles are relatively hypoechoic. In short-axis, nerves appear honeycomb like (Fig. 6.3), and in long-axis a hyperechoic epineurium is seen at the edges, with discontinuous internal hypoechoic tubular structures representing the fascicles (Fig. 6.4). This ultrasonographic appearance has been closely correlated to the histologic appearance.4 Transducer manipulation, particularly tilting, may also influence the hyper- or hypoechogenicity of nerve tissue, and this property is known as anisotropy. Pathologic nerves generally are enlarged, with a homogeneous hypoechoic appearance; the internal hyperechoic connective tissue pattern is lost and this is suggested to be because of peri- or intraneural edema.3 Tendon has an appearance most like nerve tissue, but with finer internal striations and greater anisotropy. Tendon may be distinguished from nerve by moving the ultrasound transducer proximal and distal along the target; nerve varies little, but tendon flattens and blends into muscle.
Normal muscle has a generally hypoechoic ultrasonographic appearance with internal hyperechoic connective tissue striations, and vascular structures are anechoic (blood-containing); arteries show pulsation and are difficult to compress, and veins are easily compressed and may show flow or size changes with respiration. Bone has a hyperechoic cortical surface and transmits little ultrasound energy such that shadowing is apparent deep to the bone surface (Fig. 6.5). Air is also a poor ultrasound conductor, and ultrasound imaging through air-containing structures is unsatisfactory.
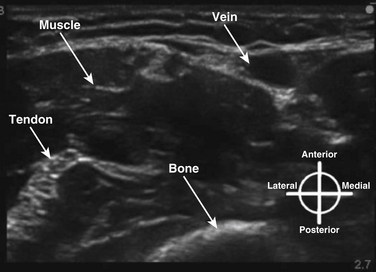
Fig. 6.5 Ultrasound appearance of nonneural tissues, with vein, muscle, tendon, and bone identified.
Supraclavicular Anatomy
The ultrasonographic appearance of the brachial plexus is best understood by first reviewing the gross anatomic three-dimensional (3D) appearance of the elements of the plexus and adjacent structures. With this knowledge, the expected appearance in sequential two-dimensional anatomic “cuts” of the brachial plexus can be predicted, and correlated with the ultrasound images at the same location. A review paper has recently shown the correlations of anatomic cross section, histology, and ultrasound appearance of the brachial plexus.5 Many of the gross anatomic cross-sectional images included here as figures were made after access of the brachial artery and basilic vein at the elbows of unembalmed cadavers. Fluid infused under pressure was used to distend and identify vessels during ultrasound imaging. Later, injection of liquid red and blue latex into the arterial and venous systems was performed prior to freezing and sectioning for cross-sectional specimens.
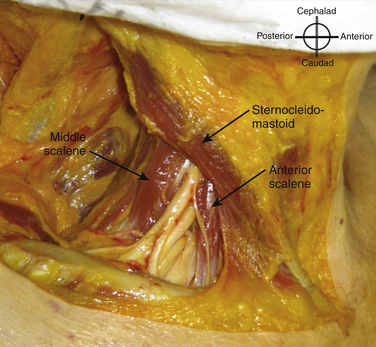
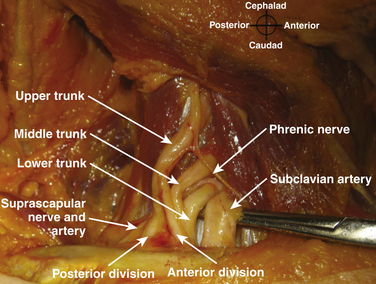
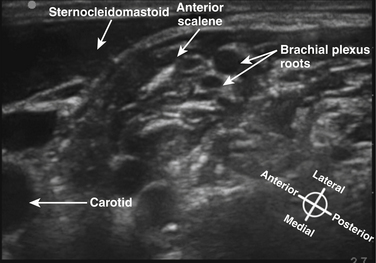
The roots exit the cervical spine sandwiched between the anterior scalene muscle, originating from the anterior tubercles of the transverse processes of C3-6 and inserting on the first rib between the subclavian artery and vein, and the middle scalene muscle, originating from the posterior tubercles of the transverse processes of C2-7 and inserting on the first rib posterior to the plexus (Fig. 6.6). Anterior to the anterior scalene muscle and deep to the sternocleidomastoid muscle is the carotid sheath containing the common carotid artery and internal jugular vein. The formation of the upper trunk from C5 and C6, the middle trunk as the continuation of C7, and the lower trunk from C8 and T1 are commonly described at the lateral edge of the scalene muscles. The three trunks cross the first rib closely approximated to the posterior surface of the subclavian artery, and this is where the plexus is most compact and readily and completely blocked by injection of local anesthetic; this is known as a supraclavicular block (see following section). Each trunk separates into anterior and posterior divisions, and these divisions may occur proximal (Fig. 6.7), on, or distal to the first rib.
