Chapter 8 Ultrasound-assisted breast reduction
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Introduction
As is known, ultrasound energy was initially used by Zocchi1–6 to emulsify fat. A special instrument, composed of an ultrasound generator, a crystal piezoelectric transducer, and a titanium probe transmitter was utilized to target adipocyte cells. This new technology was first applied to body fat to emulsify only fat cells while sparing the other supporting vascular and connective components of the cutaneous vascular network. More recently, Goes,7 Zocchi,1–6 Benelli8 and di Giuseppe,9–12 have started to apply this technology to breast tissue to achieve breast reduction and correction of mild to medium-degree breast ptosis.
Preoperative Preparation
Preoperative Mammography
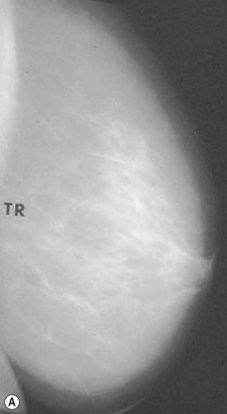
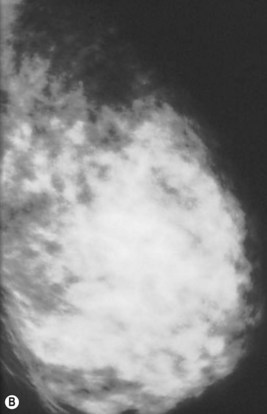
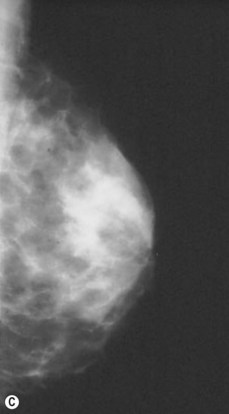
Preoperative mammograms (anteroposterior and lateral views), the so-called Ecklund view, are taken to evaluate the nature and consistency of the breast tissue (fibrotic, mixed or fatty parenchyma ), distribution of the fat, presence of calcifications, and areas of dysplasia or nodularity that might necessitate further studies (Fig. 8.1). The presence of fibroadenomas, calcifications, and other suspected or doubtful radiologic findings should be double-checked with ultrasound and a radiologist experienced in breast tissue resonance.
Contraindications
Furthermore, because the amount and distribution of the breast fat is variable, not all women are candidates for breast volume reduction with UAL. If fat and glandular tissue are mixed, penetration of the tissue may be impossible, as noted by Lejour13 and Lejour and Abboud.14 If the breast tissue is primarily glandular, the technique is not indicated.
Surgical Technique
Infiltration
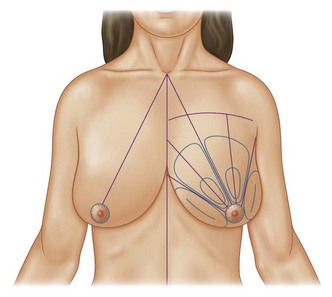
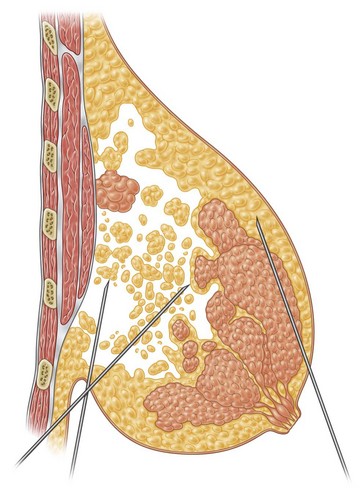
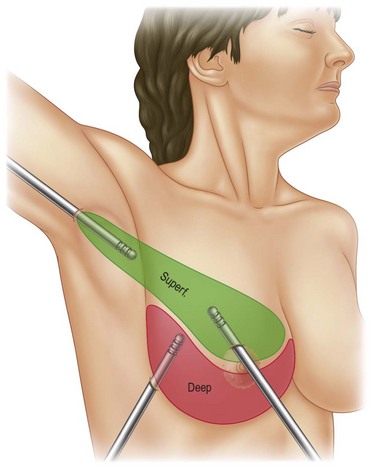
Infiltration should be divided into the three different layers of the breast: deep, intermediate, and superficial (Fig. 8.3).
