Chapter 12 Tympanoplasty—Undersurface Graft Technique
Postauricular Approach
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
Since the fundamental principles of tympanoplasty were first introduced by Wullstein1 and Zollner,2 there has been great diversity in the accepted surgical techniques used for repair of the tympanic membrane. The multitude of graft materials employed is a testimony to the difficulty of middle ear reconstruction. With advanced microsurgical techniques, the state of the art has now developed to the extent that graft success rates of 90% to 97% are to be expected.3–5 Two basic grafting techniques have evolved based on where the graft material is placed in relation to the drum remnant (overlay versus underlay techniques). This chapter presents a method of undersurface grafting. Detailed surgical techniques and appropriate preoperative and postoperative care are presented.
HISTORICAL ASPECTS
Modern middle ear reconstructive surgery represents a culmination of more than a century of contributions by numerous dedicated and innovative otologic surgeons. The term tympanoplasty was originally defined in 1964 by what was then known as the American Academy of Ophthalmology and Otolaryngology’s Committee on Conservation of Hearing as “an operation to eradicate disease in the middle ear and to reconstruct the hearing mechanism without mastoid surgery, with or without tympanic membrane grafting.”6 If a mastoid procedure is included, the term tympanoplasty with mastoidectomy is used.
The era of surgical repair of the tympanic membrane dates as far back as the 19th century. In 1853, Toynbee7 described closure of a perforation of the tympanic membrane using a small rubber disk attached to a silver wire. Ten years later, Yearsley8 advocated placing a cotton ball over the perforation; in 1887, Blake9 introduced the concept of placing a thin paper patch over the membrane. The use of cautery to promote spontaneous healing of tympanic membrane perforations was introduced by Roosa in 187610; he used silver nitrate. Later, Joynt,11 Linn,12 and Derlacki13 described modifications of this technique using various forms of cautery and patches. Closure of tympanic membrane perforations was considered appropriate only for dry central perforations, however. At this point, no one advocated the use of drum closure for the chronically draining ear.
It was not until 1952 that Wullstein1 and Zollner2 revolutionized middle ear surgery by advocating reconstructive grafting of the chronically diseased ear through the use of full-thickness or split-thickness skin grafts. House and Sheehy14 and Plester15 later used canal skin, believing that it more closely resembled the squamous layer of the tympanic membrane. The overall poor success rates of these grafts and the development of iatrogenic cholesteatomas prompted the search for alternative grafting materials.
Shea16 and Tabb,17 working independently, described the use of autogenous vein to close the tympanic membrane. Goodhill18 advocated tragal perichondrium in the mid-1960s, and tympanic membrane homografts became popular a few years later. Glasscock and House19 reported the first sizable series of homograft tympanic membrane transplants in 1968. Interest in homografts has waned, however, largely because of the fear of transmission of infectious diseases. Storrs20 performed the first fascia graft in the United States. Although vein, perichondrium, and homografts still have their advocates, autogenous fascia has now become the standard by which all other grafting materials are measured.
The use of skin grafts required that the tympanic membrane perforation be repaired by laying the graft on top of the denuded drum remnant. This method of repair eventually became known as the overlay technique and was carried over to other forms of grafting material. With the use of connective tissue grafts, the graft material could be placed medial to the tympanic membrane remnant. The success of this approach eventually gave rise to the underlay technique of tympanic membrane grafting; Austin and Shea3 reported a large series. Proponents of the underlay procedure submit that it eliminates many of the problems associated with overlay grafts, such as anterior blunting, epithelial pearl formation, and lateralization of the new drum.
In 1973, Glasscock4 described an underlay grafting technique that relied on a postauricular approach. With minor modifications, this approach continues to be the preferred method of dealing with disorders of the tympanic membrane and the middle ear.
FUNDAMENTALS
Traditional Objectives
The traditional objectives of tympanoplasty have not changed in 50 years and remain:
The goals, in this order of priority, allow for patient counseling and reasonable expectations.
