Chapter 5 Ultrasound of Focal Neuropathies
Focal neuropathy is a broad term that refers to a discrete peripheral nerve lesion that causes pain, numbness, or weakness. Focal neuropathies are classified by either location or etiology. The location refers to the specific site along the peripheral nerve. The etiology can be further divided into extrinsic and intrinsic causes. Extrinsic causes include any structure compressing and damaging the nerve, such as ganglion cysts, inflamed tendon sheaths (tenosynovitis), anomalous muscles, aberrant or dilated vessels, and benign or malignant tumors. The most common extrinsic cause of focal neuropathy is entrapment of a nerve as it passes through a stiff fibro-osseous tunnel, such as the carpal tunnel in the wrist or the cubital tunnel near the elbow.1 Extrinsic etiologies also include trauma to the nerve, which can result from prolonged compression, acute blunt injury, or penetrating injury. Intrinsic etiologies are less common than extrinsic. Examples of intrinsic causes include neuromas, nerve sheath tumors, and nerve infarct (often associated with vasculitis).
The epidemiology of all focal neuropathies taken together is not known because they are coded and evaluated separately, based on location or etiology. However, by assessing the epidemiology of only the most common types, it is clear that focal neuropathies are prevalent and cause significant morbidity and medical expense. Carpal tunnel syndrome (CTS), or median mononeuropathy at the wrist, is the most common entrapment neuropathy, with an age-adjusted incidence of 105 cases per 100,000 person-years and a prevalence of 3% to 10% in the general population.1,2 It accounts for more than $500 million annually in health-care and indemnity costs in the United States, and individuals with CTS have an average of 84 work days lost.3,4 Ulnar neuropathy at the elbow is the second most common focal neuropathy, with a standardized incidence of 20.9 cases per 100,000 person-years.5 Other common focal neuropathies include ulnar neuropathy at the wrist, radial neuropathy in the spiral groove, and peroneal neuropathy at the fibular head.6,7
The diagnostic approach for an individual with a potential focal neuropathy varies based on presentation, site of neuropathy, suspected etiology, local standard of care, and physician preference. For the most common focal neuropathies, such as CTS or ulnar neuropathy at the elbow, some physicians will make the diagnosis and treatment decisions based on history and examination alone, whereas most prefer to confirm their clinical opinion with nerve conduction studies (NCS) and electromyography (EMG) for any type of focal neuropathy. For cases with an atypical presentation, or neuropathy at a nonentrapment site, electrodiagnostic studies (NCS and EMG) are recommended.8
High-resolution ultrasound has evolved over the past two decades such that small structures, like nerves, are now easily visualized.9,10 It also allows visualization of surrounding structures, such as muscles, tendons, bones, and vessels, as discussed in Chapter 4. Ultrasound addresses the shortcomings of electrodiagnostic studies because it permits direct assessment of extrinsic and intrinsic causes of focal neuropathy and helps confirm localization inferences made from NCS and EMG. In addition, ultrasound is painless.
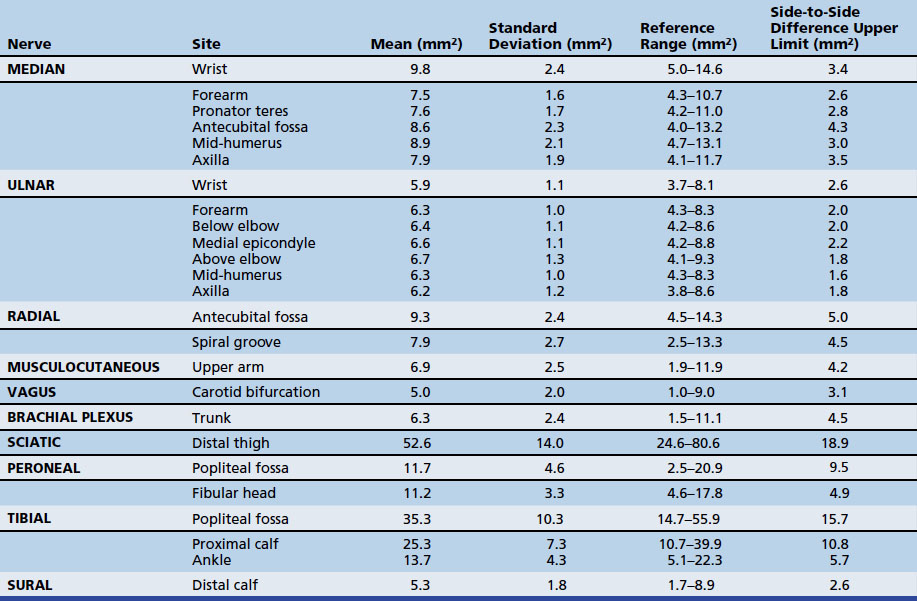
The first study to use ultrasound to assess a focal neuropathy occurred in the early 1990s and was in individuals with CTS.11 Since then, ultrasound has been studied for the diagnosis of focal neuropathies at sites throughout the arm and leg. This chapter describes the techniques used to image focal neuropathies with ultrasound as well as the data supporting its use. The techniques described in this chapter are brief, because the chapter focuses more on pathologic conditions, but more detailed protocols are listed in the Appendix. In addition, it should be noted that the imaging sequences in this chapter often describe the longitudinal view of the nerve first, but the protocols typically suggest starting with a cross-sectional view. As is described later, changes in nerve cross-sectional area are often seen with focal neuropathies, so cross-sectional area reference values are provided (Table 5.1).
Median Nerve
Wrist (Carpal Tunnel Syndrome)
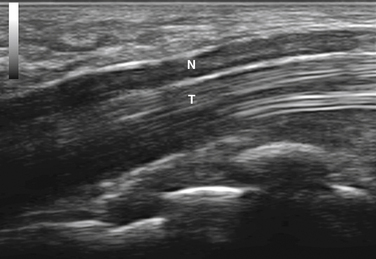
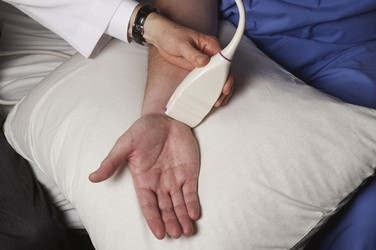
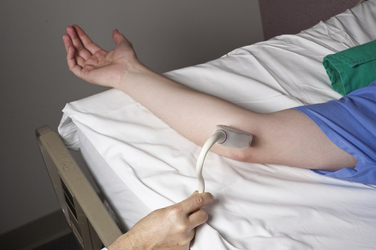
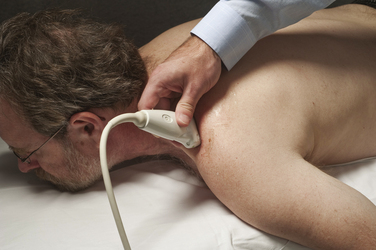
Imaging starts with a sagittal view, by placing the linear array transducer across the wrist (Fig. 5.1). The image obtained in this position includes the median nerve in a longitudinal view, as well as the flexor digitorum superficialis and profundus tendons (Fig. 5.2). This view allows the physician to obtain a general overview of the median nerve and other wrist structures. It also provides an image in which pinching of the median nerve, as it passes under the rigid flexor retinaculum, can be assessed subjectively and objectively.12 In this sagittal view, flexion of the fingers causes sliding of the tendons in a distal-to-proximal plane. In normal conditions, the median nerve also slides in this same plane13 but with less displacement than the tendons (Video 5.1). One study demonstrated decreased longitudinal sliding of the median nerve in those with CTS compared with controls,14 but this finding was not confirmed in a separate report.15
