CHAPTER 85 Tumors of the Spine
General Information
The spinal tumors may arise from local lesions occurring within or adjacent to the spinal column or from distant malignancies spreading to the spine or paraspinous tissues by hematogenous or lymphatic routes. Local involvement of the spine may result from primary tumors of bone, primary lesions arising in the spinal cord or its coverings, or contiguous spread of tumors of the paraspinous soft tissues and lymphatics. Metastatic disease may occur in the spine with almost any of the solid tumors of the body, with osseous malignancies of the appendicular skeleton, and with systemic lymphoreticular malignancies such as multiple myeloma and lymphoma. Metastatic tumors are far more common than primary lesions in the spine, accounting for 97% of all spinal column tumors. Patients with adenocarcinoma are especially prone to spinal metastases, with primaries from lung, breast, prostate, kidney, gastrointestinal tract, and thyroid making up the majority. It has been estimated that between 50% and 70% of patients with carcinoma will develop skeletal metastases before death, and this number may be as high as 85% for women with breast carcinoma.1 Primary tumors of the spine are rare, and for the most part their relative incidence reflects that seen in the skeleton in general. Some tumors (chordoma, osteoblastoma) do show a predilection for the spinal column, but these still make up a small proportion of all spinal tumors.
Symptoms
The clinical presentation usually provides clues that alert the physician to the presence of a spinal neoplasm. Pain and weakness are the most common presenting complaints. Pain is present in nearly 85% of patients including 20% who report radicular symptoms.2 At the time of initial evaluation more than 40% of patients have subjective complaints of weakness, and objective neurologic deficits can be identified in 35% of patients with benign tumors and 55% of patients with malignancies. A palpable mass is detectable in only 16% of patients.
Although back pain is an exceedingly common and nonspecific complaint, pain associated with neoplasia tends to be progressive and unrelenting and does not have such a close association with activity as does mechanical back pain. Pain at night is particularly worrisome. Pain symptoms may localize to a specific spinal segment and may be reproduced by pressure or percussion over the involved segment. Radicular symptoms are less common but are still frequently seen in patients with cervical or lumbar involvement. Radicular pain in the thoracic region may result in “girdle” pain forming a belt of dysesthesias and paresthesias circumferentially from the level of vertebral involvement. These radicular symptoms may mimic a herniated nucleus pulposus, leading to confusion in diagnosis and treatment. Pain from neoplasia, however, is unremitting, progressive, and not relieved by rest or recumbency.3
Pain may arise from one of a number of causes. Local tumor growth may expand the cortex of the vertebral body, resulting first in thinning and remodeling of the cortex and later in pathologic fracture and invasion of paravertebral soft tissues. As the cortex expands, the overlying periosteum is distorted and stretched, stimulating pain receptors in that tissue. Local tumor extension from a paravertebral mass or following fracture may compress or invade adjacent nerve roots, resulting in radicular symptoms of pain or paresthesias. Pathologic fracture resulting from extensive vertebral body destruction may produce acute pain symptoms similar to those seen in traumatic compression fractures. Fractures may also produce mechanical instability and subsequent pain. Finally, any of these causes of back pain may be associated with acute or chronic compression of the spinal cord or nerve roots, resulting in focal and radicular symptoms of pain, paraparesis, or paraplegia.4
Neurologic deficits are fairly common in patients presenting with spinal tumors; they are most common in rapidly expanding malignant lesions, but any slowly progressive, expansile neoplasm may produce a deficit if left alone long enough. Weakness, usually in the lower extremities, may become apparent months or years after the onset of pain and is rarely the first symptom seen. Nonetheless, up to 70% of patients will manifest clinical weakness by the time the correct diagnosis is made. To avoid this complication, the clinician should maintain a high index of suspicion for patients with persistent back or radicular pain, particularly those with a history of known systemic malignancies.5
Bowel and bladder dysfunction may develop before diagnosis in up to half of patients with cord compression.6 Patients with compression at the level of the conus medullaris may present with isolated sphincter dysfunction, but it is far more common to see associated lower extremity impairments. The neurologic assessment in these patients must include an evaluation of bladder function.
Imaging Techniques
Plain Radiographs
Plain roentgenograms should be the first test in any case where a neoplasm is suspected. Anteroposterior (AP) and lateral views of the involved vertebra can provide considerable information about the nature and behavior of the lesion and may be sufficient to identify some characteristic tumor types. Even when the specific tumor type is not implicated, the benign or malignant nature of the lesion may be deduced from the pattern of bony destruction. Geographic patterns of bone destruction suggest a slowly expanding lesion, typically benign; more rapidly growing tumors produce a moth-eaten appearance; highly malignant, aggressive lesions produce a permeative pattern of destruction.7 However, because radiographic evidence of bone destruction is not apparent until between 30% and 50% of the trabecular bone has been destroyed, early lesions may be hard to detect.8 Twenty-six percent of patients with spinal metastases will have occult lesions undetectable on plain radiographs.9
Bone Scan
99mTechnetium bone scans are commonly used to detect neoplastic disease of the musculoskeletal system. Their superior sensitivity makes them ideal to detect lesions in symptomatic patients with negative or equivocal radiographs. The ability to scan the entire body makes bone scans advantageous to determine the extent of dissemination in patients with known systemic disease and to define the most accessible lesion to biopsy in patients with an unknown primary malignancy. More recently, intraoperative bone scans (IOBSs) have been used for osteoid osteomas. These benign lesions of the spine can cause significant pain and spinal deformity in the pediatric population and are often surgically elusive. IOBSs have been shown to minimize the need for multiple surgical procedures to ensure complete resection. As such, IOBSs have been shown to be highly sensitive for lesion localization and verification of complete surgical extirpation.10 However, the main disadvantage of bone scans is their poor specificity because non-neoplastic pathology, most often osteoarthritis, can also cause focal areas of increased uptake. The addition of single photon emission computed tomography (SPECT), a recent technologic advancement, however, improves the predictive value of planar scans by better defining the anatomic location of uptake.11,12
Computed Tomography Imaging
With the advent of portable CT scanners, expanded use of intraoperative CT scanning for spinal surgery has been reported. Because CT scanning provides excellent visualization of osseous pathology, it can be extremely helpful in the evaluation of tumor resections during complex spinal operations.13
Magnetic Resonance Imaging
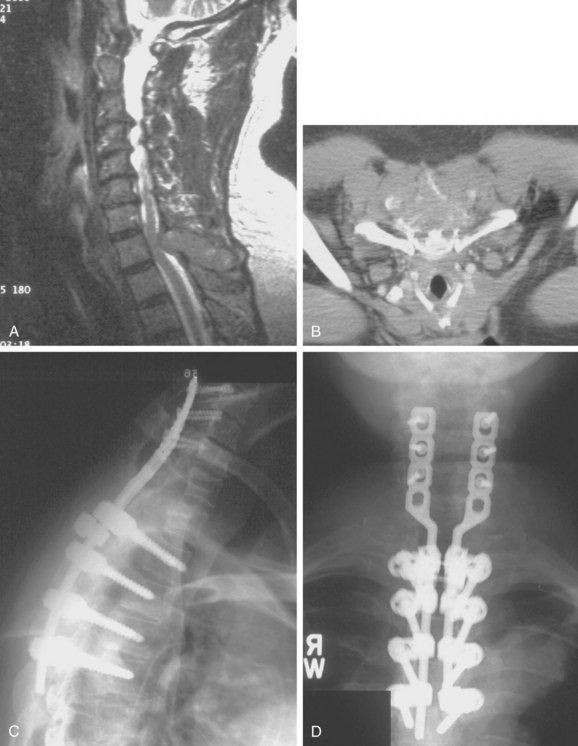
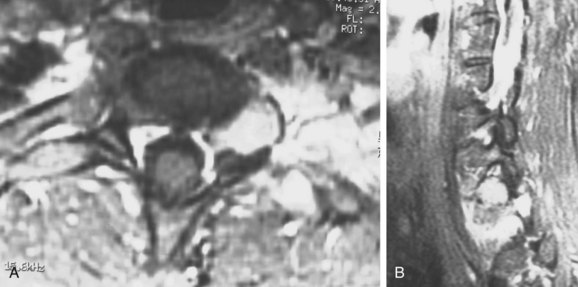
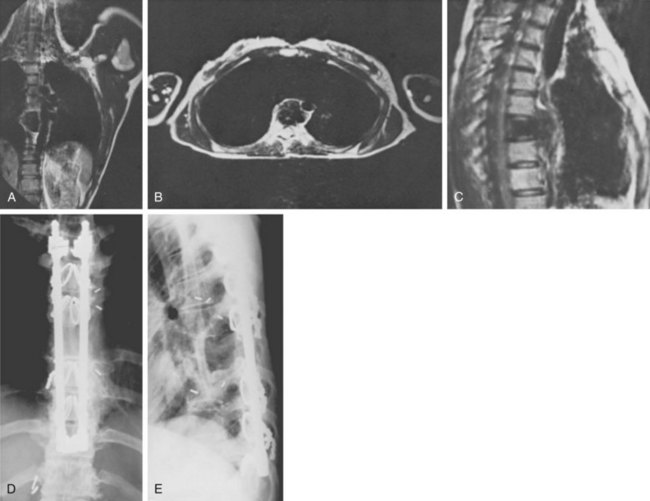
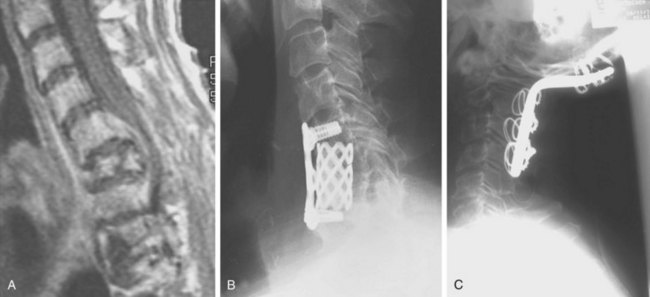
Because of its superior sensitivity and specificity, MRI has become the method of choice to evaluate the spine. It is well tolerated and safe with no exposure to radiation. MRI provides the best imaging of neural structures and can clearly define intramedullary, intradural, and extradural compressive lesions, as well as the extent of the lesion causing the compression, things that myelography cannot do (Fig. 85–1). The ability to obtain multiplanar images and to better delineate soft tissue structures make it invaluable in diagnosis and treatment planning. Whole spine MRI is also the best method to evaluate and direct treatment in a patient with suspected spinal cord compression and known malignancy.
MRI can detect neoplasms in the spine because normal marrow is replaced with tumor tissue with increased cellularity and extracellular water content.14 Thus most neoplasms have low signal intensity on T1 and high signal on T2. Fat suppression techniques combined with intravenous gadolinium further increase the contrast between normal fatty marrow and tumor tissue, making MRI the imaging technique with the earliest ability to detect neoplasm.15
These methods can also help to distinguish pathologic fracture due to osteoporosis versus malignancy. Malignant lesions usually have a more ill-defined margin, involvement of the pedicle, marked enhancement, and frequent paravertebral soft tissue extension while benign causes of vertebral compression usually still have fat present within the body, do not involve the pedicle, demonstrate more focal edema, and do not have an associated soft tissue mass.16,17 Infection can also be differentiated from neoplasm on MRI because infectious processes usually involve the disc space and endplate and incite a significant amount of edema. Neoplastic lesions are often more defined, do not involve the disc or endplates, and are associated with limited edema.14
Summary Imaging Appearance
The imaging appearance of primary bony tumors of the spine and simulating lesions of the spine has been described recently. There are specific imaging characteristics of benign and malignant lesions in the spine that can be described as follows. Benign bone tumors commonly appear as well circumscribed. As slow-growing lesions they may present with a calcified or sclerotic matrix. Malignancy on the other hand is often characterized by aggressive permeative lesions with bone destruction, cortical invasion, and associated soft tissue mass. CT may be helpful in characterization of the tumor matrix, exact location, extension and osseous changes, whereas MRI is superior for evaluation of the associated soft tissue mass, bone marrow infiltration, and intraspinal extension.18
Biopsy Techniques
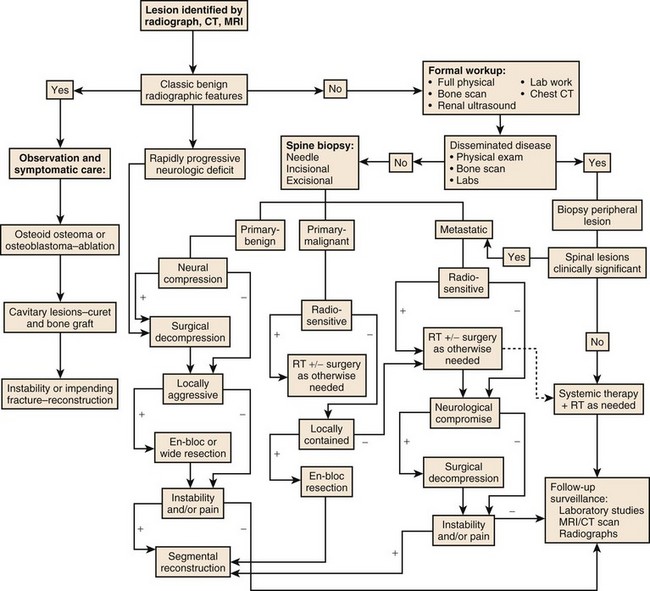
The importance of a carefully planned biopsy cannot be overemphasized. Once the biopsy incision has been made, there are few choices left in planning the definitive tumor removal. When the surgeon selects an approach for biopsy, he or she is committed to that approach from thereon. The hazards of biopsy seen in musculoskeletal tumors of the extremities are doubly apparent in the axial skeleton. The incidence of inadequate or inappropriate biopsy that significantly alters a patient’s care is greater than one in three overall and probably higher in lesions of the spine.19 This risk is reduced significantly when the biopsy is performed by the treating, rather than the referring, physician.