BASIC TECHNICAL PRINCIPLES
Infection Control
In the only study of statistical power on this subject, Jackson23 concluded that prophylactic antibiotics are harmless, but useless. In common uncomplicated tympanoplasty, antimicrobial prophylaxis is unwarranted. An indication for prophylaxis exists in the draining ear, which, intuitively, has a high postinfection rate with graft failure. Despite this fact, no protocol exists to prevent such an outcome. This is an ideal indication for intraoperative irrigation, yet ototoxicity and medicolegal concerns have impeded human study design to address this issue.
Grafting Techniques and Exposure
Two approaches to tympanic membrane grafting have evolved over the years. The overlay technique previously was popular because of its high success rate and reproducibility.24 Experience has recognized recurring downsides, however. Blunting of the anterior sulcus, when significant, can result in conductive hearing loss by malleus fixation, as can lateralization of the graft away from the malleus. Inability to denude the drum of epithelium completely could and has resulted in epithelial pearls or cholesteatoma or both. Because the EAC skin is removed and replaced, delays in healing occur.
SURGICAL TECHNIQUE
Surgical Preparation
The postauricular region and the tragus are injected with a 2% lidocaine and 1:100,000 epinephrine solution. After injection, the patient is draped with two layers of sterile sheets. The first layer consists of a 3M plastic ear drape (No. 1030). This waterproof sheet effectively isolates the patient and prevents contamination if the paper drapes become soaked with irrigation solution. Finally, a custom-designed paper otologic drape and a plastic drainage bag are applied (Fig. 12-1). Suction-irrigation tubing and cautery lines are secured to the field. The scrub nurse attaches a compartmentalized plastic pouch to the Mayo stand to hold suction tips and electrocauteries.
Anesthesia
All patients undergo general endotracheal anesthesia with an epinephrine-compatible anesthetic agent. Long circuits are used on the anesthesia machine to enable the anesthesiologist to be seated at the foot of the table, opposite the surgeon (Fig. 12-2). The scrub nurse is positioned at the head of the table across from the surgeon.
Incisions
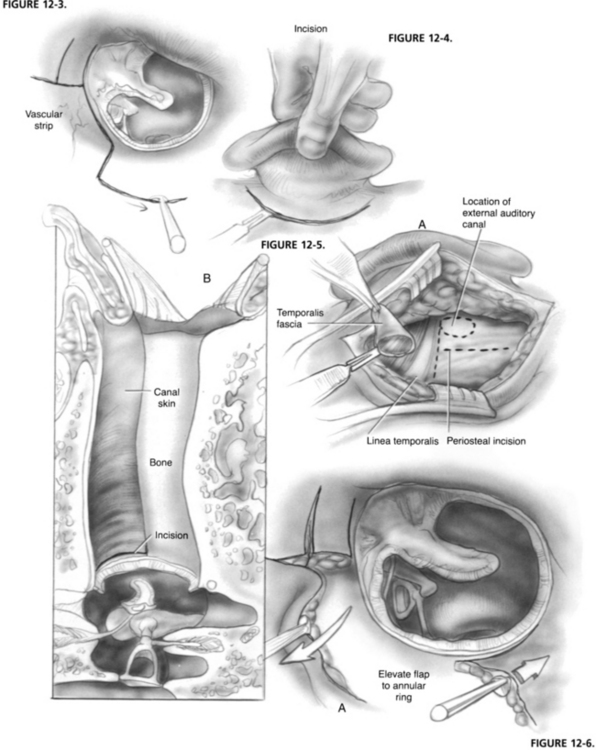
The vascular strip is outlined by making incisions at the tympanosquamous and tympanomastoid suture lines using a No. 67 Beaver knife blade. In addition, small inferiorly and superiorly based flaps are created by making right angle incisions to the vascular strip incisions. The medial end of the vascular strip is formed by connecting the two primary incisions with a No. 72 Beaver blade approximately 2 mm lateral to the annulus (Fig. 12-3).
A postauricular incision is made approximately 5 mm behind the postauricular crease (Fig. 12-4). The surgeon firmly grasps the auricle in the left hand and forcefully pulls forward and outward. Constant tension allows identification of the loose areolar tissue overlying the temporalis fascia and creates a bloodless surgical plane. Incisional bleeding is controlled with electric cautery.