In deeper and intermediate layers I expect a 1.5 : 1 ratio between infiltration and aspirate; in the superficial layer I normally infiltrate twice what I expect to extract (2 : 1 ratio). I use blunt infiltration cannulas, as described by Klein,15 15–20 cm long.
Probes
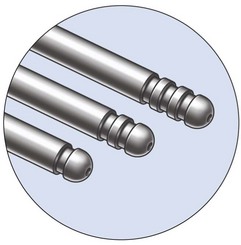
The Vaser system (Sound Surgical Technologies, Denver, CO, USA) provides different sizes and lengths of solid titanium probes (Box 8.1) expressly designed to fulfill all purposes in body contouring, as well as being capable of emulsification through the cavitation effect produced by the ultrasound energy. The piezoelectric transducer transforms electric energy into “vibration energy”, thus allowing the solid titanium probe to emulsify the target fat cells.
Technique
The Vaser surgeon may now utilize two further options:
1. The 4.5 mm large probe, for larger volumes, with a higher percentage of fat emulsification, for 1 minute,depending on the diameter of the probe.
2. The “cone” tip probe, which is very aggressive in fibrous tissue, and has been designed for male breast tissue (gynecomastia) destruction (Fig. 8.4).
Fat Emulsification
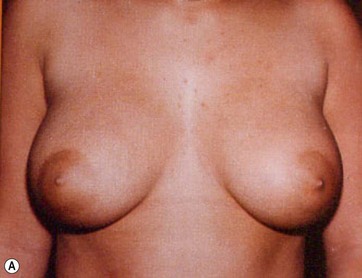
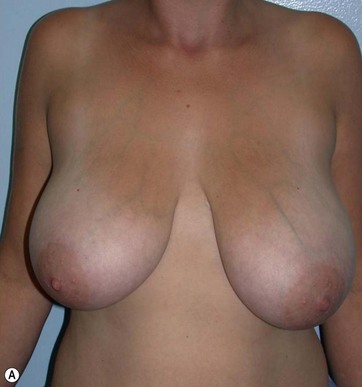
In breast reduction with UAL the duration of the procedure varies depending on the volume of reduction, the type of tissue encountered, and the amount of skin retraction required. A breast with purely fatty tissue is easier to treat than one with mixed glandular tissue, in which fat cells are smaller, stronger, and denser (Fig. 8.5).
The surgical planes, with good criss-cross tunneling and adequate undermining, are planned in the preoperative drawings. If large undermining is required for skin retraction, the superficial layers are treated initially. Then the deeper planes are reached, and more time is spent in thicker areas. Surgeons inexperienced in the procedure should be especially cautious when performing the technique, particularly in the subdermal planes.9–12,15–19
Subcutaneous UAL Undermining
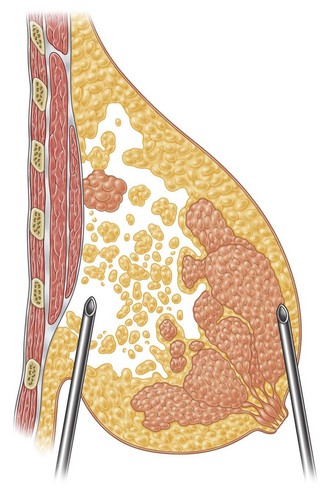
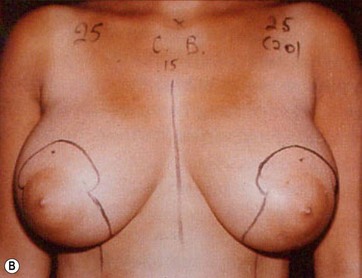
Together with UAL application to the fat layers, starting from the deeper layers and progressing to the more superficial ones, it is advisable to thin the superficial layer of the subcutaneous tissue of the upper and lower quadrants by using a different angle pattern, as in standard lipoplasty.20,21 This superficial undermining with low-frequency ultrasound energy helps to enhance the retraction of the breast skin and to redrape the breast skin to the newly shaped and reduced mammary cone (Fig. 8.6).