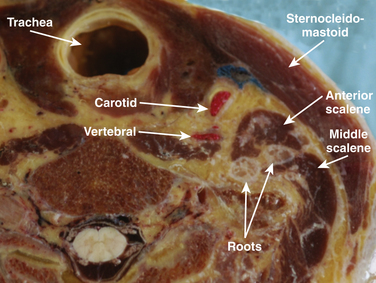
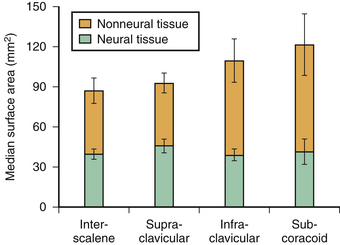
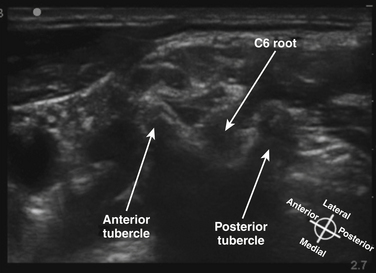
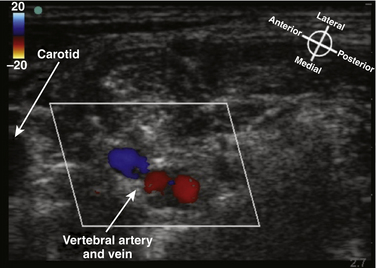
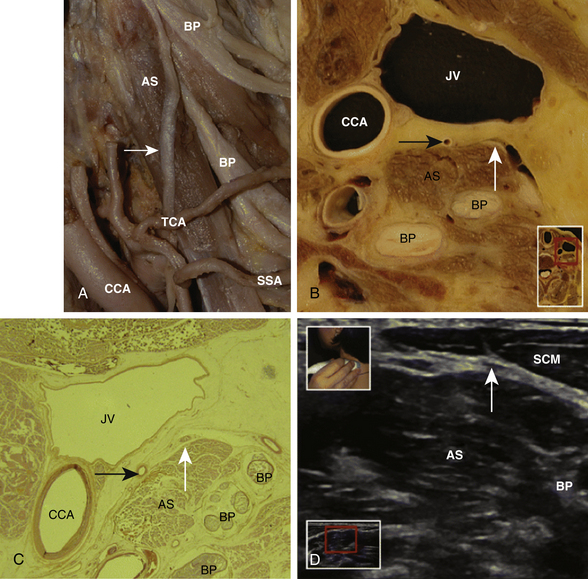
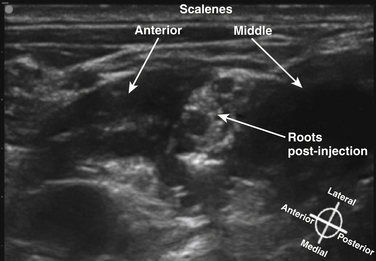
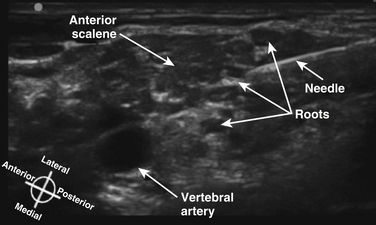
The cross-sectional anatomic appearance of the neck can be predicted from the previously described relationships, and correlated with the ultrasound appearance. If an anatomic section is made, or a high-frequency ultrasound probe positioned short-axis to the course of the brachial plexus roots at approximately the level of the cricoid cartilage (C6 level) (Fig. 6.8), the trachea is seen anteriorly, adjacent to the (usually) homogeneous hyperechoic thyroid gland. Posterolateral to the thyroid is the carotid sheath, and the common carotid artery, internal jugular vein, and vagus nerve may be identified. Posterolateral to the carotid sheath, the scalene muscles and brachial plexus are located deep to the sternocleidomastoid. The cross-sectional appearance of the scalene muscles is characteristic and helps identify the cervical roots passing between them (Fig. 6.9). The best ultrasound image is usually obtained with a modest caudad inclination of the transducer such that it is perpendicular to the plexus. The roots appear as round or oval hypoechoic structures with few internal echoes. A significantly higher fascicle–to–connective tissue ratio above the clavicle compared with below has been demonstrated (Fig. 6.10), and because the epineurium and connective tissue separating the hypoechoic fascicles is hyperechoic, this may explain the relative hypoechoic appearance of brachial plexus elements above the clavicle compared with a hyperechoic appearance distal to the clavicle (Fig. 6.11).6 The transverse processes of the cervical spine can be identified by their hyperechoic cortical edge and shadowing deep to their surface, with the root seen between the anterior and posterior tubercles (Fig. 6.12). The characteristic absence of the anterior tubercle of C7 has been used to identify the level of the roots imaged.7 The vertebral artery and vein are often seen adjacent to the C7 transverse process and can also be useful in identifying root level (Fig. 6.13). Once the root level is determined, the probe may be rotated 90 degrees to achieve a long-axis view and visualize the root exit zones (Fig. 6.14). The phrenic nerve is sometimes seen adjacent to the C5 root, diverging from it anteriorly and traveling just superficial to the anterior scalene fascia as the transducer is moved caudad (Fig. 6.15).8
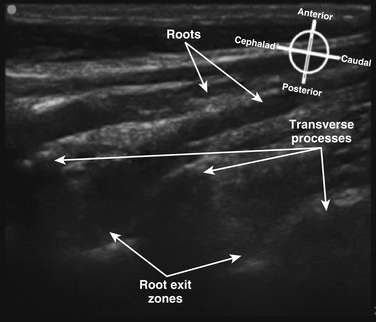
Fig. 6.14 Ultrasound long-axis view of the brachial plexus roots in the neck, achieved by 90-degree rotation of the probe after obtaining the short-axis view shown in Figure 6.11. The roots curve out from the transverse processes and become more superficial distally.
