Chapter 24 Tumors of the Uterine Corpus
Malignant tumors of the uterine corpus can be divided into epithelial and mesenchymal types (sarcomas). In 2011, there were an estimated 46,470 cases of cancer involving the uterine corpus in the United States, of which 3% were uterine sarcomas with an estimated 8120 deaths.1,2
I Endometrial Cancer
Introduction
The most common epithelial tumor of the uterine corpus is endometrial cancer, accounting for approximately 50% of all new gynecologic cancers. In the United States, it is the fourth most common malignancy in women, following breast, lung, and colorectal cancers.1
In the majority of women, endometrial cancer is confined to the uterus, as stage I (71%) and stage II (24%) at time of presentation owing to the appearance of abnormal uterine bleeding.3 This clinical presentation and diagnosis allow for a favorable prognosis.
Epidemiology and Risk Factors
In the development of endometrial cancer, age is the most important risk factor. This is a disease predominantly seen in the postmenopausal female, with a median age at diagnosis of 60 years.3 Other risk factors that have been associated in developing endometrial cancer are long-term exposure to unopposed estrogens, residence in North America and Europe, metabolic syndrome (obesity, diabetes), years of menstruation, nulliparity, history of breast cancer, long-term use of tamoxifen, hereditary nonpolyposis colorectal cancer (HNPCC) family syndrome, hormone-replacement therapy with less than 12 to 14 days of progestagens, and first-degree relative with endometrial cancer.4
African American women tend to be diagnosed with higher stage, grade, and risk histologies than non-Hispanic white women.5 Although some cases of endometrial cancer have a hereditary basis, the vast majority are sporadic. HNPCC, Lynch’s syndrome, is an autosomal dominant cancer presenting with early-onset colon, rectal, ovary, small bowel, ureter/renal pelvis, and endometrial cancers. Two to 5% of endometrial cancers are associated with the Lynch syndrome.6 The gynecologic cancer (endometrial or ovarian) presents earlier than colon cancer in about half of the patients with Lynch’s syndrome.4,6,,7
Anatomy and Pathology
On immunohistochemical staining, PTEN mutations are common in type 1 tumors and uncommon in serous tumors. TP53 mutations are not common in type 1 tumors but are common in serous carcinoma.4 Other histologic types of endometrial carcinomas include mucinous, mixed, squamous cell, transitional cell, and undifferentiated.4 Women taking tamoxifen for the treatment of breast cancer are at an increased risk of developing endometrial cancer; however, the risk-benefit ratio favors the use of tamoxifen. Newer aromatase inhibitors are now available and are being used instead of tamoxifen.
Key Points Anatomy and pathology
• Two types of endometrial cancer have been described.
• Type 1 endometrial cancers are the most common, occur in younger women, develop in a background of endometrial hyperplasia and have a favorable prognosis.
• Type 2 endometrial cancers occur in older women, are associated with endometrial atrophy, and are at high risk of relapse and metastases.
Patterns of Tumor Spread
Type 1 tumors primarily spread to pelvic lymph nodes. More positive lymph nodes are seen around the obturator nerve than the external or common iliac vessels. Infrarenal para-aortic adenopathy is seen when there are positive pelvic lymph nodes, adnexal metastases, and serosal infiltration. Type 2 tumors have a higher incidence of lymphatic involvement and extrauterine spread.4 The adnexa and cervix can also be invaded by endometrial carcinoma.
Staging Evaluation
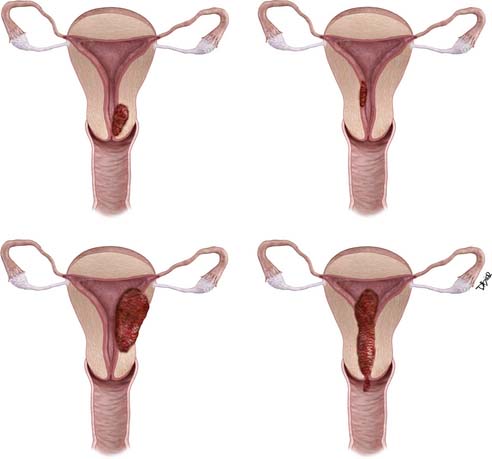
Surgery is used to stage endometrial cancer. The most recent FIGO staging is also used to manage these patients. Stage I is endometrial carcinoma confined to the corpus uteri, whereas stage II involves the corpus uteri and the cervix but has not extended outside of the uterus. Stage III endometrial carcinoma extends outside of the uterus but is confined to the true pelvis. In stage IV, endometrial cancer involves the bladder or bowel mucosa or has metastasized to distant sites. These stages are divided into further subgroups (Table 24-1 and Figure 24-1).8
Table 24-1 AJCC Tumor-Node-Metastases (TNM) and International Federation of Gynecology and Obstetrics (FIGO) Surgical Staging Systems for Endometrial Cancer
| TNM CATEGORIES | FIGO* STAGES | SURGICAL-PATHOLOGIC FINDINGS |
|---|---|---|
| Primary Tumor (T) | ||
| TX | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | |
| Tis† | Carcinoma in situ (preinvasive carcinoma) | |
| T1 | I | Tumor confined to the corpus uteri |
| T1a | IA | Tumor limited to endometrium or invades less than one-half of the myometrium |
| T1b | IB | Tumor invades one-half or more of the myometrium |
| T2 | II | Tumor invades stromal connective tissue of the cervix but does not extend beyond uterus‡ |
| T3a | IIIA | Tumor involves serosa and/or adnexa (direct extension or metastasis)# |
| T3b | IIIB | Vaginal involvement (direct extension or metastasis) or parametrial involvement# |
| IIIC | Metastases to pelvic and/or para-aortic lymph nodes# | |
| IV | Tumor invades bladder and/or bowel mucosa, and/or distant metastases | |
| T4 | IVA | Tumor invades bladder mucosa and/or bowel (bullous edema is not sufficient to classify a tumor as T4) |
| Regional Lymph Nodes (N) | ||
| NX | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | IIIC1 | Regional lymph node metastasis to pelvic lymph nodes (positive pelvic nodes) |
| N2 | IIIC2 | Regional lymph node metastasis to para-aortic lymph nodes, with or without positive pelvic lymph nodes |
| Distant Metastasis (M) | ||
| M0 | No distant metastasis | |
| M1 | IVB | Distant metastasis (includes metastasis to inguinal lymph nodes, intra-peritoneal disease, or lung, liver, or bone. It excludes metastasis to para-aortic lymph nodes, vagina, pelvic serosa, or adnexa) |
† Note: FIGO no longer includes Stage 0 (Tis).
