Tumors of the Pancreas
David S. Klimstra
N. Volkan Adsay
Introduction
The diagnosis of pancreatic neoplasms requires differentiation of invasive ductal adenocarcinoma from its mimics and preoperative evaluation of cystic lesions. Solid, cellular pancreatic neoplasms present a different type of diagnostic challenge. The relative inaccessibility of the pancreas and the potential complications from substantial biopsies means that small tissue samples (i.e., core biopsies or fine-needle aspirations) are often the only specimens available for diagnosis.
Research has helped to clarify the diagnostic features of many pancreatic tumors, including cystic and intraductal neoplasms, neuroendocrine tumors, acinar cell neoplasms, and rare variants of ductal adenocarcinoma. An accurate diagnosis is usually possible, particularly when ancillary diagnostic and radiographic tests are used. The use of sensitive imaging techniques, such as spiral computed tomography (CT), endoscopic ultrasound, and magnetic resonance cholangiopancreatography (MRCP), has increased detection of less common types of pancreatic tumors. Advances in surgical techniques and improvements in postoperative care have rendered pancreatectomy a relatively commonplace operation, with markedly decreased rates of mortality and morbidity associated with pancreatic tumors and tumor-like lesions.
Classification
The past few decades have witnessed the characterization of several previously unrecognized types of pancreatic neoplasms and tumor-like lesions (e.g., autoimmune pancreatitis). The classification of pancreatic tumors and delineation of the mechanisms of pancreatic neoplasia changed somewhat with the publication of the 2010 World Health Organization (WHO) classification.1 The current classification, which is outlined in Box 40.1,1,2 is based on three features: the lines of cellular differentiation reflected in the neoplasm,3 the gross configuration of the tumor (i.e., solid, cystic, or intraductal), and for an important subgroup of potentially noninvasive neoplasms, the degree of dysplasia (i.e., low, intermediate, or high grade).
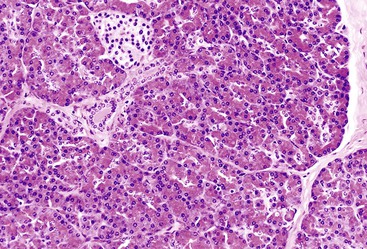
The line of differentiation refers to the cellular phenotype of the neoplasm.4 Most pancreatic tumors recapitulate one or more of the normal epithelial cell lines of the pancreas: ductal, acinar, or neuroendocrine (Fig. 40.1). The defining pathologic features of each of these normal cell types are reflected to various degrees in their corresponding neoplasms (Table 40.1).
Table 40.1
Characteristics of Pancreatic Epithelial Cells and Corresponding Neoplasms
| Cell Line | Light Microscopy | Histochemistry | Immunohistochemistry | Ultrastructure | Genetic Changes |
| Ductal | Glands, papillae, lumina, mucin | Mucin stains positive | Glycoprotein markers (TAG72, CEA, DUPAN2, MUC1, CA 19-9) | Mucigen granules | KRAS, TP53, SMAD4, CDKN2A |
| Acinar | Solid sheets, nests, acini; granular eosinophilic cytoplasm; prominent nucleoli | d-PAS–positive granules | Enzyme markers (trypsin, lipase, chymotrypsin) | Zymogen granules, irregular fibrillary granules | APC/β-catenin pathway |
| Neuroendocrine | Nested, trabecular, gyriform patterns; cytologic uniformity; salt-and-pepper chromatin | Argyrophil (grimelius)–positive granules | Neuroendocrine markers (chromogranin, synaptophysin, CD56) | Neurosecretory granules | MEN1 gene, DAXX and ATRX genes, mTOR pathway genes |
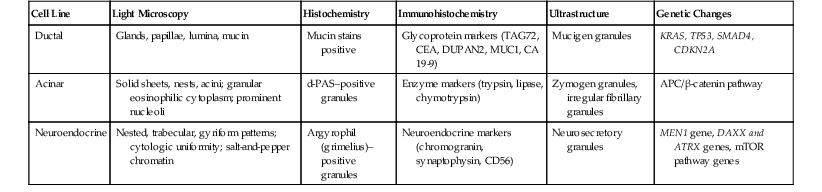
APC, Adenomatous polyposis coli protein that helps to control cell division; CA, cancer antigen; CDKN2A, formerly known as p16 or INK4A; CEA, carcinoembryonic antigen; d-PAS, periodic acid–Schiff (PAS) stain with diastase digestion; DUPAN2, monoclonal antibody to human pancreatic adenocarcinoma cells; mTOR, mammalian target of rapamycin cell signaling pathway; MUC1, cell surface–associated mucin 1 protein; SMAD4, formerly known as DPC4; TAG72, tumor-associated glycoprotein 72 (clone B72.3).
Ductal differentiation in pancreatic neoplasia is defined as recapitulation of the characteristics of normal ducts (i.e., gland or tubule formation and mucin production). Mucin can be demonstrated histochemically with stains, such as periodic acid–Schiff (PAS), mucicarmine, or high-iron diamine and Alcian blue, and it is regarded as a hallmark of ductal differentiation in the pancreas, although it is not invariably identified in ductal-type neoplasms.
Many immunohistochemical markers of ductal differentiation, such as cancer antigen (CA) 19-9, carcinoembryonic antigen (CEA), tumor-associated glycoprotein 72 (TAG72 [clone B72.3]), the gastrointestinal cancer–associated protein DUPAN2, and cell surface–associated mucin 1 protein (MUC1), detect mucin-related antigens or oncoproteins.5,6 Mutations at codon 12 of the KRAS gene are common (>90%) in ductal adenocarcinomas and in many related carcinomas and ductal-type preinvasive neoplasms. Because KRAS mutations are unusual in nonductal neoplasms, they may be considered evidence of ductal differentiation in certain situations. Expression of specific keratin subtypes, including cytokeratins 7 and 19 (CK7 and CK19), is characteristic of ductal neoplasms but is not lineage specific.
Neuroendocrine differentiation is defined as the production of peptide hormones or bioamines by tumor cells.3 Well-differentiated pancreatic neuroendocrine tumors (PanNETs) often have an organoid growth pattern and a characteristic appearance of the nuclear chromatin. Neuroendocrine differentiation is documented mainly by immunohistochemistry. The general neuroendocrine markers, chromogranin and synaptophysin, are considered the most specific. Other neuroendocrine markers include neuron-specific enolase (NSE), β-1,3-glucuronyltransferase 1 (B3GAT1, formerly called Leu-7 or CD57), and neural cell adhesion molecule 1 (NCAM1 or CD56), but their specificity has been questioned, and they are no longer recommended for the diagnostic confirmation of neuroendocrine differentiation.7 Production of specific peptides or bioamines may be demonstrable in certain PanNETs, but it is not necessary diagnostically. Electron microscopy may be used to identify dense-core secretory granules, but this technique has been largely supplanted by immunohistochemistry.
Despite the preponderance of acinar cells among the epithelial elements of the pancreas, pancreatic neoplasms with acinar differentiation are uncommon.3 In addition to having characteristic light microscopic features, acinar cell neoplasms produce zymogen granules containing pancreatic enzymes that can be detected by immunohistochemistry. Antibodies directed against trypsin and chymotrypsin are the most widely used, and lipase, elastase, and a few others can be detected.8 Zymogen granules can be demonstrated with the PAS stain in combination with diastase (d-PAS), which typically reveals small, positively staining cytoplasmic granules. Zymogen granules also can be visualized ultrastructurally. A second type, the irregular fibrillary granule, is considered a highly specific ultrastructural feature of acinar cell neoplasms.8
Many primary tumors of the pancreas have a characteristic radiographic and gross appearance. Most can be divided into primarily solid or primarily cystic categories (Table 40.2). Several types of tumors are inherently cystic, with each locule lined by neoplastic epithelial cells. Others develop a cystic change through a process of degeneration or necrosis, or both. This feature is characteristic of some entities, but it may also occur in typically solid tumor types. Intraductal tumors often appear cystic because of massive dilation of the native pancreatic ducts.
Table 40.2
Solid and Cystic Pancreatic Neoplasms
| Typically Solid Neoplasms | Typically Cystic Neoplasms |

Although most pancreatic tumors can be divided into frankly benign or malignant categories, the concept of borderline malignant potential was applied to certain families of pancreatic tumors that showed a spectrum of dysplasia,5–20 such as intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms that demonstrate intermediate-grade dysplasia. It was later recognized that most neoplasms in the borderline category (which lack invasive carcinoma) have a benign clinical course similar to that of tumors with low-grade dysplasia. The most recent Armed Forces Institute of Pathology (AFIP) fascicle on tumors of the pancreas and the 2010 WHO classification proposed eliminating the borderline category in favor of a system that uses the degree (grade) of dysplasia (see Box 40.1).1,2
Ductal Adenocarcinoma and Variants
The ductal system of the pancreas, which is responsible for carrying acinar secretions to the duodenum, is not the most abundant epithelial component of the organ. However, most pancreatic neoplasms (>90%) have a ductal origin, and 80% to 90% of these lesions are invasive ductal adenocarcinomas.9 The term pancreatic cancer is used synonymously with ductal adenocarcinoma, despite the fact that many other carcinoma types arise in this organ. This type of neoplasm is responsible for the dismal prognosis of pancreatic cancer, and the diagnosis of ductal adenocarcinoma remains problematic for pathologists.
Ductal-type pancreatic carcinomas are separated into three general categories:
Carcinomas in the third category often have an associated conventional ductal adenocarcinoma component, which provides evidence in favor of their ductal origin.
Invasive Ductal Adenocarcinoma
Clinical Features
Invasive ductal adenocarcinoma (i.e., conventional ductal adenocarcinoma, tubular adenocarcinoma) is the most common type of pancreatic neoplasm and is one of the most fatal of all human cancers. In the United States, there were 43,900 new cases and 37,400 deaths in 2012, and the number of new cases appears to have risen over the past decade. The age-adjusted incidence rate is 12.3 cases per 100,000 persons.10 Pancreatic cancer is the fourth leading cause of death from cancer.