Optimizing Outcomes
Vaser is selective, and does not interfere with the vascular network of the dermal tissue, if properly made. It divides the connective and supporting structures of the skin. Rudolph (1991)22 showed the great potential of the dermal layer in wound contracture. As much as the dermis is thinned, so much contraction will result, providing the tissue vascularization is preserved.
Clinical Results
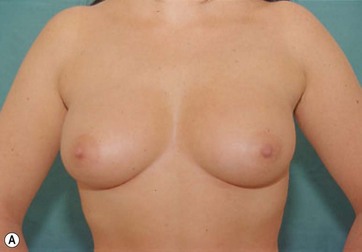
The major postoperative nipple–areola complex elevation was 5 cm.
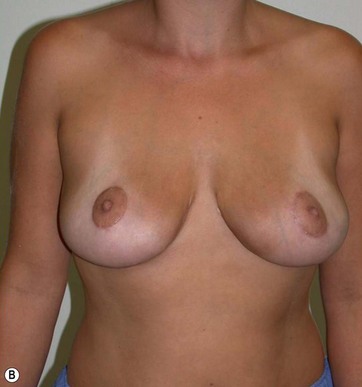
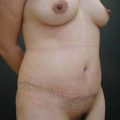
There was no evidence of suspicious calcification resulting from surgery at the 5-year postoperative follow up. Essentially, an increase in breast tissue fibrosis was noticeable in the postoperative mammograms, which was responsible for the new consistency, texture and tone of the breasts, and also responsible for the lifting of the breasts (Figs 8.7–8.9).
Mastopexy
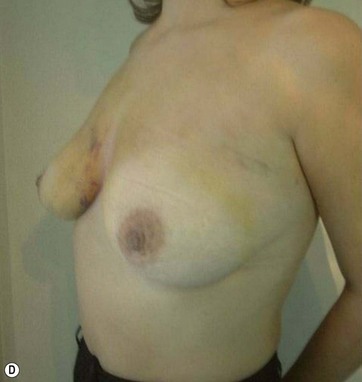
I have applied the technique in clinical cases with the patient expressively refused visible breast cutaneous scars, and the potential correction of ptosis with an anatomical implant. The upward rotation of the breast and the retraction can finally elevate the breast by 2–4 cm from its initial position (Figs 8.9 and 8.10). A supporting bra has to be applied for the first 4 weeks after surgery; despite this, I strongly believe that a similar bra should be advised forever in patients with large breasts or a tendency to ptosis (Fig. 8.11).
Histologic Changes
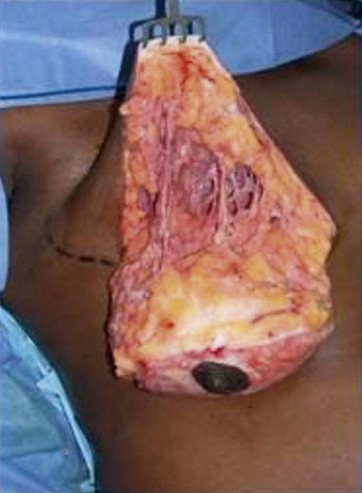
The breast tissue that underwent emulsification with UAL was analyzed histologically by Chun, Taylor and Van Meter (2002)23 who presented a paper at the American Society for Aesthetic Plastic Surgery (ASAPS) meeting in Las Vegas. They operated on 10 patients with large breasts using the Genesis Contour device (Mentor HS, Santa Barbara, CA, US), with breast UAL. Then, with open surgery, a breast specimen was removed, weighing 430–1530 g.