‡ Endocervical glandular involvement only should be considered as Stage I and no longer as Stage II.
# Positive cytology has to be reported separately without changing the stage.
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC (SBM). (For complete information and data supporting the staging tables, visit ww.springer.com.) Reprinted from Pecorelli S, Denny L, Ngan H, et al. Revised FIGO staging for carcinoma of the vulva, cervix and endometrium. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2009;105:103-104. Copyright 2009, with permission from International Federation of Gynecology and Obstetrics.
Imaging Evaluation
Primary Tumor
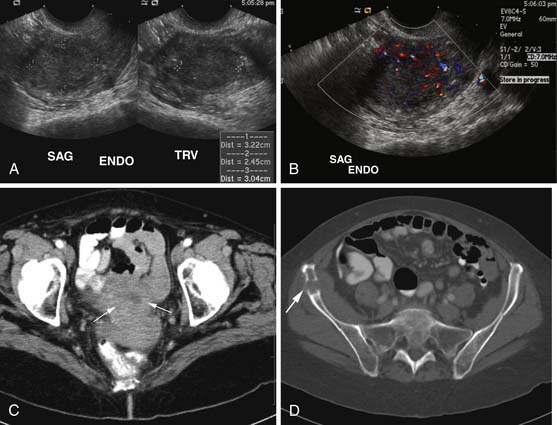
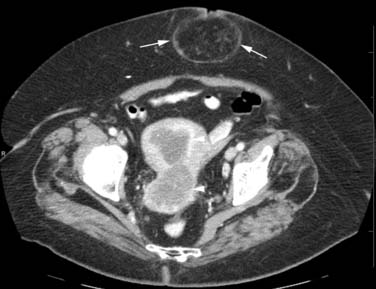
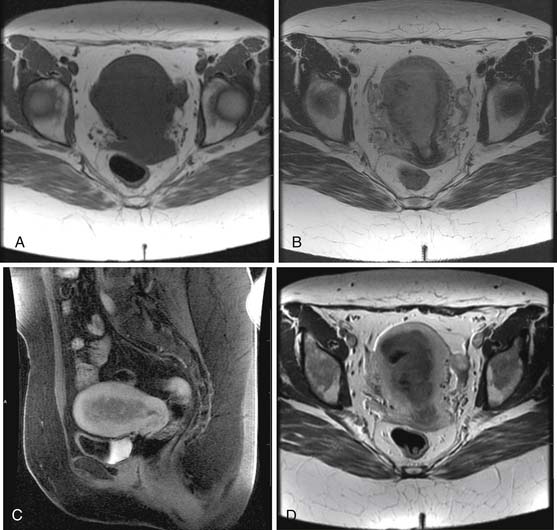
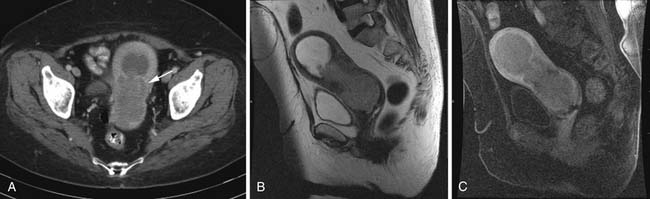
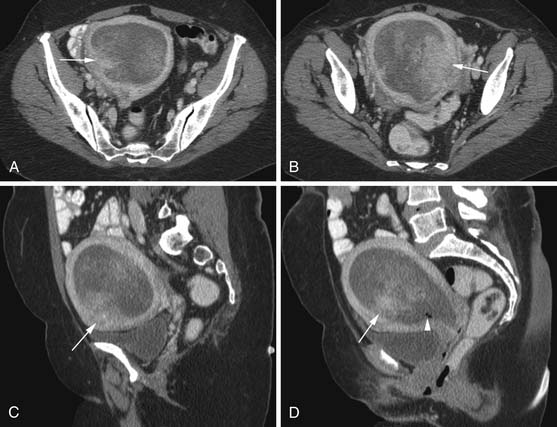
Various imaging modalities (Figures 24-2 to 24-6) can be used to help the attending physician evaluate the patient with endometrial cancer preoperatively, postoperatively, and during surveillance visits.
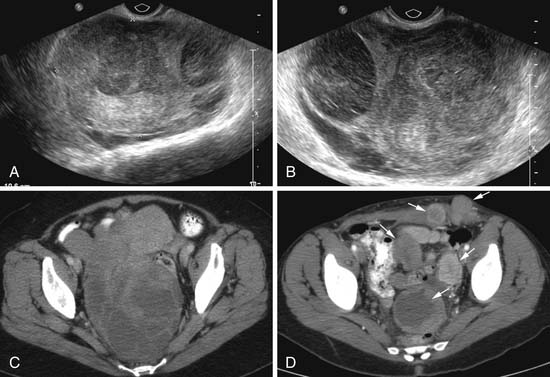
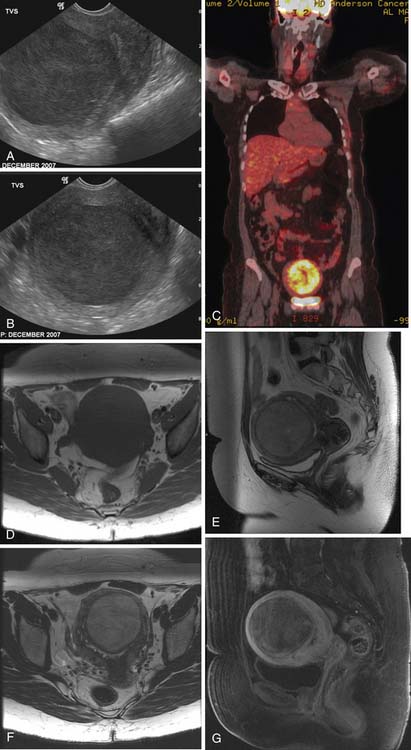
Ultrasound, especially transvaginal sonography, is noninvasive, is readily available, is less expensive, uses no ionizing radiation, and in some studies, can image the tumor and myometrial invasion, but it can do poorly in evaluating the spread of tumor to adjacent organs.9,10 Endometrial thickness (double layer) greater than 4 to 5 mm in a postmenopausal woman is considered abnormal and raises a flag when there is postmenopausal bleeding. However, the endometrium in this setting is nonspecific and can be seen in hyperplasia, polyps, and cancer.