Harvesting Fascia
When hemostasis is achieved, a small Weitlaner retractor is positioned to hold the auricle forward. The scrub nurse places a small Senn retractor under the upper part of the incision and pulls laterally, exposing the temporalis fascia. The loose areolar tissue overlying the temporalis fascia is ballooned up with a local anesthetic to facilitate its removal. An incision is made at the level of the linea temporalis, and the areolar tissue is dissected free from the temporalis fascia using Metzenbaum scissors (Fig. 12-5). This tissue is pressed onto a polytef (Teflon) block and placed on the back table under a gooseneck lamp to dehydrate it.
Exposing the Middle Ear
The retractor is removed, and an incision is made along the linea temporalis extending anterior and superior to the EAC. A T-shaped incision is created by dropping a vertical limb from the midpoint of the linea temporalis to the mastoid tip (see Fig. 12-5). A Lempert elevator is used to mobilize the periosteum to the level of the ear canal. The vascular strip is identified from posteriorly, grasped with Adson forceps, and held forward in the blade of a Weitlaner retractor along with the auricle (see Fig. 12-5). A second Weitlaner retractor is placed between the temporalis muscle and the mastoid tip at right angles to the first retractor.
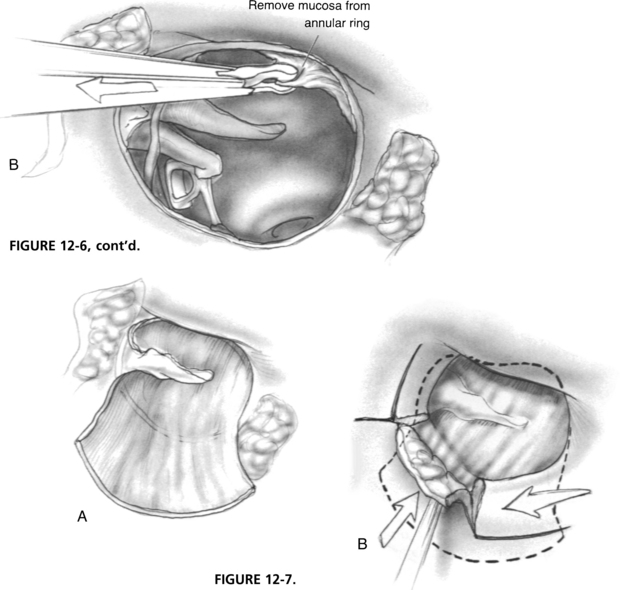
The ear canal is copiously irrigated with a physiologic saline solution to remove blood debris. With a 20 gauge needle suction in the surgeon’s left hand and a House No. 2 lancet knife in his or her right hand, the skin of the inferior ear canal is elevated down to the fibrous annulus (Fig. 12-6), creating an inferiorly based flap. Next, a House No. 1 sickle knife is used to develop a superior flap just above the short process of the malleus. The fibrous annulus is mobilized out of its sulcus anterior to the malleus.
Preparation of the Tympanic Membrane Remnant
When the disease of the middle ear and mastoid has been eradicated, the drum remnant is prepared for grafting. Small attic, marginal, or central perforations are prepared to preserve the normal drum remnant. In the case of an extensive perforation of a severely diseased membrane, the entire drum remnant is removed to the level of the annulus. The manubrium of the malleus is denuded, preserving the fibrous annulus. The mucosa of the undersurface of the drum remnant or annulus is abraded through the use of a House No. 1 sickle knife and cup forceps to ensure further an adequate recipient surface for the graft (see Fig. 12-6).
Placement of the Graft
The graft is grasped using cup forceps, rehydrated in a physiologic saline solution, such as Tis-U-Sol irrigating solution, and placed in the middle ear. With a 22 gauge suction in the surgeon’s left hand and a right angle hook in his or her right hand, the graft is slid under the manubrium of the malleus onto the lateral attic wall. A House annulus elevator is used to tuck the fascia under the drum remnant anteriorly and inferiorly (Fig. 12-7).