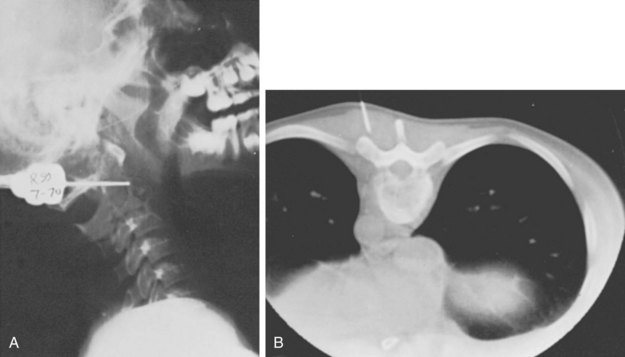
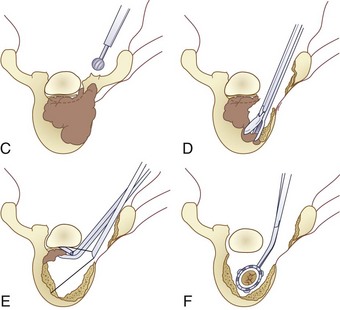
Three forms of biopsy are available to the surgeon: excisional, incisional, and needle biopsy or aspiration. On occasion a posterior lesion may prove suitable for an excisional biopsy, but most lesions of the spinal column will require either an incisional or needle biopsy. Needle biopsies are subject to sampling errors and provide a small specimen for evaluation (Fig. 85–2). The primary role of needle biopsy is confirmation—confirmation of metastatic disease, of recurrence of a known lesion, or of sarcomatous histology in an otherwise classic clinicoradiologic presentation of osteosarcoma.20 Culture results may also be obtained when infection must be ruled out.
The incisional biopsy should be the last step in the staging of the patient, performed just before the definitive surgical resection.21 A number of basic principles should be observed when performing the biopsy. The biopsy incision should be placed so that it may be excised with the tumor during the definitive procedure. Transverse incisions must be avoided, and the tumor approached in the most direct manner possible. Tissues should be handled carefully, and hemostasis should be meticulous so that hematoma does not carry tumor cells distant from the primary site. Bone should not be removed or windowed unless absolutely necessary. The specimen obtained should be large enough to allow histologic and ultrastructural analysis, as well as immunologic stains. The margin of the soft tissue mass is often most diagnostic because central portions are often necrotic. The surgeon should take care not to crush or distort the specimen, so as to maintain its architecture. If a soft tissue component exists, a frozen section should be obtained. Finally, if the definitive excision is to follow the biopsy under the same anesthetic, it is essential that all instruments used during the biopsy be discarded. The field should be redraped, and the surgeons should change gowns and gloves before the excision is begun. This same precaution should be followed in obtaining the bone graft for spinal reconstruction.
A recent review of the literature determined that the Weinstein-Boriani-Biagini tumor staging system accurately predicted the attainment of wide or marginal en bloc resection in 88% of cases.22 There was a clear increase in tumor recurrence when intralesional procedures were performed before the definitive en bloc resection. Tumor recurrence significantly shortened patient survival. Surgical complication rates ranged from 13% to 56%, and mortality ranged from 0% to 7.7%. The authors concluded that (1) incisional biopsy or intralesional resection significantly increases the risk of local recurrence. Therefore transcutaneous CT-guided trocar biopsy was recommended instead.
Tumors
Primary Tumors of Bone
Primary bone or soft tissue tumors arising in the spine are uncommon. In reviewing 82 primary neoplasms of the spine seen over a 50-year period at one center, 31 benign and 51 malignant lesions were identified, representing 8 benign and 9 malignant tumor types.2 All six cervical neoplasms in this series were benign, whereas two thirds of all thoracic, lumbar, and sacral primary tumors were malignant. Seventy-five percent of tumors found in the vertebral body were malignant, compared with only 35% of those in the posterior elements. For patients older than 18 years, 80% of primary tumors proved to be malignant but in children and adolescents only 32% of lesions were malignant. The 5-year survival in this series was 86% in patients with benign tumors and 24% in patients with malignancies.2 Bohlman’s review of 23 patients with primary neoplasms of the cervical spine also showed a marked difference in tumor type with patient age. In that series all patients younger than 21 years old had benign tumors, whereas 10 of 14 (71%) patients older than 21 had a malignancy.23
Benign
Osteochondroma
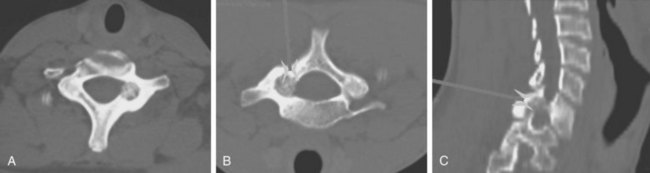
Although osteochondromas are the most common benign tumor of bone, only 3% occur in the spine in the solitary form and only slightly more in multiple osteochondromatosis.24 They are cartilage-capped bony projections arising from an abnormality in the perichondral ring. These lesions are usually asymptomatic in patients in the second and third decades of life, although patients may present with a painless mass. Rarely, patients may have neurologic compromise as a result of the lesion impinging on the canal or a nerve root. This scenario is more common in patients with multiple osteochondromatosis and in cervical lesions. Of osteochondromas presenting with spinal cord compression, 56% were in the cervical spine, 38% were thoracic, and 6% lumbar.25 Most of these lesions are not apparent on plain films. CT is usually necessary to determine the point of origin of the lesion, but MRI is necessary to define the cartilage cap and the proximity of the cord and other soft tissues. Because of the slow progression of the compressive cord lesion, excision of the tumor, en bloc or piecemeal, provides excellent recovery of neurologic function with little likelihood of recurrence (Fig. 85–3).
Although solitary spinal osteochondromas are uncommon lesions of the maturing adolescent skeleton, this lesion is much less common in older individuals. Only a few reports are available on osteochondromas in adults. In rare cases, lesions involving the spinal canal can cause low back pain and neurogenic claudication due to expansion of the lamina and extension into the spinal canal and adjacent facet joints.26 When treating osteochondroma in adolescents, one has to consider the possibility of recurrence if symptoms do not abate. This rare complication of recurrence has only been reported in three cases of cervical osteochondroma.27
Multiple hereditary exostoses with vertebral exostosis into the spinal canal are rare but must be considered if other skeletal lesions are present and multiple cervical exostotic lesions are found. This has been described in 68 of patients in whom exostoses arising from the spinal column have been described. Twenty percent of patients with multiple hereditary exostoses had lesions encroaching into the spinal canal. Patients with lesions inside the spinal canal were typically asymptomatic and neurologically normal, with radiographs that did not demonstrate the lesion. Compared with female patients, male patients were more likely to have spinal lesions and more likely to have lesions encroaching into the spinal canal (P = 0.014). Because of risk of neurologic injury, routine MRI to screen all patients with multiple hereditary exostoses has been recommended at least once during the growing years.28
Aggressive “Benign” Primary Spine Neoplasms
Osteoblastoma, Aneurysmal Bone Cyst, and Giant Cell Tumor
In order to define an improved clinical care plan for osteoblastoma, aneurysmal bone cyst, and giant cell tumor, these lesions have been grouped together in a category of “aggressive, benign, osseous neoplasms.”29 A panel of spine experts including oncologists, surgeons, and radiation oncologists graded the literature as either strong or weak. No prospective or randomized studies were found, reflecting the limited number of patients with aggressive primary osseous tumors. However, the osteoblastoma initial search identified 211 articles, of which 17 addressed spinal issues. The initial search for aneurysmal bone cysts revealed only 6 of 482 articles were pertinent, and only 8 of 178 articles on giant cell tumors focused on the treatment of spinal lesions. Authors found differences in clinical care but concluded that “surgical treatment should be directed at gross resection of the tumor, understanding that this may be limited by anatomic confines and the potential for morbidity.”
Osteoblastoma and Osteoid Osteoma
Osteoblastoma and osteoid osteoma are osteoblastic lesions differentiated primarily by size. These benign lesions show a propensity for spinal involvement, usually the posterior elements. Osteoid osteoma occurs in the spine in approximately 10% to 25% of cases, and osteoblastoma occurs in roughly 32% to 46% of cases.30–33 Patients usually present in their second or third decade, and the most common complaint is of back pain. The pain is typically persistent and unrelated to activity and often is most noticeable at night. Although aspirin classically provides dramatic relief of symptoms, this is not universal. Patients with osteoblastomas may have dull, achy pain typical of other benign lesions.
Painful scoliosis can be another presenting symptom. In a large review,34 78% of patients with osteoid osteoma and 54% of those with osteoblastoma were found to have scoliosis. The lesions were almost entirely on the concave side of the curve and were more commonly in the thoracic and lumbar rathern than cervical regions. The scoliosis is thought to result from muscle spasm on the side of the lesion. Most curves will improve or resolve after removing the lesion. This may not be the case, however, if the scoliosis has been present for a prolonged period of time.
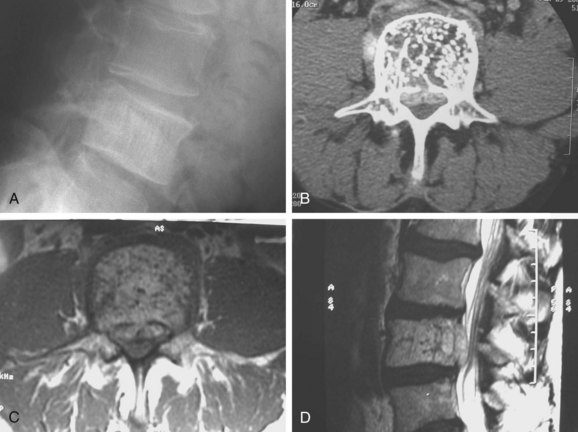
Radiographic demonstration of osteoid osteoma is difficult (Fig. 85–4). The lesion, by definition less than 2.0 cm in diameter, is easily obscured among the overlapping shadows of the vertebral column. CT demonstrates the lesion well, but if the cuts are at the wrong level or too wide, the tumor may be missed completely. The most sensitive method of locating an osteoid osteoma is by bone scan. The technetium bone scan provides accurate localization of the lesion, decreasing the average duration of symptoms by providing an early diagnosis and allowing prompt treatment.35
Osteoblastomas become considerably larger than osteoid osteomas and may be quite apparent on plain radiographs. The lesion is characterized radiographically by the expansion of the cortical bone, maintaining a thin rim of reactive bone between the lesion and the surrounding soft tissue. There is often a rim of reactive bone separating the tumor from the rest of the medullary bone. Although the tumor may show stippling in some cases, there is rarely if ever a lobulated or soap-bubble appearance to these lesions.32 When involvement of the vertebral body is seen, it is limited and results from extension of tumor into the body from the pedicle (Fig. 85–5).
MRI appearance of osteoblastoma is characterized by the localized inflammatory response the tumor causes. These inflammatory features can simulate malignant behavior on MRI, and an aggressive resection could be prompted for an otherwise benign lesion. Therefore it is critical to corroborate MRI findings, with other imaging and clinical data. Osteoblastoma is characterized by classic benign features on CT. In a recently reported case, a lumbar MRI revealed a sacral tumor with malignant features of marrow edema and enhancement, whereas a CT showed a well-circumscribed lesion without lytic changes or malignant bone formation.36 A conservative resection was performed, and histopathology confirmed the diagnosis of osteoblastoma. The authors concluded that the MRI findings for osteoblastoma can be misleading and cautioned that CT should be used when evaluating benign tumors with known inflammatory responses on MRI.
