84. Tyrosinemia
Definition
Tyrosinemia is an aminoacidopathy of tyrosine metabolism with elevated serum and urinary tyrosine excretion and related metabolites. Type I results in inhibition of some renal tubular function. The renal implications are similar to the renal dysfunction observed with Fanconi syndrome (see p. 132). Tyrosinemia is also known as hereditary tyrosinemia type I (HTI).
Incidence
The overall incidence of tyrosinemia is estimated at 1:100,000. Northern European populations demonstrate an approximate incidence of 1:8000, whereas the population of Quebec, Canada, demonstrates an incidence of 1:1846. There is no gender preference.
Etiology
HTI results from the homozygous, autosomal recessive inheritance of a genetic mutation on chromosome 15, loci q23-q25. To date 30 distinct mutations at these loci have been documented.
Signs and Symptoms
• Anorexia
• Bloody stool
• Cabbage-like body odor
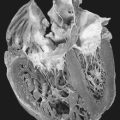
• Cirrhosis
• Diarrhea
• Diminished nutritional intake
• Epistaxis
• Failure to thrive
• Hepatic nodules
• Hepatomegaly
• Jaundice
• Lethargy
• Marked edema
• Melena
• Polyneuropathy
• Purpuric lesions
• Vomiting
Medical Management
Treatment of HTI is directed toward the patient’s acute hepatic decompensation, which may result in greatly reduced or even absent critical coagulation factors. Replacement or replenishment of inadequate and/or absent coagulation factors is necessary to prevent inordinate blood volume losses from even seemingly benign injuries.
The patient with HTI has some dietary restrictions. Specifically, the patient must minimize intake of phenylalanine and tyrosine to allow for only the essential daily requirements.
The child with HTI is typically critically ill on presentation. Stabilization is essential. Currently, surgical interventions are limited. Development of severe cirrhosis, hepatocarcinoma, or other hepatic lesion(s) may necessitate liver transplantation, but this extreme measure is typically a treatment of last resort.
Complications
• Abdominal crisis
• Hepatic cirrhosis
• Hepatocellular carcinoma
• Hepatoma
• Peripheral neuropathy
• Renal Fanconi syndrome (see p. 132)
• Renal tubular acidosis type 2
• Rickets secondary to renal tubular acidosis
• Seizures
Anesthesia Implications
Dehydration is a real risk at any time for the patient with tyrosinemia. As a result, the patient’s NPO duration should be kept to the safest tolerable minimum time.
The patient being treated with diuretic therapy should also have an electrolyte level determined immediately before surgery, with attention focused on the potassium concentration. Potassium supplementation may be necessary and may cause a nonemergent and/or elective operative procedure to be delayed until the potassium concentration has been corrected.
Arterial blood gas analysis may be needed in conjunction with analysis of electrolyte levels to determine the current state of acid-base equilibrium. The patient may attempt to compensate by inducing a respiratory alkalosis. Anesthetists must watch for this possibility to ensure that the balance is not altered significantly, which could exacerbate the metabolic acidosis that arises as a result of the patient’s altered respiratory pattern during general anesthesia. Adequate ventilation during general anesthesia may necessitate supplemental administration of sodium bicarbonate and should be guided by serial arterial blood gas analyses during anesthesia.
Because of the potential need for obtaining multiple blood samples, insertion of an arterial pressure line may be appropriate—even considerate—for the patient.
Liver function testing is of particular concern for the patient with tyrosinemia, especially one with concurrent Wilson’s disease (see p. 355) or Fanconi syndrome (see p. 132).
Patients may be developmentally delayed and have significant speech impairment as a result of associated galactosemia. Therefore, extra effort and patience may be required of the anesthetist to foster patient understanding and cooperation before administering the anesthetic.
Because the patient’s NPO status may be altered and renal failure is possible, general anesthesia should be induced via rapid sequence induction.
Selection of a volatile agent for maintenance of general anesthesia should be guided by the patient’s degree of renal involvement or dysfunction. Regional anesthesia may be appropriate, but fluid resuscitation must be tempered by the degree of renal dysfunction that may be present.
Metabolism of various anesthesia-related medications, from nondepolarizing muscle relaxants to midazolam to opioid analgesics, may be altered as a result of the degree of the patient’s liver dysfunction.