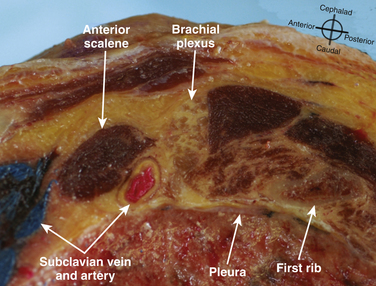
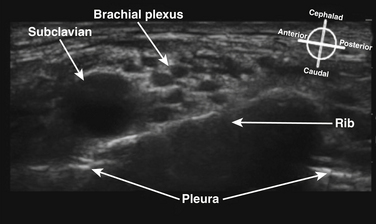
The cross-sectional appearance of the plexus at the base of the neck with the transducer aimed somewhat medial but mostly caudad demonstrates the first rib, pleura, scalene muscles, and subclavian artery and vein (Fig. 6.16). The long axis of the transducer is more parallel to the base of the neck than the clavicle, but adjustments in rotation and inclination are necessary to obtain the best image (Fig. 6.17). At this site, although three distinct trunks are often seen at dissection, the ultrasound appearance is more commonly similar to a cluster of grapes without clear distinction between the trunks (Fig. 6.18). Three arteries may also be seen at this level: the transverse cervical and suprascapular arteries superficial to (sometimes through) the plexus (Fig. 6.19), and the dorsal scapular artery, which most commonly passes through the plexus between the C7 and C8 roots (middle and lower trunk) (Fig. 6.20).
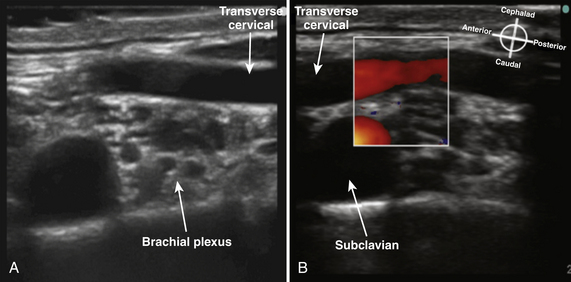
Fig. 6.19 Ultrasound short-axis view of the brachial plexus as in Figure 6.18, with (B) and without (A) Doppler to demonstrate the large transverse cervical artery crossing superficial to the plexus.
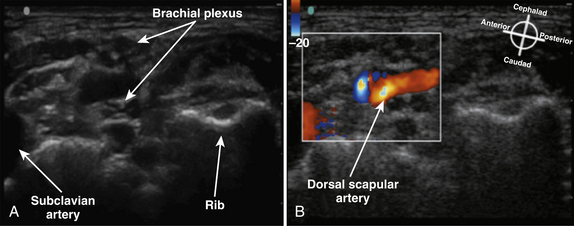
Fig. 6.20 Ultrasound short-axis view of the brachial plexus as in Figure 6.18, with (B) and without (A) Doppler to demonstrate the dorsal scapular artery crossing through the brachial plexus.
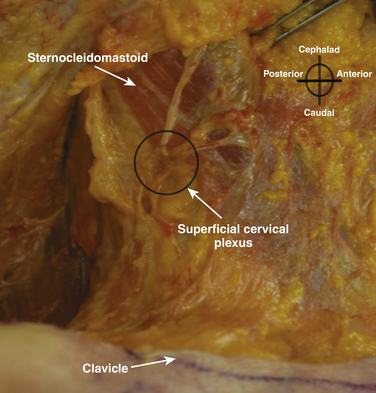
Although not part of the brachial plexus, the superficial cervical plexus is also important for cutaneous innervation of the neck, clavicle, and proximal shoulder. It originates from the anterior rami of C2-4, and is tightly bundled as it passes around the posterior midpoint of the sternocleidomastoid before its terminal branches diverge (Fig. 6.21). The sternocleidomastoid is readily identified in cross section by its hypoechoic appearance, internal striations, and sharp posterior edge. Although the actual branches of the superficial cervical plexus may be challenging to identify, ultrasound is used to guide local anesthetic injection deep to the posterior edge of the sternocleidomastoid to produce block (Fig. 6.22).