Magnetic resonance imaging (MRI) is the imaging modality of choice to preoperatively evaluate the depth of myometrial invasion, the presence or absence of adenopathy, and adjacent soft tissue invasion. It has a high soft tissue resolution and accuracy (~90%), especially if it is contrast-enhanced.11,12 However, it is more expensive and, in some locations, is limited in number and availability. This is an ideal imaging modality to help stage a patient who is not a surgical patient.
MRI protocols vary as the number and type of pulse sequences, body orientations, field of view (FOV), matrix and slice thickness, use or lack of use of dynamic imaging, and other factors. Myometrial invasion is best seen with disruption of the junctional zone on T2-weighted images and subendometrial enhancement on dynamic imaging. Adenopathy, cervical and adnexal involvement, and signal change in the bladder wall and rectum can also be seen. In addition, vaginal involvement can be identified. Functional imaging using diffusion-weighted MRI, although not ready for prime time, shows promise and great potential in managing patients with endometrial cancer.13,14
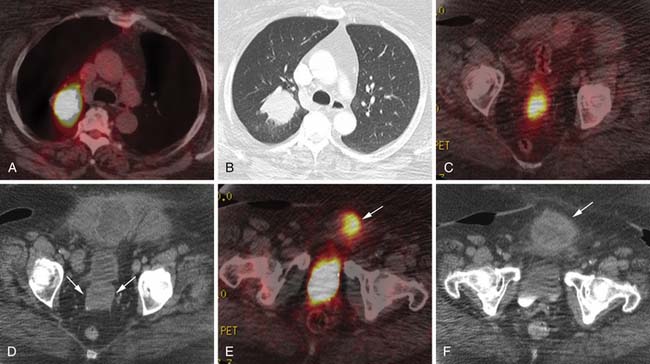
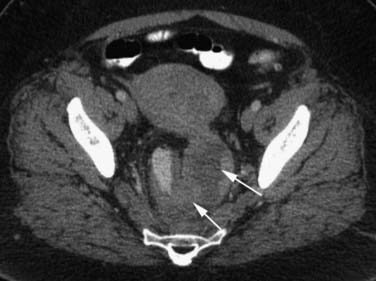
Computed tomography (CT) of the chest and abdomen is best used to evaluate for extrapelvic metastatic disease and possible hydronephrosis. This imaging tool is more readily available, and with newer multidetector systems, the image quality has improved with faster patient throughput; however, it does utilize radiation. More recently, fluoro-2-deoxy-D-glucose (FDG)–positron-emission tomography (PET) and FDG-PET/CT have been used to stage, assist in radiation therapy planning, and evaluate response to therapy and in surveillance. Higher standard uptake values (SUVs) are seen with deep invasion of the myometrium. It is also useful for identifying nodal involvement, given that it may dictate the radiation port.15,16 These imaging modalities are of limited use in diagnosing endometrial carcinoma. Molecular imaging shows great promise for future evaluation in the clinical management of patients with endometrial cancer.
Nodes
Key Points Imaging
• The preoperative imaging evaluation should point out to the surgeon the tumor location, penetration of the myometrium and maybe the cervix, suspected locations of adenopathy, extent into adjacent soft tissues, and any metastatic disease.
• For those patients who undergo chemotherapy, the location of recurrent or metastatic disease and lymphadenopathy needs to be presented to the surgeon and the medical oncologist if the latter is involved with the management of the patient.
• The location of recurrent or metastatic disease and lymphadenopathy can be used for radiation therapy ports.
Treatment
The decision for a lymph node dissection of the pelvis and para-aortic nodal groups, superior and inferior to the inferior mesenteric artery, is made at the time of surgery and is a controversial issue among different centers and geographic regions. In the United States, when the endometrial carcinoma is confined to the uterus, a limited staging procedure is done in that pelvis and para-aortic lymphadenectomy is performed.16
Arguments for and against a pelvic and para-aortic lymphadenectomy are described in the literature with thorough lymph node dissection in patients with type 2 and selected patients with type 1 endometrial cancers.4,17–20
Several trials and various treatment options that use cytotoxic and biologic agents are published in the literature.4,18,21–23
For certain patients, radiation therapy is used to manage their endometrial cancer. The goal of adjuvant radiation therapy is to treat microscopic disease. However, this is controversial worldwide, as is the use of external beam radiation (pelvic radiation, extended field, or whole abdominal radiation) versus vaginal brachytherapy, especially in the high intermediate-risk endometrial carcinomas. Part of the dilemma that leads to controversy is patient selection, experience in grading, and depth of myometrial invasion of these neoplasms by various pathologists.4,24–27
Key Points Therapy
• Surgery is the standard of treatment for those surgical candidates with endometrial cancer.
• Radiation therapy is used in the postoperative state to manage recurrence and adenopathy.
• Chemotherapy is used for palliation; with newer biologic agents, a longer period of survival with less toxicity may be possible.
New Therapies
The mainstay of therapy for endometrial cancer is surgery followed by radiation therapy and chemotherapy. Advances have been made into the understanding of the molecular biology of endometrial cancer.21–23,28 These discoveries have clinical and therapeutic implications. Some of the genetic alterations seen with endometrial cancer involve among others the tumor suppressor protein PTEN and associated pathways, microsatellite instability, K-ras mutations, p53 mutations, and HER-2/neu expression. Some of the target therapies have been directed to the mammalian target of rapamycin (mTOR) inhibitors, human epithelial growth factor receptor (EGFR), and angiogenesis inhibition of the vascular endothelial growth factor (VEGF). With time, these target therapies and others to be discovered should lead to better patient care and survival and minimizing postoperative therapeutic complications. A full discussion on these therapies is beyond the scope of this chapter.
Epidemiology and Risk Factors
Certain risk factors, such as obesity, age, and parity, have been associated with these uterine sarcomas.2,29 A small number of women with uterine sarcoma have undergone prior pelvic radiotherapy.30,31 An increased risk of uterine sarcoma has been identified with the use of tamoxifen.32–34
Clinical Presentation
The most common pure mesenchymal uterine sarcoma is the LMS, which accounts for 1% to 2% of uterine malignancies and tends to occur in women older than 40 years. Preoperatively, LMS is usually not suspected because the surgeon presumes that the clinical pattern is that of benign leiomyomata discovered during the surgical exploration or at the time of pathologic review. However, malignancy should be suspected in an enlarging pelvic mass in a postmenopausal woman who is not on hormonal supplemental replacement therapy. Typically, LMS is a solitary intramural necrotic mass that is poorly circumscribed, has an infiltrating margin, and often does not project outside of the myometrium. These tumors classically have severe nuclear atypia, hypercellularity, and a high mitotic rate exceeding 15 mitotic figures per 10 high-power fields.2 Nodal metastases are uncommon unless the LMS extends outside of the uterus, in which case the regional nodal basins and the para-aortic nodes may become involved. At times, LMS can have positive lymph nodes despite palpably normal lymph node tissue and lack of evidence of extrauterine spread.2,35,36 Hematogenously, LMS spreads to the lungs, liver, brain, kidney, and bone.