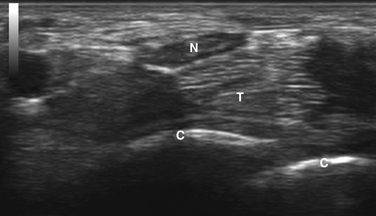
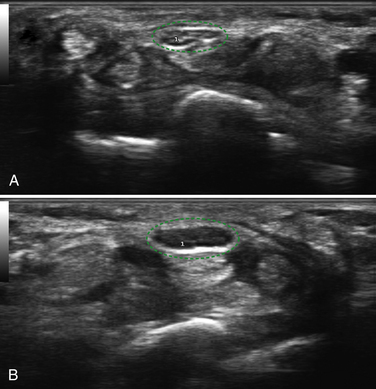
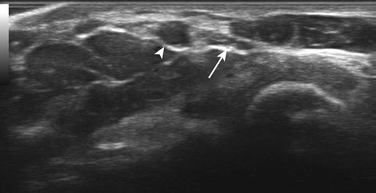
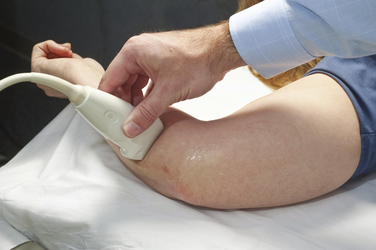
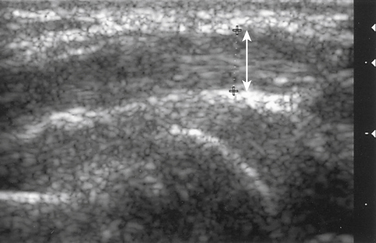
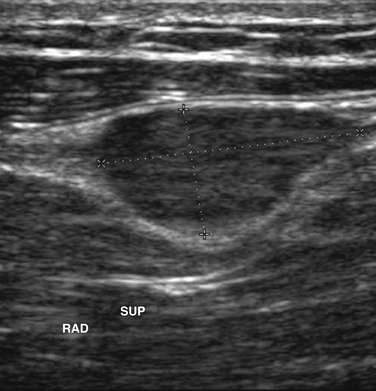
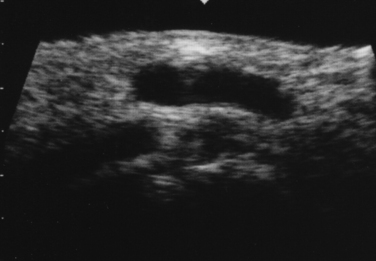
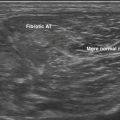
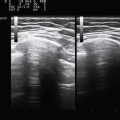
Next, the linear array transducer is rotated 90 degrees and a cross-sectional image is obtained at the level of the distal wrist crease (Fig. 5.3). In this image, the transverse carpal ligament, median nerve, flexor digitorum tendons, and carpal bones can be seen (Fig. 5.4). This is the view in which the majority of objective measurements have been reported. As will be outlined, many different techniques have been described for acquiring measurements that accurately differentiate those with CTS from healthy controls, but the universal finding is that the cross-sectional area of the median nerve is enlarged at the entrance to the carpal tunnel in those with CTS (see Chapter 2 for a list of pathologic processes known to result in nerve enlargement). Initial studies suggested that internal landmarks, such as the hook of the hamate or level of the pisiform bone be used to determine the site at which the cross-sectional area measurement is obtained.11,16 However, subsequent studies have shown that finding the area of maximal median nerve enlargement proximal to the entrance of the tunnel and obtaining the cross-sectional area of the median nerve at this site (Fig. 5.5), are sensitive and specific and require only one cross-sectional area measurement be obtained.17 Some clinicians have suggested that demyelinating polyneuropathies, such as Charcot-Marie-Tooth, which cause diffuse nerve swelling (see Chapter 7), could be mistaken for CTS if only a single measurement of the median nerve cross-sectional area at the wrist is obtained. Therefore, Hobson-Webb and colleagues showed that a ratio of the median nerve area at the wrist to the area of the nerve in the forearm is an accurate method to diagnose CTS and handle the potential problem of diffuse nerve enlargement.18 Their study showed that a ratio greater than 1.4 resulted in a sensitivity of 100% for the diagnosis of CTS. (A similar ratio of greater than 1.4 is also used to diagnose aneurysmal enlargement of the arteries, as described in Chapter 4.)
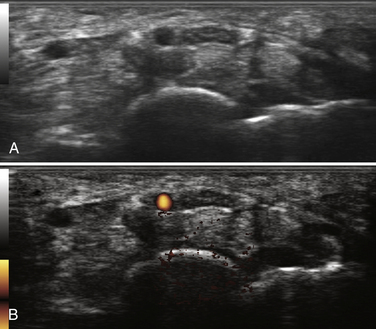
In addition to the cross-sectional area of the median nerve at the wrist, other measurements have been proposed to diagnose CTS with neuromuscular ultrasound (Box 5.1). Nerve flattening, which can be numerically demonstrated by comparing the maximum with the minimum nerve diameter, is present in CTS.19 Bowing of the flexor retinaculum is also reported in CTS.11 Similar to the findings in the longitudinal plane, decreased nerve mobility has been reported with dynamic imaging in a transverse view.20 Median nerve hypervascularity, as detected with color flow Doppler, has been described as a sensitive (95%), and relatively specific (71%) technique for the detection of carpal tunnel syndrome.21 This finding has not yet been confirmed in subsequent studies, and anecdotal experience in the author’s laboratory suggests that this degree of accuracy may be somewhat overinflated, but the observation is valid. The finding of median nerve hypervascularity, which has also been noted with magnetic resonance imaging (MRI),22 may provide insight into the pathophysiology of CTS because it suggests there is a circulatory disturbance in the median nerves of those with CTS. Finally, the median nerve of those with CTS is frequently found to be hypoechoic; however, this finding has not been systemically studied or quantified.23
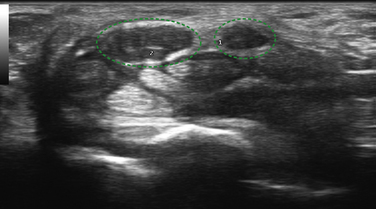
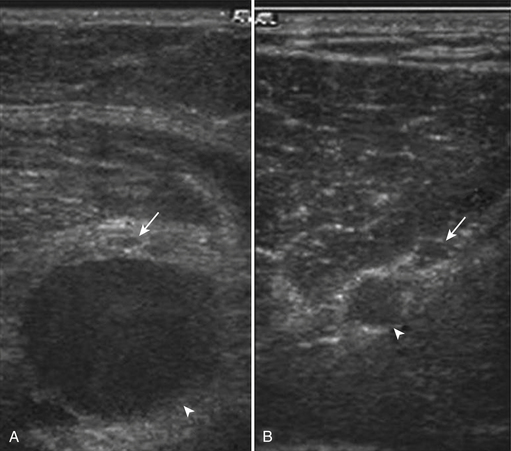
Other anatomic findings have been detected using ultrasound to examine the wrists of those with CTS. One finding that is noted rather commonly, and is estimated to occur in 16% of wrists,24 is the presence of a persistent median artery running through the carpal tunnel (Fig. 5.6; Table 5.2). The relevance of this patent vessel regarding CTS is not known, but there are case reports of thrombosed persistent median arteries leading to CTS.25,26 A finding that at times coexists with a persistent median artery is that of a bifid median nerve (Fig. 5.7). The median nerve typically branches distal to the carpal tunnel, but in 9% of healthy controls and 19% of those with CTS a bifid median nerve is present proximal to, or within, the carpal tunnel (see Table 5.2).27 It is postulated that individuals with bifid median nerves are predisposed to the development of CTS, but this has not been confirmed in a prospective fashion. Of note, hand surgeons are often unable to confirm the presence of a bifid median nerve during open carpal tunnel release, and it is thought that perhaps the epineurium is not completely divided, so it obscures the presence of the early median nerve division.
| Finding | Frequency in Healthy Controls | Frequency in Disease State |
|---|---|---|
| Persistent median artery | 16% | NA |
| Bifid median nerve | 9% | 19% in CTS |
| Ulnar subluxation | 25% | NA |
| Peroneal ganglia | NA | 18% in peroneal neuropathy at fibular head |
CTS, Carpal tunnel syndrome; NA, not available.