Infraclavicular Anatomy
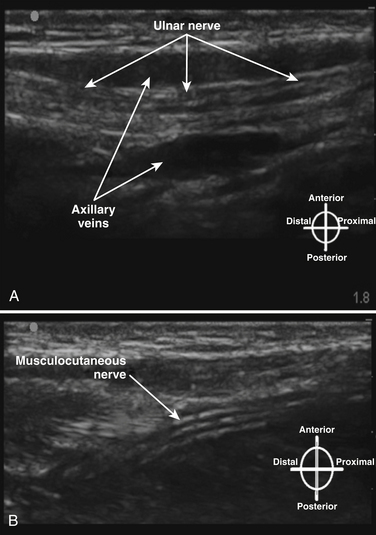
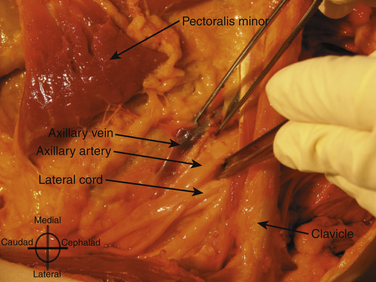
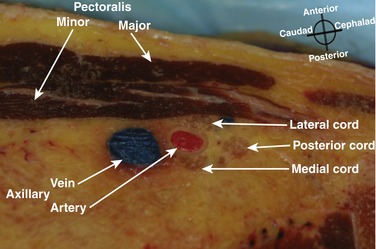
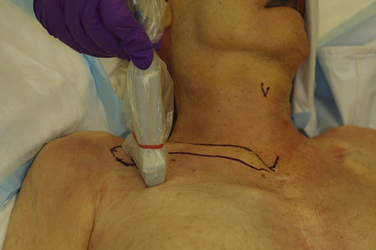
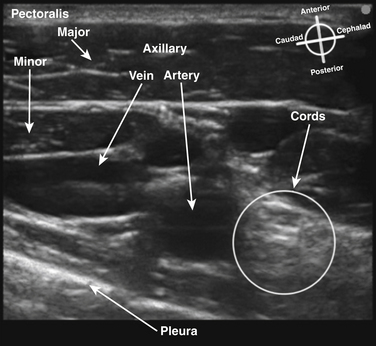
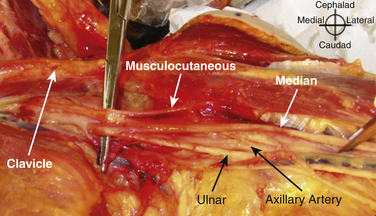
The brachial plexus trunks or divisions at or beyond the level of the first rib reform into the cords in the infraclavicular fossa. These cords twist around the axillary artery in a characteristic pattern deep to the pectoralis major and minor muscles; ultimately 13 terminal branches arise from the infraclavicular brachial plexus. The lateral cord, formed from the anterior divisions of the upper and middle trunk, and, therefore carrying mainly fibers from the C5 to C7 cervical roots, follows the superolateral surface of the axillary artery until dividing into the musculocutaneous nerve and the lateral branch to the median nerve (Fig. 6.23). The lateral cord also gives off the lateral pectoral nerve, which innervates the pectoralis major muscle. The medial cord, formed from the anterior division of the lower trunk and carrying fibers from C8 and T1, twists around the artery from its deep to its anteromedial surface to lie between the axillary vein and artery distal to the coracoid process. It gives rise to the medial brachial and antebrachial cutaneous nerves, the medial pectoral nerve to the pectoralis minor muscle, the medial branch to the median nerve, and its terminal branch, the ulnar nerve. The posterior cord, formed from the posterior divisions of all three trunks and carrying fibers from C5 to T1, also twists around the artery from its position deep to the artery but cephalad to the lateral and medial cords near the first rib, and ultimately is positioned posterior to the axillary artery. It gives rise to the axillary nerve, the thoracodorsal and subscapular nerves, the terminal branch, and the radial nerve. This pattern of the medial and posterior cords twisting around the artery was described after MRI of the plexus from the clavicle to the base of the axilla.9 The ultrasound appearance is predicted from the anatomic cross section showing pectoralis muscles, axillary vessels, and the three cords (Fig. 6.24). The probe is positioned short-axis to the course of the artery (Fig. 6.25), and the ultrasound image readily demonstrates artery, vein, pectoralis muscles, and lateral cord, but only rarely shows all three cords (Fig. 6.26).