The second most common type of pure malignant mesenchymal uterine tumors is the ESS. These neoplasms have cells that resemble endometrial stromal cells of the proliferative endometrium. Currently, they are classified as endometrial stromal nodules, low-grade ESSs, and the undifferentiated endometrial sarcoma. The latter used to be classified as the high-grade ESS.2 The term ESS is now restricted to the low-grade ESS. The endometrial stromal nodule, the least common of the pure endometrial stromal tumors, is usually a solitary, well-circumscribed, expansile but not infiltrating tumor that has a smooth margin.2 These tumors are cured by hysterectomy and have a good prognosis.
The ESSs constitute approximately 10% to 15% of uterine malignancies that have a mesenchymal component. These neoplasms are more common in the 40- to 55-year-old age group. Approximately 25% of these patients may be asymptomatic. These tumors have mild nuclear atypia, are usually not necrotic, have a low mitotic index (<5 mitotic figures per 10 high power fields), have variable degrees of myometrial penetration and irregular margins, and tend to involve the parametrial veins and lymphatics.2 Some of these neoplasms fill the uterine cavity and may extend into the cervical canal. Recurrences tend to develop in the abdomen and pelvis and also in the lungs.2,35,37,38 The undifferentiated endometrial sarcoma is very aggressive; has severe nuclear pleomorphism, a high degree of destructive myometrial invasion, necrosis, and a high mitotic activity (>10 mitotic figures per high-power field), and lacks smooth muscle or endometrial differentiation.2 This biologic behavior leads to local recurrences and distant metastasis.
These tumors are more commonly found in postmenopausal women, more often nonwhite, and present with more advanced disease. The spread of this disease is mainly via lymphatic channels and less likely hematogenously. Most deaths are due to recurrent disease in the pelvis and abdomen rather than metastatic disease. Metastatic and recurrent tumors are more often carcinomatous.2,39–44 The more rare adenosarcoma is a mixed tumor that tends to occur in postmenopausal women but has been reported in adolescents and young adults. These neoplasms are of a low malignant potential and arise from the endometrium. The uterine cavity can be filled by this tumor and can project through the cervical os. These tumors can have focal hemorrhage, necrosis, and cystic changes. These adenosarcomas are a mixture of benign epithelium and low-grade sarcoma usually of the endometrial stromal type.2
Depending on the percentage of the sarcomatous component, the spread of disease is mainly to the pelvic lymph nodes and recurrences tend to occur in the pelvis and vagina.2,17,18
Key Points Clinical presentation
• The uterine sarcomas are aggressive and are either pure or mixed types.
• The pure types are the LMS and ESS.
• The mixed types are the adenosarcoma and MMMT.
• The most common presentation is that of abnormal vaginal bleeding and a pelvic mass in a peri- or postmenopausal patient.
• The uterine sarcomas tend to be large masses with foci of necrosis and hemorrhage.
Staging Evaluation
Historically, the World Health Organization (WHO) classification was used to stage these uterine sarcomas. However, more recently, the FIGO staging criteria has been developed to better reflect their different biologic behavior2 (Table 24-2).
Table 24-2 AJCC Tumor-Node-Metastases (TNM) and International Federation of Gynecology and Obstetrics (FIGO) Surgical Staging Systems for Uterine Sarcomas (includes Leiomyosarcoma and Endometrial Stromal Sarcoma)*
| Leiomyosarcoma and Endometrial Stromal Sarcoma | ||
|---|---|---|
| TNM CATEGORIES | FIGO STAGES | DEFINITION |
| Primary Tumor (T) | ||
| TX | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | |
| T1 | I | Tumor limited to the uterus |
| T1a | IA | Tumor 5 cm or less in greatest dimension |
| T1b | IB | Tumor more than 5 cm |
| T2 | II | Tumor extends beyond the uterus, within the pelvis |
| T2a | IIA | Tumor involves adnexa |
| T2b | IIB | Tumor involves other pelvic tissues |
| T3 | III† | Tumor infiltrates abdominal tissues (not just protruding into the abdomen) |
| T3a | IIIA | One site |
| T3b | IIIB | More than one site |
| T4 | IVA | Tumor invades bladder or rectum |
| Regional Lymph Nodes (N) | ||
| NX | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | IIIC | Regional lymph node metastasis |
| Distant Metastasis (M) | ||
| M0 | No distant metastasis | |
| M1 | IVB | Distant metastasis (excluding adnexa, pelvic and abdominal tissues) |
Note: Simultaneous tumors of the uterine corpus and ovary/pelvis in association with ovarian/pelvic endometriosis should be classified as independent primary tumors.
* Carcinosarcomas should be staged as carcinomas of the endometrium (see Table 24-1).
† In this stage, lesions must infiltrate abdominal tissues and not just protrude into the abdominal cavity.
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC (SBM). (For complete information and data supporting the staging tables, visit ww.springer.com.) Reprinted from D’Angelo E, Prat J. Uterine sarcomas: a review. Int J Gynaecol Obstet. 2010;116:131-139. Copyright 2010, with permission from International Federation of Gynecology and Obstetrics.
Imaging
At the present time, there are no pathognomonic imaging features to identify these uterine sarcomas. The main concern is that of a rapidly enlarging mass in a peri- or postmenopausal woman who is not undergoing hormonal replacement therapy. Multiple imaging modalities are used initially and in follow-up to evaluate the status of these patients (Figures 24-7 to 24-10). These include chest radiographs to evaluate mainly for pulmonary metastatic disease, and an abdominal series to evaluate for a bowel obstruction with or without barium studies, the latter yielding. Bone scans are used to evaluate for osseous metastatic disease.