With this technique, there is a point in the inferior canal at approximately the 6 o’clock position where the graft makes a transition from lying medial to the annulus to being lateral to it. The remaining graft is draped along the posterior canal wall, and the inferior canal flap with attached annulus is repositioned over the graft. Similarly, the superior flap is placed, covering the fascia lying anterior to the malleus (see Fig. 12-7). A House annulus elevator is used to even all edges of the annulus and smooth out the graft. The surgeon must ensure that flap margins are flat because buried skin may result in pearl formation. Polymyxin B and bacitracin (Polysporin) ointment is placed over the fascia graft filling the anterior sulcus. The retractors are removed, and the vascular strip is carefully replaced to its original position. The mastoid periosteum incision is closed with 3-0 polyglactin 910 (Vicryl) suture in interrupted fashion.
The postauricular incision is closed with the same 4-0 suture in a subcuticular fashion. No skin sutures are used, obviating later removal. Proper position of the vascular strip is confirmed again under direct vision through an ear speculum, and the remainder of the ear canal is filled with antibiotic ointment. A cotton ball is placed in the meatus, and a sterile, prepackaged plastic mastoid bubble dressing is applied (Fig. 12-8).
RESULTS
In 1982, Glasscock and associates5 reported their experience in 1556 cases of tympanic membrane repair using the postauricular undersurface grafting technique described here. Of these cases, 663 were simple tympanoplasties, 687 involved a mastoidectomy, 54 were performed to repair a graft failure at a second stage, 38 involved a tympanoplasty with mastoid revision, and 114 were canal wall down mastoidectomies in which the tympanic membrane was grafted. Of ears, 463 (34%) had undergone at least one previous surgery. All tympanic membranes were repaired using areolar tissue, temporalis fascia, or tragal perichondrium.
A more recent study examined results from revision chronic ear surgery.25 The authors found that control of recurrent cholesteatoma was achieved in 93% of cases. Hearing also can be significantly improved by closure of recurrent perforations or reconstruction of the ossicular chain. These authors found that hearing results after ossicular chain reconstruction were not as good for revision surgery as seen for primary surgery.25 Another study by the same authors compared surgical outcomes for chronic ear surgery between smokers, former smokers, and nonsmokers.26 This study showed that smokers have significantly more cholesteatomas, more canal wall down mastoidectomies, and overall worse hearing results than nonsmokers. Smokers also had significantly more postoperative complications than nonsmokers. Patients who quit smoking within 5 years of their surgery had the same risks as current smokers. In patients who quit smoking more than 5 years before surgery, the results of chronic ear surgery were no different than in patients who never smoked.
CARTILAGE GRAFT TYMPANOPLASTY
In cases of severe atelectasis of the tympanic membrane, a cartilage tympanoplasty is often indicated. Cartilage autografts have long been used in repair of canal wall defects and ossiculoplasty.27–30 In 1982, Glasscock and associates5 first described the successful use of cartilage/perichondrial autografts for severe atelectasis, attic cholesteatoma, and posterior retraction pockets.5 Since that time, other authors have reported their results with this technique.31,32
Surgical Techniques
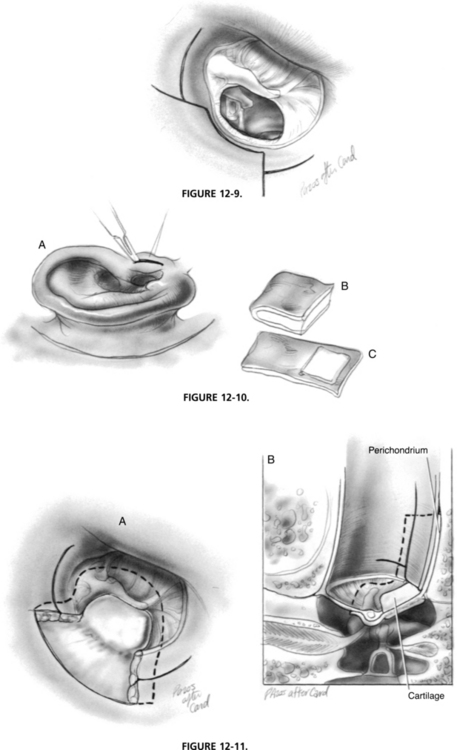
After exposure of the middle ear is obtained, the next step is to excise all diseased and atelectatic tympanic membrane. The posterior fibrous annulus is elevated from its bony sulcus with a House No. 2 lancet knife. Careful dissection is required to avoid tearing the atelectatic drum to ensure that no epithelium is left in the middle ear. To verify complete removal of an attic retraction, it is often necessary to perform mastoidectomy. Posterosuperior retractions must be elevated in continuity with the remainder of the tympanic membrane. When elevating the drum off the lenticular process and the stapes suprastructure, applying force in the posterior-to-anterior direction allows the stapedius tendon to provide countertraction, preventing inadvertent stapes subluxation. A House No. 1 sickle knife is used to elevate the diseased membrane off the manubrium and lateral process of the malleus. Fibrous adhesions are lysed, and diseased middle ear mucosa is removed with cup forceps (Fig. 12-9).