The treatment for both osteoblastoma and osteoid osteoma is by excision. Excision provides reliable pain relief, and the spinal deformity is improved or disappears in the majority of patients.30,37 Although a complete excision is desirable, it is not always feasible in the spine. Curettage and bone grafting of vertebral osteoblastomas have provided satisfactory long-term results in the vast majority of cases.32,37 Intraoperative CT guidance or preoperative CT localization may aid in complete resection of these lesions with the least amount of destruction to normal tissues. Osteoid osteomas in particular are treated commonly in other areas of the body with radiofrequency ablation. Although concern for thermal damage to neural structures is quite valid, recent reports of its safe use in spinal lesions may lead to its careful use in this setting.38 In other instances, stereotactic guidance systems may be used to provide a precise excision through a minimally invasive approach.39
Aggressive osteoblastoma is another variant of osteoblastoma. It is more aggressive and has higher recurrence rates and is considered to be a borderline or intermediate osteoblastic tumor. Although the true incidence and distribution of this rare tumor is not known, complete surgical excision by en bloc resection and spondylectomy is indicated because recurrence rates of 10% to 15% have been reported. Transformation to osteosarcoma has been reported and is associated with multiple recurrences. A recent article reported a rare case of recurrent aggressive osteoblastoma of the thoracic spine and accomplished successful treatment by total en bloc spondylectomy.40
Aneurysmal Bone Cyst
Aneurysmal bone cysts (ABCs) involving the spine are rare lesions that may be found at any level. They involve the posterior elements nearly 100% of the time (Fig. 85–6), but they may also commonly extend into the vertebral body. These benign, highly vascular osseous lesions of unknown origin may present difficult diagnostic and therapeutic challenges.41,42 ABCs are often expansile lesions containing thin-walled, blood-filled cystic cavities that cause bone destruction and sometimes spinal deformity and neurologic compromise. ABCs occur mostly in children and young adults, and 75% of patients are younger than 20 years old at presentation. In the lumbar spine, vertebra plana as a result of an ABC has been described and the authors pointed out that an ABC should be considered in the differential diagnosis when vertebra plana is seen in pediatric patients.43
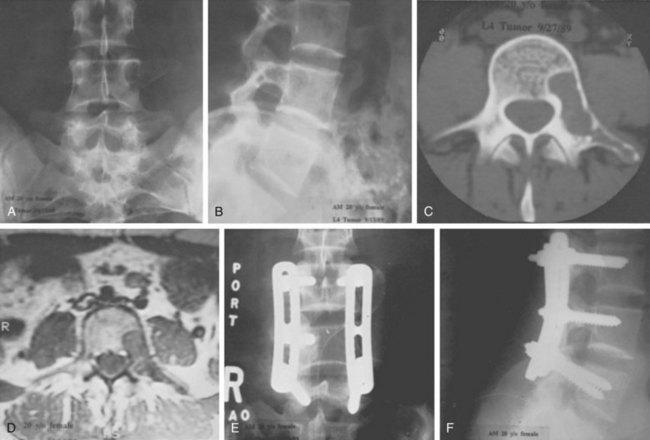
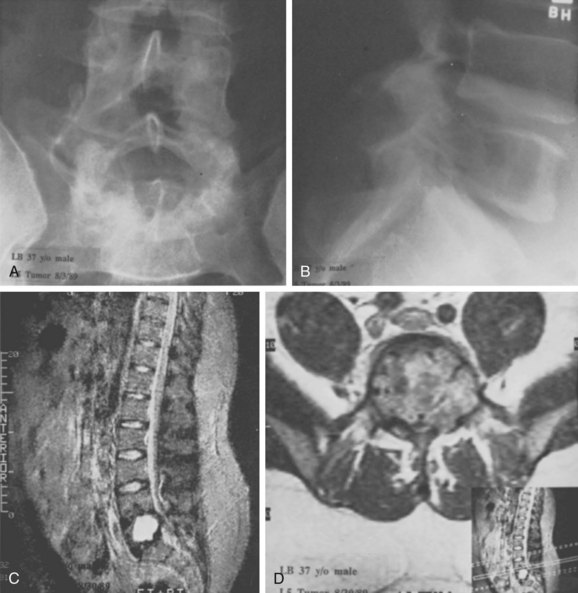
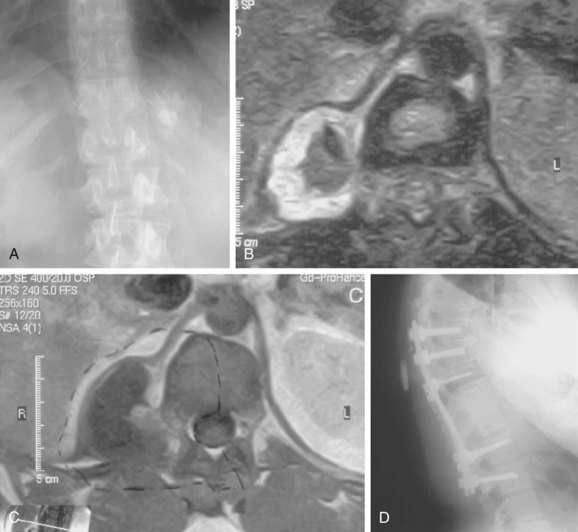
FIGURE 85–6 A-B, Plain anteroposterior and lateral radiographs of L4 tumor involving the left L4 transverse process and pedicle. Diagnosis was osteoblastoma with elements of aneurysmal bone cyst (solid ABC). C-D, Preoperative computed tomography and magnetic resonance imaging scans of lesion. E-F, Anterior posterior and lateral radiograph views after resection of tumor involving zones II, III, and IVA (see Fig. 85–12).
The treatment of spinal ABCs remains controversial according to the literature. Treatment options include simple curettage with bone grafting, complete excision, embolization, and radiation therapy. Reconstruction and stabilization of the spine may be warranted if deformity and instability are present. Preservation of neurologic function may dictate surgical management. Nonetheless, complete excision offers the best chance of cure and spinal decompression if neurologic deficits are present. Treatment strategy should be aimed at complete excision whenever possible. Aggressive intralesional curettage and bone grafting may have higher local recurrence rates than complete excision of the lesion.42 Instrumented fusion will be necessary if the lesion or surgical excision compromises the stability of the spine. Preoperative selective embolization is helpful, especially in larger lesions.44
More recently, selective arterial embolization with N-2-butyl cyanoacrylate has been described.45 The study population comprised 20 male and 16 female patients with an age range of 3.3 to 60.8 years.5 Nine lesions were localized in the appendicular skeleton (1 in the upper and 8 in the lower limb), 4 in the thoracic cage (1 rib lesion and 3 scapular lesions), 17 in the pelvis and 6 in the spine (1 thoracic and 5 sacral localizations). Of the 55 embolizations performed, only 1 embolization was necessary in 22 cases (61%), 2 were necessary in 9 cases (25%), and 3 were necessary in the remaining 5 patients (14%). In ninety-four percent of patients, treatment was found to be effective with follow-up ranging from 0.9 to 5 years. Only seven patients underwent surgery during the embolization study. The authors reported on three complications (5%)—two cases of skin necrosis and one case of transient paresis—and concluded that arterial embolization with cyanoacrylate was a viable treatment for aneurysmal bone cysts. They recommended embolization as a less invasive, lower-cost, easily repeated, simpler procedure than surgery and it does not preclude surgery later.45
In children, aneurysmal bone cysts may represent a particular challenge because fusion and stabilization with instrumentation are often avoided due to concerns over instability following future axial skeleton growth. Recently, two case reports of aneurysmal bone cyst in the cervical spine of children were published.46 They both demonstrated deeply involved lesions with extensive bone destruction. Both were treated aggressively with resection, fusion, and stabilization with instrumentation. Both patients were reported to have had an uncomplicated postoperative course. At 36- and 18-month follow-up, both patients had no cervical spine instability or recurrence of tumor. The authors concluded that treatment of aneurysmal bone cyst in the cervical spine is challenging when it occurs in close proximity to neural and vascular structures.
Hemangioma
Vertebral hemangiomas are common lesions, occurring in approximately 10% of all patients, but they are rarely symptomatic or of clinical importance. They usually occur in the vertebral body but can extend to the posterior elements. Reports of deformity or pain associated with vertebral hemangiomas are uncommon, but cases of nerve root and cord compression have been documented, predominantly in the thoracic region (Fig. 85–7).47 The diagnosis of vertebral body hemangioma can usually be made on plain radiographs, although cross-sectional imaging will provide more information about cord impingement or fracture. Plain films classically show prominent vertical striations produced by the abnormally thickened trabeculae of the involved vertebral body.
Occasionally, spinal osseous hemangioma can mimic metastasis of a malignant tumor. This has been reported recently in a 44-year-old woman where the lesion was located in the seventh cervical vertebra.48 On histopathologic examination the lesion showed reactive osteoid formation and nontypical osteolytic radiologic findings, strongly suggesting a metastatic process. This case report underlined the importance of including hemangioma into the differential diagnosis even if it is found in the cervical spine. Although hemangioma is rarely found in the cervical spine, treatment of supposedly malignant metastatic lesions could expose the patient to the risks of unneeded aggressive local and adjuvant treatments.
Spinal hemangiomas are radiosensitive and frequently respond to radiotherapy alone. Alternatively, ethanol injection into the lesion or traditional embolization are other minimally invasive methods of treatment, although careful technique is required.49 When cord compression develops and surgical treatment is considered,50 angiography is indicated to establish the vascular source for the tumor, to identify the primary vascular supply to the cord (the artery of Adamkiewicz), and for consideration of preoperative embolization. Smaller symptomatic lesions may be managed with vertebral augmentation. Recently, percutaneous treatment with vertebroplasty for aggressive and symptomatic vertebral hemangiomas has been reported in 24 patients.51 The authors reported the utility of vertebroplasty with good clinical results in all patients with pain relief within 24 to 72 hours even when epidural extension was present. They concluded that percutaneous vertebroplasty “is a valuable, mini-invasive, and quick method that allows a complete and enduring resolution of the painful vertebral symptoms without findings of fracture of a vertebral body adjacent or distant to the one treated.” Large destructive lesions occasionally require anterior reconstruction.
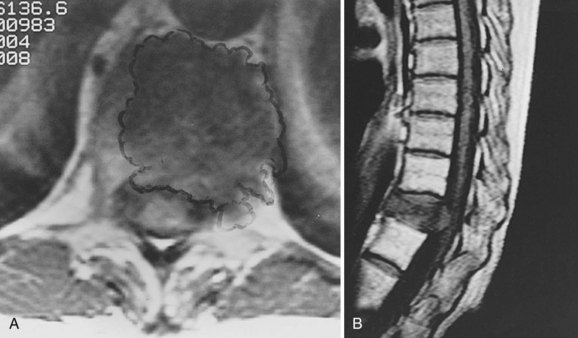
Giant Cell Tumor
Although rare reports of malignant giant cell tumors exist, the vast majority of these lesions are slow-growing, locally aggressive tumors that do not metastasize. Spinal involvement is usually seen in patients in their third and fourth decades, and symptoms may exist for many months before the patient sees a physician. Plain radiographs usually demonstrate an area of focal rarification, though some present with a more geographic lytic appearance and marginal sclerosis. Giant cell tumors are most commonly found in the vertebral body and may expand the surrounding cortical bone extensively as the tumor enlarges (Fig. 85–8). CT is especially important in the evaluation of these tumors preoperatively because complete resection is critical to the eradication of local disease. CT is also crucial in the early identification of recurrences and should be used in postoperative follow-up on a routine basis. Complete excision is the treatment of choice and should be attempted en bloc whenever feasible in the spine.
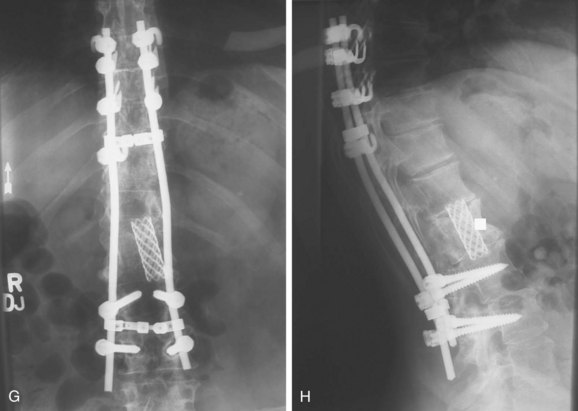
Giant cell tumors involving the spine have a poorer prognosis than extremity lesions due to their locally invasive nature and the difficulty in obtaining a wide margin. This results in high local recurrence rates.2,52 Adjuvant treatment with radiation therapy was associated with unacceptably high rates of postirradiation sarcoma, often leading to the patient’s death. Recent reports have demonstrated favorable responses using megavoltage radiation therapy with low or no risk of radiation-induced sarcoma.53,54 With more aggressive surgical resection, radiotherapy is unnecessary and good disease-free survivals have been obtained without the risks of irradiation (Fig. 85–9).52,55
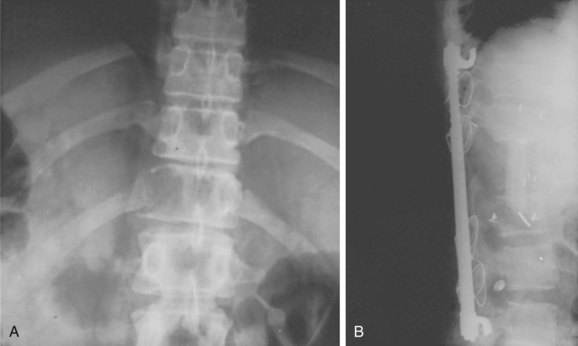
FIGURE 85–9 A, Giant cell tumor at T12. B, Lateral view radiograph 5 years after total vertebrectomy with no recurrence.