Transabdominal and transvaginal ultrasound (TVU) studies have been used to evaluate these uterine sarcomas with better tissue definition by TVU. However, the myometrial/endometrial interface can be distorted by benign and malignant processes. The ultrasound findings can be nonspecific. The uterine sarcomas tend to be heterogeneous with mixed echogenicity, with poorly defined margins, filling the uterine cavity. The endometrial and myometrial junction is not well seen. If the uterine mass is very large, the FOV may not completely image the uterine mass and penetration of the mass may be limited by the ultrasound frequency. The yield from vascular evaluation of these masses by color Doppler and power Doppler is mixed and nonspecific. Low impedance of these tumoral vessels may or may not aid in suggesting a malignant process.35,45–48
CT scanning of these uterine sarcomas cannot differentiate between them. The CT findings include an enlarged uterus, heterogeneous enhancement, low-density areas of hemorrhage, and necrosis. However, CT is a good imaging modality to evaluate for ascites, adenopathy, and local and distant metastatic disease.49,50
The ESS on MRI can show a heterogeneous mass of variable signal intensity on both T1- and T2-weighted pulse sequences. The endometrial/myometrial interface is irregular owing to variable degrees of myometrial invasion. Heterogeneous enhancement is also seen following the administration of intravenous contrast. An advanced ESS involving vessels and ligaments can also be seen. As with LMS, a large degenerating leiomyoma may be difficult to separate from an ESS.12,35,49,51,52
Heterogeneous signal intensity of the MMMTs can be seen in MRI studies. These tumors can expand the uterine cavity and show invasion of the myometrium with loss of the junctional zone. Areas of hemorrhage show high signal intensity on T1-weighted images with high signal intensity in areas of necrosis on T2-weighted images.12,49,52,53
On MRI, the adenosarcomas are heterogeneous enhancing masses. Polypoid components can be seen along with potential myometrial invasion and cystic changes. The mass can protrude through the cervical os and is best seen on the sagittal views.54
Diffusion-weighted MRI is a functional tool that has been applied for abdominal and pelvic use. It is noninvasive, can help to identify benign from malignant lesions, and identify the spread of disease.14
Currently, molecular imaging of the uterine sarcomas uses PET or PET/CT. These studies use FDG. Other radiolabeled compounds have been described.15 PET/CT can simultaneously evaluate the anatomic and metabolic data, although the CT scans are not for diagnosis, and is preferred over PET alone imaging. Patient preparation is a must along with attention to detail involving the imaging protocol. The details involving the mechanics and physics of PET and PET/CT are beyond the limits of this chapter and the reader is advised to consult outstanding textbooks on these topics. Very few PET or PET/CT studies have been published involving the uterine sarcomas. Cervical and ovarian cancer has been reported more frequently using this imaging modality. However, PET and PET/CT evaluation of the uterine sarcomas can be useful for staging and assessing for lymph node metastases, recurrence, and possible local, regional, or distant metastases.15,16,55
Metastatic disease to the uterus can alter the shape and size of the organ with heterogeneity of the involved myometrium. Metastatic disease to the uterus has been reported from various extragenital cancers.56–60,31–35
Key Points Imaging
• No pathognomonic imaging features are available to identify these uterine sarcomas.
• The best imaging modality to evaluate the tumors is MRI.
• The uterus will tend to be enlarged with a mass that can have foci of necrosis and hemorrhage.
• Other imaging modalities can be used to determine the full length of metastatic spread outside of the pelvis that, in the future, can be consolidated with molecular imaging.
Treatment
Compared with endometrial carcinomas, uterine sarcomas behave more aggressively and are associated with a poorer prognosis. All uterine sarcomas are staged surgically using the 2009 FIGO staging system for uterine sarcomas (see Table 24-2).8 There are separate staging systems for (1) LMSs and ESSs, (2) adenosarcomas, and (3) carcinosarcomas. The staging system used for carcinosarcomas is the same as that used for epithelial endometrial carcinomas. Treatment recommendations vary by histologic subtypes.
Leiomyosarcoma
LMSs are very aggressive tumors with recurrence rates of 53% to 71% even if diagnosed at an early stage.61,62 Standard treatment includes hysterectomy and tumor debulking if disease is present outside of the uterus. In women with LMS, the rate of lymph node involvement in the absence of visible extrauterine disease has been shown to be extremely low, and lymphadenectomy is therefore not recommended.41–43 In addition, removal of the ovaries is controversial. In a large retrospective study of 1396 women with LMS, a subset analysis was performed on 341 women younger than 50 years with stage I and II disease, and no differences in survival were found between the women who underwent BSO and those who did not.63 Ovarian preservation is, therefore, acceptable in premenopausal women with early-stage LMS.
Observation after surgical resection is believed to be reasonable in patients with LMS confined to the uterus. However, adjuvant therapy is often considered. Although postoperative radiation therapy may reduce the rate of local recurrence, improvement in survival has not been demonstrated.36,64–67 Given these findings, and the propensity of LMS to relapse distantly, adjuvant radiation therapy is generally not recommended. However, adjuvant chemotherapy is commonly used for women with LMS, with docetaxel and gemcitabine the preferred combination.35
In patients with locally recurrent LMS, radiation therapy is sometimes used. If metastatic disease or multiple sites of recurrence are noted, systemic therapy with chemotherapy or hormonal therapy is used. Response rates of 15% to 42% have been reported using the combination of docetaxel and gemcitabine for metastatic LMS in both the first-line and the second-line setting.68,69 In addition, hormonal therapy may be beneficial in women with recurrent or metastatic LMS. Approximately 50% to 60% of patients have tumors that express hormone receptors.70,71 Responses have been described in these patients using agents such as aromatase inhibitors and mifepristone.72,73
Endometrial Stromal Sarcomas
ESSs are less aggressive tumors with a favorable prognosis.37 Standard treatment is primarily surgical and includes hysterectomy with BSO because these tumors are often hormonally sensitive. Whether or not to perform lymphadenectomy in patients with ESS is controversial. The incidence of nodal involvement in ESSs has been reported to range from 0% to 33%.74–77 However, these studies have not shown a survival benefit associated with lymphadenectomy, and it therefore remains unclear whether it should be performed in women with ESS.