To harvest the cartilage perichondrial graft, an incision is made on the posteromedial surface of the tragus (Fig. 12-10). This incision is carried through the tragal cartilage, preserving the dome of the tragal cartilage for cosmesis. A House No. 2 lancet elevator is used to elevate the perichondrium from one surface of the cartilage, leaving it hinged on the other side, similar to a book cover. The cartilage is trimmed to the proper dimensions, depending on the degree of disease present. A posterosuperior quadrant retraction often requires a cartilage graft of approximately 4 mm in diameter. For cases of atelectasis of the entire tympanic membrane, the cartilage can be incorporated into the entire pars tensa. In this situation, a wedge-shaped area of cartilage is accessed to accommodate the manubrium, if present. When using large cartilage grafts not likely to move in healing, perichondrium is not needed and is detached.
When the middle ear has been packed with moistened Gelfoam, the cartilage/perichondrial graft is placed with the perichondrium side facing laterally. The perichondrium is tucked under the manubrium and draped over the ear canal posteriorly. The cartilage should not overlap the posterior canal wall. The areolar tissue graft is trimmed to size and placed lateral to the cartilage perichondrial graft and medial to the fibrous annulus and manubrium (Fig. 12-11). This areolar graft serves to cover any remaining defects in the tympanic membrane or exposed bone in the external canal. When perichondrium is not used, the cartilage graft is placed atop the middle ear Gelfoam and medial to the tympanic membrane graft. The cartilage is ultimately enveloped by the neo–tympanic membrane. The superior-based and inferior-based canal flaps are returned to their original positions, covering the grafts as they extend onto the posterior canal wall. The external canal is filled with Polysporin ointment, and closure proceeds in the manner previously described.
Results
Results in 100 ears using cartilage tympanoplasty were reported more recently.34 The retracted portion of the tympanic membrane was in the posterosuperior quadrant in 37%, the entire drum in 34%, the posterior half in 18%, the pars flaccida alone in 16%, the pars flaccida and pars tensa combined in 4%, and the anterior half in 1%. Cholesteatoma was present in 33 ears. Ossicular reconstruction was required in 52 ears; 31 of these procedures were performed simultaneously with the cartilage tympanoplasty, and 21 were staged.
1. Wullstein H. Funktionelle Operationen im Mittelokr mit Hilfe des Freven Spalthappen-Transplantes. Arch Ohr Nas Kehlhopfheilk. 1952;161:422.
2. Zollner F. The principles of plastic surgery of the sound-conducting apparatus. J Laryngol Otol. 1955;69:637.
3. Austin D.F., Shea J.J. A new system of tympanoplasty using vein graft. Laryngoscope. 1961;71:596.
4. Glasscock M.E. Tympanic membrane grafting with fascia: Overlay versus underlay technique. Laryngoscope. 1973;5:754.
5. Glasscock M.E., Jackson C.J., Nissen A.J., et al. Postauricular undersurface tympanic membrane grafting: A follow-up report. Laryngoscope. 1982;92:718.
6. Committee on Conservation of Hearing of the American Academy of Ophthalmology and Otolaryngology. Standard Classification for Surgery of Chronic Ear Infection. Arch Otolaryngol Head Neck Surg. 1964;81:204.