A series of 22 consecutive giant cell tumors (GCTs) of the cervical spine reviewed56 the clinical patterns and follow-up data of GCT arising in the cervical spine, correlating treatment and outcomes over time.1 Patients underwent surgical treatment from 1990-2003. The choice of surgical intervention was based on the Weinstein-Boriani-Biagini tumor staging system. The authors employed two different protocols of surgical treatment: Eight patients underwent subtotal resection, there were 13 cases of total spondylectomy, and one additional patient had en bloc resection for a lesion in the posterior element of C7. Spinal reconstruction was done with autograft and anterior instrumented fusion or anterior and posterior combined instrumented fusion. Eighteen patients received postoperative adjunctive therapies. Twenty-one patients were available for mid- and long-term follow-up (average of 67.8 months, ranging from 36 to 124 months). They reported satisfactory neurologic recovery, and fusion rates were 100%. The local recurrence rate was 71.4% in patients who underwent subtotal resection but only 7.7% for total spondylectomy. Four patients died within follow-up, and all of these deaths were in recurrent cases. One patient developed pulmonary metastases. The authors recommended strictly en bloc resection but recognized that it is often not a feasible option in the cervical spine because of the involvement of critical neurovascular structures.
Eosinophilic Granuloma
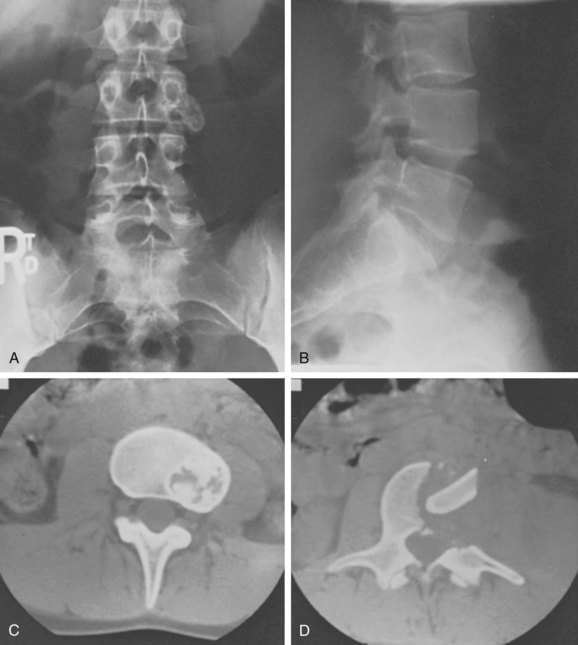
Eosinophilic granuloma is a benign, self-limiting condition that produces focal destruction of bone. A process of unproven etiology, eosinophilic granuloma is most commonly seen in children before the age of 10 years, when treatment can be challenging because preservation of neurologic function is of imminent concern.57 It can, however, occur in the adult spine as well, where it may present with local osteolysis.58 Lesions of the skull are most common, but any bone may be affected; vertebral involvement occurs in approximately 10% to 15% of cases. Spinal involvement can be seen in any of the triad of syndromes in which the disease manifests itself—isolated eosinophilic granuloma; the multifocal, chronic, and disseminated form, Hand-Schuller-Christian disease; or the acute disseminated or infantile form, Letterer-Siwe disease. The vertebral body is typically involved, usually in the thoracic or lumbar spine. Patients usually present with pain, focal spasm (torticollis is common in cervical lesions), and mild to moderate kyphosis. Neurologic compromise develops in a significant number of patients and may be severe.
Early in the disease process radiographs show a central lytic lesion with poorly defined margins and permeative bone destruction. At this point the lesion may produce a marked periosteal reaction, and distinguishing it from osteomyelitis or Ewing sarcoma may be impossible. As the vertebral body collapses and settles, radiographs demonstrate the flattened disc of dense cortical bone retained between the two intact intervertebral discs. This “coin-on-end” appearance of vertebra plana (Fig. 85–10) is a classic finding in eosinophilic granuloma but is not pathopneumonic; a similar appearance can be produced by either infection or high-grade sarcoma. With such a broad differential, the importance of obtaining an adequate biopsy specimen before beginning treatment cannot be overstated.59
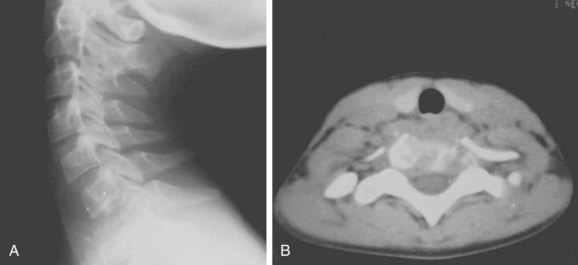
FIGURE 85–10 A, Lateral radiograph view of eosinophilic granuloma at C7. B, Computed tomography scan.
The treatment of eosinophilic granuloma is somewhat controversial, but it is clear that many patients will heal their lesions without any treatment other than biopsy, often reconstituting much vertebral height.60 Low-dose radiotherapy (500 to 1000 rads) has been advocated in the past, but this may be avoided in most patients. In those who present with a neurologic deficit, the established course of biopsy followed by irradiation and immobilization remains the most widely accepted.61
A retrospective case review of seven children with eosinophilic granuloma of the cervical spine identified therapeutic goals as spinal stability, preservation of neurologic function, and relief of pain, while keeping in mind that patients are still growing.57 There were five boys and two girls (mean age: 10 years; range 4 to 16) with a mean follow-up of 19 years (range 8 to 29). The symptoms at presentation varied according to the localization of the tumor and treatment varied from observation alone, prolonged immobilization, systemic chemotherapy, curettage with or without bone grafting, corticosteroid injection, and low-dose radiation therapy depending on the severity of clinical presentation. The authors recommended surgery for a child presenting with neurologic involvement. On the basis of their experience, they concluded that children with vertebral interbody fusion can show normal alignment of the neck and maintain normal motion at long-term follow-up.
Malignant
Osteosarcoma
Approximately 2% of all primary osteogenic sarcomas arise in the spine, 95% from the anterior elements. Patients present with pain and often with neurologic compromise. A palpable mass may be a late finding. Metastatic lesions are also commonly present on diagnosis. Vertebral osteosarcoma (especially in the sacrum) is frequently secondary to previous Paget disease or radiation therapy, in which case prognosis is even more grim.5,62,63 The radiographic findings include both lytic and sclerotic lesions, with cortical destruction, soft tissue calcification, and collapse in advanced cases. CT and MRI demonstrate intraspinal and paraspinal soft tissue masses more clearly, allowing more accurate preoperative planning.
Treatment of lesions in the spinal column is usually difficult, and the outcome in these tumors has traditionally been poor. Therapy consisting of limited tumor excision and radiotherapy provided median survival ranging from 6 to 10 months.5,62 In hopes of improving survival in this and other spinal malignancies, a more aggressive surgical approach has been advocated in recent years. For osteosarcoma, this has resulted in longer survival times and some cures.2,63–65
A systematic review of the literature and consensus recommendations by an international expert focus group has recently reviewed and classified “evidence in the literature regarding (1) the role of neoadjuvant chemotherapy and (2) impact of extent of surgical resection on clinical outcome, particularly survival and local control, in patients with spinal Ewing sarcoma (ES) and osteosarcoma (OS).” This study revealed moderate-level evidence suggesting that neoadjuvant chemotherapy offers significant improvements in local control and long-term survival in osteosarcoma of the spine. This multimodality management is essential in lieu of weak evidence suggesting that en bloc resection provides improved local control and potentially improved overall survival. Neoadjuvant therapy portends the most favorable local control and long-term survival. En bloc surgical resection may improve overall survival and decrease risk of recurrence.66
Ewing Sarcoma Family Tumors
Approximately 3.5% of all Ewing tumors arise in the spinal column, with the majority originating in the sacrum.67 Patients are usually in the second or third decade of life and present with pain with or without constitutional symptoms. Neurologic compromise is reportedly present approximately 60% of the time.67 These tumors are usually centered in the vertebral body, but extension into the posterior elements is common. On occasion, Ewing tumors can be mistaken for psoas abscess.2 The permeative appearance of Ewing tumors on radiographs can make diagnosis difficult, even in advanced disease, and collapse of the vertebral body may produce a vertebra plana indistinguishable from that seen in eosinophilic granuloma.68 MRI is the best modality to differentiate these neoplasms and to define the extent of the lesion including occasional epidural metastases. Surgical treatment is indicated for decompression of neural elements and stabilization of the vertebral column, but the treatment of choice for Ewing sarcoma involves multiagent chemotherapy and high-dose radiotherapy. Recent studies indicate that attempts at surgical extirpation in combination with systemic therapy and radiotherapy may improve prognosis.69
Similar to osteosarcoma, moderate-level evidence has been found to suggest that neoadjuvant chemotherapy and multimodality management offer significant improvements in local control and long-term survival in patients with Ewing sarcoma.66 Investigators found low-level evidence supported a weak recommendation that en bloc surgical resection provides improved local control but does not improve overall survival. Radiation therapy for spinal ES may also be used for local control either alone or to supplement incomplete resection.
A recent study attempted to determine the best local treatment combined with neoadjuvant chemotherapy for Ewing sarcoma family tumors (ESFTs) of the spine and sacrum.70 The rate of local recurrence of ESFTs in the spine and sacrum was investigated when treated with neoadjuvant chemotherapy and locally by radiotherapy alone, or surgery, followed by reduced doses of radiotherapy. The authors followed 43 patients, 26 of whom were treated with neoadjuvant chemotherapy and locally by radiotherapy alone, and 17 by surgery followed by radiotherapy at reduced doses. The 5- and 10-year disease-free survival (EFS) rates were 37% and 30%, respectively, and the 5- and 10-year overall survival rates were 42% and 32%. Prognosis was unrelated to gender or age, tumor volume, chemotherapy protocol, or local treatment. Instead, outcomes seemed worse for patients with primary tumors located in the sacrum than for patients with tumors located in other parts of the spine (5-year survival ≥ 23% vs. 46%). Surgery seemed to have little beneficial effect because there were no differences between patients treated with radiotherapy alone and those treated by radiotherapy and surgery. Authors concluded that ESFTs in the spine and sacrum have a poor outcome, and prognosis is significantly worse than that of primary ESFT in other sites.
Chordoma
Chordoma is a relatively rare malignancy occurring in all adult age groups but is predominantly found in patients in the fifth or sixth decade of life. The tumor arises from remnants of the primitive notochord71 found in the sacrococcygeal and suboccipital regions of the spine and occasionally from notochordal rests within the vertebral body in the thoracic or lumbar region.72 Although this tumor is characterized by its slow, relentless local spread, it is a fully malignant lesion capable of distant metastases. Initial symptoms are usually mild and progress slowly as the tumor expands. Chordomas may reach a considerable size before metastasizing, and symptoms of constipation, urinary frequency, or nerve root compression may appear before patients present to their physician. A firm, fixed presacral mass can usually be palpated on rectal examination.
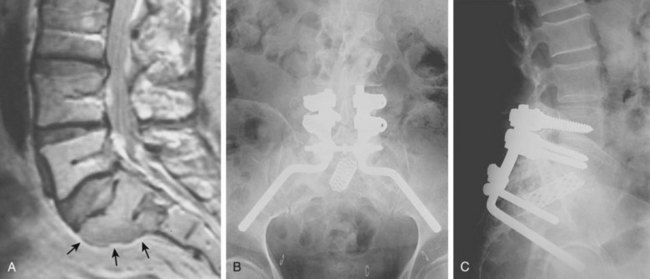
Radiographically, chordomas are lytic lesions in the midline of the vertebral body or sacrum, usually associated with a soft tissue mass. CT is helpful to define the destruction of bony architecture, and MRI is mandatory to define the extent of the tumor for presurgical planning. Patient survival depends on local control of the tumor. Local recurrence of a chordoma is a grim prognostic sign, dramatically reducing the likelihood of cure. Surgical extirpation of the tumor is the only curative procedure, and a wide margin must be obtained (Fig. 85–11). En bloc excision is associated with the least risk of local recurrence but may be a terrifically challenging undertaking.72,73
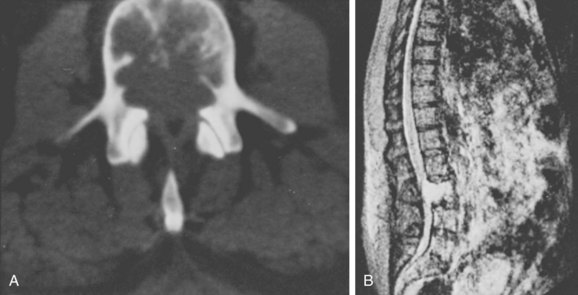
FIGURE 85–11 A, Computed tomography scan of L3 chordoma involving zones IIIA and IVA and B (see Fig. 85–12). B, Magnetic resonance imaging scan of the same patient, T2 image (sagittal).