Given the favorable prognosis and long remissions associated with ESS, observation is generally recommended for early-stage disease after surgery. Adjuvant radiation therapy and cytotoxic chemotherapy are not usually used. However, most ESS tumors express estrogen (ER) and progesterone (PR) receptors, so hormonal therapy may be considered.78–80 Agents used include progestins, gonadotropin-releasing hormone (GnRH) analogues, and aromatase inhibitors.77,80–83 There is no consensus regarding which patients with ESS should be treated with hormonal therapy and/or which agents should be used. In general, it is recommended that patients who are postmenopausal or who have undergone BSO and whose tumors express ER and PR, aromatase inhibitors or progestins should be used. In premenopausal women with retained ovaries, adjuvant treatment with GnRH analogues is recommended.84 Treatment should be continued for 5 years. Given the high level of hormone sensitivity of ESS tumors, estrogen therapy and tamoxifen should be avoided in these patients because they may increase the risk of recurrence.85–87
Uterine Carcinosarcoma/Malignant Mixed Müllerian Tumor
Uterine carcinosarcomas are rare and highly aggressive tumors with a poor prognosis. Approximately 30% of patients have extrauterine disease at the time of diagnosis.88–90 The standard treatment includes hysterectomy, BSO, and staging with pelvic washings, pelvic and para-aortic lymphadenectomy, and omental biopsy. The incidence of lymph node metastases is approximately 20%, and performing a lymphadenectomy has been shown to have a survival benefit.91,92 In advanced carcinosarcoma, cytoreduction is recommended.
Owing to the high risk of recurrence associated with uterine carcinosarcoma, adjuvant treatment with radiation and/or chemotherapy is usually given. Adjuvant radiation therapy has been reported to decrease local recurrences that occur in the pelvis, but a survival benefit has not been shown.90,93,94 Because of the high rate of recurrence outside the pelvis, adjuvant chemotherapy is often used. Regimens that have been shown to have activity include cisplatin and ifosfamide; cisplatin and doxorubicin; cisplatin, doxorubicin, and paclitaxel (TAP); as well as paclitaxel and carboplatin.95–97 For patients who are suitable for aggressive management, a combination of chemotherapy and radiation therapy is used. Whole abdominal radiation is an alternative, but less favorable, treatment option. Recurrent or metastatic disease is treated with palliative chemotherapy. Paclitaxel and carboplatin is generally favored owing to the ease of administration, lower toxicity, and response rates comparable with those of other regimens.98,99
Prognosis
In general, uterine sarcomas have a much poorer prognosis compared with other gynecologic malignancies. The most important prognostic factor for each type of uterine sarcoma is stage. The 5-year survival rates are 76% for stage I, 60% for stage II, 45% for stage III, and 29% for stage IV disease according to the FIGO staging system for endometrial cancer, which was used for uterine sarcomas prior to 2009.63,100 Additional factors associated with poor prognosis include high histologic grade, suboptimal tumor reduction, lymphatic and/or vascular space invasion, and high mitotic count.63,101 Although survival differences have been difficult to demonstrate between histologic types, ESSs and adenosarcomas have a better prognosis, and LMS tumors a worse prognosis compared with other uterine sarcomas.
1. American Cancer Society. Cancer Facts & Figures 2011. Atlanta: American Cancer Society; 2011.
2. D’Angelo E., Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131-139.
3. Creaseman W., Odicino F., Maisonneuve P., et al. Carcinoma of the corpus uteri. Int J Gynecol Oncol. 2006;95(Suppl 1):S105-S143.
4. Amant F., Moerman P., Neven P., et al. Endometrial cancer. Lancet. 2005;366:491-505.
5. Trimble E.L., Harlan L.C., Clegg L.X., et al. Pre-operative imaging, surgery and adjuvant therapy for women diagnosed with cancer of the corpus uteri in community practice in the United States. Gynecol Oncol. 2005;96:741-748.
6. Watson P., Lynch H. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993;71:677-685.
7. Aarnio M., Mecklin J.P., Aaltonen L.A., et al. Life-time risk of different cancers in hereditary non-polyposis CRC (LS) syndrome. Int J Cancer. 1995;64:430-433.
8. Edge SB., Byrd D.R., Compton C.C., et al. AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2010.
9. Fotopoulou C., Sehouli J., Schefold J.C., et al. Preoperative transvaginal ultrasound (TVS) in the description of pelvic tumor spread in endometrial cancer: Results of a prospective study. Anticancer Res. 2008;28:2453-2458.
10. Lane B.F., Wong Y.C., Jade J. Imaging of endometrial pathology. Clin Obset Gynecol. 2009;52:57-72.
11. Barwick T.D., Rockall A.G., Barton D.P., et al. Imaging of endometrial carcinoma. Clin Radiol. 2006;61:545-555.
12. Whitten C.R., deSouza N.M. Magnetic resonance imaging of uterine malignancies. Top Magn Reson Imaging. 2006;17:365-377.
13. Jujii S., Matsusue E., Kigawa J., et al. Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions. Eur Radiol. 2008;18:18-23.
14. Whittaker C.S., Coady A., Culver L., et al. Diffusion-weighted MR imaging of female pelvic tumors: a pictorial review. Radiographics. 2009;29:759-774.
15. Lai C.H., Yen T.C., Chang T.C. Positron emission tomography imaging for gynecologic malignancy. Curr Opin Obstet Gynecol. 2007;19:37-41.
16. De Gaetano A.M., Calcagni M.L., Rufini V., et al. Imaging of gynecologic malignancies with FDG PET-CT: case examples, physiologic activity, and pitfalls. Abdom Imaging. 2009;34:696-711.
17. Masciullo V., Amadio G., Lo Russo D., et al. Controversies in the management of endometrial cancer. Obstet Gynceol Int. 2010:638165.
18. Pacini P.B., Basile S., Maneschi F., et al. Systematic pelvic lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707-1716.
19. Lu K.H. Management of early-stage endometrial cancer. Semin Oncol. 2009;36:137-144.
20. Bassarak N., Blankestein T., Bruning A., et al. Is lymphadenectomy a prognostic marker in endometriod adenocarcinoma of the human endometrium? BMC Cancer. 2010;10:224-236.
21. Dellinger T.H., Monk B.J. Systematic therapy for recurrent endometrial cancer: a review of North American trials. Expert Rev Anticancer Ther. 2009;9:905-916.
22. Dizon D.S. Treatment options for advanced endometrial carcinoma. Gynecol Oncol. 2010;117:373-381.
23. Zagouri F., Bozas G., Kafantari E., et al. Endometrial cancer: What is new in adjuvant and molecularly targeted therapy. Obstet Gynecol Int. 2010:749579.
24. Naumann R.W. The role of radition therapy in early endometrial cancer. Curr Opin Onstet Gyncol. 2002;14:75-79.
25. Look K. Stage I-II endometrial adenocarcinoma evolution of therapeutic paradigms: the role of surgery and adjuvant radiation. Int J Gyncecol Cancer. 2002;12:237-249.
26. Shaeffer D.T., Randall M.E. Adjuvant radiotherapy in endometrial carcinoma. Oncologist. 2005;10:623-631.
27. Eifel P.J. Raditherapy: Intermediate-risk endometrial cancer – adjuvant treatment. Nat Rev Clin Oncol. 2010;7:553-554.