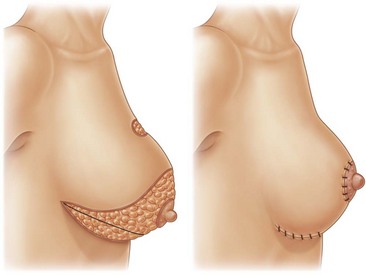
In 2006, at the ASAPS meeting in Orlando (FL, USA), Nagy and McCraw (Mississipi University Medical School, St Louis, Missouri, US) first presented the combination of breast fat emulsification by Vaser with open surgery breast reduction. They re-introduced the technique of Raymond Passot, a French surgeon, who in 1925 published the so-called “BUTTON” mammoplasty or the “ no vertical scar” reduction, which became the most common method of breast shaping in Europe before World War II (Fig. 8.12).
Markings
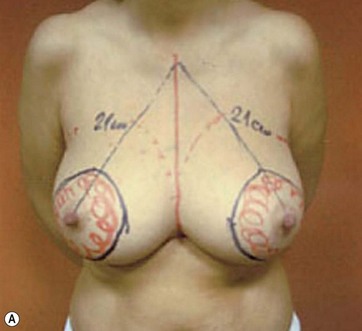
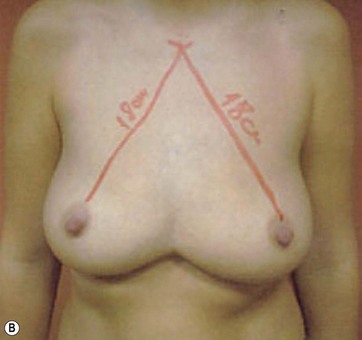
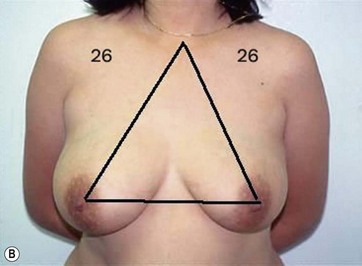
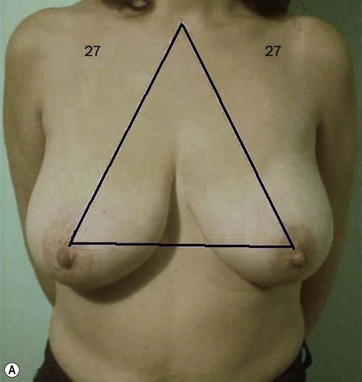
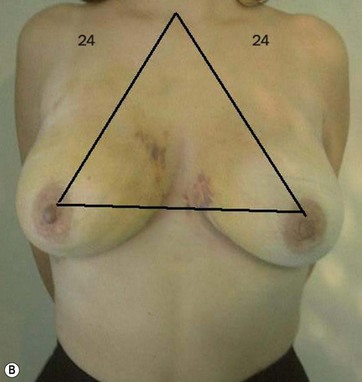
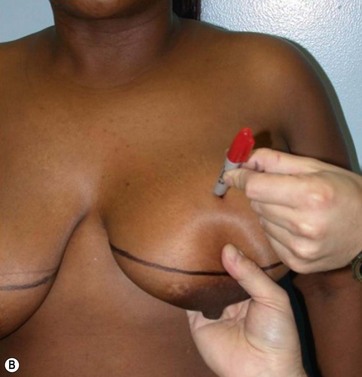
I start marking the new nipple position, which is from 19–21 cm from the midclavicular point (as in all classic measurements – this is the Pitanguy referral point). Then I mark the inframammary fold, which ranges from 15–23 cm (Fig. 8.13). The flap margin is 8–9 cm below the new nipple site.
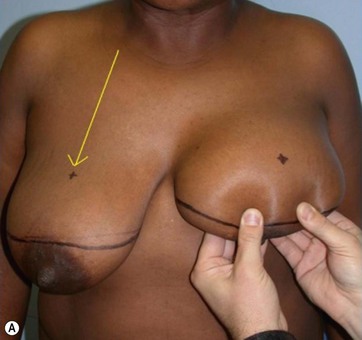
After completing aspiration of emulsified fat, the lower flap is detached from the chest wall, with a central large inferior pedicle based on perforators from the pectoralis muscle. The upper quadrants, already treated with Vaser (Fig. 8.14), show the network of the subcutaneous breast tissue, as it appears after emulsification of fat and aspiration. All the supporting structures of the skin (elastic bundles, vessels, nerves, connective supports) are conserved. This pattern is similar to what happens in closed breast reduction. As the flap is reduced, it is advanced to fill the empty space. The new nipple is positioned and centered on its pedicle.