A recent study evaluated factors that contribute to improved local control and survival with chordoma. Forty-two patients underwent resection for sacral chordoma with 12 female and 30 male patients. The proximal extent of the sacrectomy was at least S2 in 32 patients. The authors reported a median survival of 84 months, and 5-year disease-free and disease-specific survival rates were 56% and 77%, respectively. Local recurrence and metastasis occurred in 17 (40%) and 13 (31%) patients, respectively. Local recurrence (P = 0.0001), metastasis (P = 0.0001), prior resection (P = 0.046), and higher grade (P = 0.05) were associated with a worse disease-free survival.74 Factors associated with poor outcome and increased local recurrence rates include prior resections (P = 0.0001) and intralesional resections (P = 0.01). The authors reported that treatment of wide contaminated margins with cryosurgery and/or radiation was not associated with a higher local recurrence rate. Surgical resection of sacral chordoma was associated with significant morbidity and complications requiring rectus abdominis flaps in some cases to decrease wound complications (P = 0.01). Thirty-one (74%) patients reported that they self-catheterize, 16 (38%) patients required bowel training, and an additional 12 (29%) patients had a colostomy. Twenty-eight (67%) patients reported sexual dysfunction. Two (5%) patients died because of sepsis. The authors recommended avoiding intralesional resection because of higher local recurrence rates and worse survival. Newer methods of radiation therapy using proton beams or using intensity-modulated radiotherapy may decrease rates of local recurrence further with less morbidity, although long-term studies are necessary.75
Chondrosarcoma
Approximately 7% to 10% of chondrosarcomas arise in the spinal column.76 Most of these tumors are low grade, so they grow slowly and are relatively resistant to radiotherapy and chemotherapy. A high propensity for local recurrence leads to the poor prognosis of spinal chondrosarcoma. Most patients present with pain, some with a palpable mass if the tumor arises from the posterior elements. Neurologic compromise is common.
Radiographically, chondrosarcoma has a fairly characteristic appearance. In advanced disease there is a large area of bone destruction and an associated soft tissue mass with flocculent calcifications within it. If there is no soft tissue mass, the vertebral lesion may be primarily lytic, with sclerotic margins, and with no mottled calcification.77 CT and MRI are invaluable in demonstrating the extent of the lesion and evaluating cord compromise.
Complete surgical excision is required to cure the patient with chondrosarcoma. This may be impossible to obtain in some vertebral lesions. Stener pioneered en bloc excision of chondrosarcoma of the spine in 1971.78 All of the larger series since then have demonstrated the importance of obtaining negative margins in an en bloc fashion to increase disease-free survival.79
When the management of sacral tumors requires partial or complete sacrectomy, reconstruction of the lumbosacral junction is necessary. Staged anterior/posterior en bloc sacrectomy with Galveston L-rod pelvic ring reconstruction or more modern variations thereof are often required. Understanding the anatomy and biomechanics of the spinopelvic apparatus and the lumbosacral junction, as well as having a familiarity with the various techniques available for carrying out sacrectomy and pelvic ring reconstruction, will enable the spine surgeon to effectively manage sacral tumors.1
Solitary Plasmacytoma
True solitary plasmacytoma is a rare entity comprising only about 3% of all plasma cell neoplasms. Though the course of multiple myeloma is usually rapidly progressive and lethal, patients with solitary plasmacytoma may have prolonged survival if local control can be obtained. The 1-year mortality rate for patients with multiple myeloma involving the spine is 76%; 100% at 4 years.80 The 5-year disease-free survival in solitary plasmacytoma is roughly 60%.81
Lymphoma
Lymphoma may present as a systemic disease with skeletal manifestations or as an isolated bony tumor referred to in the past as a reticulum cell sarcoma. Because the lesion has been considered a metastatic lesion by some authors, it is not consistently included in reviews of primary bone tumors. There is a distinction between primary and secondary lymphoma in terms of survival, with a distinctly better prognosis in the former than the latter.82
Metastatic Tumors
Metastases are by far the most common skeletal tumors seen by the orthopedist, and the spine is the most common site of skeletal involvement.75 Skeletal metastases are produced by almost all forms of malignant disease but are most commonly secondary to carcinomas of the breast, lung, or prostate and less frequently from renal, thyroid, or gastrointestinal carcinomas (Table 85–1). Multiple myeloma and lymphoma are common sources of disseminated skeletal lesions, though whether they are considered metastatic or primary lesions has varied from author to author. Breast, lung, prostate, and lymphoreticular disease account for approximately 60% of all spinal column tumors.
TABLE 85–1 Location of Primary Neoplasms Producing Metastatic Lesions of Bone: a Review of 5006 Cases
| Primary Site | Totals (%) |
|---|---|
| Breast | 2020 (40) |
| Lung | 646 (13) |
| Prostate | 296 (6) |
| Kidney | 284 (6) |
| Gastrointestinal | 255 (5) |
| Thyroid | 110 (2) |
| Bladder | 160 (3) |
| Total | 5006 |
Pathophysiology of Metastasis
Tumor emboli entering the bloodstream tend to lodge in the natural filters of the vascular tree—the capillary beds of the liver, lungs, and bone marrow. To become established in the medullary canal tumor, emboli must either bypass the capillary beds of the liver and lungs, usually by establishing a metastasis there first, or circumvent these filters and reach the medullary sinusoids by an entirely different route. Tumors of the lung may seed the vertebral column directly through the segmental arteries, whereas carcinomas of the breast, gastrointestinal tract, and prostate are thought to reach the vertebral system through communications with the paravertebral venous plexus originally described by Batson.83 Venous drainage from the breast by the azygous veins communicates with the paravertebral venous plexus in the thoracic region, whereas the prostate drains through the pelvic plexus in the lumbar region. Retrograde flow through Batson plexus has been shown to occur during Valsalva maneuver and may allow direct implantation of tumor cells in the vascular sinusoids of the vertebral body without passing through the usual capillary networks. Recent autopsy and animal studies have verified Batson’s original work.84
Finally, there are intrinsic factors inherent to the tumor cells themselves, which may give one cell line a particular advantage in surviving and growing in the medullary space. This is an exciting area of current research that may not only lead to a better understanding of the pathogenesis of skeletal metastasis but also to more effective treatment.85
Prognosis
In patients with spinal cord compression, the neurologic status before treatment correlates strongly with posttreatment outcome whether in terms of likelihood of recovery, extent of recovery, or the ability to maintain or regain ambulation or bowel and bladder function.86 Further discussion of this aspect of treatment can be found later in “Decompression.”
Approach to Treatment
Patients who have pain but no spinal instability and no neurologic deficit can usually be managed with nonoperative measures. This includes hormonal or chemotherapy treatment, especially for breast and prostate cancer, bisphosphonates, and radiation therapy. Newer treatments include radioisotopes87 and minimally invasive vertebroplasty or kyphoplasty.88 Bracing provides effective palliation for patients with pain and mild instability, and the halo vest is an important option for patients with cervical metastases and more significant instability.89
While radiation therapy is the mainstay for treating spinal metastases, if mechanical instability is present, surgery is indicated. For radiosensitive tumors, treat patients with intralesional excision and posterior instrumentation in combination with postoperative radiation therapy. For radioresistant tumors or significant bony destruction, perform an anterior/posterior or posterolateral decompression and stabilization.90 The patient with a solitary metastasis and the potential for long-term survival represents a special circumstance in which the surgical team should consider en bloc spondylectomy similar to a primary malignant lesion.91
Tumors in Children
Tumors of the immature spine differ in type from those seen in adults, particularly in terms of the malignant lesions. Nearly 70% of primary bone tumors seen in children are benign.92 Osteoid osteomas and osteoblastomas, osteochondromas, and aneurysmal bone cysts account for more than 40% of all primary spinal lesions seen in pediatric patients. Malignant lesions, although less common, are predominantly from metastasis or contiguous invasion from neuroblastoma, embryonal carcinoma, and sarcoma.93–94 These highly aggressive lesions have a poor prognosis regardless of treatment. Ewing sarcoma is the most common primary malignancy (Table 85–2),2 but even more common is a metastatic lesion. Diagnosis in these patients may be challenging because presenting complaints may be nonspecific.
| Tumor | Number (%) |
|---|---|
| Benign | |
| Osteoblastoma | 4 |
| Osteochondroma | 4 |
| Aneurysmal bone cyst | 4 |
| Giant cell tumor | 3 |
| Eosinophilic granuloma | 2 |
| Osteoid osteoma | 2 |
| Hemangioma | 1 |
| Angiolipoma | 1 |
| Malignant | |
| Ewing sarcoma | 3 |
| Chordoma | 1 |
| Osteosarcoma | 1 |
| Malignant giant cell tumor | 1 |
| Chondrosarcoma | 1 |
| Others | 3 |
| Total | 31 |
* Primary bone tumors found in the spinal column in 31 patients younger than 18 years of age.
Leukemia
Another disease presenting in pediatric patients is leukemia. In 6% of children with leukemia, back pain and vertebral collapse are the initial findings at the time of presentation. During the course of the disease 10% will sustain a pathologic vertebral fracture, sometimes involving multiple levels.95 When first seen, these children manifest a variety of nonspecific constitutional symptoms and the correct diagnosis is often hard to make. Lethargy, anemia, and fever occur commonly and often in combination. The peripheral leukocyte count is elevated in 60% of patients, and the erythrocyte sedimentation rate is also elevated. Radiographs may not demonstrate any focal abnormality, or they may show focal lytic lesions, occasional sclerotic lesions, or isolated periosteal reactions. It is important to keep in mind that radionucleotide scans are unreliable in patients with leukemia, in some cases showing no uptake in areas of obvious bony destruction. The presenting symptoms and signs mimic those seen in patients with osteomyelitis or joint sepsis, and this misdiagnosis is common. Keys to making the correct diagnosis in these confusing presentations are the identification of anemia, the recognition of those 40% of patients who are leukopenic at presentation, the presence of inconsistent bone scan results, and a high index of suspicion on the part of the examining physician.
Spinal Deformity
Progressive deformity may occur for any of a number of reasons after treatment of pediatric spinal tumors. As in adults, deformity may result from structural deficiencies caused by the erosion of bone by tumor or by aggressive surgical resection. These deformities may be more severe or progressive in children, however, particularly in the case of postlaminectomy kyphosis and particularly in the thoracic spine. The younger the child at the time of laminectomy, the more severe the eventual deformity is likely to be.93 Irradiation and rib resection can cause iatrogenic scoliosis, as can a higher number of laminectomy levels. Surgical management of pediatric tumor patients must anticipate the later development of deformity and seek to minimize it. Deformities that are certain to progress must be identified early on, and treatment instituted to halt this progression.
Treatment
General
The goals of treatment for patients with spine tumors are to provide cure if possible, palliation and early return to activity if not, and a stable spinal column and normal or improved neurologic function in either case. Table 85–3 provides a general outline for the treatment of spine tumors.
| Therapy | Indication |
|---|---|
| Observation | Indolent and clearly benign tumors (hemangioma, osteochondroma, bone island, bone infarct) |
| Radiotherapy | Metastatic lesions from a known radiosensitive primary (multiple myeloma, breast carcinoma) |
| Chemotherapy | Metastatic lesions from a known chemosensitive primary (thyroid) |
| Intralesional excision, curettage | Benign tumors with limited potential for recurrence (aneurysmal bone cyst, osteoblastoma), radiosensitive metastatic lesions with adjuvant radiation therapy |
| Marginal excision ± adjuvant cryotherapy or radiotherapy | Locally aggressive benign lesions (giant cell tumor), radiosensitive primary and metastatic lesions (plasmacytoma, breast and prostate carcinoma), low-grade malignancies |
| Wide excision | All primary malignancies without known metastases (osteosarcoma, chondrosarcoma, chondroma); solitary metastases with likelihood of prolonged survival (breast, prostate, renal cell carcinoma); locally aggressive benign tumors (giant cell tumor) |
Staging
To treat spinal neoplasms appropriately, an organized, calculated approach must be applied in preoperative planning. The surgeon must understand the anatomic extent of the lesion in three dimensions and anticipate the stabilization and reconstruction that will be necessary. Although true anatomic compartments (as defined by Enneking96) do not exist in the spinal column, anatomic structures do provide natural planes for dissection and wide excision. The vertebral body, anterior and posterior longitudinal ligaments, intervertebral discs, and dura may all be resected to avoid leaving residual tumor behind. Neural, muscular, and vascular structures may all be sacrificed to obtain an adequate surgical margin in primary malignancies. Such an aggressive approach is justified: as in extremity surgery, a complete resection provides the best prognosis for both local control and cure of the disease.