Other Sites
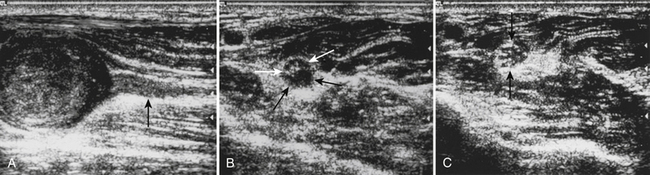
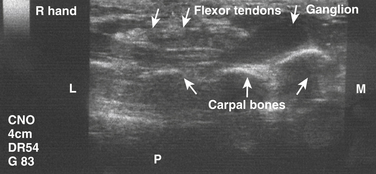
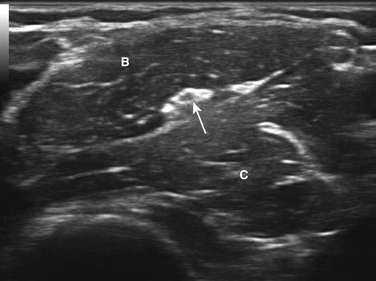
Although the majority of neuromuscular ultrasound research involving the median nerve has focused on the wrist and CTS, there are scattered reports of focal median nerve pathology detected at sites outside the wrist. The technique for imaging these proximal sites varies based on the location of interest, but in general a cross-sectional view is used. In the author’s lab, imaging typically starts with a cross-sectional view at either the wrist or the antecubital fossa, and then the median nerve is tracked farther distal or proximal to the area of interest (Video 5.2). As the median nerve is traced distal into the palm it splits into several branches, which can be challenging to image (Video 5.3). There are two case reports of mid-palm ganglion cysts causing isolated mononeuropathies of the motor branch of the median nerve, which were detected by ultrasound (Fig. 5.8).28,29 The median nerve is more easily seen as it is traced proximal from the wrist. Several case reports have described pathology in the forearm leading to median mononeuropathies, including traction injury, neurilemmoma, and a tendon sheath fibroma (Figs. 5.9 and 5.10).30–32 Other pathologic processes, detected with ultrasound, also have been reported to cause median mononeuropathies in the upper arm, proximal to the antecubital fossa. These include compressive vascular anomalies, neuromas, and hamartomas (Figs. 5.11 and 5.12).33–37
Ulnar Nerve
Wrist (Guyon’s Canal)
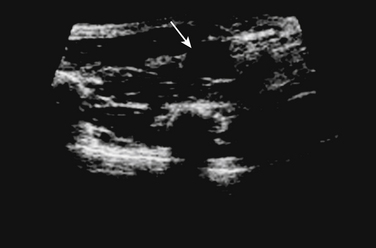
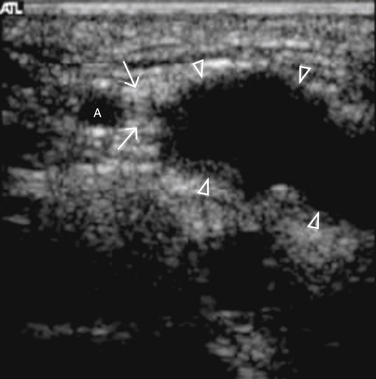
The ulnar nerve is more challenging to visualize at the wrist with ultrasound than the median nerve, but with experience the ulnar nerve can be reliably detected at this site. A sagittal view of the ulnar nerve can be difficult to obtain, therefore a cross-sectional image at the wrist only may be acceptable. The ulnar nerve lies just medial to the ulnar artery at the wrist, which provides a consistent landmark to assist in identifying the nerve (Figs. 5.13 and 5.14). Only case reports and series exist in the literature describing pathology affecting the ulnar nerve at the wrist, but several different pathologies are described. Ultrasound has been used to detect ulnar neuropathy at the wrist (Guyon’s canal) secondary to ganglion cysts,38–40 anomalous muscles,41,42 abnormal ulnar arteries,43,44 and other structural abnormalities such as lipomas (Fig. 5.15; also see Fig. 5.16 online at www.expertconsult.com)45
Elbow
Ulnar neuropathy at the elbow is the second most common entrapment neuropathy, after CTS.5 Nerve conduction studies and EMG are sensitive and specific for the diagnosis of ulnar neuropathy at the elbow,46 but they are not as accurate as they are in CTS, which makes ultrasound an even more attractive diagnostic tool for ulnar neuropathy at the elbow.
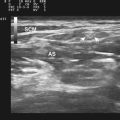
Imaging of the ulnar nerve at the elbow starts with a longitudinal view of the ulnar nerve in the groove between the medial epicondyle and the olecranon process (Fig. 5.17). This view is used to assess for focal nerve enlargement and for abnormal structures extrinsic to the ulnar nerve. As with imaging of the median nerve, the most relevant information is typically obtained with a cross-sectional view of the ulnar nerve. This starts with the transducer placed about 5 cm distal to the medial epicondyle (Fig. 5.18). At this level, the ulnar nerve often has a triangular shape and it is in the cubital tunnel. A cross-sectional nerve measurement can be taken at this site, and then the nerve is followed proximally to about 5 cm proximal to the medial epicondyle, where another cross-sectional measurement is obtained (Video 5.4). As the ulnar nerve is followed over this 10-cm segment, care is made to note the area of maximal enlargement of the ulnar nerve, and a cross-sectional area measurement is obtained at the site of maximal enlargement. Focal enlargement may occur at more than one site in this segment.
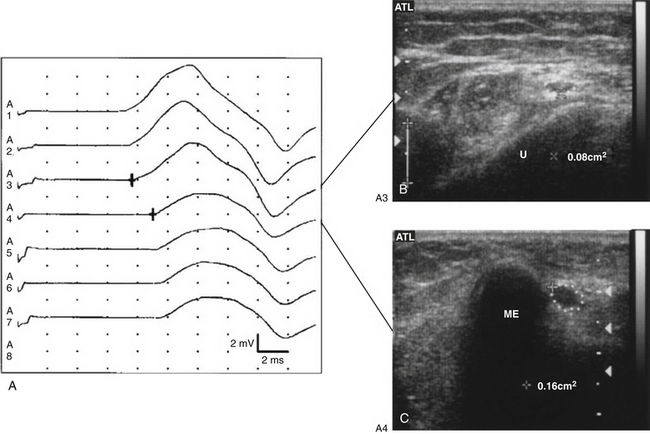
Several studies have shown that individuals with ulnar neuropathy at the elbow have ulnar nerve enlargement at a focal site around the elbow, and ultrasound has sensitivity and specificity greater than 80% for the diagnosis of ulnar neuropathy at the elbow.17,47 Beekman and colleagues demonstrated that the addition of ultrasound to electrodiagnostic studies alone increases the sensitivity from 78% to 98% for the diagnosis of ulnar neuropathy at the elbow.48 Nerve enlargement correlates closely with electrodiagnostic data,49,50 and it has been shown that the site of nerve enlargement with ulnar neuropathy at the elbow may match with the site of conduction block (Fig. 5.19).51
As with electrodiagnostic studies, ultrasound can help localize the site of ulnar nerve entrapment around the elbow. Park and colleagues demonstrated that ultrasound can differentiate cubital tunnel syndrome from supracondylar ulnar neuropathy, because the site of maximal nerve enlargement is found just proximal to the site of entrapment.52 Yoon and associates showed that the site of maximal nerve enlargement in ulnar neuropathy at the elbow varies considerably over the course of the nerve, both below and above the elbow, with the most common site of maximal enlargement at the medial epicondyle.53 In addition to a single measurement of maximal enlargement, a ratio of the site of maximal enlargement to a distal or proximal uninvolved portion of the ulnar nerve may improve accuracy in cases in which nerves are diffusely enlarged, such as obesity or polyneuropathy.53 Ultrasound also can be used to measure the area of the cubital tunnel, but studies have shown that a ratio of ulnar nerve-to-tunnel area does not improve diagnostic accuracy in the average patient.54,55
The ability of ultrasound to add anatomic information to the electrical information obtained with nerve conduction studies and EMG is perhaps the main benefit of combining these two diagnostic techniques, and that benefit is, as expected, also seen when evaluating ulnar neuropathy at the elbow. Kato and colleagues found that 8% of their cases of ulnar neuropathy at the elbow resulted from arthritis and medial elbow ganglia, which can be detected with ultrasound.56 Perineuromas, detected with ultrasound, also have been reported to cause ulnar neuropathy at the elbow (Fig. 5.20).57 Perhaps one of the most interesting anatomic findings is that ulnar nerve subluxation or dislocation, during elbow flexion, occurs in about one quarter of healthy volunteers (see Video 5.5 and Table 5.2).58 When the ulnar nerve is displaced from its normal location in the ulnar groove around the elbow, measurements used for nerve conduction studies are inaccurate, which leads to inaccurate results, and in particular false-negative nerve conduction study results in those with ulnar neuropathy at the elbow.58,59 Therefore, in those with classic symptoms of ulnar neuropathy at the elbow but normal electrodiagnostic studies, ultrasound to assess for nerve enlargement and subluxation can be particularly informative.60
Other Sites
Ultrasound can be used to image the ulnar nerve from the wrist up to the axilla (Video 5.6). As has been noted, the majority of pathologies are found at the elbow and the wrist, but there are reports of focal ulnar neuropathies, assessed with ultrasound, at sites other than the elbow and wrist. These cases include an accessory forearm muscle (Fig. 5.21), a ruptured triceps tendon, and an arteriovenous hemangioma in the upper arm.61–63 Of note, anastomotic connections between the ulnar and median nerves have yet to be identified by ultrasound.