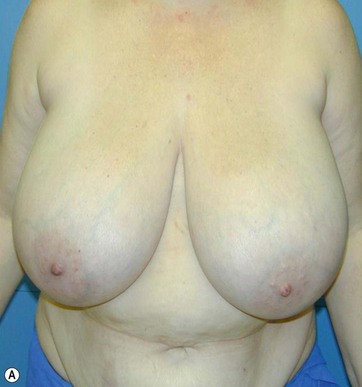
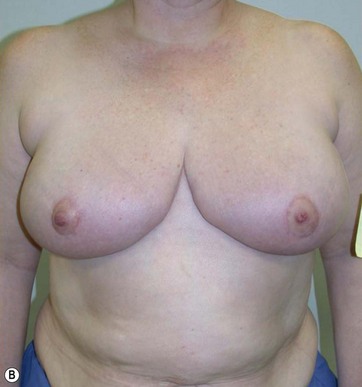
The Passot technique combined with Vaser has been applied to several types of breast ptosis. I have performed large reductions with this technique (up to 2900 g per side) (Fig. 8.15), or operated on the so-called “long breast”.
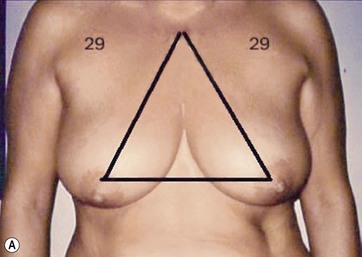
The typical case where I actually combine the Passot technique with Vaser is a moderate degree of ptosis, 26–28 cm from the midclavicular point, with a mild to moderate hypertrophy (Fig. 8.16). Results are satisfactory and tend to improve with time (Box 8.2).
Box 8.2
The Secrets of the Success of the Passot Technique Combined with Breast Reduction
1. Shaping, under control, the upper and lower quadrants of the breast
2. Maintaining the vascularization of the upper quadrants by using Vaser, as this is a selective technique of emulsification
3. Repositioning of the nipple–areola complex without tension, which ensures a good scar (no distortion, no widening)
4. Surgeons must possibly reconsider the priority in scar selection for breast reduction. For most women, the inframammary scar is preferable to the vertical scar, as it is less visible, despite being longer
Complications and Their Management
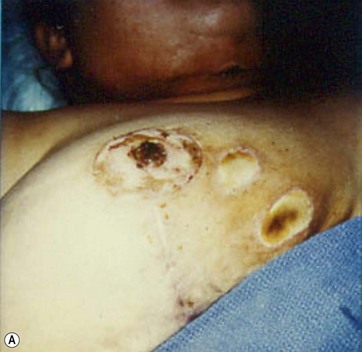
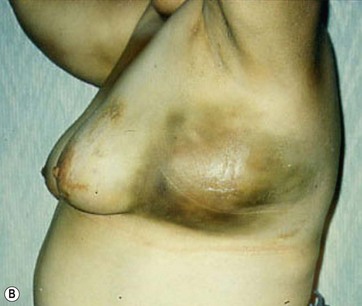
Hematoma
Hematoma formation is another potential complication, though no cases occurred in this series. A photograph of a case of hematoma in a patient treated by another surgeon is shown in Fig. 8.17. This hematoma was localized in the subaxillary region, where the tumescent infiltration was initially administered. The surgeon who performed the operation revealed that the anesthesiologist incorrectly used standard sharp needles rather than blunt infiltration cannulas. The formation of the hematoma, which appeared immediately after the infiltration, was thus related to an incorrect tumescent infiltration technique and not to the breast reduction with UAL.