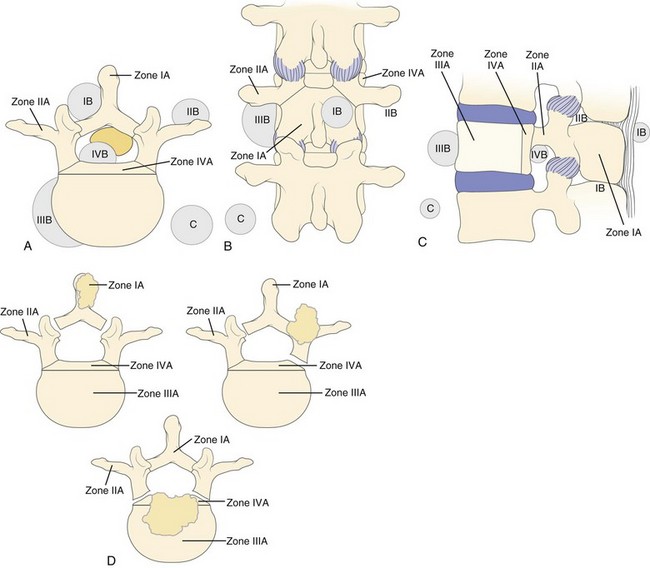
The vertebral body may be divided into four zones, I to IV. Tumor extension is designated as A, B, and C for intraosseous, extraosseous, and distant tumor spread (Fig. 85–12).97 Zone IA includes the spinous process and pars interarticularis along with the inferior facets. Zone IIA includes the superior articular facet, the transverse process, and the pedicle from the level of the pars to its junction with the vertebral body. Zone IIIA includes the anterior three fourths of the vertebral body, whereas zone IVA designates involvement of the posterior one fourth of the body, that segment immediately anterior to the cord. Zones IB to IVB are the extraosseous extensions of tumor beyond the boundaries of the cortical bone, and zones IC to IVC designate associated regional or distant metastatic involvement. Surgical approach is determined by the zones involved and the extent of the local tumor spread, while overall outcome depends on the degree of extension to other body systems, as well as the type and grade of tumor.
Surgical Approach
Many different surgical approaches are available to the spine surgeon, and variations to each have been described (Table 85–4). Choosing the correct approach for the given situation is, perhaps, the most important step in treating these conditions. The surgical approach selected must provide sufficient access for both tumor excision and stabilization of the spine thereafter. If both operations cannot be performed through the same incision, the surgeon must plan for a combined approach. An ill-planned approach may leave the surgeon unable to complete the excision of the tumor, a situation to be avoided if at all possible.
| Level | Anterior | Posterior |
|---|---|---|
| Upper cervical | Transoral | Midline |
| Extraoral | ||
| Extreme lateral | ||
| Lower cervical | Southwick-Robinson | Midline |
| Cervicothoracic/upper thoracic | Sternal splitting | Midline |
| Low anterior costotransversectomy ± endoscopy | Costotransversectomy | |
| Thoracic | Thoracotomy | Midline |
| Costotransversectomy ± endoscopy | Costotransversectomy | |
| Transpedicular | ||
| Thoracolumbar | 11th rib extrapleural-retroperitoneal | Midline |
| Posterolateral | ||
| Lumbar | Retroperitoneal | Midline |
| Transabdominal | Transpedicular | |
| Posterolateral |
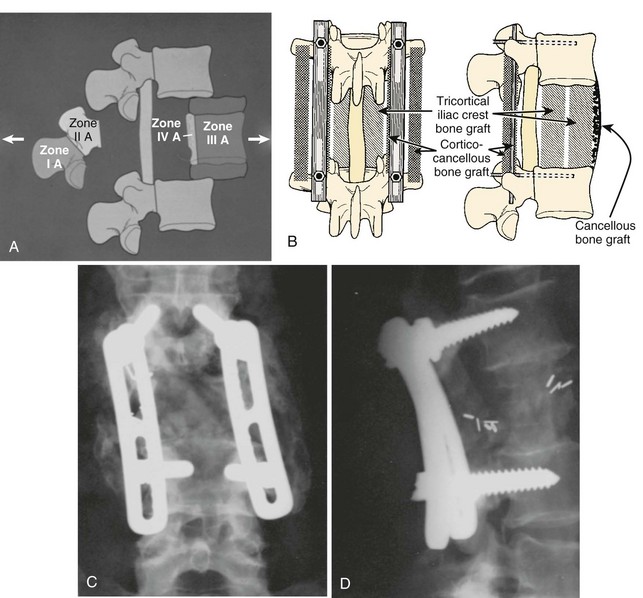
Zone IV lesions that require a complete or en bloc excision must be managed through a combined anterior and posterior surgical approach. These lesions involve the most inaccessible region of the vertebral body and are the most difficult lesions to reconstruct; they provide major technical challenges to the surgeon before, during, and after the actual tumor resection. Zones I, II, and/or III must be crossed at some point to provide access to zone IV lesions, and frequently more than one zone may be involved with tumor. Complete excision can be obtained, though tumor margins must often be crossed. Excision requires vertebrectomy, essentially separating zone II from zones III and IV through combined approaches (Figs. 85-13 and 85-14), and in such cases both anterior and posterior stabilization is usually necessary. Failure to provide sure fixation and an adequate bone graft may result in loss of fixation, with catastrophic neurologic complications if hardware migrates into the canal or if excessive kyphosis develops.76,98
The best approach is also dictated by the level within the spinal column in which the tumor is found (see Table 85–4). Upper cervical lesions can be approached anteriorly via transoral, extraoral,99 or extreme lateral100 approaches. This allows resection, decompression, and reconstruction for anterior tumors. Most surgeons use the Southwick-Robinson anterior approach for lower cervical tumors.101 The anterior cervicothoracic junction and upper thoracic spine present an anatomic challenge to surgeons. Options are the sternal splitting approach, sterno-clavicular excision, the low anterior approach (a caudal extension of the Southwick-Robinson approach), and the posterolateral costotransversectomy. Splitting the sternum causes much morbidity, but the latter two approaches provide limited exposure. Endoscopic assistance provides a considerable advantage in these cases to allow complete vertebrectomy and anterior decompression in cases where en bloc excision is not indicated.102 Midline posterior approaches are used in the cervical and upper thoracic spine for posterior tumors and for instrumentation that is often required for adequate stabilization either in combination with an anterior approach or alone.
The standard approach to the anterior thoracic spine is a thoracotomy with rib resection. Good results have been obtained using this approach for anterior resection, decompression, and reconstruction in oncologic procedures.103 Endoscopic assistance is also quite helpful as an adjunct to posterolateral approaches in the thoracic spine. Use of the eleventh rib extrapleural-retroperitoneal approach to the thoracolumbar junction gives the surgeon adequate exposure in most cases in this area.104 The anterior lumbar spine can be approached via a retroperitoneal or transabdominal approach. The successful use of minimally invasive retroperitoneal procedures has allowed many surgeons to lean away from endoscopic assistance in the lumbar spine.105
The thoracolumbar spine can be approached posteriorly via a midline incision for tumors of the posterior elements and for instrumentation and stabilization. The posterior transpedicular approach to the anterior thoracolumbar spine can be used for decompression and subtotal vertebrectomy. A simultaneous anterior and posterior approach,106 a staged posterior and then anterior approach,107,108 or a posterior approach alone109,110 can be used for total en bloc spondylectomy. (See “Resection” later for a more detailed discussion.)
Complete radiographic evaluation including CT and MRI allows accurate determination of the tumor location and extension and a more informed prediction of the tumor grade if not its actual tissue type. Additional laboratory and screening studies focus the differential further, allowing the surgeon to plan an operation that will adequately treat the tumor without exposing the patient to needless risks. Accurately determining the most likely tumor type in each case before surgery is important; overtreatment of benign disease can be nearly as disastrous as undertreatment of malignancy. The surgeon also needs to assess whether preoperative embolization of the tumor will be helpful, as it is in many cases such as metastatic renal cell carcinoma, hemangioma, and aneurysmal bone cyst. An algorithm has been developed for the evaluation of patients presenting for the first time with a spine lesion (Fig. 85–15).
Resection
The biology of the tumor and its stage largely determine the necessary resection margin and surgical approach (see Tables 85-3 and 85-4). Several studies have shown that obtaining a wide or marginal margin in both primary malignancies111 and metastases112–114 improves survival and local recurrence over intralesional margins. Obtaining the widest margin possible is essential in many locally aggressive or malignant primary tumors, particularly those that do not respond well to irradiation. A wide margin can be obtained in most isolated lesions in zones IA through IIIA because the tumor can usually be completely resected. Type IVA lesions can often be resected cleanly but only by removing the surrounding compartments as well. An adequate margin can be extremely difficult to obtain in type B lesions. IB to IVB lesions may not be completely resectable without producing serious neurologic deficits. In these cases surgery is marginal at best and often intralesional. The decision to attempt a wide or radical resection in these cases must be weighed against the risk of neurologic deficit.
Special mention should be made of sacral tumors. Partial or total sacrectomy with a combined anterior and posterior approach is effectively an amputation with the potential for a wide margin. Often the spine surgeon is assisted by general and/or vascular surgeons with this demanding procedure. The complication rate is high, mostly because of wound problems and colorectal complications. If nerve roots can be salvaged at least unilaterally, bowel and bladder function can be retained.115,116
Decompression
Spinal cord compression is reported to occur in between 5% and 20% of patients with widespread cancer.9,117 Although cord compression can also result from primary benign and malignant tumors of the spinal column, this occurs less frequently, and often the canal is effectively decompressed during the resection of the tumor. In all, spinal cord compression may result from one of four types of processes: (1) direct compression from an enlarging soft tissue mass, (2) pressure due to fracture and retropulsion of bony fragments into the canal, (3) severe kyphosis following vertebral collapse, and (4) pressure due to intradural metastases. The most common cause of cord compression is mechanical pressure from tumor tissue or bone extruded from the collapsing vertebral body.
Tumor Biology
The intrinsic nature of each specific primary or metastatic neoplasm determines which will have slow or rapid growth, which will be invasive, which will produce metastases, and how long a patient is likely to survive after metastases are present.87,113,117,118 Although metastatic lesions usually demonstrate behavior similar to their parent lesions, this is not always true; some metastases are more invasive or rapid growing than the primary lesions they come from. Rapid tumor expansion or vertebral erosion and fracture can produce acute cord compression and a poorer prognosis for improvement. Slower expansion produces gradual cord impingement, from which the patient has a much better chance of recovering. Understanding the tumor type and its biology allows the surgeon to reasonably predict if and when a specific lesion will endanger neurologic structures.
Neurologic Status
The neurologic status before treatment correlates strongly with post-treatment outcome whether in terms of likelihood of recovery, extent of recovery, or the ability to maintain or regain ambulation or bowel and bladder function. Between 60% and 95% of the patients who can walk at the time of diagnosis will retain that ability following treatment. By comparison only 35% to 65% of paraparetic patients will regain the ability to walk, and less than 30% of paraplegic patients will regain ambulation.87,113,119–121 The rate of progression of the neurologic deficit also has clear prognostic significance. A patient who progresses from the earliest onset of symptoms to a major deficit in less than 24 hours has a poor prognosis for recovery irrespective of treatment. Conversely, compression that has evolved over a course of months has a far more favorable prognosis for recovery following treatment.