Patients are usually between the ages of 60 and 80 years at diagnosis; occurrence in patients younger than 40 years is unusual.11 At clinical presentation, patients usually have jaundice (caused by invasion and obstruction of the common bile duct) when the carcinoma is in the head of the gland, or they may have nonspecific symptoms such as back pain and weight loss.12
The cause of ductal adenocarcinoma is complex and probably multifactorial. Smoking and high intake of dietary saturated fat are considered risk factors.13–16 Whether acquired chronic pancreatitis and diabetes mellitus constitute risk factors is a subject of ongoing debate, although patients with hereditary chronic pancreatitis and tropical calcifying pancreatitis are considered at increased risk.17–20
Most ductal adenocarcinomas are sporadic, but approximately 10% of pancreatic cancer cases are familial. The genetic basis for 80% of familial cases is unknown, but the risk is increased by heritable genetic syndromes such as hereditary breast cancer syndrome (resulting from mutations in BRCA2 or, less commonly, BRCA1), familial atypical multiple mole–melanoma (FAMMM) syndrome (resulting from mutations in CDKN2A [p16], a tumor suppressor gene), Peutz-Jeghers syndrome (resulting from STK11 [LKB1] mutations), familial pancreatitis (resulting from PRSS1 mutations), hereditary nonpolyposis colorectal cancer (HNPCC) syndrome (resulting from mutations in DNA mismatch repair genes), and Fanconi anemia (resulting from FANCC and FANCG mutations).21–28 Some families also carry germline mutations in PALB2 and ATM.29–31 IPMNs also appear to develop in patients with familial pancreatic carcinoma at an increased frequency.32,33
More than 75% of ductal adenocarcinomas are solid tumors, and 60% to 70% develop in the head of the pancreas.34 Possibly because of the lack of a capsule surrounding the pancreas, ductal adenocarcinomas typically involve surrounding structures, especially the common bile duct, duodenum, and peripancreatic soft tissues, even when the tumors are relatively small. Ductal adenocarcinomas that develop in the tail of the pancreas may spread to surrounding organs (i.e., spleen, kidney, stomach, and colon) when they are still relatively small.4–36 If a solid pancreatic tumor is larger than 5 cm in its greatest dimension and is still resectable, it is unlikely to represent a ductal adenocarcinoma.
Almost 80% of ductal adenocarcinomas are unresectable at the time of diagnosis,35 largely because of encasement of major mesenteric vessels36 or metastases to the liver, peritoneum, or other distant sites. Many ductal adenocarcinomas disseminate very early in the course of disease, although some data suggest that the subset of cases lacking mutations in SMAD4 (formerly DPC4) remains more localized to the pancreas and adjacent structures.37
Pathologic Features
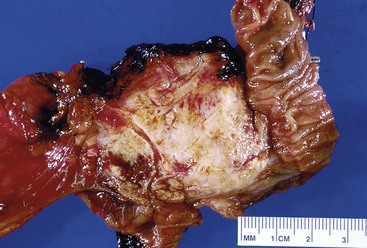
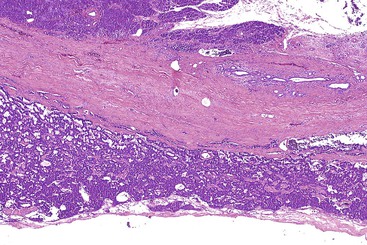
Grossly, most ductal adenocarcinomas are solid, firm, infiltrative tumors (Fig. 40.2) with ill-defined borders. The cut surface is often gritty or slightly gelatinous.38,39 Less commonly, gross areas of necrosis may be seen. It is often difficult to distinguish carcinoma from adjacent areas of fibrosing chronic pancreatitis.
For tumors in the head of the gland, direct invasion of the common bile duct and duodenum is common. The major pancreatic ducts coursing through the carcinoma are usually narrowed or even completely obliterated. Recognition that the center of the tumor is located in the head of the pancreas helps to distinguish pancreatic ductal adenocarcinomas from primary carcinomas of the common bile duct, duodenum, or ampulla of Vater. Some ductal adenocarcinomas exhibit cystic changes due to necrosis with cystic degeneration, as a result of cystic dilation of obstructed ducts (i.e., retention cyst formation), or because of cystic dilation of the neoplastic glands (i.e., large duct pattern).40 In some cases, invasive ductal adenocarcinomas may be associated with a preexisting benign cystic or intraductal neoplasm, such as a mucinous cystic neoplasm or an IPMN.
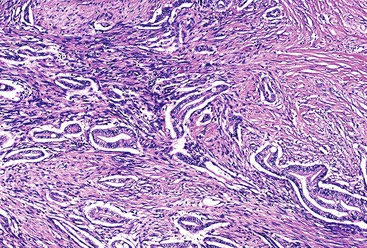
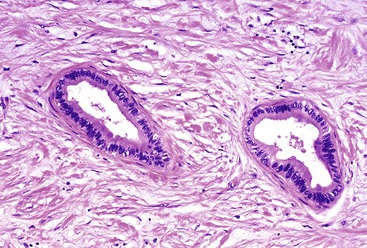
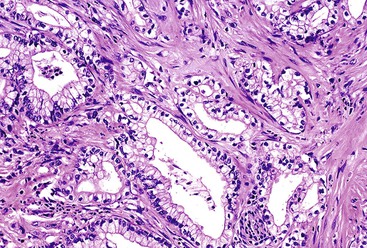
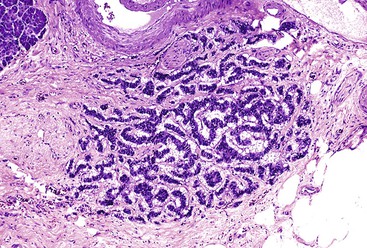
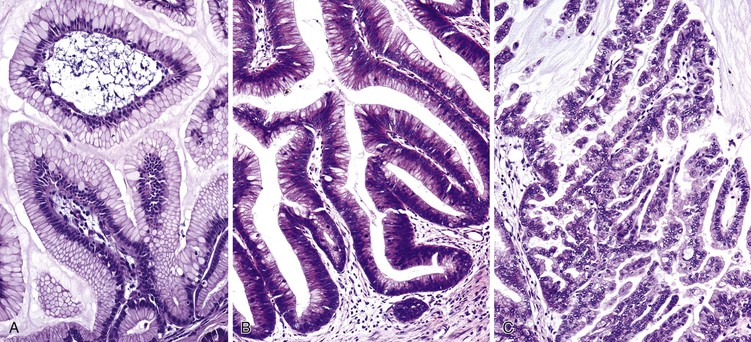
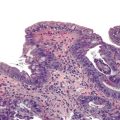
Microscopically, in its conventional form, ductal adenocarcinoma is characterized by a proliferation of small tubular structures lined by cuboidal mucinous cells in abundant desmoplastic stroma (Fig. 40.3).41 In well-differentiated ductal adenocarcinomas, the growth pattern and cytologic appearance of the cells may be deceptively benign, closely mimicking non-neoplastic ductules characteristic of chronic pancreatitis (Fig. 40.4). Malignant glands usually replace the normal lobular arrangement of benign ducts with haphazardly arranged tubules. The cells that line malignant glands typically form a single regular layer, but stratification and irregular papillae may be prominent in some cases.
The cytoplasm of the tumor cells may be abundant and usually contains mucin; clear cell change is also common. The nuclei may retain a basal orientation in the cells, but loss of polarity in some of the glands is typical. The nuclei vary in size, shape, and intracellular location between cells in each gland (Fig. 40.5). A variation in size of more than fourfold between adjacent nuclei suggests carcinoma.
Perineural invasion and vascular invasion are common and diagnostically useful, although these features often are not detected in core-needle biopsies. In some cases, tumor cells infiltrate adjacent normal islets. Invasion into peripancreatic adipose tissue is also common. The finding of an immediate juxtaposition of a gland with adipocytes without intervening stroma (i.e., naked glands in fat) is a strong sign of malignancy.42 Another helpful feature in the differential diagnosis of benign from malignant glands is the finding of glands situated adjacent to muscular blood vessels, because normal pancreatic ducts are usually separated from large blood vessels by a considerable amount of acinar parenchyma.43,44 However, this feature is less helpful when the pancreas is profoundly atrophic.
In poorly differentiated ductal adenocarcinomas, the neoplastic glands are usually admixed with small clusters of cells with ill-formed lumina, pleomorphic nuclei, and abundant mitotic figures (Fig. 40.6). It is common for ductal adenocarcinomas to exhibit a mixture of well-formed glands along with individual cells or solid clusters.
The periphery of ductal adenocarcinomas is often indistinct, and neoplastic glands may be found well beyond the apparent gross extent of the tumor. The finding of isolated glands in otherwise normal peripancreatic adipose tissue several millimeters from the nearest edge of the carcinoma confounds the evaluation of the soft tissue margins.42 Invasion of preexisting epithelial structures, such as the common bile duct, duodenal mucosa, blood vessels, or native pancreatic ducts, can result in colonization of the basement membrane of the invaded structure,45 which may simulate a preinvasive neoplasm, such as a duodenal adenoma or pancreatic intraepithelial neoplasia (PanIN). Continuity of intraductal neoplastic cells with frankly invasive elements supports an interpretation of colonization.