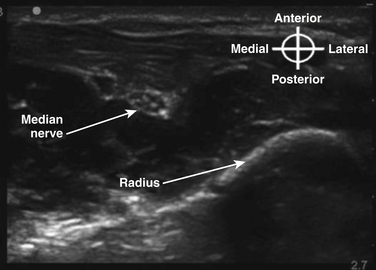
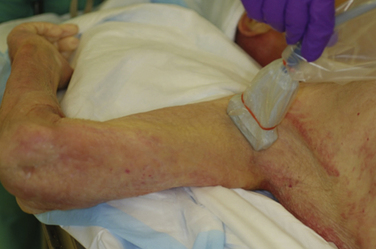
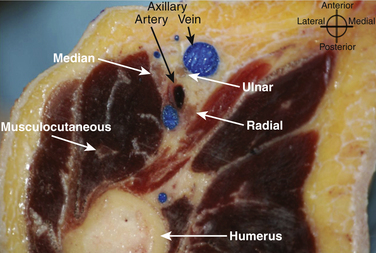
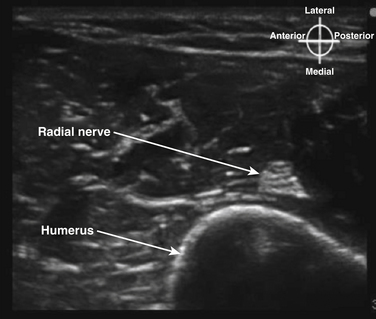
In the axilla, the terminal branches of the brachial plexus can be identified using a short-axis transducer position proximal on the arm (Fig. 6.27). The terminal nerves are followed distal from the axilla toward the elbow for positive identification (Fig. 6.28). The four nerves can be seen in anatomic cross section (Fig. 6.29). The axillary artery and multiple axillary veins are identified, and the median nerve may be found superficial and lateral from the artery just below the deep fascia of the arm. If the nerve is followed caudad, it remains adjacent to the artery and crosses superficial to it to be found on the ulnar side of the artery at the elbow. The ulnar nerve is superficial and usually medial or caudad to the artery in the axilla, sometimes separated from it by an axillary vein. If followed distal, it remains just below the deep fascia of the arm but diverges from the artery and is followed to the olecranon groove at the elbow. The radial nerve is usually deep to the artery, and if followed distal it diverges from the artery and moves deep to pass around the medial side of the humerus, usually accompanied by the deep brachial artery. The radial nerve can be located again lateral to the lateral epicondyle just above the elbow between the brachialis and brachioradialis muscles and prior to its division into deep and superficial branches (Fig. 6.30). The musculocutaneous nerve is located at the axilla in the fascial plane between the biceps brachii and coracobrachialis muscles. On ultrasound examination, all four terminal branches of the plexus have a multifascicular, oval, or round appearance with hyperechoic epineurium (Fig. 6.31).
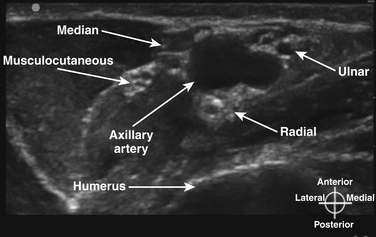
Fig. 6.31 Ultrasound short-axis view with the probe positioned as in Figure 6.27, demonstrating the four terminal branches of the plexus with the most common orientation relative to the axillary artery.
The anatomic location and appearance of the terminal branches of the brachial plexus described above is the most typical pattern, but “most typical” is seen in only 65% of individuals, with frequent variations in the relative position of the four nerves around the artery.10 In fact, there is considerable variability in the anatomy of the vessels, muscles, and nerves from the cervical spine to the axilla; ultrasound is useful in identifying such variation once the “normal” pattern is appreciated by the ultrasonographer.11
Solution Injection and Effects on Imaging and Stimulation
The injection of any fluid into the perineural tissue often tracks around the nerve, and the difference in acoustic impedance between the nerve and the injected fluid enhances the ability to image the nerve (Fig. 6.32). This property is routinely used during local anesthetic injection to ensure the nerve is completely surrounded by the anesthetic. Conversely, the injection of any air degrades the image substantially, because of the poor acoustic transmission properties of air and should be avoided during interventional techniques.
During ultrasound-guided nerve block, anesthesiologists commonly also use nerve stimulation to confirm the identity of mixed motor and sensory nerves. If saline is injected, it has been determined that current is dispersed and the motor stimulation is inhibited, but that 5% dextrose in water (D5W) injection has the opposite effect of eliminating current dispersion at the needle tip and enhancing motor stimulation.12
Indications for Ultrasound Imaging of the Brachial Plexus
Ultrasound-Guided Brachial Plexus Block Techniques Above the Clavicle
Major local anesthetic block of the brachial plexus typically requires a volume of local anesthetic that could, if injected intravenously, result in seizure or cardiovascular collapse. In addition, there are complications resulting from needle injury of structures adjacent to the plexus such as the lungs. Practitioners undertaking such techniques should be well versed in the indications for nerve block at various levels of the plexus, and well trained or supervised and skilled in cardiopulmonary support, treatment of local anesthetic toxicity, and treatment of other complications of neural blockade.
The interscalene block uses the injection of local anesthetic (or catheter placement) at the upper roots or upper trunk level between the scalene muscles for anesthesia or analgesia of the shoulder or proximal humerus (Fig. 6.33). In the postinjection figure, the enhancement of the upper trunk after injection of local anesthetic is readily apparent (Fig. 6.34). The lower trunk is not reliably blocked with this approach. The block has historically resulted in a 100% incidence of hemidiaphragmatic paralysis from phrenic blockade. In addition, the proximity of the needle to the neuraxis has resulted in rare epidural or intracord injection using nerve stimulator techniques13 and, although controversial, the performance of this block in unconscious patients is generally discouraged. Ultrasound-guided interscalene block commonly uses a short-axis, in-plane technique with the needle directed tangential to the cervical spine rather than toward it, and may convey some safety advantage but this will be challenging to prove.14 Studies comparing ultrasound guidance with nerve stimulation have shown success of a reduced dose of local anesthetic using ultrasound, and a lower risk of phrenic block.15,16 Unfortunately even if the phrenic block risk is less likely, it cannot be predicted which patients will develop this side effect, and the block is relatively contraindicated in patients who cannot tolerate a 25% to 30% reduction in vital capacity. Interscalene block also sometimes results in spread of local anesthetic to the sympathetic chain with resultant Horner’s syndrome, and spread to the recurrent laryngeal nerve with ipsilateral vocal cord paresis or paralysis.