Radial Nerve
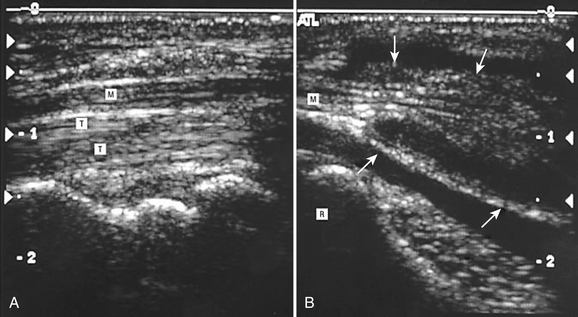
Unlike the median and ulnar nerves, the radial nerve is not easily imaged with ultrasound throughout the entire length of the arm. The radial nerve can be reliably seen just proximal to the antecubital fossa and from there it can be followed to the axilla (Video 5.7). However, the radial nerve is quite difficult to see when following it distally from the antecubital fossa into the forearm. The main reason it is more difficult to image distally is because the nerve splits into the superficial radial and posterior interosseous nerves, and the caliber of each is much smaller than the median or ulnar nerve. With practice and patience, both the superficial radial and posterior interosseous nerves can be tracked with ultrasound in thin individuals, but in some cases these nerves just cannot be seen throughout their entire course.
The most common cause of radial nerve injury in the upper arm is fracture of the humerus, and this condition has been studied with ultrasound.64 Bodner and colleagues showed that ultrasonographic findings in individuals with radial nerve injury following humerus fracture helped determine the severity of the radial nerve injury, and these data guided the decision to pursue surgery over conservative treatment.65 In addition, the ultrasonographic images correlated closely with the intraoperative findings. It was similarly shown that ultrasound of radial nerve injury at the spiral groove not associated with humerus fracture is an accurate technique. The majority of radial nerve injuries in this study were secondary to prolonged compression (“Saturday night palsy”), and ultrasound reliably demonstrated abnormal radial nerve enlargement at the spiral groove in these individuals (Fig. 5.22).66 Finally, case reports have shown that ultrasound can detect radial neuropathy in the upper arm from entrapment in a humerus fracture callus and from cephalic vein aneurysm.67,68
Although the radial nerve in the forearm can be difficult to image as it splits into the superficial radial and posterior interosseous nerves, there are still reports of ultrasound improving diagnostic utility for focal neuropathies in these nerves. Visser examined the superficial radial nerves of 20 healthy controls and found the normal cross-sectional area to be 2 mm2. He then used ultrasound to examine two individuals with superficial radial neuropathies based on history and examination, and ultrasound found one to have a traumatic neuroma and the other to have a schwannoma (Fig. 5.23).69 Another report showed focal enlargement of the superficial radial nerve in a woman with mononeuritis multiplex, and the ultrasound was used to guide a nerve biopsy.70 Neuropathy of the posterior interosseous nerve has also been explored with ultrasound. One series of four patients, along with two other case reports, demonstrated ultrasonographic evidence of focal swelling of the posterior interosseous nerve near the supinator muscle in those with weakness in finger extension.71–73
Other Upper Extremity Nerves
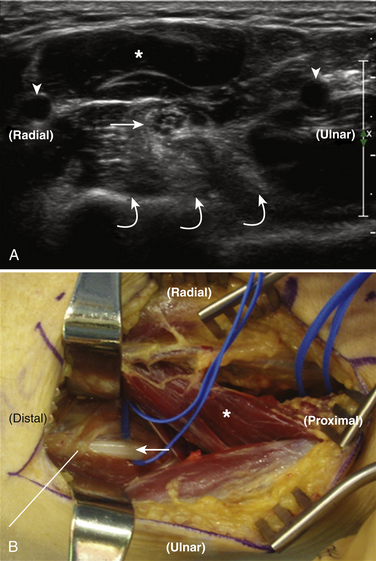
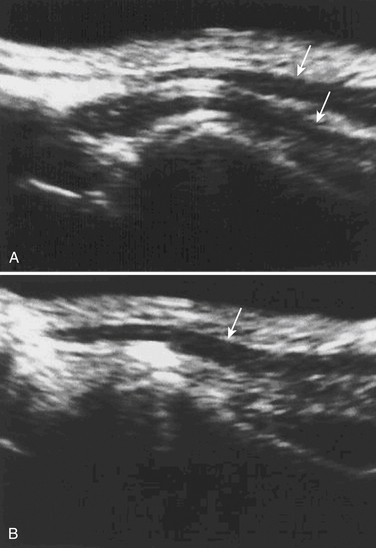
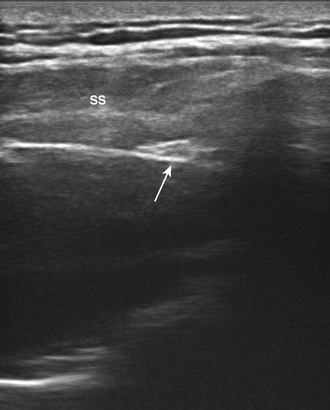
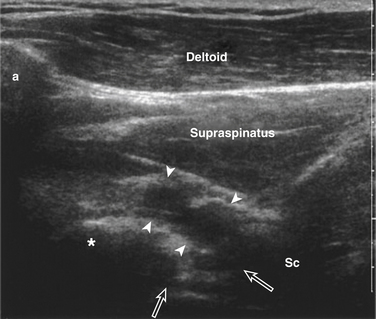
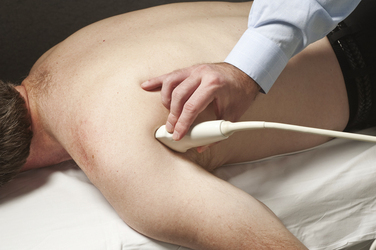
The suprascapular nerve arises from the upper trunk of the brachial plexus and innervates the supra- and infraspinatus muscles. In most individuals, high-resolution ultrasound can be used to image the suprascapular nerve in the supraspinatus fossa.74 The nerve is best seen with the transducer placed to obtain a coronal image (Figs. 5.24 and 5.25), and Doppler can be used to identify the adjacent suprascapular artery. Several case reports and series have documented ganglion cysts, identified with ultrasound, in the spinoglenoid notch as causes of suprascapular neuropathy (Fig. 5.26).74–76
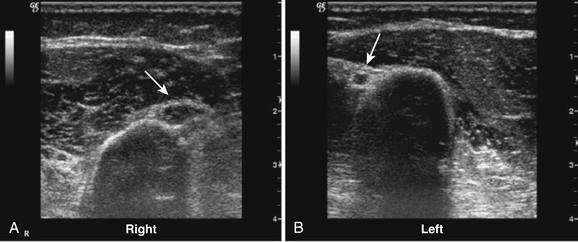
Little has been published regarding diagnostic ultrasound for neuropathy of either of the axillary or musculocutaneous nerves, but the anesthesia literature is robust regarding ultrasound imaging of these nerves to guide local blocks.77 Despite the lack of diagnostic studies, both nerves can be imaged with ultrasound in most individuals.74 To visualize the axillary nerve, the subject’s arm is abducted and the transducer is placed in the posterior axillary fold (Fig. 5.27). In this position, the axillary nerve can be detected adjacent to the posterior circumflex artery (Fig. 5.28). The musculocutaneous nerve is also imaged with the subject’s arm abducted (Fig. 5.29). The nerve is seen in cross section at the level of the intersection between the anterior deltoid and the biceps brachii muscles (Fig. 5.30).