28. Bansal N., Yendluri V., Wenham R.M. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8-13.
29. Albrektsen G., Heuch I., Wik E., et al. Prognostic impact of parity in 493 uterine sarcoma patients. Int J Gynecol Cancer. 2009;19:1062-1067.
30. Varela-Duran J., Nochomovitz L.E., Prem K.A., et al. Post irradiation mixed müllerian tumors of the uterus. Cancer. 1980;45:1625-1631.
31. Meredith R.J., Eisert D.R., Kaka Z., et al. An excess of uterine sarcomas after pelvic irradiation. Cancer. 1986;58:2003-2007.
32. Treilleux T., Mignotte H., Clement-Chassagne C., et al. Tamoxifen and malignant epithelial-nonepithelial tumors of the endometrium: report of six cases and review of the literature. Eur Surg Oncol. 1999;25:477-482.
33. McCluggage W.G., Abdulkader M., Price J.H., et al. Uterine carcinosarcomas in patients receiving tamoxifen: a report of 19 cases. Int J Gynecol Cancer. 2000;10:280-284.
34. Wickerham D.L., Fisher B., Wolmark N., et al. Association of tamoxifen and uterine sarcoma. J Clin Oncol. 2002;20:2758-2760.
35. Amant F., Coosemans A., Debiec-Rychter M., et al. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188-1198.
36. Leitao M., Sonoda Y., Brennan M., et al. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol. 2003;91:209-212.
37. Dionigi A., Oliva E., Clement P.B., et al. Endometrial stromal nodules and endometrial stromal tumors with limited infiltration: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2002;26:567-581.
38. Chang K.L., Crabtree G.S., Kim Lim-Tan S., et al. Primary uterine endometrial stromal neoplasms: a clinicopathologic study of 117 cases. Am J Surg Pathol. 1990;14:415-438.
39. McCluggage W.G. Uterine sarcomas (malignant mixed müllerian tumors) are metaplastic carcinomas. J Gynecol Cancer. 2002;10:687-690.
40. Kernochan L.E., Garcia R.L. Carcinosarcomas (malignant mixed müllerian tumor) of the uterus: advances in elucidation of biologic and clinical characteristics. J Natl Cancer Compr Netw. 2009;7:550-557.
41. Brooks S.E., Zhan M., Cote T., et al. Survival epidemiology and end results analysis of 267 cases of uterine sarcomas, 1989-1999. Gynecol Oncol. 2004;93:204-208.
42. Silverberg S.G., Major F.J., Blessing J.A., et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol. 1990;9:1-19.
43. Doss L.L., Llorenas A.S., Henriquez E.M. Carcinosarcoma of the uterus: a 40-year experience from the state of Missouri. Gynecol Oncol. 1984;18:43-53.
44. Yamada D.S., Burger R.A., Brewster W.R., et al. Pathologic variables and survival for patients with surgically evaluated carcinosarcoma of the uterus. Cancer. 2000;88:2782-2786.
45. Clement P.B., Scully R.E. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Path. 1990;21:363-381.
46. Kim J.A., Lee M.S., Choi J.S. Sonographic findings of uterine endometrial stromal sarcoma. Korean J Radiol. 2006;7:281-286.
47. Chen C.D., Huang C.C., Wu C.C., et al. Sonographic characteristics in low-grade endometrial stromal sarcoma: report of two cases. J Ultrasound Med. 1995;14:165-168.
48. Gandolfo N., Gandolfo N.G., Serafini G., et al. Endometrial stromal sarcoma of the uterus: MR and US findings. Eur Radiol. 2000;10:776-779.
49. Rha S.E., Byun J.Y., Jung S.E., et al. CT and MRI of uterine sarcomas and their mimickers. AJR Am J Roentgenol. 2003;181:1369-1374.
50. Smith T., Moy L., Runowicz C. Müllerian mixed tumors: CT characteristics with clinical and pathologic observations. AJR Am J Roentgenol. 1997;169:531-535.
51. Saia E., Wakely S., Senior E., et al. MRI of malignant neoplasms of the uterine corpus and cervix. AJR Am J Roentgenol. 2007;188:1577-1587.
52. Ueda M., Otsuka M., Hatakenaka S., et al. MR imaging findings of uterine endometrial stromal sarcoma: differentiation from endometrial carcinoma. Eur Radiol. 2001;11:28-33.
53. Ohguri T., Aoki T., Watanabe H., et al. MRI findings including gadolinium-enhanced dynamic studies of malignant mixed mesodermal tumors of the uterus: differentiation from endometrial carcinomas. Eur Radiol. 2002;12:2737-2742.
54. Lee H.K., Kim S.H., Cho J.Y., et al. Uterine adenofibroma and adenosarcoma: CT and MR findings. J Comput Assist Tomogr. 1998;22:314-316.
55. Iyer R.B., Balachandran A., Devine C.E., et al. PET/CT and cross sectional imaging of gynecologic malignancy. Cancer Imaging. 2007;7(Spec No A):S130-S138.
56. Kumar N.B., Hart W.R. Metastases to the uterine corpus from extragenital cancers. A clinicopathologic study of 63 cases. Cancer. 2006;50:2163-2169.
57. Young R.H., Scully R.E. Ovarian metastases from cancer of the lung: problems in interpretation—a report of seven cases. Gynecol Oncol. 1985;21:337-350.
58. Tsoi D., Buck M., Hammond I., et al. Gastric adenocarcinoma presenting as uterine metastasis – a case report. Gynecol Oncol. 2005;97:932-934.
59. Le Bouedec G., Kauffman P., De Latour M., et al. Uterine metastasis of breast cancer. Report of 8 cases. J Gynecol Obstet Biol Reprod (Paris). 1991;20:349-354.
60. Parini C.L., Mathis D., Leath C.A. Occult metastatic lung carcinoma presenting as locally advanced uterine carcinoma on positron emission tomography/computed tomography imaging. Int J Gynecol Oncol. 2007;17:731-734.
61. Major F.J., Blessing J.A., Silverberg S.G., et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71(Suppl 4):1702-1709.
62. Abeler V.M., Royne O., Thoresen S., et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 54, 2009. 355–564
63. Kapp D.S., Shin J.Y., Chan J.K. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112:820-830.
64. Giuntoli R.L.2nd, Metzinger D.S., DiMarco C.S., et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89:460-469.
65. Sorbe B. Radiotherapy and/or chemotherapy as adjuvant treatment of uterine sarcomas. Gynecol Oncol. 1985;20:281-289.
66. Ferrer F., Sabater S., Farrus B., et al. Impact of radiotherapy on local control and survival in uterine sarcomas: a retrospective study from the Grup Oncologic Catala-Occita. Int J Radiat Oncol Biol Phys. 1999;44:47-52.