The current grading system for pancreatic ductal adenocarcinoma (i.e., Klöppel’s grading scheme endorsed by the WHO) entails evaluation of glandular differentiation, mucin production, mitoses, and nuclear atypia.46 This scheme correlates well with prognosis but is cumbersome and not widely used. Another proposal advocates assigning two scores to the two most prevalent patterns, mirroring Gleason grading for prostate carcinoma. This system is more independently significant in predicting outcome.47 The American Joint Committee on Cancer (AJCC) staging parameters for ductal adenocarcinoma are outlined in Table 40.3.48
Table 40.3
2010 American Joint Committee on Cancer Staging for Pancreatic Carcinoma
| Stage | Definition |
| Primary Tumor | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ (PanIN-3) |
| T1 | Tumor limited to pancreas, 2 cm or less |
| T2 | Tumor limited to pancreas, more than 2 cm |
| T3 | Tumor extends beyond the pancreas, no involvement of celiac axis or superior mesenteric artery |
| T4 | Tumor involves the celiac axis or superior mesenteric artery |
| Regional Lymph Nodes | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| Distant Metastasis | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
PanIN-3, High-grade pancreatic intraepithelial neoplasia.
From Compton CC, Fritz AG, Greene FL, Trotti A, eds, for the American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010.
Chronic pancreatitis (i.e., interstitial fibrosis, atrophy, and modestly dense inflammation) is often seen in the pancreatic tissue adjacent to ductal adenocarcinomas. Differentiating chronic pancreatitis from carcinoma is discussed later (see Differential Diagnosis). The lining of native pancreatic ducts may show proliferative changes (i.e., PanINs). Squamous, transitional, intestinal, and, rarely, oncocytic49 metaplasia may be seen in the non-neoplastic ducts.50
Ancillary Diagnostic Tests and Molecular Properties
Mucin histochemistry and immunohistochemical markers of ductal differentiation (i.e., glycoproteins) invariably show at least focal positivity in typical ductal adenocarcinomas. Staining for CK7, CK8, CK18, and CK19 and epithelial membrane antigen (EMA) is usually positive. CK20 is detected in about 25% of cases, and its expression is usually less widespread and intense than that of CK7. The exception to this cytokeratin expression pattern is colloid carcinoma, in which CK20 expression is strong and diffuse (discussed later).51,52 Immunohistochemical markers of glycoproteins that are often detectable in ductal adenocarcinoma include CA 19-9, CEA, TAG72, CA 125, and DUPAN2. Of these, CEA, TAG72, and CA 125 are regarded as tumor-associated glycoproteins that are not significantly expressed in normal ductal cells and are expressed only to a limited degree in low-grade PanIN.53
Cell surface–associated mucin proteins are expressed in various degrees in different types of ductal neoplasms. Most conventional ductal adenocarcinomas express MUC1, MUC3, MUC4, and MUC5AC.51,54–57 In contrast, MUC2 is not expressed in ductal adenocarcinomas other than those with intestinal differentiation, such as colloid carcinoma. MUC6, a marker of pyloric gland differentiation, is expressed in 35% of ductal adenocarcinomas.58
Stains for chromogranin or synaptophysin may demonstrate a minor neuroendocrine cell component associated with the neoplastic glands. Some neuroendocrine cells are non-neoplastic islet cells entrapped in the tumor, because ductal adenocarcinomas have a propensity to invade islets. However, neoplastic neuroendocrine cells also occur in ductal adenocarcinomas.59,60
The molecular alterations in ductal carcinomas have been studied extensively in the past decade. A comprehensive genomic analysis of more than 20,000 genes disclosed that most cases have primarily point mutations, which average 63 per tumor.61 Twelve core signaling pathways were altered in 67% to 100% of the tumors. The most commonly mutated genes include KRAS, CDKN2A (formerly called p16, INK4A, or CDKN), TP53, and SMAD4.62,63 Mutations in codon 12 of the KRAS oncogene are detected in more than 90% of ductal adenocarcinomas, and TP53 mutation occurs in 50%. Most ductal adenocarcinomas also harbor abnormalities in CDKN2A because of mutation or hypermethylation of the promoter region of DNA.64 Loss of SMAD4 expression is found in 55% of invasive ductal adenocarcinomas.65,66 Although many other molecular abnormalities occur in ductal adenocarcinoma,64 this constellation of genetic changes is characteristic and distinguishes ductal adenocarcinomas from pancreatic neoplasms of other cell lineages.
Other pathways commonly altered include apoptosis, regulation of the G1/S phase transition, hedgehog signaling, DNA damage control, cell adhesion, integrin signaling, MAPK8 (JNK) signaling, control of invasion, GTPase signaling, transforming growth factor-β (TGF-β) signaling, and NOTCH signaling.61 Mutations in mismatch repair genes, BRCA2, STK11, BRAF, TGFBR2, and MAP2K4 are less common, but some of them underlie the hereditary cases of pancreatic ductal carcinoma.63
Analysis of gene expression has revealed other overexpressed molecules (e.g., fascin, mesothelin, claudin 4, S100AP, S100A6, S100P) in ductal adenocarcinomas. Some have been used as potential immunohistochemical markers to help distinguish reactive (non-neoplastic) glands from carcinoma.67–73 Strong immunoexpression of TP53 or complete loss of SMAD4 expression can be used to support a diagnosis of carcinoma, although both markers lack a high degree of sensitivity.
Differential Diagnosis
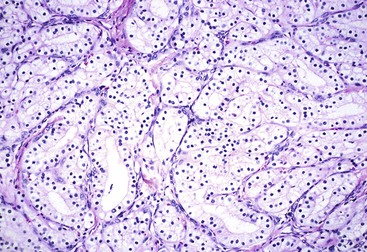
The main diagnostic consideration for conventional ductal adenocarcinoma is the distinction of a well-differentiated carcinoma from benign ductules in areas of atrophic chronic pancreatitis.2,74 Ductal adenocarcinoma may appear deceptively benign, and ductules in chronic pancreatitis may appear infiltrative.
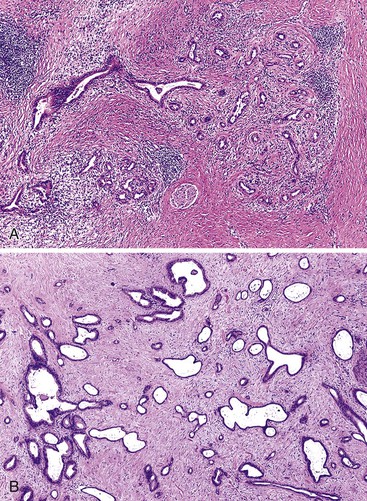
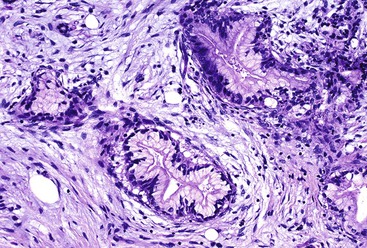
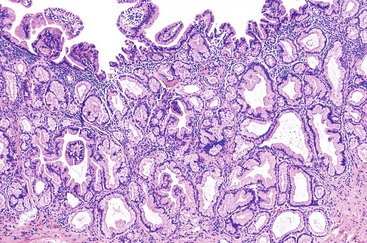
On low-power examination, ductules in areas of chronic pancreatitis retain the original lobular configuration of the normal pancreas, because they represent the residual intralobular ductules remaining after the loss of acinar elements during atrophy. Each lobular cluster of ductules contains a central, larger, branched, slightly dilated duct surrounded by small, round ductules (Fig. 40.7). The stroma in each lobule often is less dense than that between lobules. In contrast, invasive glands lack a lobular growth pattern and are usually distributed more haphazardly (see Fig. 40.7). The contours of individual glands also are usually irregular and angulated. Some glands may be incomplete, with epithelium lining only part of the neoplastic lumen and stroma lining the rest.
Cytologically, nuclear enlargement, nuclear contour irregularities, and loss of nuclear polarity are important clues to a diagnosis of carcinoma. Variations in size and shape and an intracellular location between adjacent nuclei in individual glands indicate malignancy. The finding of a nucleus-to-cytoplasm ratio of 4 : 1 is considered highly suspicious for carcinoma. Dense acidophilic cytoplasm is more common in carcinomas than in benign conditions.
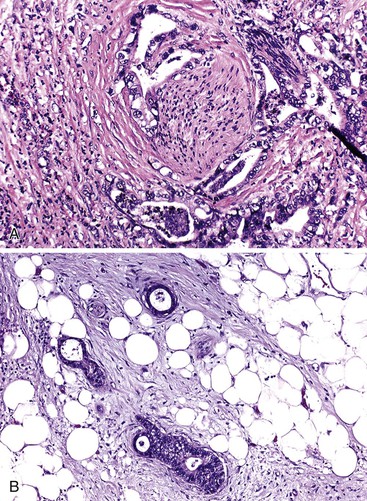
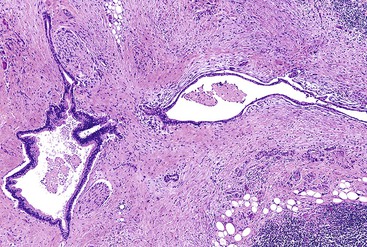
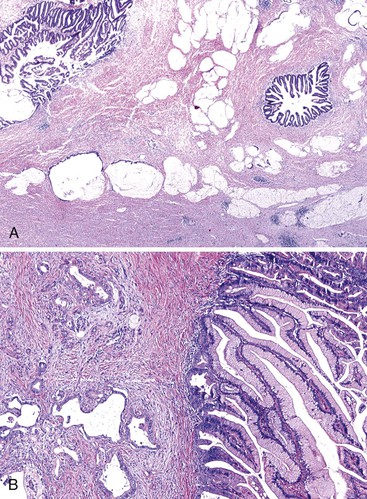
Unlike other sites where reactive stromal cells may display significant nuclear atypia, pleomorphism and bizarre nuclei are uncommon in the stroma of inflammatory conditions of the pancreas. Individual atypical cells in the stroma of the pancreas are much more likely to be cancer cells, especially if they contain dense acidophilic cytoplasm. The finding of atypical glands in abnormal locations is also helpful. For instance, perineural or vascular invasion, invasion of the duodenal muscularis propria, invasion of stroma closely adjacent to a muscular vessel,43,44 and immediate juxtaposition of glands to adipocytes42 are features considered to be almost 100% diagnostic of carcinoma (Fig. 40.8).