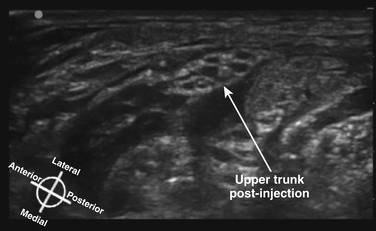
Fig. 6.34 Ultrasound short-axis view of the same patient shown in Figure 6.33, after the injection of local anesthetic, which surrounds and enhances the upper trunk.
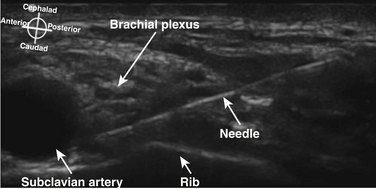
There has been a great resurgence of interest in supraclavicular block, or brachial plexus block at the level of the first rib and subclavian artery since the introduction of ultrasound guidance (Fig. 6.35). Historical approaches to supraclavicular block prior to ultrasound guidance had a reported risk of pneumothorax from 0.6% to 6%. The ability to visualize the first rib and pleura has almost certainly reduced the likelihood of pneumothorax with this approach, but proof of this assumption would require a very large study. In addition, elimination of the risk of pneumothorax requires the ability to image the rib and pleura well, and the ability to maintain continuous awareness of the needle tip location. Supraclavicular block provides complete block of T1 to C5 routinely, but still carries some risk of phrenic, recurrent laryngeal nerve and sympathetic chain blockade. One study showed a specific ultrasound-guided technique eliminated the risk of phrenic block.17
Ultrasound-Guided Brachial Plexus Block Techniques Below the Clavicle
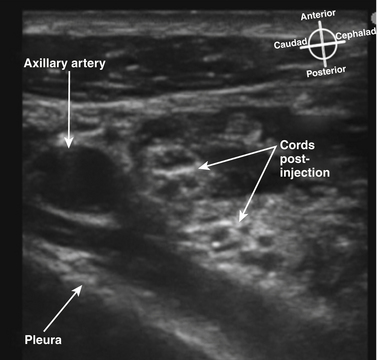
The infraclavicular block is performed at the level of the cords of the plexus, and provides surgical anesthesia of the elbow, forearm, hand, and upper inner arm. The axillary artery, vein, and pleura are readily visualized with ultrasound, and the lateral cord superficial and cephalad to the artery and deep to the pectoralis major and minor is usually apparent (Fig. 6.36). In larger patients, these structures may be at a depth of 5 to 8 cm from the skin, however, and require a lower-frequency transducer for adequate penetration. The posterior and medial cords, unlike the lateral cord, are more difficult to image, and the block is performed as a periarterial injection rather than an injection requiring visualization of specific nerve tissue. Because the cords are closely apposed to the artery, injection of local anesthetic at the posterior surface of the artery with spread around the cephalad surface reliably results in successful blockade.18 In many studies, the use of ultrasound guidance has resulted in reduced rate of vascular puncture when compared with nerve stimulator guidance, and this advantage of ultrasound guidance has been demonstrated at other block sites as well. There is almost no risk of phrenic or recurrent laryngeal nerve block with this approach, because local anesthetic does not appear to spread proximally above the first rib. Pneumothorax has, however, been reported with ultrasound-guided infraclavicular block when the operator failed to continuously visualize the needle tip.19
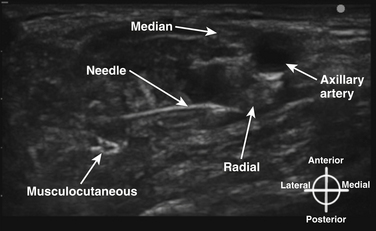
The axillary block is a block of the terminal nerves of the brachial plexus, and because these branches begin to diverge in the axilla, it requires multiple injections whether or not ultrasound guidance is used. Complete axillary blockade results in anesthesia of the medial arm and medial elbow, as well as the forearm and hand. The axillary artery is first identified with ultrasound, and each of the four terminal nerves is visualized and surrounded with a small amount of local anesthetic (Fig. 6.37). Nerve identity is confirmed by following the distal course of each nerve, or by nerve stimulation. Ultrasound-guided axillary block has been shown to have a higher success rate than a transvascular approach20 and reduced paresthesia during performance.21 Historically, this block has been performed with large volumes of local anesthetic such as 10 mL per nerve. It has recently been shown, with an up-down titration approach in volunteers, that only 1 mL of local anesthetic per nerve is needed to produce successful axillary block using ultrasound guidance.22
Ultrasound for Evaluation of Brachial Plexus Pathology
Ultrasound demonstrated a surgical injury to the upper trunk, which was not evident on MRI scanning and led to reexploration and repair.23 A report of ultrasound evaluation of brachial plexus traction injuries in 4 patients concluded it was “useful” as a bedside evaluation tool.24 A series of 28 patients with proximal brachial plexus pathology including trauma and tumor was reported, and characteristic ultrasound appearance of abnormal plexus elements was described; the technique was again felt to be useful, but it was concluded that MRI was necessary in most cases.25
A large prospective evaluation demonstrated the usefulness of ultrasound for assessment of the supraclavicular brachial plexus. The study included 221 consecutive patients over 3.5 years referred for brachial plexus ultrasound by a group of three radiologists. A high-frequency (12-5 and 17-9 MHz) standardized ultrasound examination was performed to assess four roots and three trunks bilaterally in each patient. A subset of 12 patients with brachial plexus trauma who were subsequently explored was evaluated to determine the accuracy of the ultrasound examination in detecting major and minor nerve lesions (Fig. 6.38). There were high sensitivity and specificity of the ultrasound examination with failure to identify only 5 minor lesions out of 89 major and minor lesions. The authors concluded the high-resolution ultrasound examination should be considered first-line for brachial plexus trauma, and should be performed early to facilitate early exploration.26
As mentioned, the necessary training, experience, and, perhaps, certification needed for nonradiologists to perform diagnostic evaluations using ultrasound are subjects that have yet to be addressed.