67. Hensley M.L., Ishill N., Soslow R., et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol Oncol. 2009;112:563-567.
68. Hensley M.L., Blessing J.A., Mannel R., Rose P.G. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329-334.
69. Hensley M.L., Blessing J.A., Degeest K., et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323-328.
70. Bodner K., Bodner-Adler B., Kimberger O., et al. Estrogen and progesterone receptor expression in patients with uterine leiomyosarcoma and correlation with different clinicopathological parameters. Anticancer Res. 2003;23:729-732.
71. Zhai Y.L., Nikaido T., Orii A., et al. Frequent occurrence of loss of heterozygosity among tumor suppressor genes in uterine leiomyosarcoma. Gynecol Oncol. 1999;75:453-459.
72. Hardman M.P., Roman J.J., Burnett A.F., Santin A.D. Metastatic uterine leiomyosarcoma regression using an aromatase inhibitor. Obstet Gynecol. 2007;110:518-520.
73. Koivisto-Korander R., Leminen A., Heikinheimo O. Mifepristone as treatment of recurrent progesterone receptor-positive uterine leiomyosarcoma. Obstet Gynecol. 2007;109:512-514.
74. Shah J.P., Bryant C.S., Kumar S., et al. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol. 2008;112:1102-1108.
75. Leath C.A.3rd, Huh W.K., Hyde J.Jr., et al. A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol. 2007;105:630-634.
76. Goff B.A., Rice L.W., Fleischhacker D., et al. Uterine leiomyosarcoma and endometrial stromal sarcoma: lymph node metastases and sites of recurrence. Gynecol Oncol. 1993;50:105-109.
77. Amant F., De Knijf A., Van Calster B., et al. Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Br J Cancer. 2007;97:1194-1199.
78. Reich O., Regauer S., Urdl W., et al. Expression of oestrogen and progesterone receptors in low-grade endometrial stromal sarcomas. Br J Cancer. 2000;82:1030-1034.
79. Chu M.C., Mor G., Lim C., et al. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 2003;90:170-176.
80. Ioffe Y.J., Li A.J., Walsh C.S., et al. Hormone receptor expression in uterine sarcomas: prognostic and therapeutic roles. Gynecol Oncol. 2009;115:466-471.
81. Reich O., Regauer S. Is tamoxifen an option for patients with endometrial stromal sarcoma? [author reply]. Gynecol Oncol. 2005;96:561.
82. Burke C., Hickey K. Treatment of endometrial stromal sarcoma with a gonadotropin-releasing hormone analogue. Obstet Gynecol. 2004;104:1182-1184.
83. Gadducci A., Cosio S., Genazzani A.R. Use of estrogen antagonists and aromatase inhibitors in breast cancer and hormonally sensitive tumors of the uterine body. Curr Opin Investig Drugs. 2004;5:1031-1044.
84. Reich O., Regauer S. Hormonal therapy of endometrial stromal sarcoma. Curr Opin Oncol. 2007;19:347-352.
85. Pink D., Lindner T., Mrozek A., et al. Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol. 2006;101:464-469.
86. Beer T.W., Buchanan R., Buckley C.H. Uterine stromal sarcoma following tamoxifen treatment. J Clin Pathol. 1995;48:596.
87. Pang L.C. Endometrial stromal sarcoma with sex cord-like differentiation associated with tamoxifen therapy. South Med J. 1998;91:592-594.
88. Arrastia C.D., Fruchter R.G., Clark M., et al. Uterine carcinosarcomas: incidence and trends in management and survival. Gynecol Oncol. 1997;65:158-163.
89. Sartori E., Bazzurini L., Gadducci A., et al. Carcinosarcoma of the uterus: a clinicopathological multicenter CTF study. Gynecol Oncol. 1997;67:70-75.
90. Yamada S.D., Burger R.A., Brewster W.R., et al. Pathologic variables and adjuvant therapy as predictors of recurrence and survival for patients with surgically evaluated carcinosarcoma of the uterus. Cancer. 2000;88:2782-2786.
91. Temkin S.M., Hellmann M., Lee Y.C., Abulafia O. Early-stage carcinosarcoma of the uterus: the significance of lymph node count. Int J Gynecol Cancer. 2007;17:215-219.
92. Nemani D., Mitra N., Guo M., Lin L. Assessing the effects of lymphadenectomy and radiation therapy in patients with uterine carcinosarcoma: a SEER analysis. Gynecol Oncol. 2008;111:82-88.
93. Reed N.S., Mangioni C., Malmstrom H., et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer. 2008;44:808-818.
94. Callister M., Ramondetta L.M., Jhingran A., et al. Malignant mixed müllerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys. 2004;58:786-796.
95. Sutton G., Kauderer J., Carson L.F., et al. Adjuvant ifosfamide and cisplatin in patients with completely resected stage I or II carcinosarcomas (mixed mesodermal tumors) of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;96:630-634.
96. Kushner D.M., Webster K.D., Belinson J.L., et al. Safety and efficacy of adjuvant single-agent ifosfamide in uterine sarcoma. Gynecol Oncol. 2000;78:221-227.
97. Makker V., Abu-Rustum N.R., Alektiar K.M., et al. A retrospective assessment of outcomes of chemotherapy-based versus radiation-only adjuvant treatment for completely resected stage I-IV uterine carcinosarcoma. Gynecol Oncol. 2008;111:249-254.
98. Hoskins P.J., Le N., Ellard S., et al. Carboplatin plus paclitaxel for advanced or recurrent uterine malignant mixed mullerian tumors. The British Columbia Cancer Agency experience. Gynecol Oncol. 2008;108:58-62.
99. Toyoshima M., Akahira J., Matsunaga G., et al. Clinical experience with combination paclitaxel and carboplatin therapy for advanced or recurrent carcinosarcoma of the uterus. Gynecol Oncol. 2004;94:774-778.
100. Brooks S.E., Zhan M., Cote T., Baquet C.R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204-208.
101. Rovirosa A., Ordi J., Ascaso C., et al. Prognostic factors in uterine sarcomas: a 21-year retrospective study at the Clinic and Provincial Hospital of Barcelona in Spanish. Med Clin (Barc). 1998;111:172-176.