Sciatic Nerve
The sciatic nerve is the largest peripheral nerve in the body, but it has a deep location within the leg, which can make portions of it difficult to visualize with ultrasound.78 The sciatic can first be seen with ultrasound at the gluteal crease, but it is deep at this site, making identification difficult. As the nerve is traced distally it becomes easier to image. Several centimeters proximal to the popliteal fossa it is reliably imaged with the transducer placed to obtain a cross-sectional view (Fig. 5.31). The cross-sectional area of the sciatic nerve at this site is large, and the nerve typically has a homogeneous appearance without distinct fascicles or outer boundaries (Fig. 5.32). Just distal to this site, the nerve splits into the common peroneal and tibial nerves.
There are few reports of diagnostic neuromuscular ultrasound of the sciatic nerve, and most of the reports involve the use of ultrasound to visualize vascular structures leading to sciatic mononeuropathy. For example, ultrasound has been used to identify a persistent sciatic artery thought to be causing sciatic neuropathy and leg weakness,79 and there are other reports of aneurysms of vessels in the thigh leading to sciatic neuropathy.80 Diagnostic ultrasound of the sciatic nerve itself is rare. There are scattered reports of ultrasound being used to identify masses compressing the proximal sciatic nerve in the pelvis,81 but MRI and computed tomography (CT) are likely better imaging modalities for intrapelvic visualization. Heinemeyer and colleagues conducted one of the few reports to examine the sciatic nerve itself in a systematic fashion, but they did so in a more diffuse condition: hereditary motor and sensory neuropathies (see Chapter 7).78 Given that the sciatic nerve is easily seen with ultrasound in the distal thigh, and that there are only a few reports of diagnostic sciatic nerve ultrasound, this is an area in which further research is needed.
Peroneal Nerve
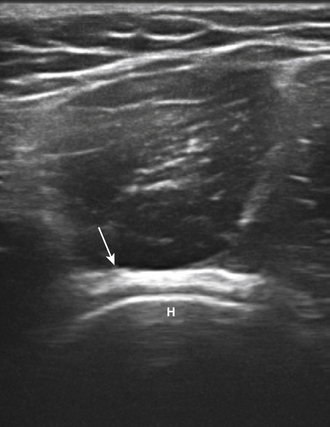
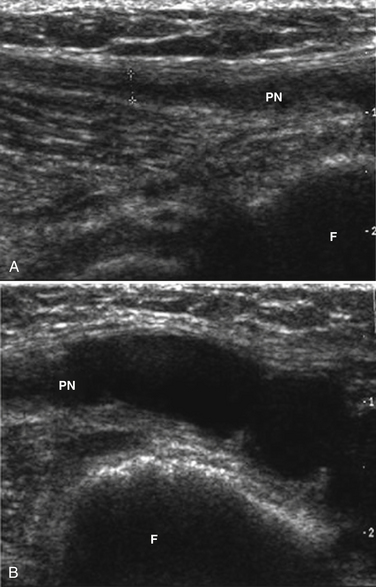
Of the nerves in the lower extremity, the peroneal is the most commonly studied with ultrasound. This occurs because it is commonly entrapped at the fibular head and the nerve is easily visualized with ultrasound at that site. In fact, the peroneal nerve can be routinely seen with ultrasound starting at the split from the sciatic nerve to just distal to the fibular head using a cross-sectional view (Fig. 5.33). The peroneal nerve is the smaller branch of the sciatic (the bulk of which becomes the tibial) and it has the typical honeycomb nerve–appearance with ultrasound (Fig. 5.34). Near the fibular head the common peroneal nerve splits into the deep and superficial peroneal nerves, and after this split the two branches of the peroneal are difficult to visualize with ultrasound.
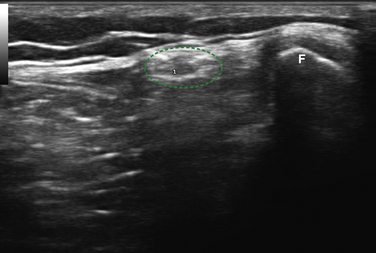
Fig. 5.34 The peroneal nerve (outlined in green) is shown in cross-section next to the fibular head (F).
As noted, most ultrasound studies of the peroneal nerve focus on its location near the fibular head. Some of the first reports were of ganglia, either intra- or extraneural, causing peroneal mononeuropathy at the fibular head, and these cysts were easily seen with ultrasound.82–84 Visser expanded on these findings with an important study of 41 individuals with footdrop; 28 of these patients were found to have peroneal neuropathy at the fibular head, and of those, 5 (18%) had intraneural ganglia detected by ultrasound within the peroneal nerve at the level of the fibular head (Fig. 5.35; also see Table 5.2).85 This is important because surgical treatment of intraneural ganglia can lead to resolution of a footdrop from peroneal neuropathy.86
Another cause of severe peroneal neuropathy around the level of the fibular head is knee dislocation. Gruber and colleagues used ultrasound to examine the peroneal nerve of nine individuals with subluxation of the knee.87 They found significant enlargement of the peroneal nerve compared with controls, and in one individual they noted a peroneal nerve stump. They concluded that ultrasound was very helpful in assessing peroneal nerve damage following knee subluxation, and it helped to determine operative management.
As with the sciatic nerve, ultrasound has been used to detect vascular abnormalities leading to peroneal mononeuropathies. This includes the detection of aneurysms and pseudoaneurysms within the popliteal fossa compressing the peroneal nerve.88,89 Finally, Lo and colleagues demonstrated nerve enlargement in five consecutive patients with idiopathic peroneal neuropathy at the fibular head, similar to the enlargement seen with idiopathic CTS.90
Tibial Nerve
The tibial nerve is reliably imaged with ultrasound in two distinct areas of the lower extremity. First, it can be seen as a continuation of the sciatic nerve, after it branches into the common peroneal, throughout the course of the popliteal fossa (Fig. 5.36). However, as the tibial nerve travels into the calf, it is difficult to image. The second site at which the tibial nerve is easily imaged is at the ankle, where it is found just distal to the medial malleolus (Fig. 5.37).
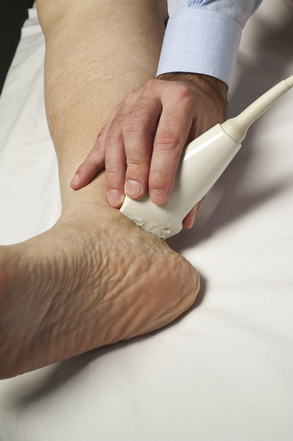
Fig. 5.37 The transducer is used to obtain a cross-sectional image of the tibial nerve at the ankle.
Popliteal Fossa
The tibial nerve is reliably seen within the popliteal fossa using a cross-sectional view, but reports of tibial mononeuropathies evaluated with ultrasound at this site are rare, especially when compared with the robust ultrasonographic literature for peroneal neuropathy at the knee. There are reports of Baker’s cysts, ruptured Baker’s cysts, intraneural ganglia, and neurilemmomas causing tibial neuropathies at the knee,91–93 but systematic studies of the tibial nerve at the knee have not been reported.
Ankle
Focal neuropathy of the tibial nerve at the ankle is often called tarsal tunnel syndrome, because the nerve passes through the fibro-osseous tarsal tunnel at this site. Unlike CTS, idiopathic tarsal tunnel syndrome is a rare condition that may in fact be overdiagnosed.94 Although idiopathic tarsal tunnel is uncommon, structural abnormalities leading to tibial neuropathy at the ankle have been reported and can be easily seen with ultrasound given the superficial nature of the structures at the medial ankle. In addition, it has been shown that ultrasound of the tibial nerve at the ankle has good inter- and intrarater reliability.95
As noted, reports of idiopathic tarsal tunnel syndrome, with ultrasonographic enlargement of the tibial nerve at this site, are rare.96 Much more common are reports of structural abnormalities, including neurilemmomas, accessory muscles, and talocalcaneal coalition.97–99 In addition, Nagaoka and colleagues reported 30 individuals with ganglia, causing tibial neuropathy at the ankle, most of which originated from the talocalcaneal joint. They followed up this study with a prospective evaluation of 17 feet with tarsal tunnel syndrome using ultrasound.100,101 In all 17 cases, ultrasound was able to make a preoperative diagnosis of the cause of tarsal tunnel syndrome: 13 were from ganglia (Fig. 5.38), 3 from varicose veins, and 1 from talocalcaneal coalition.