Conclusion
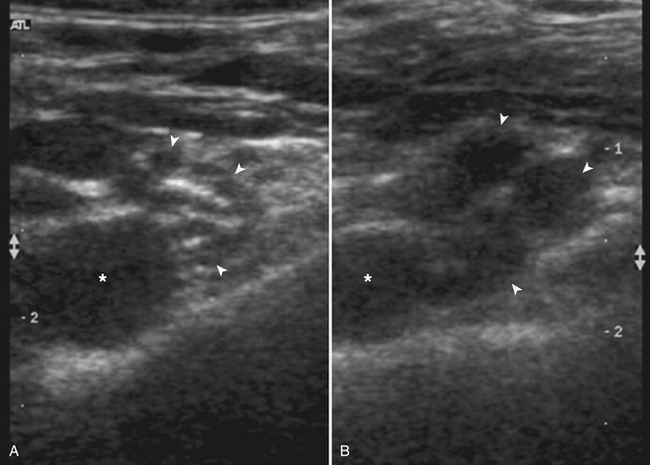
Interventional ultrasound for local anesthetic injection around the brachial plexus (and other nerves) has contributed substantially to our knowledge of older nerve localization techniques, and will continue to do so. Over the past two decades nerve localization was performed using electrical stimulation of mixed sensorimotor nerves, or the elicitation of a sensory paresthesia as a needle contacted a nerve. It was assumed that current density was homogeneous and inversely proportional to the square of the distance from the needle to the nerve, such that closer and closer approximation of needle tip to nerve resulted in stronger motor response prior to needle to nerve contact. Both of these “truths” have been disproved, and needle-nerve contact has been readily demonstrated using ultrasound imaging without either paresthesia or motor response.27 Similar findings were shown in a study of radial and ulnar nerve stimulation and needle-to-nerve proximity as measured by ultrasound. Contact of the needle with both nerves did not reliably produce motor stimulation at low stimulating current.28 It is now accepted that epineurial puncture may be relatively common using older localization techniques, but fortunately does not typically result in fascicular puncture or nerve injury. In fact, in a study of axillary block using a paresthesia-seeking technique but monitoring needle position and local anesthetic injection using ultrasound, a full 69% of nerves were “punctured,” as evidenced by nerve swelling with injection (Fig. 6.39). On careful postoperative follow-up at 48 and 96 hours, 3 weeks and 6 months, respectively, none of the 26 patients demonstrated any nerve dysfunction.29
Of course there will be technological advances in ultrasound equipment and processing. Improved tissue resolution will enhance interventional techniques, but the greatest challenge in this area is needle visualization, particularly in deeper structures or with steeper needle angles to the ultrasound plane. Acoustic radiation force impulse imaging has been studied and shown to improve nerve and needle visualization,30 and spatial positioning has been introduced to track needle placement on-screen. It would seem intuitive that 3D or volume imaging would eliminate the challenge of visualizing in-plane needle placement, but current 3D transducers are costly and have not simplified or improved ultrasound-guided nerve block and are continually being refined. The current limitation of 7 MHz for 3D transducers reduces resolution and the quality of the image, but higher-frequency transducers are likely to soon be available.31 Alternatives to piezoelectric crystals for ultrasound generation are in development and may affect transducer size, and eventually wireless transducers should make scanning much less cumbersome.
1. Ting P.L., Sivagnanaratnam V. Ultrasonographic study of the spread of local anaesthetic during axillary brachial plexus block. Br J Anaesth. 1989;63:326-329.
2. Sites B.D., Chan V.W., Neal J.M., et al. The American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anaesthesia and Pain Therapy Joint Committee recommendations for education and training in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2009;34:40-46.