Invasive ductal adenocarcinoma should be differentiated from noninvasive PanIN, which can be challenging because high-grade PanIN displays most of the cytologic abnormalities of invasive carcinoma. Evaluation of the number, distribution, and location of the individual glandular units in relation to the lobular architecture helps to make this pathologic distinction. Desmoplastic stroma and irregularity of ductal contours are characteristic features of invasive carcinoma. Because pancreatic ducts do not contain a basal or myoepithelial cell layer and because many of the abnormalities of invasive carcinoma are also found in high-grade PanINs, there are no immunohistochemical stains that can reliably determine whether a specific atypical gland is invasive. When the differential diagnosis for a core-needle biopsy specimen includes high-grade PanIN and invasive carcinoma, it is worth considering that high-grade PanIN is rarely encountered in the absence of invasive carcinoma somewhere in the gland, especially when there is a pancreatic mass.
Another potential pitfall in the differential diagnosis of ductal adenocarcinoma is aggregation of islets in atrophic chronic pancreatitis (Fig. 40.9).75 Clustered islets may form small, irregular nests or cords of cells that have an infiltrative appearance. Occasionally, these islet cells are situated around small nerve fibers, simulating perineural invasion by carcinoma. However, the uniformity of cells, round nuclei, lack of glandular lumina, and neuroendocrine-type chromatin point to a benign reactive process. Immunolabeling for neuroendocrine markers (i.e., chromogranin and synaptophysin) can be helpful in problematic cases.
Adenocarcinomas of other organs can resemble pancreatic ductal adenocarcinoma, especially carcinomas arising in the biliary tree or upper gastrointestinal tract. Metastases to the pancreas from sites such as the lung, breast, or gynecologic organs can be mistaken for pancreatic primaries, but more commonly, a pancreatic primary is considered for metastases to the peritoneum, the liver, or other organs.
Although the previously described immunophenotype and molecular profile of pancreatic ducal adenocarcinoma is characteristic, it is not sufficiently specific to be useful to distinguish pancreatic primaries from adenocarcinomas of other sites. Coexpression of CK7, CK19, CEA, CA 19-9, TAG72 and CA 125 can occur in multiple primary adenocarcinomas, and the lack of expression of lung markers (e.g., thyroid transcription factor 1 [TTF-1], napsin A) or hormone receptors does not exclude origin in the lung, breast, or gynecologic tract. Clinical and radiographic correlation is often needed to define the primary site in these cases.
Natural History
The prognosis of patients with pancreatic ductal adenocarcinoma is dismal.76 The overall 5-year survival rate is less than 5%, and the median survival time after diagnosis is approximately 9 months.
Surgical resection is considered the best hope for long-term survival.77 However, only approximately 20% of ductal adenocarcinomas are resectable at the time of diagnosis; the remainder are locally advanced (mostly invading neighboring large mesenteric vessels) or metastatic (usually to the liver or peritoneum). In addition to high-resolution CT scanning, laparoscopy is increasingly used to identify radiographically occult metastatic disease before laparotomy.35
Postresection survival has not improved significantly during the past several decades.78 The median survival time of patients after resection is 12 to 18 months, with a reported 5-year survival rate as high as 20%, although patients may still die of their disease after 5 years.79. The survival rate beyond 8 years is approximately 3%. Some authorities advocate adjuvant therapy (i.e., chemotherapy and radiation) after surgery. Cases that are unresectable are usually treated with chemotherapy. Current chemotherapy protocols include mainly gemcitabine or 5-fluorouracil, often in combination with other agents.80
Morphologic Patterns of Ductal Adenocarcinoma
In addition to the conventional tubular pattern, ductal adenocarcinomas of the pancreas include several morphologic variations. The clinical and biologic characteristics of these patterns are not significantly different from those of conventional ductal adenocarcinoma, but the morphologic features are distinctive and may raise differential diagnostic issues. These patterns are often found focally in otherwise typical ductal adenocarcinomas.
Foamy Gland Pattern
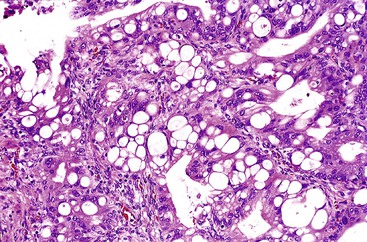
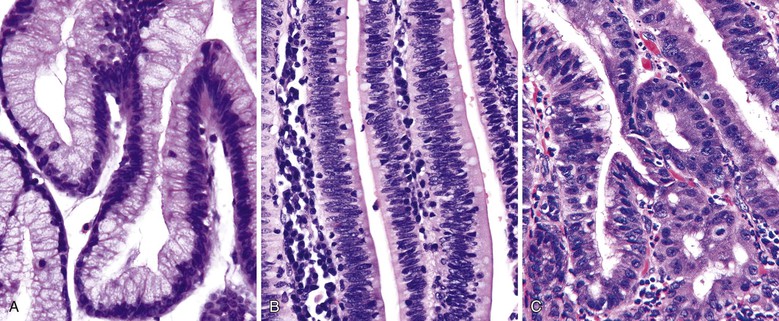
The foamy gland pattern represents a subtle, well-differentiated carcinoma that mimics benign glands with low-grade PanIN.81 This variant is characterized by infiltrating, well-formed glands lined by columnar cells that contain abundant, pale cytoplasm, resembling PanIN-1. The nuclei are typically well polarized but wrinkled in appearance, with indentations caused by the cytoplasmic contents (Fig. 40.10).
The most characteristic features that help to distinguish this variant from low-grade PanIN are a foamy, microvesicular, pale cytoplasm with vesicles that are small, fine, and evenly sized and an apical chromophilic condensation of the cytoplasm that forms a thin, well-delineated band reminiscent of a brush border. Although the apical condensation is strongly positive for mucin markers, the microvesicular component of the cytoplasm is typically negative (in contrast to ducts involved by PanIN-1 that are often PAS positive). This staining pattern parallels that of small, nonmucinous, intralobular ducts, which contain apical mucin granules that do not form a distinct band, but rather demonstrate a gradual acquisition. The tumor protein TP53 is often abnormally expressed in the nuclei of these malignant glands.
Large Duct Pattern
Occasionally, invasive tubular adenocarcinomas have a microcystic appearance because of ectasia of infiltrating neoplastic glands (Fig. 40.11).82 For unclear reasons, this phenomenon may be particularly pronounced in regions of the tumor that infiltrate the muscularis propria of the duodenum. Although microcystic glands may be detected grossly, the cystic change is usually not pronounced enough to be detected radiographically. The cytologic appearance of the epithelium may be deceptively bland.
Patients with tumors that have a large duct pattern have only a slightly higher survival rate than patients with conventional ductal adenocarcinoma. Cystic dilation of an invasive gland should be distinguished from a noninvasive component of a mucinous cystic neoplasm or intraductal neoplasm, both of which have a much better prognosis.
Features that favor large, duct–type adenocarcinomas are clustering of glands, irregularity of the contour of the ducts, and a desmoplastic stroma. Tumor cells often contain intraluminal necrotic debris with neutrophils.
Vacuolated Pattern
Lesions with a vacuolated pattern have a gland-in-gland architecture in which tumor cells form cribriform nests punctuated by many large vacuoles (i.e., microcysts) that contain cellular debris and mucin (Fig. 40.12). The vacuoles appear to form by the merging of multiple intracytoplasmic lumina. Focally, vacuolated cells have the morphologic features of adipocytes or signet ring cells.83 This pattern may be helpful in the differential diagnosis of metastatic tumors of unknown origin because it is seen only rarely in other types of adenocarcinoma.
Lobular Carcinoma–like Pattern
Occasionally, ductal adenocarcinoma may display a pattern of growth similar to that of mammary lobular carcinoma.84,85 Instead of forming tubules, the tumor cells form cords and may have a single-file arrangement (Fig. 40.13). Targetoid patterns and individual cell infiltration, often with signet ring cell formation, may be seen.86 This growth pattern may mimic diffuse-type gastric adenocarcinoma and is often associated with conventional tubular-type ductal adenocarcinoma elsewhere in the tumor.
Solid Nested Pattern
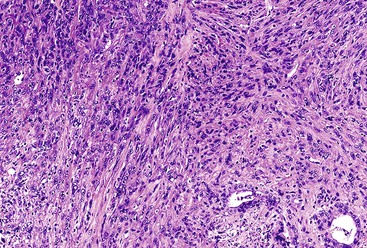
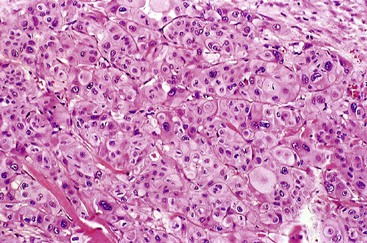
Poorly differentiated pancreatic ductal adenocarcinomas may infiltrate in a nested pattern without prominent gland formation, mimicking a neuroendocrine neoplasm or squamous cell carcinoma (Fig. 40.14). However, most of these cases contain foci of typical ductal adenocarcinoma.
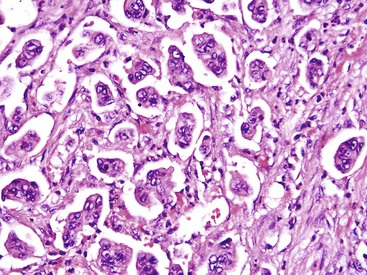
In some cases, the cells display abundant acidophilic cytoplasm and single, prominent nucleoli. This pattern creates a picture reminiscent of a hepatocellular carcinoma (i.e., hepatoid pattern) or an oncocytic neoplasm.87,88 However, the immunohistochemical staining pattern is different from that of hepatocellular carcinoma. In some cases, cells with a nested pattern have clear cytoplasm, resembling renal cell carcinoma. Some authorities refer to this variant as clear cell carcinoma.89,90
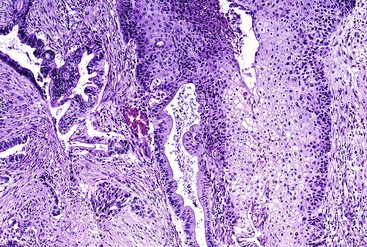
Micropapillary Pattern
Some poorly differentiated ductal adenocarcinomas exhibit a micropapillary pattern, with solid clusters of cells suspended in lacunar spaces (Fig. 40.15). Neutrophils infiltrate regions in a micropapillary pattern.91 As in other anatomic locations, pancreatic carcinomas with a micropapillary pattern are particularly aggressive.