Decompression Surgery
The cumulative experience of many authors has led to the following recommendations for decompression surgery in the spine: (1) Use the posterior approach in the upper cervical spine89; (2) use the posterior approach in the lower cervical, thoracic, and lumbar spine only for tumors of the posterior elements; (3) use anterior approaches for the majority of lower cervical, thoracic, and lumbar lesions because most tumors are located in the body in these regions103,119,121; (4) use alternative approaches in patients in whom medical factors preclude anterior approaches: costotransversectomy with or without endoscopic assistance in the thoracic spine (Fig. 85–16) and posterolateral transpedicular vertebrectomy in the thoracic and lumbar spine102,106; and (5) use a combined anterior and posterior approach or an extended posterior approach for en bloc spondylectomy or when the tumor involves many levels.78,107–110
Reconstruction
After resection of a tumor, the spinal column will often need some form of reconstruction in order to restore the mechanical stability of the spine and compensate for the loss of bony elements. The surgeon must observe several key principles (Fig. 85–17). First, definitive treatment must restore the anterior weight-bearing column. This is mandatory in any case in which a significant amount of the anterior column has been resected at either one level or over many levels. Without reconstruction, collapse and kyphosis will result. Second, use posterior instrumentation to restore the posterior tension band after extensive laminectomy, especially in cases where facet joints have been removed. This will help to prevent kyphosis and can compensate for resected musculature. Third, combine anterior reconstruction with posterior instrumentation for larger resections. This includes subtotal and total spondylectomy and multilevel resections. Fourth, anticipate disease progression, especially in cases of metastasis. Problems can be avoided by including more levels in the fixation construct above and below the tumor, combining anterior and posterior instrumentation, and maximizing fixation points. Last, strive for biologic fusion in patients who are likely to survive more than 3 to 6 months.
Posterior Instrumentation
Although Harrington distraction rods and Luque rods with sublaminar wires were used successfully in the past, they have been supplanted by newer segmental instrumentation systems. These systems are versatile, with hook and screw fixation possible at multiple levels. The superior strength and resiliency of these constructs allows them to be used even in cases where the posterior elements have been completely resected or destroyed by tumor. The surgeon can contour rods or plates to restore sagittal alignment and can either compress or distract separately at each intercalary level. Pedicle screws offer more secure anchorage and can be used in the thoracic and lumbar spine with good results (Fig. 85–18).122
These systems are not infallible, however. In areas of greater stress, like the transitional areas between the occiput and upper cervical spine and the cervicothoracic and thoracolumbar junctions, fixation may have to be extended over more levels to provide enough stability. Also, in cases where a significant portion of the anterior and middle columns is missing or collapsed, these systems can fatigue and fail.123 Anterior reconstruction is required. Further, pedicle screws can pull out when bone quality is poor. This is frequently a risk in these patients because they often have or will receive radiotherapy, may already have osteoporosis, and may have diffuse metastatic disease. Augmentation of screw fixation with polymethylmethacrylate (PMMA) in poor-quality bone can be helpful.124
Anterior Reconstruction
Anterior spinal reconstruction using PMMA remains somewhat controversial. Although use in traumatic spinal instability has led to significant complications and failure, most authors agree there is a role for cement in stabilizing metastatic spinal lesions. This role has become more limited, however, as better alternatives have become available. PMMA is resilient in compression but has no potential for biologic fixation. It should be used only as a spacer, providing a temporary internal splint in anticipation of eventual bony arthrodesis or inevitable demise. If arthrodesis is not obtained, it is only a matter of time before the methacrylate construct fails; only patients with a limited life expectancy should be treated with methacrylate fixation without bone grafting. Longitudinal Steinmann pins may be incorporated into the PMMA mass to enhance both the bending resistance of the construct and its fixation to the adjacent vertebral bodies. Anterior plates can also be used to increase the rigidity of this construct. Care must be taken to avoid contact of the PMMA with the dura, to maintain enough space for the sac, and to prevent thermal injury. This is especially important when the patient is supine, in which case a sheet of Gelfoam may be used to shield the thecal sac while the cement polymerizes under a constant flow of saline irrigation.125
Prosthetic cages with morcellized autograft, tricortical strut grafts, and allografts are used for patients with a longer anticipated survival. Grafts should be keyed into the vertebral endplates, whereas the endplates should not be violated when using titanium cages. These can be used alone for one- or two-level resections but need to be combined with anterior or posterior instrumentation for larger defects. The goal is complete bony arthrodesis, and results in attaining this have been good.126,127
Anterior plate fixation may be combined with anterior column reconstruction to restore sagittal, coronal, and torsional rigidity following vertebrectomy, eliminating the need for posterior instrumentation in some patients (Fig. 85–19).128 Plate fixation also minimizes the likelihood that the strut graft or cage will displace. There is less need to key the graft into the adjacent vertebral bodies, and the graft can be impacted directly into the hard vertebral endplates. Because the graft or cage rests on the endplates, there is less chance of subsidence over time.
Several carbon fiber vertebral replacement prostheses have become available recently. They can provide both the mechanical support necessary for axial stability and the potential for bone ingrowth or arthrodesis, without the morbidity of harvesting large tricortical autografts. These prostheses can be connected to the posterior instrumentation, providing a stable construct for large defects. Modularity and radiolucency are other advantages.129
Summary
Pearls
Pitfalls
Key Points
1 Weinstein JN, McLain RF. Primary tumors of the spine. Spine. 1987;12:843-851.
2 Bohlman HH, Sachs BL, Carter JR, et al. Primary neoplasms of the cervical spine. J Bone Joint Surg. 1986;68A:483-494.
3 Hart RA, Boriani S, Biagini R, et al. A system for surgical staging and management of spine tumors: a clinical outcome study of giant cell tumors of the spine. Spine. 1997;22:1773-1783.
4 Sundaresan N, Rothman A, Manhart K, et al. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine. 2002;27:1802-1806.
5 Weinstein JN. Surgical approach to spine tumors. Orthopaedics. 1989;12:897-905.
6 Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599-609.
1 Jaffe HL. Tumors and Tumorous Conditions of the Bones and Joints. Philadelphia: Lea and Febiger; 1958.
2 Weinstein JN, McLain RF. Primary tumors of the spine. Spine. 1987;12:843-851.
3 Sim FH, Dahlin DC, Stauffer RN, Laws ER. Primary bone tumors simulating lumbar disc syndrome. Spine. 1977;2:65-74.
4 Harrington KD. Current concepts review: Metastatic disease of the spine. J Bone Joint Surg. 1986;68A:1110-1115.
5 Shives TC, Dahlin DC, Sim FH, et al. Osteosarcoma of the spine. J Bone Joint Surg. 1986;68A:660-668.
6 Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: Diagnosis and treatment. Ann Neurol. 1978;3:40-51.
7 Lodewick GS. Determining growth rates of focal lesions of bone from radiographs. Radiol. 1980;134:577-583.
8 Edelstyn GA, Gillespie PJ, Grebell ES. The radiologic demonstration of osseous metastases: Experimental observations. Clin Radiol. 1967;18:158-164.
9 Wong DA, Fornasier VL, MacNab I. Spinal metastases: The obvious, the occult, and the imposters. Spine. 1990;15:1-4.
10 Blaskiewicz DJ, Sure DR, Hedequist DJ, et al. Osteoid osteomas: intraoperative bone scan-assisted resection. J Neurosurg Pediatr. 2009;4:237-244.
11 Savelli G, Chiti A, Grasselli G, et al. The role of bone SPET study in diagnosis of single vertebral metastases. Anticancer Res. 2000;20:1115-1120.
12 Gates GF. SPECT bone scanning of the spine. Semin Nucl Med. 1998;28:78-94.
13 Hum B, Feigenbaum F, Cleary K, Henderson FC. Intraoperative computed tomography for complex craniocervical operations and spinal tumor resections. Neurosurgery. 2000;47:374-380.
14 Vacarro AR, Shah SH, Schweitzer ME, et al. MRI description of vertebral osteomyelitis, neoplasm, and compression fracture. Orthopedics. 1999;22:67-73.
15 Yamaguchi T. Intertrabecular vertebral metastases: Metastases only detectable on MR Imaging. Semin Muscoloskel Radiol. 2001;5:171-175.
16 Chan JH, Peh WC, Tsui EY, et al. Acute vertebral body compression fractures: Discrimination between benign and malignant causes using apparent diffusion coefficients. Br J Radiol. 2002;75:207-214.
17 Shih TT, Huang KM, Li YW. Solitary vertebral collapse: Distinction between benign and malignant causes using MR patterns. J Magn Reson Imaging. 1999;9:635-642.
18 Abdel Razek AA, Castillo M. Imaging appearance of primary bony tumors and pseudotumors of the spine. J Neuroradiol. 2010;37:37-50.
19 Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited: members of the Musculoskeletal Tumor Society. J Bone Joint Surg. 1996;78A:656-663.
20 Mirra JM, Gold RH, Picci P. Osseous tumors of intramedullary origin. In: Mirra JM, editor. Bone Tumors. Philadelphia: Lea & Febiger; 1989:31-33.
21 Talac R, McLain RF. Biopsy Principles and Techniques for Spinal Tumors. Semin Spine Surg. 2009;21:70-75.
22 Yamazaki T, McLoughlin GS, Patel S, et al. Feasibility and safety of en bloc resection for primary spine tumors: a systematic review by the Spine Oncology Study Group. Spine (Phila Pa 1976). 2009;34:S31-S38.
23 Bohlman HH, Sachs BL, Carter JR, et al. Primary neoplasms of the cervical spine. J Bone Joint Surg. 1986;68A:483-494.
24 Calhoun JM, Chadduck WM, Smith JL. Single cervical exostosis: report of a case and review of the literature. Surg Neurol. 1992;37:26-29.
25 Khosla A, Martin DS, Awwad EE. The solitary intraspinal vertebral osteochondroma. An unusual cause of compressive myelopathy: features and literature review. Spine. 1999;24:77-81.
26 Barsa P, Benes V, Suchomel P. Solitary spinal osteochondroma. A case report. Acta Chir Orthop Traumatol Cech. 2009;76:424-427.
27 Tubbs RS, Maddox GE, Grabb PA, et al. Cervical osteochondroma with postoperative recurrence: case report and review of the literature. Childs Nerv Syst. 2010;26:101-104.
28 Roach JW, Klatt JW, Faulkner ND. Involvement of the spine in patients with multiple hereditary exostosies. J Bone Joint Surg Am. 2009;91:1942-1948.
29 Harrop JS, Schmidt MH, Boriani S, Shaffrey CI. Aggressive “benign” primary spine neoplasms:osteoblastoma, aneuysmal bone cyst, and giant cell tumor. Spine. 2009;34(22 Suppl):S39-S47.
30 Keim HA, Reina EG. Osteoid-osteoma as a cause of scoliosis. J Bone Joint Sur. 1975;57A:159-163.
31 Kransdorf MJ, Stull MA, Gilkey FW, et al. Osteoid osteoma. R adiographics. 1991;11:671-696.
32 Marsh BW, Bonfiglio M, Brady LP, et al. Benign osteoblastoma: range of manifestations. J Bone Joint Surg. 1975;57A:1-9.
33 Kroon HM, Schurmans J. Osteoblastoma: clinical and radiographic findings in 98 new cases. Radiology. 1990;175:783-790.
34 Saifuddin A, White J, Sherazi Z, et al. Osteoid osteoma and osteblastoma of the spine: factors associated with the presence of scoliosis. Spine. 1998;23:47-53.
35 Pettine KA, Klassen RA. Osteoid-osteoma and osteoblastoma of the spine. J Bone Joint Surg. 1986;68A:354-361.
36 Chakrapani SD, Grim K, Kaimaktchiev V, Anderson JC. Osteoblastoma of the spine with discordant magnetic resonance imaging and computed tomography imaging in a child. Spine. 2008;33:E968-E970.
37 Ozaki T, Likjenqvist U, Hillmann A, et al. Osteoid osteoma and osteoblastoma: experiences with 22 patients. Clin Orthop. 2002;397:394-402.
38 Cove JA, Taminiau AH, Obermann WR, et al. Osteoid osteoma of the spine treated with percutaneous computed tomography-guided thermocoagulation. Spine. 2000;25:1283-1286.
39 Moore T, McLain RF. Image-Guided Surgery in Resection of Benign Cervicothoracic Spinal Tumors. The Spine Journal. 2005;5:109-114.
40 Nishida K, Doita M, Kawahara N, et al. Total en-bloc spondylectomy in the treatment of aggressive osteoblastoma of the thoracic spine. Orthopedics. 2008;31:403.
41 Burch S, Hu S, Berven S. Aneurysmal bone cysts of the spine. Neurosurg Clin N Am. 2008;19:41-47.
42 Liu JK, Brockmeyer DL, Dailey AT, Schmidt MH. Surgical management of aneurismal bone cysts of the spine. Neurosurg Focus. 2003;15:E4.
43 Codd PJ, Riesenburger RI, Klimo PJr, et al. Vertebra plana due to an aneurismal bone cyst of the lumbar spine. Case report and review of the literature. J Neurosurg. 2006;105:S490-S495.
44 Papagelopoulos PJ, Currier BL, Shanghnessy WJ, et al. Aneurysmal bone cyst of the spine: Management and outcome. Spine. 1998;23:621-628.
45 Rossi G, Rimondi E, Bartalena T, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2 cyanoacrylate. Skeletal Radiol. 2010;39:161-167.
46 Perlmutter DH, Campbell S, Rubery PT, et al. Aneurysmal bone cyst: surgical management in the pediatric cervical spine. Spine. 2009;34:E50-E53.