Other Lower Extremity Nerves
The sural nerve can be imaged from just above the lateral malleolus to the inferior edge of the gastrocnemius, and it is best seen with a cross-sectional view (Fig. 5.39). No studies of sural neuropathies, diagnosed with ultrasound, are reported, but two interesting series have been published in which high-resolution ultrasound was used to prevent focal sural nerve damage. The first series reported that ultrasonographic mapping of the sural nerve, prior to repair of ruptured Achilles tendon, could prevent nerve injury.102 The second was a similar report in which mapping of the sural nerve and its relationship to the small saphenous vein was used to prevent nerve damage prior to vein stripping or thermal ablation for varicose veins.103
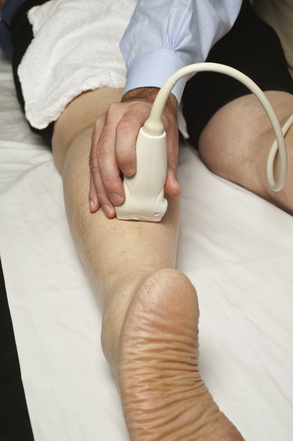
Fig. 5.39 The transducer is placed on the distal calf to obtain a cross-sectional image of the sural nerve.
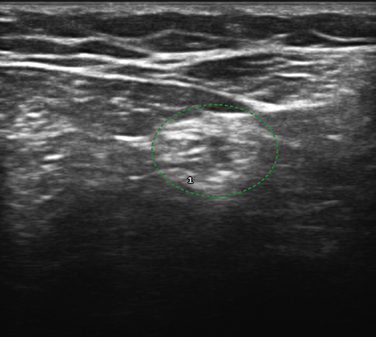
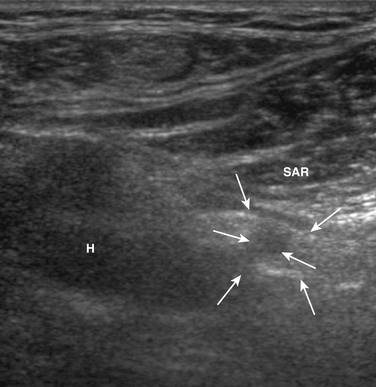
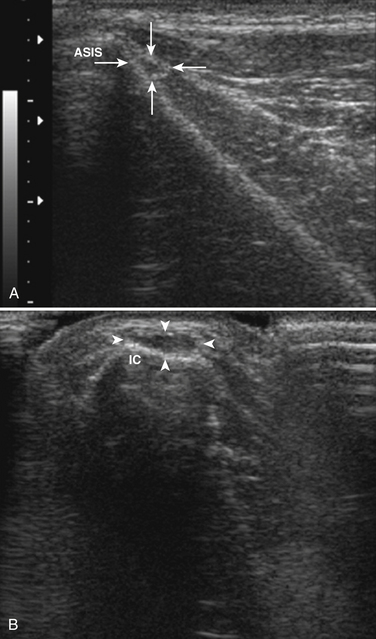
The femoral nerve can be difficult to image with ultrasound because it branches extensively, leaving many small nerve branches, just distal to the inguinal ligament. Despite this limitation, Gruber and colleagues used ultrasound to examine the femoral nerve of seven individuals with iatrogenic femoral neuropathy, and they noted enlargement of the femoral in five of these individuals.104 In the others, one had a hematoma surrounding the nerve (Fig. 5.40) and in the other nerve transection was ruled out. A proximal branch of the femoral nerve, the lateral femoral cutaneous nerve of the thigh is known to be easily entrapped, typically in obese individuals, which causes a condition called meralgia paresthetica. The lateral femoral cutaneous nerve can be imaged by placing the ultrasound transducer over the anterior superior iliac spine, just distal to the lateral inguinal ligament (Fig. 5.41). There is one report of nerve enlargement, demonstrated with ultrasound, in an individual with meralgia paresthetica secondary to wearing very tight jeans (Fig. 5.42).105
Conclusion
As detailed in this chapter, neuromuscular ultrasound is a helpful complement to electrodiagnostic studies in the diagnosis of focal neuropathies. The ability of ultrasound to demonstrate nerve cross-sectional area enlargement in entrapment neuropathies, to detect peripheral nerve anatomic variants, and to identify intrinsic and extrinsic causes of focal neuropathies makes it an invaluable addition to the clinical neurophysiology laboratory. Further research continues to refine the use of neuromuscular ultrasound, and as ultrasound technology continues to rapidly improve it will allow for higher resolution images, increased diagnostic accuracy, and, ultimately, improved patient outcomes.
1. Atroshi I., Gummesson C., Johnsson R., et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153-158.
2. Stevens J.C., Sun S., Beard C.M., et al. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134-138.
3. Webster B.S., Snook S.H. The cost of compensable upper extremity cumulative trauma disorders. J Occup Med. 1994;36:713-717.
4. Feuerstein M., Miller V.L., Burrell L.M., Berger R. Occupational upper extremity disorders in the federal workforce: prevalence, health care expenditures, and patterns of work disability. J Occup Environ Med. 1998;40:546-555.
5. Mondelli M., Giannini F., Ballerini M., et al. Incidence of ulnar neuropathy at the elbow in the province of Siena (Italy). J Neurol Sci. 2005;234:5-10.
6. Latinovic R., Gulliford M.C., Hughes R.A. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263-265.
7. Aprile I., Padua L., Padua R., et al. Peroneal mononeuropathy: predisposing factors, and clinical and neurophysiological relationships. Neurol Sci. 2000;21:367-371.
8. Campion D. Electrodiagnostic testing in hand surgery. J Hand Surg Am. 1996;21:947-956.
9. Kim S., Choi J.Y., Huh Y.M., et al. Role of magnetic resonance imaging in entrapment and compressive neuropathy: what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity. Eur Radiol. 2007;17:509-522.
10. Fornage B.D. Peripheral nerves of the extremities: imaging with US. Radiology. 1988;167:179-182.
11. Buchberger W., Judmaier W., Birbamer G., et al. Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol. 1992;159:793-798.
12. Kele H., Verheggen R., Bittermann H.J., Reimers C.D. The potential value of ultrasonography in the evaluation of carpal tunnel syndrome. Neurology. 2003;61:389-391.
13. Nakamichi K., Tachibana S. Transverse sliding of the median nerve beneath the flexor retinaculum. J Hand Surg Br. 1992;17:213-216.
14. Hough A.D., Moore A.P., Jones M.P. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88:569-576.
15. Erel E., Dilley A., Greening J., et al. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J Hand Surg Br. 2003;28:439-443.
16. Nakamichi K., Tachibana S. Ultrasonographic measurement of median nerve cross-sectional area in idiopathic carpal tunnel syndrome: diagnostic accuracy. Muscle Nerve. 2002;26:798-803.
17. Wiesler E.R., Chloros G.D., Cartwright M.S., et al. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;31:726-732.
18. Hobson-Webb L.D., Massey J.M., Juel V.C., Sanders D.B. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:1353-1357.
19. Nakamichi K.I., Tachibana S. Enlarged median nerve in idiopathic carpal tunnel syndrome. Muscle Nerve. 2000;23:1713-1718.
20. Nakamichi K., Tachibana S. Restricted motion of the median nerve in carpal tunnel syndrome. J Hand Surg Br. 1995;20:460-464.
21. Mallouhi A., Pulzl P., Trieb T., et al. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240-1245.
22. Sugimoto H., Miyaji N., Ohsawa T. Carpal tunnel syndrome: evaluation of median nerve circulation with dynamic contrast-enhanced MR imaging. Radiology. 1994;190:459-466.