Selectivity and Specificity of Ultrasound
Large amounts of fat are often found in patients with breast hypertrophy, even among thin adolescents. Lejour and Abboud14 emphasized that once the fat is removed by lipoplasty before breast reduction, the proportion of glandular tissue, connective tissue vessels, and nerves is increased.
These structures are important for maintaining vascularity, sensitivity, and lactation potential. Lejour13 affirmed that if the breasts contain substantial fat, weight loss may result in breast ptosis. The degree of recurrent ptosis can be minimized if lipoplasty is performed preoperatively to reduce the fatty component of the breasts. This observation anticipated the great potential of UAL for breast surgery.
Because it is a selective technique, UAL may be applied in breast surgery to destroy and emulsify only the fatty component of the breast tissue without affecting the breast parenchyma for which the ultrasound energy has no specificity. The specificity of the technique is connected with the cavitation phenomenon and the efficiency of the system hinges on the type of the titanium probe used and the energy level selected. Lejour13 argued that the suctioning of breast fat also made the breast suppler and more pliable, which facilitates shaping, especially when the areola pedicle is long. This consideration is particularly important with fatty breasts, which have a less reliable blood supply. These benefits are significantly increased by the use of UAL because the specificity of this technique spares the vessel network.
The selectivity of UAL was demonstrated by Fisher,24,25 and Palmieri26 in their studies on the action of the ultrasound probe in rat mesenteric vessels. Later, Scheflan and Tazi25 introduced endoscopic evaluation of UAL. They used a Stortz endoscopic system and camera (Stortz, Tuttlingen, Germany) to videotape the action of the titanium probe within the ultrasound device in the superficial layers, verified by needle depth, after standard infiltration with the tumescent technique. UAL was performed with criss-cross tunnels, and the procedure was recorded on videotape. An adjacent area was treated with standard lipoplasty. The technique was compared with standard lipoplasty, which was also endoscopically assisted and monitored. The authors found that standard lipoplasty appears to be the more aggressive technique, with the mechanical destruction of the entire subcutaneous tissue, despite the use of 2–3 mm wide blunt cannulas.
As noted by Lejour,13 retraction of the skin after standard lipoplasty cannot be expected to be sufficient to produce a satisfactory breast shape. Subcutaneous aspiration must be extensive to obtain the necessary skin retraction, and the risk of localized skin necrosis resulting from excessive superficial liposuction cannot be ignored.28
Calcifications
Lejour13 and Lejour and Abboud14 argued that the risk of postoperative fat necrosis or calcifications was the reason many surgeons avoided the use of lipoplasty in the breast. The main cause of fat necrosis is breast ischemia brought about by extensive dissection or direct mechanical damage, with resultant venous drainage. Calcification in breast reduction surgery may derive from an area of fat necrosis or breast necrosis and subsequent scarring. Such calcifications are most often located at the incision lines (periareolar, or vertical scar in the inverted-T approach), where more tension is placed in approximating the lateral and medial flaps. However, when the tension is too high, areas of necrosis could arise from the approximating suture and later cause calcifications that are visible on mammography. However, the risk of such complications in UAL procedures is quite low.
Calcifications in breast parenchyma are to be expected after any mammoplasty procedure. In reduction mammoplasty, it is preferable that they be localized along the breast scars.29 When lipoplasty is performed in addition to the mammoplasty procedure, benign macro-calcifications are slightly more numerous in the parenchyma than they are in breasts reduced without lipoplasty. This may occur because of the trauma caused by lipoplasty or because lipoplasty suction is applied to the most fatty breasts, which are more prone to liponecrosis.30 However, 1 year after fatty breast reduction with UAL, follow up mammography revealed only a slight increase of small microcalcifications, similar to those found after other mammary procedures.