47 Friedman DP. Symptomatic vertebral hemangiomas: MR findings. AJR. 1996;167:359-364.
48 Lakemeier S, Westhoff CC, Fuchs-Winkelmann S, et al. Osseous hemangioma of the seventh cervical vertebra with osteoid formation mimicking metastasis: a case report. J Med Case Reports. 2009;3:92.
49 Bas T, Aparisi F, Bas JL. Efficacy and safety of ethanol injections in 18 cases of vertebral hemangioma: a mean follow-up of 2 years. Spine. 2001;26:1577-1582.
50 Aksu G, Fayda M, Saynak M, Karadeniz A. Spinal cord compression due to vertebral hemangioma. Orthopedics. 2008;31:169.
51 Guarnieri G, Ambrosanio G, Vassallo P, et al. Vertebroplasty as a treatment of aggressive and symptomatic vertebral hemangiomas: up to 4 years of follow-up. Neuroradiology. 2009;51:471-476.
52 Hart RA, Boriani S, Biagini R, et al. A system for surgical staging and management of spine tumors: a clinical outcome study of giant cell tumors of the spine. Spine. 1997;22:1773-1783.
53 Malone S, O’Sullivan B, Catton C, et al. Long term follow-up of efficacy and safety of megavoltage radiotherapy in high-risk giant cell tumors of bone. Int J Radiat Oncol Biol Phys. 1995;33:689-694.
54 Chakravarti A, Spiro IJ, Hug EB, et al. Megavoltage radiation therapy for axial and inoperable giant-cell tumor of bone. J Bone Joint Surg. 1999;81(A):1566-1573.
55 Stener B, Johnsen OE. Complete removal of three vertebrae for giant cell tumour. J Bone Joint Surg. 1971;53B:278-287.
56 Junming M, Cheng Y, Dong C, et al. Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine. 2008;33:280-288.
57 Denaro L, Longo UG, Papalia R, et al. Eosinophilic granuloma of the pediatric cervical spine. Spine. 2008;33:E936-E941.
58 Nakamura H, Nagayama R. Eosinophilic granuloma presenting with local osteolysis in an adult lumbar spine. J Clin Neurosci. 2008;15:1398-1400.
59 Baghaie M, Gillet P, Dondelinger RF, et al. Vertebra plana: benign or malignant lesion? Pediatr Radiol. 1996;26:431-433.
60 Nesbit ME, Kieffer S, D’Angelo GJ. Reconstitution of vertebral height in histiocytosis X: a long-term follow-up. J Bone Joint Surg. 1969;51A:1360-1368.
61 Grubb MR, Currier BL, Pritchard J, et al. Primary Ewing’s sarcoma of the spine. Spine. 1994;19:309-313.
62 Barwick KW, Huvos AG, Smith J. Primary osteogenic sarcoma of the vertebral column. Cancer. 1980;45:595-604.
63 Spiegel DA, Richardson WJ, Scully SP, et al. Long-term survival following total sacrectomy with reconstruction for the treatment of primary osteosarcoma of the sacrum. A case report. J Bone Joint Surg. 1999;81(A):848-855.
64 Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069-1077.
65 Sundaresan N, Rosen G, Huvos AG, et al. Combined treatment of osteosarcoma of the spine. Neurosurgery. 1988;23:714-719.
66 Sciubba DM, Okuno SH, Dekutoski MG, Gokaslan ZL. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine. 2009;34(22 Suppl):S-58-68.
67 Grubb Mr, Currier BL, Pritchard J, et al. Primary Ewing’s sarcoma of the spine. Spine. 1994;19:309-313.
68 Poulsen JO, Jensen JT, Tommesen P. Ewing’s sarcoma simulating vertebra plana. Acta Orthop Scand. 1975;46:211-215.
69 Hadfield MG, Zuezado MM, Williams RL, et al. Ewing’s family of tumors involving structures related to the central nervous system: a review. Pediatric Develop Pathol. 2000;3:203-210.
70 Bacci G, Boriani S, Balladelli A, et al. Treatment of nonmetastatic Ewing’s sarcoma family tumors of the spine and sacrum: the experience from a single institution. Eur Spine J. 2009;18:1091-1095.
71 Yamaguchi T, Yamato M, Saotome K. First histologically confirmed case of a classic chordoma arising in a precursor benign notochordal lesion: differential diagnosis of benign and malignant notochordal lesions. Skeletal Radiology. 2002;31:413-418.
72 Boriani S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the sacrum: Treatment and outcome in 21 cases. Spine. 1996;21:1569-1577.
73 Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122-2134.
74 Schwab JH, Healey JH, Rose P, et al. The surgical management of sacral chordomas. Spine. 2009;34:2700-2704.
75 Newman CB, Keshavarzi S, Aryan HE. En bloc sacretomy and reconstruction: technique modification for pelvic fixation. Surg Neurol. 2009;72:752-756. discussion, 756
76 Shives TC, McLeod RA, Unni KK, et al. Chondrosarcoma of the spine. J Bone Joint Surg. 1989;71(A):1158-1165.
77 Hermann G, Sacher M, Lanzieri CF, et al. Chrondrosarcoma of the spine: an unusual radiographic presentation. Skeletal Radiol. 1985;14:178-183.
78 Stener B. Total spondylectomy in chondrosarcoma arising from the seventh thoracic vertebra. J Bone Joint Surg. 1971;53B:288-295.
79 Boriani S, De lure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine. 2000;25:804-812.
80 Valderrama JAF, Bullough PG. Solitary myeloma of the spine. J Bone Joint Surg. 1988;50B:82-90.
81 McLain RF, Weinstein JN. Solitary plasmacytomas of the spine: a review of 84 cases. J Spinal Disord. 1989;2:69-74.
82 Ching HY, Horsman JM, Radstone CR, et al. Non-Hodgkin’s lymphoma presenting with spinal involvement: the Sheffiels Lymphoma Group experience (1970-2000). Int J Oncol. 2001;19:149-156.
83 Batson OV. The role of the vertebral veins in metastatic processes. Ann Intern Med. 1942;16:38-45.
84 Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578-583.
85 Clohisy DR, Perkins SL, Ramnaraine ML. Review of cellular mechanisms of tumor Osteolysis. Clin Orthop. 2000;373:104-114.
86 Helweg-Larsen S, Sorensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46:1163-1169.
87 Resche I, Chatal J-F, Pecking A, et al. A dose-controlled study of 153 Sm-ethylenediaminetetramethylene-phosphonate (EDTMP) in the treatment of patients with painful bone metastasis. Eur J Cancer. 1997;33:1583-1591.
88 Dudeney S, Lieberman IH, Reinhardt MK, et al. Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol. 2002;20:2382-2387.
89 Bilsky MH, Shannon FJ, Sheppard S, et al. Diagnosis and management of a metastatic tumor in the atlantoaxial spine. Spine. 2002;27:1062-1069.
90 Jackson RJ, Gokaslan ZL, Loh SC. Metastatic renal cell carcinoma of the spine: surgical treatment and results. J Neurosurg. 2001;94(1 Suppl):18-24.
91 Sundaresan N, Rothman A, Manhart K, et al. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine. 2002;27:1802-1806.
92 Beer SJ, Menezes AH. Primary tumors of the spine in children: natural history, management and long-term follow-up. Spine. 1997;22:649-659.
93 Fraser RD, Paterson DC, Simpson DA. Orthopaedic aspects of spinal tumours in children. J Bone Joint Surg. 1977;59B:143-151.
94 Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease in the pediatric population. J Pediatr Orthop. 1985;5:261-267.
95 Rogalsky RJ, Black GB, Reed MH. Orthopaedic manifestations of leukemia in children. J Bone Joint Surg. 1986;68A:494-501.
96 Enneking WF, Spanier SS, Goodmann MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop. 1980;153:106-120.
97 Weinstein JN. Surgical approach to spine tumors. Orthopaedics. 1989;12:897-905.
98 McAfee PC, Bohlman HH, Ducker T, et al. Failure of stabilization of the spine with methylmethacrylate. J Bone Joint Surg. 1986;68(A):1145-1157.
99 DeAndrade JR, MacNab I. Anterior occipitocervical fusion using an extra pharyngeal exposure. J Bone Joint Surg. 1969;51(A):1621.
100 Salas E, Sekhar LN, Ziyal IM, et al. Variations of the extreme-lateral craniocervical approach: anatomical and clinical analysis of 69 patients. J Neurosurg. 1999;90(4 Suppl):206-219.
101 Southwick WO, Robinson RA. Surgical approaches to vertebral bodies in the cervical and lumbar regions. J Bone Joint Surg. 1957;39(A):631.
102 McLain RF. Endoscopically assisted decompression for metastatic thoracic neoplasms. Spine. 1998;23:1130-1135.
103 Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599-609.
104 Kim M, Nolan P, Finkelstein JA. Evaluation of the 11thrib extrapleural-retroperitoneal approach to the thoracolumbar junction. Technical note. J Neurosurg. 2000;93(1 Suppl):168-174.
105 Muhlbauer M, Pfisterer W, Eyb R, et al. Minimally invasive retroperitoneal approach for lumbar corpectomy and anterior reconstruction. Technical note. J Neurosurg. 2000;93(1 Suppl):161-167.
106 Bilsky MH, Boland P, Lis E, et al. Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine. 2000;25:2240-2250.
107 Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg. 2001;94(2 Suppl):232-244.
108 Heary RF, Vaccaro AR, Benevenia J, et al. En bloc vertebrectomy in the mobile lumbar spine. Surg Neurol. 1998;50:548-556.
109 Krepler P, Windhager R, Bretschneider W, et al. Total vertebrectomy for primary malignant tumours of the spine. J Bone Joint Surg. 2002;84(B):712-715.
110 Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy: a new surgical technique for primary malignant vertebral tumors. Spine. 1997;22:324-333.
111 Talac R, Yaszemski MJ, Currier BL, et al. Relationship between surgical margins and local recurrence in sarcomas of the spine. Clin Orthop. 2002;397:127-132.
112 Abdel-Wanis MS, Kawahara N, Murata A, et al. Thyroid cancer spinal metastases: report on 25 operations in 14 patients. Anticancer Res. 2002;22:2509-2516.
113 Bohm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg. 2002;84(B):521-529.
114 Jackson RJ, Gokaslan ZL, Loh SC. Metastatic renal cell carcinoma of the spine: surgical treatment and results. J Neurosurg. 2001;94(1 Suppl):18-24.
115 Nakai S, Yoshizawa H, Kobayashi S, et al. Anorectal and bladder function after sacrifice of the sacral nerves. Spine. 2000;25:2234-2239.
116 Raque GHJr, Vitaz TW, Shields CB. Treatment of neoplastic diseases of the sacrum. J Surg Oncol. 2001;76(4 Suppl):301-307.
117 Constans JP, Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations: review of 600 Cases. J Neurosurg. 1983;59:111-118.
118 Tatsui H, Onomura T, Morishita S, et al. Survival rates of patients with metastatic spinal cancer after scintigraphic detection of abnormal radioactive accumulation. Spine. 1996;21:2143-2148.
119 Harrington KD. Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Ortho. 1988;233:177-197.
120 Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir. 1998;140:957-967.
121 Kostuik JP. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine, techniques, new methods of internal fixation, results. Spine. 1983;8:512-531.
122 Fourney DR, Abi-Said D, Lang FF, et al. Use of pedicle screw fixation in the management of malignant spinal disease: experience in 100 consecutive procedures. J Neurosurg. 2001;94(1 Suppl):25-37.
123 McLain RF, Sparling E, Benson DR. Failure of short segment pedicle instrumentation in thoracolumbar fractures: complications of Cotrel-Dubousset instrumentation. J Bone Joint Surg. 1993;75(A):162.
124 Jang JS, Lee SH, Rhee CH, et al. Polymethylmethacrylate-augmented screw fixation for stabilization in metastatic spinal tumors. Technical note. J Neurosurg. 2002;96(1 Suppl):131-134.
125 Miller DJ, Lang FF, Walsh GL, et al. Coaxial double-lumen methylmethacrylate reconstruction in the anterior cervical and upper thoracic spine after tumor resection. J Neurosurg. 2000;93(2 Suppl):181-190.
126 Akamaru T, Kawahara N, Tsuchiya H, et al. Healing of autologous bone in a titanium mesh cage used in anterior column reconstruction after total spondylectomy. Spine. 2002;27:E329-E333.
127 Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10(Suppl 2):S153-S157.
128 Hall DJ, Webb JK. Anterior plate fixation in spine tumor surgery. Indications, technique, and results. Spine. 1991;16:580-583.
129 Boriani S, Biagini R, Bandiera S, et al. Reconstruction of the anterior column of the thoracic and lumbar spine with a carbon fiber stackable cage system. Orthopedics. 2002;25:37-42.