Other Pancreatic Carcinomas of Ductal Origin
Colloid Carcinoma
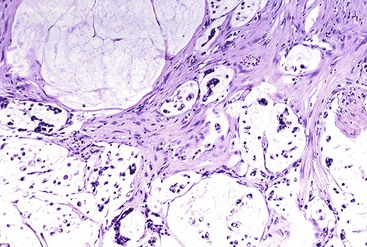
Colloid carcinoma is a type of pancreatic ductal carcinoma clinically and biologically distinct from the others discussed earlier.92 Whereas those tumor types are clinically aggressive, colloid carcinomas have a more protracted clinical course. Colloid carcinoma (i.e., mucinous noncystic carcinoma) is characterized by stromal pools of mucin-containing, scant, malignant epithelial cells arranged as strips; stellate or cribriform clusters; small, round tubules; or individual signet ring cells (Fig. 40.16). Colloid carcinomas are usually associated with an IPMN or a mucinous cystic neoplasm.93
Data suggest that colloid carcinomas have a more favorable clinical course than conventional, ductal-type invasive carcinomas. In one report, the 5-year survival rate for resected cases of colloid carcinoma was 55%, compared with 12% to 15% for conventional ductal adenocarcinomas.92 Some patients died of thromboembolic complications. In the same study, all patients who died had a history of an incisional biopsy, which raises the possibility that disruption of the integrity of the tumor may cause dissemination of these highly mucinous lesions.
Colloid carcinomas share intestinal differentiation (i.e., expression of CK20, MUC2, and CDX2) with intestinal-type IPMNs.94 Although these tumors harbor molecular abnormalities similar to those of conventional ductal adenocarcinoma (i.e., mutations in KRAS and TP53), the defects occur less frequently in colloid carcinomas. Loss of SMAD4 expression never occurs in colloid carcinomas.92,95
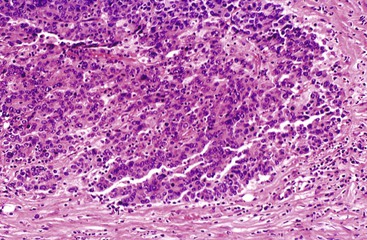
Medullary Carcinoma
Similar to medullary carcinomas in the breast and large intestine, medullary carcinoma of the pancreas is defined as a tumor with a syncytial pattern of growth of poorly differentiated epithelial cells with pushing borders, which is mostly devoid of a desmoplastic reaction but often accompanied by an inflammatory infiltrate (Fig. 40.17).96,97 Experience with these tumors is limited, and their natural history and prognosis are poorly understood. However, they do not appear to be significantly different from conventional ductal adenocarcinomas.
In contrast to conventional ductal adenocarcinoma, some medullary carcinomas are associated with genetic alterations commonly seen in medullary carcinomas of the colon (i.e., microsatellite instability), and the rate of KRAS mutations is very low.98 Some patients have a personal or family history of colon cancer, which raises the possibility of an associated inherited cancer syndrome.
Squamous Cell Carcinoma and Adenosquamous Carcinoma
Squamous cell and adenosquamous carcinomas constitute approximately 2% of all pancreatic cancers.99–102 They are more prevalent in the tail of the pancreas.34 The clinical outcome of these tumors is similar to that of conventional ductal adenocarcinoma or possibly worse.
Some cases have the appearance of an adenoacanthoma (i.e., adenocarcinoma with benign-appearing squamous elements), whereas others may have a combination of adenocarcinoma with a well-differentiated keratinizing squamous cell carcinoma, poorly differentiated squamous carcinoma without keratinization, or basaloid squamous cell carcinoma. Because small foci of squamous differentiation may occur in ductal adenocarcinomas, an arbitrary squamous component of 30% has been proposed for a carcinoma to be regarded as adenosquamous.2 Pure squamous cell carcinoma (without a glandular component) is rare. Most cases contain a glandular component (e.g., adenosquamous carcinoma) on careful histologic examination (Fig. 40.18), and any amount of glandular differentiation is sufficient for a predominantly squamous carcinoma to be regarded as adenosquamous.
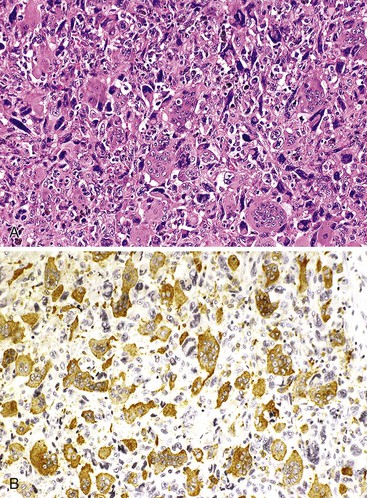
Hepatoid Carcinoma
Rarely, pancreatic carcinomas may have bona fide hepatocellular differentiation. Hepatoid carcinomas have a solid, nested, or trabecular architecture and are composed of polygonal cells with granular eosinophilic cytoplasm, centrally located nuclei, and prominent nucleoli.103–106 Bile pigment may be identified.
Staining for hepatocellular differentiation is typically positive, including hepatocyte paraffin 1 (HepPar1), polyclonal CEA (canalicular), and CD10 (canalicular). α-Fetoprotein (AFP) also may be expressed in these tumors. The differential diagnosis of hepatoid carcinoma includes acinar cell carcinoma and pancreatoblastoma, both of which can express AFP,107 and intraductal oncocytic papillary neoplasm, which consistently stains for HepPar1 but is not thought to possess true hepatocellular differentiation.108
Undifferentiated Carcinoma
Similar to other organs, the pancreas may give rise to a family of neoplasms that show little or no morphologic evidence of epithelial differentiation. In theory, undifferentiated carcinomas may arise from any of the epithelial cell lines in the pancreas. However, most undifferentiated carcinomas are thought to represent ductal neoplasms. In some instances, evidence for a ductal origin stems from the finding of a ductal adenocarcinoma component in an otherwise undifferentiated carcinoma.109 Oncogenic mutations in genes typical of ductal carcinomas (e.g., KRAS) have been identified in some undifferentiated carcinomas.96,110
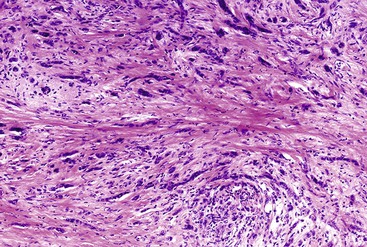
The histologic types of undifferentiated carcinomas include spindle cell (sarcomatoid) carcinoma, carcinosarcoma, anaplastic giant cell carcinoma, and undifferentiated carcinoma with osteoclast-like giant cells.111 The histologic features of the first three of these tumors overlap; the sarcomatoid components consist of anaplastic giant cells or spindle cells (Fig. 40.19) and sometimes contain heterologous stromal differentiation (e.g., bone, cartilage, and skeletal muscle).112 The designation carcinosarcoma is used when a tumor contains a separate glandular component, which results in a biphasic appearance. In some cases, the undifferentiated elements retain immunohistochemical positivity for epithelial markers, such as keratin or EMA. Others lack evidence of epithelial differentiation even at the immunohistochemical and ultrastructural levels. If these tumors lack an associated glandular component, they are indistinguishable from undifferentiated sarcoma. Cases with differentiation along a definable mesenchymal line may be classified as primary sarcomas of the pancreas, even though they may have originated from an epithelial precursor.
With the possible exception of undifferentiated carcinoma with osteoclast-like giant cells, the prognosis for undifferentiated carcinomas is poor, possibly worse than that for conventional ductal adenocarcinoma.
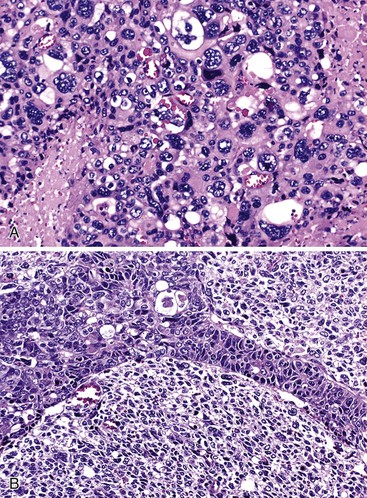
Undifferentiated Carcinoma with Osteoclast-like Giant Cells
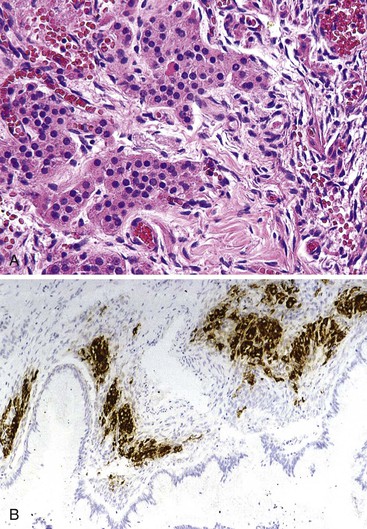
Undifferentiated carcinoma with osteoclast-like giant cells shares histologic features with certain poorly differentiated neoplasms of other epithelial organs, sarcomas, and melanoma. In all these situations, undifferentiated neoplasms contain a combination of neoplastic, plump spindle cells and non-neoplastic osteoclast-like giant cells.113–116 In the pancreas, many undifferentiated carcinomas with osteoclast-like giant cells contain a separate glandular component or are associated with a preinvasive neoplasm, such as a mucinous cystic neoplasm109,117 or PanIN. The neoplastic elements are usually moderately to markedly atypical, noncohesive, and somewhat epithelioid. Various numbers of osteoclast-like giant cells, with many nuclei showing only mild nuclear atypia (Fig. 40.20), are typically scattered randomly among the neoplastic elements. A component of mononuclear histiocytic cells can be identified in these tumors.118
Some cases also contain anaplastic giant tumor cells in addition to osteoclast-like giant cells. The osteoclast-like cells have phagocytic capability and may contain neoplastic cells engulfed in the cytoplasm. The giant cells have a histiocytic origin as demonstrated by positive immunohistochemical staining for CD68 (see Fig. 40.20, B) and other histiocytic markers, as are the mononuclear histiocytic cells.119,120 Anaplastic giant cells and mononuclear neoplastic cells are usually undifferentiated at the immunohistochemical level, and keratin expression often is restricted to only an associated glandular component. Oncogenic mutations in KRAS have been detected in the neoplastic elements of undifferentiated carcinomas with osteoclast-like giant cells.119,120
Some cases have a less aggressive clinical course than expected, but undifferentiated carcinomas with osteoclast-like giant cells are not an indolent type of neoplasm. Most patients die of the disease within 2 years of diagnosis.