7. Toynbee J. On the Use of an Artificial Membrane Tympanic in Cases of Deafness Dependent Upon Perforations or Destruction of the Natural Organ. London: J Churchill & Sons; 1853.
8. Yearsley J. Deafness, Practically Illustrated. London: J Churchill & Sons; 1863. Ecl 6
9. Blake C.J. Transactions of the First Congress of the International Otological Society. New York: D Appleton; 1887.
10. Roosa D.B. St. J: Disease of the Ear, 3rd ed. New York: William Wood; 1876.
11. Joynt J.A. Repair of the drum. J Iowa Med Soc. 1919;9:51.
12. Linn E.G. Closure of tympanic membrane perforations. Arch Otolaryngol Head Neck Surg. 1953;58:405.
13. Derlacki E.L. Repair of central perforations of the tympanic membrane. Arch Otolaryngol Head Neck Surg. 1953;58:405.
14. House W.F., Sheehy J.L. Myringoplasty. Arch Otolaryngol. 1961;73:407.
15. Plester D. Myringoplasty methods. Arch Otolaryngol. 1963;78:310.
16. Shea J.J. Vein graft closure of eardrum perforations. J Otolaryngol. 1960;74:358.
17. Tabb H.G. Closure of perforations of the tympanic membrane by vein grafts: A preliminary report of 20 cases. Laryngoscope. 1960;70:271.
18. Goodhill V. Tragal perichondrium and cartilage in tympanoplasty. Arch Otolaryngol. 1967;85:480.
19. Glasscock M.E., House W.F. Homograft reconstruction of the middle ear. Laryngoscope. 1968;78:1219.
20. Storrs L.A. Myringoplasty with the use of fascia grafts. Arch Otolaryngol. 1961;74:65.
21. Gates G.A., Avery C.A., Prihoda T.J., Cooper J.C.Jr. Effectiveness of adenoidectomy and tympanostomy tubes in treatment of chronic otitis media with effusion. N Engl J Med. 1987;317:1444-1451.
22. Fry T.L., Pillsbury H.C. The implications of controlled studies of tonsillectomy and adenoidectomy. Otolaryngol Clin North Am. 1987;20:409-413.
23. Jackson C.G. Antimicrobial prophylaxis in ear surgery. Laryngoscope. 1988;98:1116-1123.
24. Sheehy J.L., Glasscock M.E. Tympanic membrane grafting with temporalis fascia. Arch Otolaryngol. 1967;86:391.
25. Kaylie D.M., Gardner E.K., Jackson C.G. Revision chronic ear surgery. Otolaryngol Head Neck Surg. 2006;134:443-450.
26. Kaylie D.M., Bennett M.L., Davis B.M., Jackson C.G. Chronic ear surgical outcomes in smokers and non-smokers. Presented at American Academy of Otolaryngology–Head and Neck Surgery Annual Meeting. Washington: DC; September 16-19, 2007.
27. Donald F.J., McCabe B.F., Loevy S.S., et al. Atticotomy: A neglected otosurgical technique. Ann Otol Rhinol Laryngol. 1974;83:652.
28. McCleve D.E. Repair of bony canal wall defects in tympanomastoid surgery. Am J Otol. 1985;6:76.
29. McCleve D.E. Tragal reconstruction of the auditory canal. Arch Otolaryngol. 1969;90:35.
30. Linda R.E. The cartilage-perichondrium graft in the treatment of posterior tympanic membrane retraction pockets. Laryngoscope. 1973;83:747.
31. Schwaber M.K. Postauricular undersurface tympanic membrane grafting: Some modifications of the “swinging door” technique. Arch Otolaryngol Head Neck Surg. 1986;95:182.
32. Levenson R.M. Cartilage-perichondrial composite graft tympanoplasty in the treatment of posterior marginal and attic retraction pockets. Laryngoscope. 1987;97:1069.
33. Adkins W. Composite autograft for tympanoplasty and tympanomastoid surgery. Laryngoscope. 1990;100:244.
34. Glasscock M.E., Hart M.J. Surgical treatment of the atelectatic ear. Otolaryngol Head Neck Surg. 1992;3:15.