Potential Risks
In November 1998, a conference on UAL safety and effects was held in St Louis, MO, USA, sponsored by the ASERF and the PSEF.31 The panel was organized in response to an article by Topaz32 that raised questions about the safety of UAL. Topaz speculated that thermal effects and the free radicals generated during UAL might result in neoplastic transformation and other long-term complications, as a consequence of the physical effect known as sonoluminescence. Those attending the conference represented multiple scientific disciplines, including plastic surgery, physics, lipid chemistry, cancer biology, and mechanical biophysics. The participants agreed that scientists did not yet understand the mechanism of UAL action, though multiple mechanisms were probably involved, such as mechanical forces, cavitations and thermal effects.
With reference to the application of UAL to breast surgery, we investigated the histology of the breast fat tissue before and after UAL breast surgery (with serial biopsies at 6 months and 1 year after surgery) and the mammographic appearance of the breast before and 1, 2 and 3 years after surgery, particularly with respect to calcification. The results were evaluated by a sonologist not directly involved with the clinical research.33 Histologic studies revealed an increased fibrotic response to thermal insult, with a prevalence of fatty scar tissue, in all specimens evaluated.
From a mammographic viewpoint, the typical appearance of a breast reduction with UAL demonstrates predictably less scarring and fewer calcifications than occur in the standard open technique. Courtiss34 reported similar mammographic evidence in a denser breast after breast reduction by lipoplasty alone. No malignancies were reported.
Conclusion
The use of UAL for reduction of fatty breasts and mastopexy is effective and safe when applied in selected patients and performed by a surgeon with expertise in ultrasound- assisted body contouring. The selectivity of UAL enables emulsification of the fatty component of the breast parenchyma while sparing the glandular tissue and vascular network. Furthermore, long-term mammographic studies have revealed no alteration of morphology of the breast parenchyma resulting from this technique. The typical mammographic appearance of breast tissue after UAL is a denser breast.35,36
1 Zocchi M. Clinical aspects of ultrasonic liposculpture. Perspect Plast Surg. 1993;7:153–174.
2 Zocchi M. The ultrasonic assisted lipectomy (UAL): physiological principles and clinical application. Lipoplasty. 1994;11:14–20.
3 Zocchi M. Ultrasonic assisted lipectomy. Advances in Plastic and Reconstructive Surgery. [Vol. 11] Mosby Year Book: St Louis, MO, 1995.
4 Zocchi M. The ultrasonic assisted lipectomy, instructional course. San Francisco: ASAPS Annual Meeting; March 1995.
5 Zocchi M. The treatment of axillary hyperadenosis and hyperhidrosis using ultrasonically assisted lipoplasty. Presented at the Meeting of the International Society of Ultrasonic Surgery, Faro, Portugal, November 1995.
6 Zocchi M. Basic physics for ultrasound assisted lipoplasty. Clin Plast Surg. 1999;26:209–220.
7 Sampaio Goes JC. Periareolar mammoplasty: double skin technique with application of polyglactine or mixed mesh. Plast Reconstr Surg. 1996;97(5):959–968.
8 Benelli L. A new periareolar mammaplasty: round block technique. Aesth Plast Surg. 1990;14:93–100.
9 Di Giuseppe A. Mammoplasty reduction and mastopexy utilizing ultrasound liposuction. Mammographic study preoperative. Venice, Italy: 46° National Congress of Italian Society of Plastic Reconstructive and Aesthetic Surgery; June 1997.
10 Di Giuseppe A. Ultrasonically assisted liposculpturing. Am J Cosm Surg. 1997;14(3):317–327.
11 Di Giuseppe A. Reducion mamaria y pexia con la asistencia de la lipoplastia ultrasonida. Lipoplastia. 1998;1(1):16–26.
12 Di Giuseppe A. UAL for face-lift and breast reduction. Abstract for World Congress on Liposuction Surgery. California: Pasadena; October 1998.
13 Lejour M. Reduction of large breasts by a combination of liposuction and vertical mammoplasty. In: Cohen M, ed. Master of Surgery: Plastic and Reconstructive Surgery. Boston: Little, Brown, 1994.
14 Lejour M, Abboud M. Vertical mammoplasty without inframammary scar and with liposuction. Perspect Plast Surg. 1990;4:67.