3. Martinoli C., Bianchi S., Derchi L.E. Ultrasonography of peripheral nerves. Semin Ultrasound CT MR. 2000;21:205-213.
4. Kubiena H., Hörmann M., Michlits W., et al. Intraoperative imaging of the brachial plexus by high-resolution ultrasound. J Reconstr Microsurg. 2005;21:429-433.
5. van Geffen G.J., Moayeri N., Bruhn J., et al. Correlation between ultrasound imaging, cross-sectional anatomy, and histology of the brachial plexus: a review. Reg Anesth Pain Med. 2009;34:490-497.
6. Moayeri N., Bigeleisen P.E., Groen G.J. Quantitative architecture of the brachial plexus and surrounding compartments, and their possible significance for plexus blocks. Anesthesiology. 2008;108:299-304.
7. Martinoli C., Bianchi S., Santacroce E., et al. Brachial plexus sonography: a technique for assessing the root level. AJR Am J Roentgenol. 2002;179:699-702.
8. Canella C., Demondion X., Delebarre A., et al. Anatomical study of phrenic nerve using ultrasound. Eur Radiol. 2010;20:659-665.
9. Bigeleisen P., Wilson M. A comparison of two techniques for ultrasound guided infraclavicular block. Br J Anaesth. 2006;96:502-507.
10. Christophe J.L., Berthier F., Boillot A., et al. Assessment of topographic brachial plexus nerves variations at the axilla using ultrasonography. Br J Anaesth. 2009;103:606-612.
11. Soeding P., Eizenberg N. Review article: anatomical considerations for ultrasound guidance for regional anesthesia of the neck and upper limb. Can J Anaesth. 2009;56:518-533.
12. Tsui B.C., Wagner A., Finucane B. Electrophysiologic effect of injectates on peripheral nerve stimulation. Reg Anesth Pain Med. 2004;29:189-193.
13. Benumof J.L. Permanent loss of cervical spinal cord function associated with interscalene block performed under general anesthesia. Anesthesiology. 2000;93:1541-1544.
14. Mariano E.R., Loland V.J., Ilfeld B.M. Interscalene perineural catheter placement using an ultrasound-guided posterior approach. Reg Anesth Pain Med. 2009;34:60-63.
15. Renes S.H., Rettig H.C., Gielen M.J., et al. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009;34:498-502.
16. Riazi S., Carmichael N., Awad I., et al. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101:549-556.
17. Renes S.H., Spoormans H.H., Gielen M.J., et al. Hemidiaphragmatic paresis can be avoided in ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med. 2009;34:595-599.
18. Dingemans E., Williams S.R., Arcand G., et al. Neurostimulation in ultrasound-guided infraclavicular block: a prospective randomized trial. Anesth Analg. 2007;104:1275-1280.
19. Koscielniak-Nielsen Z.J., Rasmussen H., Hesselbjerg L. Pneumothorax after an ultrasound-guided lateral sagittal infraclavicular block. Acta Anaesthesiol Scand. 2008;52:1176-1777.
20. Sites B.D., Beach M.L., Spence B.C., et al. Ultrasound guidance improves the success rate of a perivascular axillary plexus block. Acta Anaesthesiol Scand. 2006;50:678-684.
21. Soeding P.F., Sha S., Royse C.E., et al. A randomized trial of ultrasound-guided brachial plexus anaesthesia in upper limb surgery. Anaesth Intensive Care. 2005;33:719-725.
22. O’Donnell B.D., Iohom G. An estimation of the minimum effective anesthetic volume of 2% lidocaine in ultrasound-guided axillary brachial plexus block. Anesthesiology. 2009;111:25-29.
23. Shafighi M., Gurunluoglu R., Ninkovic M., et al. Ultrasonography for depiction of brachial plexus injury. J Ultrasound Med. 2003;22:631-634.
24. Haber H.P., Sinis N., Haerle M., et al. Sonography of brachial plexus traction injuries. AJR Am J Roentgenol. 2006;186:1787-1791.
25. Graif M., Martinoli C., Rochkind S., et al. Sonographic evaluation of brachial plexus pathology. Eur Radiol. 2004;14:193-200.
26. Gruber H., Glodny B., Galiano K., et al. High-resolution ultrasound of the supraclavicular brachial plexus: can it improve therapeutic decisions in patients with plexus trauma? Eur Radiol. 2007;17:1611-1620.
27. Perlas A., Niazi A., McCartney C., et al. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31:445-450.
28. Sauter A.R., Dodgson M.S., Stubhaug A., et al. Ultrasound controlled nerve stimulation in the elbow region: high currents and short distances needed to obtain motor responses. Acta Anaesthesiol Scand. 2007;51:942-948.
29. Bigeleisen P.E. Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology. 2006;105:779-783.
30. Palmeri M.L., Dahl J.J., MacLeod D.B., et al. On the feasibility of imaging peripheral nerves using acoustic radiation force impulse imaging. Ultrason Imaging. 2009;31:172-182.
31. Clendenen S.R., Riutort K.T., Feinglass N.G., et al. Real-time three-dimensional ultrasound for continuous interscalene brachial plexus blockade. J Anesth. 2009;23:466-468.