23. Walker F.O., Cartwright M.S., Wiesler E.R., Caress J. Ultrasound of nerve and muscle. Clin Neurophysiol. 2004;115:495-507.
24. Gassner E.M., Schocke M., Peer S., et al. Persistent median artery in the carpal tunnel: color Doppler ultrasonographic findings. J Ultrasound Med. 2002;21:455-461.
25. Kele H., Verheggen R., Reimers C.D. Carpal tunnel syndrome caused by thrombosis of the median artery: the importance of high-resolution ultrasonography for diagnosis. Case report. J Neurosurg. 2002;97:471-473.
26. Dutly-Guinand M., Muller M., Bleuler P., Steiger R. Carpal tunnel syndrome from a thrombosed median artery: four case reports and review of the literature. Handchir Mikrochir Plast Chir. 2009;41:179-182.
27. Bayrak I.K., Bayrak A.O., Kale M., et al. Bifid median nerve in patients with carpal tunnel syndrome. J Ultrasound Med. 2008;27:1129-1136.
28. Kato H., Ogino T., Nanbu T., Nakamura K. Compression neuropathy of the motor branch of the median nerve caused by palmar ganglion. J Hand Surg Am. 1991;16:751-752.
29. Kobayashi N., Koshino T., Nakazawa A., Saito T. Neuropathy of motor branch of median or ulnar nerve induced by midpalm ganglion. J Hand Surg Am. 2001;26:474-477.
30. Ginn S.D., Cartwright M.S., Chloros G.D., et al. Ultrasound in the diagnosis of a median neuropathy in the forearm: case report. J Brachial Plex Peripher Nerve Inj. 2007;2:23.
31. Bertolotto M., Rosenberg I., Parodi R.C., et al. Case report: fibroma of tendon sheath in the distal forearm with associated median nerve neuropathy: US, CT and MR appearances. Clin Radiol. 1996;51:370-372.
32. Kuo Y.L., Yao W.J., Chiu H.Y. Role of sonography in the preoperative assessment of neurilemmoma. J Clin Ultrasound. 2005;33:87-89.
33. Cartwright M.S., Donofrio P.D., Ybema K.D., Walker F.O. Detection of a brachial artery pseudoaneurysm using ultrasonography and EMG. Neurology. 2005;65:649.
34. Marquardt G., Angles S.M., Leheta F.D., Seifert V. Median nerve compression caused by a venous aneurysm: case report. J Neurosurg. 2001;94:624-626.
35. Lazaro-Blazquez D., Soto O. Combined median and medial antebrachial cutaneous neuropathies: an upper-arm neurovascular syndrome. Electromyogr Clin Neurophysiol. 2004;44:187-191.
36. Hobson-Webb L.D., Walker F.O. Traumatic neuroma diagnosed by ultrasonography. Arch Neurol. 2004;61:1322-1323.
37. Elsaidi G.A., Wiesler E.R. Lipofibromatous hamartoma of the median nerve: case presentation of MRI, ultrasound, electrodiagnostic, histologic, and surgical findings. Am J Orthop. 2004;33:514-516.
38. Elias D.A., Lax M.J., Anastakis D.J. Musculoskeletal images: ganglion cyst of Guyon’s canal causing ulnar nerve compression. Can J Surg. 2001;44:331-332.
39. Inaparthy P.K., Anwar F., Botchu R., et al. Compression of the deep branch of the ulnar nerve in Guyon’s canal by a ganglion: two cases. Arch Orthop Trauma Surg. 2008;128:641-643.
40. Jacob A., Moorthy T.K., Thomas S.V., Sarada C. Compression of the deep motor branch of the ulnar nerve: an unusual cause of pure motor neuropathy and hand wasting. Arch Neurol. 2005;62:826-827.
41. Harvie P., Patel N., Ostlere S.J. Ulnar nerve compression at Guyon’s canal by an anomalous abductor digiti minimi muscle: the role of ultrasound in clinical diagnosis. Hand Surg. 2003;8:271-275.
42. Zeiss J., Jakab E. MR demonstration of an anomalous muscle in a patient with coexistent carpal and ulnar tunnel syndrome: case report and literature summary. Clin Imaging. 1995;19:102-105.
43. Moss D.P., Forthman C.L. Ulnar artery thrombosis associated with anomalous hypothenar muscle. J Surg Orthop Adv. 2008;17:85-88.
44. Coulier B., Goffin D., Malbecq S., Mairy Y. Colour duplex sonographic and multislice spiral CT angiographic diagnosis of ulnar artery aneurysm in hypothenar hammer syndrome. JBR-BTR. 2003;86:211-214.
45. Sakai K., Tsutsui T., Aoi M., et al. Ulnar neuropathy caused by a lipoma in Guyon’s canal: case report. Neurol Med Chir (Tokyo). 2000;40:335-338.
46. Campbell W.W. Ulnar neuropathy at the elbow. Muscle Nerve. 2000;23:450-452.
47. Beekman R., Schoemaker M.C., Van Der Plas J.P., et al. Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology. 2004;62:767-773. 17
48. Beekman R., Van Der Plas J.P., Uitdehaag B.M., et al. Clinical, electrodiagnostic, and sonographic studies in ulnar neuropathy at the elbow. Muscle Nerve. 2004;30:202-208.
49. Bayrak A.O., Bayrak I.K., Turker H., et al. Ultrasonography in patients with ulnar neuropathy at the elbow: comparison of cross-sectional area and swelling ratio with electrophysiological severity. Muscle Nerve. 2009;41:661-666.
50. Volpe A., Rossato G., Bottanelli M., et al. Ultrasound evaluation of ulnar neuropathy at the elbow: correlation with electrophysiological studies. Rheumatology (Oxford). 2009;48:1098-1101.
51. Caress J.B., Becker C.E., Cartwright M.S., Walker F.O. Ultrasound in the diagnosis of ulnar neuropathy at the elbow. J Clin Neuromuscul Dis. 2003;4:161-162.
52. Park G.Y., Kim J.M., Lee S.M. The ultrasonographic and electrodiagnostic findings of ulnar neuropathy at the elbow. Arch Phys Med Rehabil. 2004;85:1000-1005.
53. Yoon J.S., Walker F.O., Cartwright M.S. Ultrasonographic swelling ratio in the diagnosis of ulnar neuropathy at the elbow. Muscle Nerve. 2008;38:1231-1235.
54. Yoon J.S., Kim B.J., Kim S.J., et al. Ultrasonographic measurements in cubital tunnel syndrome. Muscle Nerve. 2007;36:853-855.
55. Yoon J.S., Hong S.J., Kim B.J., et al. Ulnar nerve and cubital tunnel ultrasound in ulnar neuropathy at the elbow. Arch Phys Med Rehabil. 2008;89:887-889.
56. Kato H., Hirayama T., Minami A., et al. Cubital tunnel syndrome associated with medial elbow ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am 84-A:1413-1419. 2002.
57. Beekman R., Slooff W.B., Van Oosterhout M.F., et al. Bilateral intraneural perineurioma presenting as ulnar neuropathy at the elbow. Muscle Nerve. 2004;30:239-243.
58. Kim B.J., Date E.S., Lee S.H., et al. Distance measure error induced by displacement of the ulnar nerve when the elbow is flexed. Arch Phys Med Rehabil. 2005;86:809-812.
59. Kim B.J., Koh S.B., Park K.W., et al. Pearls and oysters: false positives in short-segment nerve conduction studies due to ulnar nerve dislocation. Neurology. 2008;70:e9-e13.
60. Yoon J.S., Walker F.O., Cartwright M.S. Ulnar neuropathy with normal electrodiagnosis and abnormal nerve ultrasound. Arch Phys Med Rehabil. 2010;91:318-320.
61. Lopez M.G., Ruiz S.F., Chamorro S.C., Canadillas B.L. Forearm soft tissue mass caused by an accessory muscle. Eur Radiol. 2001;11:1487-1489.
62. Pulidori M., Capuano C., Mouchaty H., et al. Intramuscular thrombosed arteriovenous hemangioma of the upper right arm mimicking a neuroma of the ulnar nerve: case report. Neurosurgery. 2004;54:770-771.