15 Di Giuseppe A. Abstract at the 3rd European Congress of Cosmetic Surgery. Themes: Ultrasound Assistance for Body Contouring, Breast Reduction and Face Lift. How to do it?. [Berlin]. April 1999. p. 23–5
16 Di Giuseppe A. Abstract at the XV Congress of the International Society of Aesthetic Plastic Surgery (ISAPS). Themes: Harmonic Lift or Ultrasonically Assisted Skin Remodelling of Face (Video). Tokyo: Ultrasonic Assisted Lipoplasty of the Breast (Poster); April 2000.
17 Di Giuseppe A. Ultrasonically assisted breast reduction and mastopexy. Int J Cosm Surg Aesth Dermatol. 2001;3(1):23–29.
18 Di Giuseppe A. Ultrasound assisted breast reduction and mastopexy. Aesth Surg J. 2001;21(6):493–506.
19 Di Giuseppe A. Breast reduction with ultrasound assisted lipoplasty. Plast Reconstr Surg. 112(1), 2003.
20 Teimourian B. Suction Lipectomy and Body Sculpturing. St. Louis, MO: CV Mosby; 1987.
21 Teimourian B, Massac E, Jr., Wiegering CE. Reduction suction mammoplasty and suction lipectomy as an adjunct to breast surgery. Aesth Plast Surg. 1985;9:97–100.
22 Rudolph, et al. Reconstructive Plastic Surgery, Volume 1. [Converse J. M.] Saunders, 1991.
23 Chun, Taylor & Van Meter 2002, ASAPS (American society Aesthetic Plastic Surgery) annual meeting in Las Vegas, communication (Abstract of meeting).
24 Gibson T, Kenedi RM. Factors affecting the mechanical characteristics of human skin. In: Proceedings of the Centennial Symposium on Repair and Regeneration. New York: McGraw-Hill Book Company; 1968:87.
25 Gibson T, Stark H, Kenedi RM. The significance of Langer’s lines. In: Hueston JT, ed. Transactions of the Fifth International Congress of Plastic And Reconstructive Surgery. Australia: Butterworths; 1971:1213.
26 Palmieri B. Studio sull’ azione degli ultrasuoni sul tessuto vasculare del ratio. Riv Ital Chir Plast. 1994;9:635–639.
27 Schleflan M, Tazi H. Ultrasonically assisted body contouring. Aesth Plast Surg. 1991;16:117–122.
28 Becker H. Liposuction of the breast. Presented at the Lipoplasty Society of North America meeting, September 1992.
29 Mitnick JS, Roses DF, Harris MN, Colen SR. Calcifications of the breast after reduction mammoplasty. Surg Gynecol Obstet. 1990;171:409–412.
30 Lejour M, Abboud M. Reduction of mammaplasty scars: from a short inframammary scar to a vertical scar. Ann Chir Plast Esthet. 1990;35(5):369–379.
31 Young VL, Schorr MV. Report from the conference on ultrasound assisted liposuction safety and effects. Clin Plast Surg. 1999;26:481–524.
32 Topaz M. Possible long-term complications in UAL induced by sonolution minescence, sonochemistry, and thermal effects. Aesth Surg J. 1998;18:19–24.
33 Di Giuseppe A, Santoli M. Ultrasonically assisted breast reduction and mastopexy. Int J Cosm Surg Aesth Dermatol. 2001;3(1):23–29.
34 Courtiss EH. Breast reduction by suction alone. In: Spear S, ed. Surgery of the Breast: Principles and Art. Philadelphia: Lippincott-Raven, 1998.
35 Di Giuseppe A. Vaser-assisted breast reduction. In: Shiffman M, ed. Mastopexy and Breast Reduction. Principles and Practice. New York: Springer, 2009.
36 Di Giuseppe A. Mastopexy (breast lift) with ultrasound assisted liposuction. In: Shiffman M, Di Giuseppe A. Liposuction Principles and Practice. New York: Springer, 2006.