Pancreatic Intraepithelial Neoplasia
Precursors of ductal adenocarcinoma have been identified as a series of increasingly atypical proliferative changes in the epithelium of pancreatic ducts. Many of these lesions have long been recognized but reported only with descriptive terminology.121–124 Early lesions—those with minimal cytologic atypia—were not originally regarded as neoplastic and instead were designated as hyperplasia or metaplasia. However, later molecular data established most ductal proliferative lesions as neoplastic.122 The spectrum of ductal proliferative lesions is now often referred to as PanIN122 and is graded on a three-tiered scale as PanIN-1 (with A and B subtypes), -2, and -3.
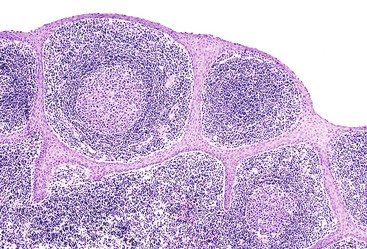
All PanIN lesions contain cytoplasmic mucin, which is not normally observed in pancreatic ductal cells without the use of histochemical staining, except occasionally in the largest interlobular ducts. PanIN-1A is a histologic change previously designated mucinous metaplasia or mucous cell hypertrophy (Fig. 40.21). Because some PanIN-1A lesions were eventually shown to harbor clonal mutations in the KRAS gene, they are now regarded as an early neoplastic change.125,126 Lesions that reveal tall columnar mucinous cells with well-polarized nuclei lacking atypia, loss of polarity, or papillary or micropapillary formations are PanIN-1As. PanIN-1B is characterized by slight nuclear stratification at the basal aspect of the epithelium and by papillary or micropapillary projections (see Fig. 40.21, B).
Previously called atypical hyperplasia or moderate dysplasia, PanIN-2 is characterized by prominent nuclear stratification involving the full thickness of the epithelium that is associated with focal loss of polarity and mild nuclear atypia (see Fig. 40.21, C). PanIN-3, previously known as carcinoma in situ or severe dysplasia, shows a significant loss of polarity, tufting of cells into the lumen, marked irregularity of nuclei, increased mitotic figures, and sometimes necrosis (see Fig. 40.21, D).
Most of the molecular abnormalities identified in invasive ductal adenocarcinomas have also been detected in PanIN. Some molecular changes occur early in the sequence (e.g., KRAS mutations), some in the middle stages (loss of CDKN2A expression), and others only in the late stage (SMAD4 and TP53 mutations).125,127
PanIN is a relatively common incidental finding. Almost 50% of older adults have foci of PanIN-1 in their pancreas.128 Higher grades of PanIN are significantly more common in pancreata that contain invasive ductal carcinoma. Foci of PanIN found in association with other tumor types or in pancreata without tumors are typically low or intermediate grade.129 Because of the difficulty of detecting PanIN in specimens other than those from resection of the pancreas, the natural history of these lesions is essentially unknown. Rare cases have been documented in which a PanIN lesion was identified before the development of invasive carcinoma.130
The development of treatment recommendations has been challenging.131 PanIN-1 is commonly identified, and PanIN-2 is frequently an incidental finding. Emerging data suggest that both lesions have a very low risk of progression to carcinoma.132 Most investigators think that PanIN-1 has negligible clinical significance. It is probably prudent not to report PanIN-1 when it is detected in a pancreas resection specimen or at a resection margin in the absence of a higher-grade PanIN lesion or invasive carcinoma because of the risk of overtreatment.
Although it is difficult to determine their biologic significance, foci of PanIN-3 identified in a pancreas resection specimen should be reported, particularly in cases that lack invasive carcinoma. Ideally, detection of pancreatic neoplasia at the PanIN-3 stage can provide an opportunity for cure that is lost after the patient develops overt clinical symptoms. Efforts to screen patients for high-grade PanIN have been thwarted by the lack of serum biomarkers and reproducible radiographic findings, although in patients with a strong family history of pancreatic carcinoma, there may be subtle radiographic findings of localized lobular atrophy that herald the development of PanIN.33
PanIN should be distinguished from well-differentiated invasive ductal adenocarcinoma, which may be difficult to do on the basis of a small biopsy specimen in which the underlying ductal architecture may be difficult to recognize. PanIN shares many cytologic features with IPMNs. Although most IPMNs are larger than PanINs and involve cystically dilated ducts at least 1 cm in diameter, differentiating these two types of lesions may be impossible in some cases. PanINs and IPMNs may exist in the same pancreas.131–134
Cystic Tumors
Cystic tumors of the pancreas are less common than ductal adenocarcinomas, representing 5% to 10% of all pancreatic neoplasms (see Table 40.2), but they constitute an important subset, because many cystic tumors are benign or low-grade malignant neoplasms.135,136 Cystic lesions are detected more commonly because of the increased use of sensitive imaging techniques. Most true cystic neoplasms of the pancreas are serous cystadenomas or mucinous cystic neoplasms. However, a variety of less common cystic tumors also may occur in the pancreas. Intraductal neoplasms are typically cystic radiographically, and solid tumor can also develop secondary cystic changes.137
Serous Cystic Neoplasm
Clinical Features
Serous cystic neoplasms are the most common type of true cystic neoplasms of the pancreas, which means that the cysts are lined by a continuous layer of neoplastic epithelium rather than being degenerative or representing dilated pancreatic ducts.138–141 Because serous cystadenomas are usually microcystic, the term microcystic adenoma is a synonym, although a macrocystic (oligocystic) type of serous cystadenoma also occurs.142,143
Microcystic serous cystadenomas are composed of numerous small cysts (>1 mm to 1 cm in diameter) that ultimately form a large (up to ≤25 cm), well-delineated tumor mass. These cystadenomas arise mostly in the body or tail of the pancreas and occur predominantly in women (female-to-male ratio of 3 : 1). The mean age of patients at diagnosis is 66 years.
Cysts (often multiple) that appear histologically similar may develop in patients with von Hippel-Lindau syndrome,144–146 although most patients with microcystic serous cystadenomas have no other associated diseases. However, this association provides some insight into the molecular biology of the neoplasm. Other concurrent pancreatic tumors described in patients with microcystic serous cystadenomas include ductal adenocarcinoma147 and PanNETs.148
Pathologic Features
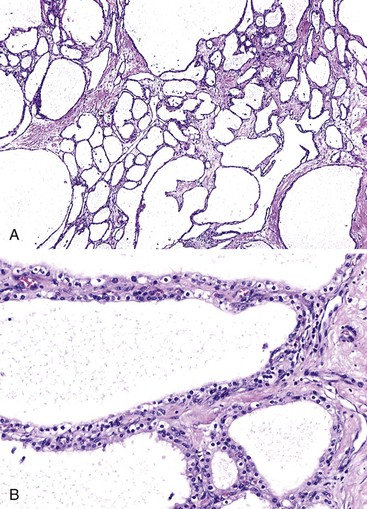
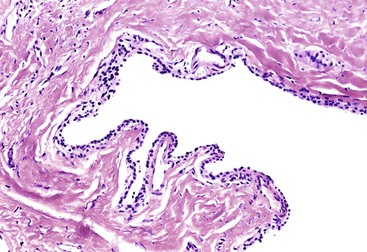
Grossly, microcystic serous cystadenomas are usually circumscribed and often contain a central, stellate, fibrous scar. The innumerable cysts produce a gross appearance resembling that of a sponge (Fig. 40.22). In some regions, the cysts may be so small that the tumor appears solid. Degenerative changes, including hemorrhage and macrocystic degeneration, may be prominent in some cases,149 mimicking the gross appearance of a pseudocyst.
Microscopically, the cysts of microcystic serous cystadenomas are lined by a single layer of flat to cuboidal cells with clear cytoplasm, well-defined cytoplasmic borders, and small, round nuclei with dense, homogeneous chromatin (Fig. 40.23). Cytoplasmic clearing results from accumulation of glycogen; mucin is not usually found in the tumors. In rare cases, the cells are more columnar and contain acidophilic, granular cytoplasm. Blunt papillary projections may be found focally, but well-formed or complex papillae are unusual. Underlying the epithelium is a continuous layer of capillary-sized vessels with plump endothelial cells.150 The stroma between cysts is hyalinized and may contain entrapped islets. These cytologic features are characteristic of microcystic serous cystadenomas and less common types of serous tumors of the pancreas, such as macrocystic serous cystadenoma and solid serous adenoma.151
Macrocystic serous cystadenomas occur more commonly in men than women. The boundaries of the tumor are often ill defined, the individual locules are 1 cm to several centimeters in diameter, and a central scar is usually absent (Fig. 40.24). Microscopically, the cyst lining is identical to that of the microcystic type, with glycogen-rich, clear cells arranged in a single, flat layer without nuclear atypia or mucin production (Fig. 40.25).