63. Duchow J., Kelm J., Kohn D. Acute ulnar nerve compression syndrome in a powerlifter with triceps tendon rupture: a case report. Int J Sports Med. 2000;21:308-310.
64. Bodner G., Huber B., Schwabegger A., et al. Sonographic detection of radial nerve entrapment within a humerus fracture. J Ultrasound Med. 1999;18:703-706.
65. Bodner G., Buchberger W., Schocke M., et al. Radial nerve palsy associated with humeral shaft fracture: evaluation with US—initial experience. Radiology. 2001;219:811-816.
66. Lo Y.L., Fook-Chong S., Leoh T.H., et al. Rapid ultrasonographic diagnosis of radial entrapment neuropathy at the spiral groove. J Neurol Sci. 2008;271:75-79.
67. Hugon S., Daubresse F., Depierreux L. Radial nerve entrapment in a humeral fracture callus. Acta Orthop Belg. 2008;74:118-121.
68. Kassabian E., Coppin T., Combes M., et al. Radial nerve compression by a large cephalic vein aneurysm: case report. J Vasc Surg. 2003;38:617-619.
69. Visser L.H. High-resolution sonography of the superficial radial nerve with two case reports. Muscle Nerve. 2009;39:392-395.
70. Chipman J.N., Mott R.T., Stanton C.A., Cartwright M.S. Ultrasonographic tinel sign. Muscle Nerve. 2009;40:1033-1035.
71. Bodner G., Harpf C., Meirer R., et al. Ultrasonographic appearance of supinator syndrome. J Ultrasound Med. 2002;21:1289-1293.
72. Joy V., Therimadasamy A., Cheun C.Y., Wilder-Smith E. Diagnostic utility of ultrasound in posterior interosseous nerve syndrome. Arch Neurol. 2009;66:902-903.
73. Chien A.J., Jamadar D.A., Jacobson J.A., et al. Sonography and MR imaging of posterior interosseous nerve syndrome with surgical correlation. AJR Am J Roentgenol. 2003;181:219-221.
74. Martinoli C., Bianchi S., Pugliese F., et al. Sonography of entrapment neuropathies in the upper limb (wrist excluded). J Clin Ultrasound. 2004;32:438-450.
75. Ogino T., Minami A., Kato H., et al. Entrapment neuropathy of the suprascapular nerve by a ganglion: a report of three cases. J Bone Joint Surg Am. 73, 1991. 141–14
76. Weiss C., Imhoff A.B. Sonographic imaging of a spinoglenoid cyst. Ultraschall Med. 2000;21:287-289.
77. Tran de Q.H., Russo G., Munoz L., et al. A prospective, randomized comparison between ultrasound-guided supraclavicular, infraclavicular, and axillary brachial plexus blocks. Reg Anesth Pain Med. 2009;34:366-371.
78. Heinemeyer O., Reimers C.D. Ultrasound of radial, ulnar, median, and sciatic nerves in healthy subjects and patients with hereditary motor and sensory neuropathies. Ultrasound Med Biol. 1999;25:481-485.
79. Kim H.J., Cho Y.S., Lim H. Persistent sciatic artery with monoplegia in right lower leg without vascular complication symptoms in an obese woman. J Emerg Med. 2008;34:291-294.
80. Eguchi K., Majima M. Sciatic neuropathy caused by disorder of a nutrient artery: a case report of thromboembolism secondary to profunda femoral artery aneurysm. Arch Phys Med Rehabil. 2001;82:253-255.
81. Benyahya E., Etaouil N., Janani S., et al. Sciatica as the first manifestation of a leiomyosarcoma of the buttock. Rev Rhum Engl Ed. 1997;64:135-137.
82. Masciocchi C., Innacoli M., Cisternino S., et al. Myxoid intraneural cysts of external popliteal ischiadic nerve: report of 2 cases studied with ultrasound, computed tomography and magnetic resonance imaging. Eur J Radiol. 1992;14:52-55.
83. Pedrazzini M., Pogliacomi F., Cusmano F., et al. Bilateral ganglion cyst of the common peroneal nerve. Eur Radiol. 2002;12:2803-2806.
84. Rawal A., Ratnam K.R., Yin Q., et al. Compression neuropathy of common peroneal nerve caused by an extraneural ganglion: a report of two cases. Microsurgery. 2004;24:63-66.
85. Visser L.H. High-resolution sonography of the common peroneal nerve: detection of intraneural ganglia. Neurology. 2006;67:1473-1475.
86. Young N.P., Sorenson E.J., Spinner R.J., Daube J.R. Clinical and electrodiagnostic correlates of peroneal intraneural ganglia. Neurology. 2009;72:447-452.
87. Gruber H., Peer S., Meirer R., Bodner G. Peroneal nerve palsy associated with knee luxation: evaluation by sonography—initial experiences. AJR Am J Roentgenol. 2005;185:1119-1125.
88. Jang S.H., Lee H., Han S.H. Common peroneal nerve compression by a popliteal venous aneurysm. Am J Phys Med Rehabil. 2009;88:947-950.
89. Megalopoulos A., Vasiliadis K., Siminas S., et al. Pseudoaneurysm of the popliteal artery complicated by peroneal mononeuropathy in a 4-year-old child: report of a case. Surg Today. 2007;37:798-801.
90. Lo Y.L., Fook-Chong S., Leoh T.H., et al. High-resolution ultrasound as a diagnostic adjunct in common peroneal neuropathy. Arch Neurol. 2007;64:1798-1800.
91. Dash S., Bheemreddy S.R., Tiku M.L. Posterior tibial neuropathy from ruptured Baker’s cyst. Semin Arthritis Rheum. 1998;27:272-276.
92. Gosk J., Rutowski R., Urban M., Reichert P., Rabczynski J. Intraneural ganglion of the tibial nerve: a case report. Neurol Neurochir Pol. 2007;41:176-180.
93. Ghaly R.F. A posterior tibial nerve neurilemoma unrecognized for 10 years: case report. Neurosurgery. 2001;48:668-672.
94. Campbell W.W., Landau M.E. Controversial entrapment neuropathies. Neurosurg Clin North Am. 2008;19:597-608.
95. Alshami A.M., Cairns C.W., Wylie B.K., et al. Reliability and size of the measurement error when determining the cross-sectional area of the tibial nerve at the tarsal tunnel with ultrasonography. Ultrasound Med Biol. 2009;35:1098-1102.
96. Vijayan J., Therimadasamy A.K., Teoh H.L., et al. Sonography as an aid to neurophysiological studies in diagnosing tarsal tunnel syndrome. Am J Phys Med Rehabil. 2009;88:500-501.
97. Tsai C.C., Lin T.M., Lai C.S., Lin S.D. Tarsal tunnel syndrome secondary to neurilemoma: a case report. Kaohsiung J Med Sci. 2001;17:216-220.
98. Kinoshita M., Okuda R., Morikawa J., Abe M. Tarsal tunnel syndrome associated with an accessory muscle. Foot Ankle Int. 2003;24:132-136.
99. Lee M.F., Chan P.T., Chau L.F., Yu K.S. Tarsal tunnel syndrome caused by talocalcaneal coalition. Clin Imaging. 2002;26:140-143.
100. Nagaoka M., Satou K. Tarsal tunnel syndrome caused by ganglia. J Bone Joint Surg Br. 1999;81:607-610.
101. Nagaoka M., Matsuzaki H. Ultrasonography in tarsal tunnel syndrome. J Ultrasound Med. 2005;24:1035-1040.
102. Flavin R., Gibney R.G., O’Rourke S.K. A clinical test to avoid sural nerve injuries in percutaneous Achilles tendon repairs. Injury. 2007;38:845-847.
103. Ricci S., Moro L., Antonelli I. Ultrasound imaging of the sural nerve: ultrasound anatomy and rationale for investigation. Eur J Vasc Endovasc Surg. 2009;39:636-641.
104. Gruber H., Peer S., Kovacs P., et al. The ultrasonographic appearance of the femoral nerve and cases of iatrogenic impairment. J Ultrasound Med. 2003;22:163-172.
105. Park J.W., Kim D.H., Hwang M., Bun H.R. Meralgia paresthetica caused by hip-huggers in a patient with aberrant course of the lateral femoral cutaneous nerve. Muscle Nerve. 2007;35:678-680.