Solid serous adenomas show a complete absence of cystic change. This tumor type is composed of back-to-back, small glands with cytologic features that are otherwise typical of a serous neoplasm (Fig. 40.26).
Histologically and immunophenotypically, all types of serous neoplasms appear to recapitulate centroacinar cells.140 For instance, they express low-molecular-weight keratins (in addition to broad-spectrum keratins), EMA, inhibin, and MLANA (MART1).152,153 Human melanoma black 45 (HMB-45) staining is typically negative in these tumors. Staining results for ductal mucin markers (i.e., TAG72, MUC1, CA 19-9, and CEA) are negative or only focally positive, although MUC6 is usually positive.152 Molecular genetic alterations include mutations of the von Hippel-Lindau tumor suppressor gene (VHL on chromosome 3p25.3) and allelic losses on chromosome 10q.154,155 As is true of other neoplasms arising in patients with von Hippel-Lindau syndrome, serous neoplasms express carbonic anhydrase IX (CA9), hypoxia-inducible factor 1α (HIF-1α), vascular endothelial growth factor (VEGF), and SLC2A1 (previously called GLUT1).150 Overexpression of the glucose transporter SLC2A1 may help to explain the accumulation of cytoplasmic glycogen in serous tumors that causes the cytoplasmic clearing.156 Genetic abnormalities typical of ductal adenocarcinoma (e.g., mutations of KRAS and TP53) have not been identified in serous tumors nor have mutations in GNAS or RNF43, which are found in other cystic or intraductal pancreatic neoplasms.155,157
Differential Diagnosis
The differential diagnosis of serous neoplasms includes other cystic tumors of the pancreas (see Differential Diagnosis of Cystic and Intraductal Lesions). The clear cell nature of the cytoplasm raises the possibility of metastatic renal cell carcinoma, a tumor that may involve the pancreas as the sole site of metastasis, may reveal a cystic change, and is common in patients with von Hippel-Lindau syndrome. Solid and acinar regions and prominent nuclear atypia combined with a more pronounced sinusoidal vascular pattern favor a diagnosis of renal cell carcinoma. Lymphangiomas also resemble serous cystic neoplasms, particularly when the latter have an attenuated epithelial lining.158 Lymphangiomas typically occur in peripancreatic tissue rather than in the pancreas, and they contain lymphoid aggregates between the cystic spaces. In difficult cases, immunohistochemical staining can help to demonstrate the endothelial nature of the lining and the absence of keratin in lymphangiomas.
Natural History
Most serous tumors of the pancreas are benign,159,160 and serous cystadenocarcinoma of the pancreas is rare.161–163 Malignancy is defined as metastasis. Primary and metastatic serous cystadenocarcinomas usually exhibit the microscopic features of microcystic serous cystadenomas, with an absence of cytoarchitectural atypia or other morphologic findings suggesting malignancy. Some authorities have questioned the malignant potential of these rare cases and have suggested instead that they develop as a form of parasitic growth of a benign neoplasm or as a manifestation of multicentricity. In the absence of demonstrable metastases, any typical serous neoplasm should be considered benign. If a definitive diagnosis can be achieved preoperatively and the tumor is asymptomatic, treatment by clinical observation and follow-up is considered a viable option.164
Another type of malignancy has been reported for serous neoplasms. In rare cases, a cytologically obvious carcinoma may arise in a microcystic serous cystadenoma (i.e., carcinoma ex microcystic adenoma).165 The carcinomas in these cases are composed of nests or clusters of clear cells, and some have large, cytoplasmic vacuoles, producing a sievelike pattern. The nuclei are enlarged and atypical relative to the residual benign serous elements, and increased mitotic activity and necrosis may be seen. The biologic behavior of these rare cases has not been defined.165
Mucinous Cystic Neoplasm
Clinical Features
Similar to their serous counterparts, mucinous cystic neoplasms are true cystic neoplasms of the pancreas.166–169 In contrast to most serous cystadenomas, mucinous cystic neoplasms are consistently macrocystic, and when defined strictly, they have very distinctive clinicopathologic characteristics. They are seen almost exclusively in women (with rare examples documented in men) in the fifth to sixth decades of life (mean age, 50 years). The tumor usually is located in the tail of the pancreas. Presenting symptoms are nonspecific and are usually the result of the effects of an enlarging mass.
Pathologic Features
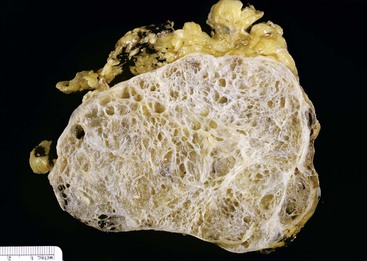
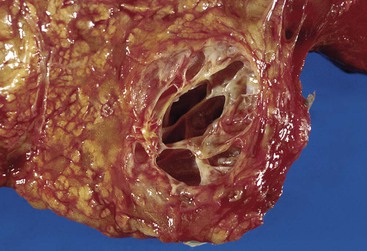
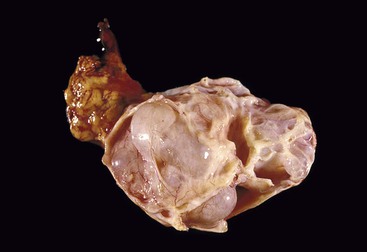
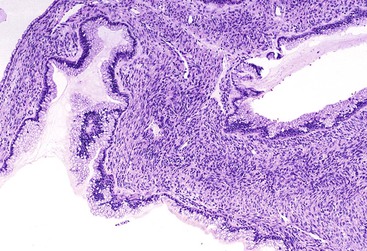
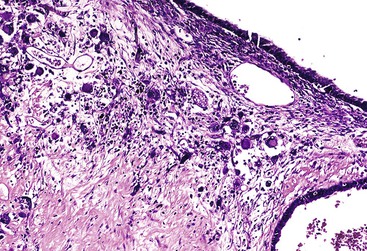
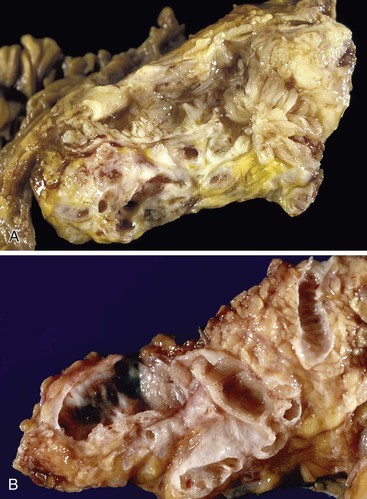
Macroscopically, mucinous cystic neoplasms are typically single, multilocular cysts surrounded by a thick, fibrotic capsule (Fig. 40.27). The mean diameter of most mucinous cystic neoplasms is greater than 10 cm. Unless there is fistula formation, these tumors do not communicate with the pancreatic ductal system. The septa between cysts are usually thin and may have velvety papillations; some appear trabeculated and thickened. The cyst contents are often mucoid, but some may have a watery consistency. Solid areas in the cysts or the peripheral capsule should be sampled extensively, because these areas may harbor an invasive carcinoma component. Degenerative changes with hemorrhage may occur, simulating the gross appearance of a pseudocyst.
Histologically, pancreatic mucinous cystic neoplasms are similar to ovarian mucinous neoplasms (Fig. 40.28). The lining epithelium consists of tall, columnar cells with abundant apical mucin, although cuboidal cells that lack obvious mucin may be seen in some cases or may predominate (Fig. 40.29). Examination sometimes reveals intestinal-type epithelium with goblet cells, but the villiform papillae resemble those of colonic villous adenomas, which are typical of intestinal-type IPMNs, are very unusual in mucinous cystic neoplasms.136 Neuroendocrine cells and Paneth cells may be detected. The epithelium may display a wide range of cytologic atypia. Some mucinous cystic neoplasms may be largely bland in appearance, containing mostly uniform, basally oriented nuclei and displaying minimal architectural complexity, whereas other tumors exhibit abundant papillary formations, pseudostratified hyperchromatic nuclei, and a cribriform architecture. Severe epithelial atypia may be focal, and numerous sections may be required to properly and thoroughly evaluate these neoplasms (Fig. 40.30).
The resemblance of pancreatic mucinous cystic neoplasms to ovarian tumors extends to a distinctive subepithelial, hypercellular, spindle cell stroma (i.e., ovarian-like stroma). This type of stroma is a diagnostic prerequisite for mucinous cystic neoplasms and is found at least focally in all bona fide examples of this neoplasm. Most contemporary authorities require it to establish a diagnosis of mucinous cystic neoplasm. In addition to spindle cell elements, the stroma often contains nests of epithelioid cells, which suggests luteinization (Fig. 40.31). Occasionally, large, circumscribed regions of stromal hyalinization may resemble corpora albicantia. The stromal cells frequently express estrogen and progesterone receptors, inhibin, and MLANA (recognized by the A103 monoclonal antibody); inhibin and MLANA are intensely expressed in epithelioid, lutea-like cells (see Fig. 40.31, B).169–171 A plausible explanation for ovarian-like stroma in mucinous cystic neoplasms has not been offered, but because the fetal pancreas contains similar hypercellular stroma surrounding developing pancreatic ducts, some authorities have suggested that the stroma of mucinous cystic neoplasms represents recapitulation of the fetal periductal mesenchyme.172
The epithelium of mucinous cystic neoplasms expresses keratin and glycoprotein markers, such as CEA and CA 19-9.167,169,173 MUC5AC is expressed diffusely, whereas the MUC2 antibody typically stains only scattered goblet cells.174 MUC1 usually is detected only in invasive carcinomas that arise in mucinous cystic neoplasms. Other than in goblet cells, intestinal differentiation is not commonly found. For instance, staining for CK20 and CDX2 is usually negative in these tumors.
Invasive carcinoma may develop in mucinous cystic neoplasms. This is a concern in cases with marked cellular atypia, because the invasive component may be focal and grossly inapparent. Solid areas of the tumor and the interface with adjacent tissues should be sampled generously and carefully. Invasive carcinoma arising in a mucinous cystic neoplasm usually resembles conventional pancreatic ductal adenocarcinoma. However, other types, such as sarcomatoid carcinoma, or undifferentiated carcinoma with osteoclast-like giant cells, may occur (Fig. 40.32). Although colloid carcinomas often are associated with IPMNs, they develop rarely in mucinous cystic neoplasms. Reports of apparent sarcomatous transformation of the cellular subepithelial stroma exist, although genetic analysis of these cases found that the sarcomatoid elements were of epithelial derivation and probably represented sarcomatoid carcinoma rather than true sarcoma.175–177
According to the 2007 Armed Forces Institute of Pathology (AFIP) fascicle on the pancreas and the 2010 WHO classification,1,2 the degree of dysplasia in mucinous cystic neoplasms should be graded as low, intermediate, or high based on the most severely dysplastic region, and the presence or absence of invasive carcinoma should be reported. Cases with low-grade dysplasia have minimal cytoarchitectural atypia of the lining epithelium. These tumors are also designated as mucinous cystadenoma, but extensive or complete tissue sectioning of the tumor should be performed to confirm this diagnosis. Mucinous cystic neoplasms with intermediate-grade dysplasia have a loss of nuclear polarity, moderate atypia, and mild architectural complexity. Mucinous cystic neoplasms with high-grade dysplasia (i.e., mucinous cystadenocarcinoma in situ) have marked cytologic atypia, mitotic figures, and larger, irregular, and hyperchromatic nuclei, usually accompanied by significant architectural complexity. Cases of mucinous cystic neoplasm with an associated invasive carcinoma are also called invasive mucinous cystadenocarcinoma.
The histologic type of invasive carcinoma should be reported, and the extent of intratumoral or extratumoral invasion should be determined. Unfortunately, the clinical relevance of this classification system is a matter of debate. Some investigators have reported recurrence or metastasis from tumors that lacked an obvious invasive carcinoma or significant cellular atypia.166,167 These investigators suggested that even benign-appearing mucinous cystadenomas have latent malignant potential. Others have suggested that rare metastasis from otherwise benign-appearing tumors may be explained on the basis of incomplete surgical removal or inadequate tissue sampling of the primary tumor and failure to identify an invasive component. Studies in which mucinous cystic neoplasms have been thoroughly sectioned have found no recurrences in cases that lack invasive carcinoma at the time of resection.169,178 Minimally invasive carcinoma confined to the stroma of the cyst septa is usually cured by surgery, provided the specimen has been thoroughly evaluated microscopically to ensure that the extent of invasive carcinoma is limited.179
DNA ploidy analysis may correlate with prognosis.180 Overall, the rate of malignancy in mucinous cystic neoplasms is approximately 10%.181 It has been suggested that invasive carcinomas that arise in mucinous cystic neoplasms may be less aggressive than conventional pancreatic ductal adenocarcinomas. Nevertheless, surgical resection is recommended for all patients with a mucinous cystic neoplasm.
Molecular analysis of mucinous cystic neoplasms has disclosed mutations or promoter methylation in many of the genes known to be abnormal in ductal adenocarcinomas, including KRAS, TP53, SMAD4, and CDKN2A. Approximately one half of mucinous cystic neoplasms have mutations in RNF43, which codes for a protein with intrinsic E3 ubiquitin ligase activity that is also mutated in IPMNs but not in ductal adenocarcinomas.155 GNAS mutations are not found in mucinous cystic neoplasms, in contrast to IPMNs.155
Differential Diagnosis
The differential diagnosis of mucinous cystic neoplasms of the pancreas is discussed later (see Differential Diagnosis of Cystic and Intraductal Lesions). Mucinous cystic neoplasms sometimes develop extensive denudation of the lining epithelium, with the stroma showing hemorrhage, fibrosis, and inflammation. Biopsy specimens from these regions of the tumor resemble pseudocysts.182
Recognition of the clinical setting (e.g., young woman, no history of pancreatitis or other cause for a pseudocyst, otherwise normal-appearing pancreas) can help to direct the pathologist to examine other regions of the cyst to find the characteristic mucinous lining. Elements of ovarian-like stroma may persist in denuded areas. Immunohistochemical demonstration of hormone receptors in stroma cells supports a diagnosis of mucinous cystic neoplasm. The term mucinous cystadenocarcinoma should not be used for a ductal adenocarcinoma that shows ectatic glands (i.e., large duct pattern), unless there is evidence that the tumor arose in a preexisting mucinous cystic neoplasm.
Some cystic lesions of the pancreas have a mucinous lining that resembles a mucinous cystic neoplasm, but they lack evidence of cellular, ovarian-like stroma. Most lesions represent a branch duct–type IPMN or retention cyst with superimposed PanIN. Because the stromal component is regarded as critical for the diagnosis of a mucinous cystic neoplasm, this designation should be made with great caution in its absence.
Cystic Change in Typically Solid Tumors
Cystic Change in Ductal Adenocarcinoma
Cystic change in ductal adenocarcinoma is uncommon and may occur by three mechanisms.40 A large, radiographically detectable cyst may develop as a result of central necrosis of the tumor.183 Diagnosis can be clinically challenging, but residual carcinoma at the periphery of the cystic cavity is usually recognizable histologically. In other cases, a ductal adenocarcinoma can produce cystic dilation of distally located obstructed ducts, which results in one or more ductal retention cysts that can be misinterpreted as the main disease process on imaging or gross pathologic evaluation. In the large duct (microcystic) pattern of ductal adenocarcinoma, infiltrating tubular units are cystically dilated and may achieve a size large enough to be visible to the naked eye.82 Cystic glands may appear to be noninvasive, mimicking foci of PanIN or IPMN, especially when the cystic change occurs in the pancreatic parenchyma.
Cystic Pancreatic Neuroendocrine Tumor
A cystic PanNET rarely occurs. It usually consists of a single, unilocular cyst that occupies most of the tumor.184–186 Cysts that develop in well-differentiated PanNETs are usually centrally located and solitary, and they are typically lined by a cuff of well-preserved tumor with overlying fibrin (Fig. 40.33). The cyst cavity is typically filled with clear fluid instead of necrotic debris.
Most cystic changes in PanNETs are degenerative, although in some cases, the cyst is partially lined by non-neoplastic ductal epithelium, suggesting the cyst may occur because of dilation of a duct surrounded by the neoplasm. Some cysts may grow as large as 25 cm. A pathologic diagnosis of cystic neuroendocrine tumor is facilitated by careful attention to the cytologic features of the tumor cells.
Cystic Acinar Neoplasms
Acinar cell cystadenoma and acinar cell cystadenocarcinoma are discussed later (see Cystic Acinar Cell Neoplasms).
Lymphoepithelial Cyst
Lymphoepithelial cyst is a tumor that occurs predominantly in men in the fifth to sixth decade of life.187 Pancreatic lymphoepithelial cysts do not appear to be associated with autoimmune conditions, human immunodeficiency virus (HIV) infection, lymphoma, or carcinoma, all of which have been associated with salivary lymphoepithelial cysts. Most lymphoepithelial cysts arise in the periphery of the pancreas, usually in the body or tail. Some appear to be peripancreatic, and the connection to the organ may be difficult to recognize.
The cyst contents vary from serous to caseous, depending on the degree of keratin formation. The cyst wall and trabecula are usually thin. The inner lining of the cyst is smooth and sometimes has focal nodularity. The lymphoid tissue is not prominent grossly because it is usually limited to a thin band that surrounds the epithelium.
Microscopically, the cysts are lined by stratified squamous epithelium that may or may not reveal prominent keratinization (Fig. 40.34). In some cases, the lining epithelium may be transitional in appearance focally, and in others, it is cuboidal or focally denuded. Sebaceous elements and mucinous cells are uncommon but can be found, and their presence should raise the possibility of a teratoma; pilar structures clearly suggest the diagnosis.
The squamous lining is surrounded by dense lymphoid tissue composed of mature T lymphocytes. Germinal centers formed by B cells are abundant in some cases. Lymphocytes are uncommon in the epithelium. Some cases have solid lymphoepithelial islands, which are microscopic clusters of epithelial cells admixed with lymphocytes, similar to the so-called epimyoepithelial islands in salivary gland counterparts. The uninvolved pancreas is usually unremarkable.
Miscellaneous Epithelial Cysts
Squamous Cysts of Pancreatic Duct
Squamous cysts of pancreatic duct have an average diameter of 2.5 cm and arise in adults.188 They are lined by flat epithelium composed of squamoid or transitional cells that can be simple or stratified. Keratinization is not found. The cells in the basal layers express tumor protein p63 (TP63), and the cells bordering the cyst lumen are positive for MUC1 and MUC6. These cysts appear to be dilated pancreatic ducts with squamous (multilayered) metaplasia.
Dermoid Cysts
Dermoid cysts (i.e., mature cystic teratomas) are rare in the pancreatic region.189 They have been reported in patients in the second to third decade of life and are morphologically similar to teratomas that develop in other sites of the body.
Epidermoid Cysts in the Intrapancreatic Accessory Spleen
Epidermoid cysts in the intrapancreatic accessory spleen are rare lesions that are seen mainly in patients in the second to third decade of life.189 They occur almost exclusively in the tail of the pancreas, a location where accessory splenic tissue is most often found. These cysts are lined by attenuated squamous cells surrounded by normal-appearing splenic tissue.
Paraampullary Duodenal Wall Cysts
Paraampullary duodenal wall cysts occur as a consequence of chronic fibrosing inflammation in the periampullary region between the duodenum and the distal common bile duct.190 Accessory pancreatic ducts may form the cysts in the duodenal wall and underlying pancreas, mimicking duodenal duplication. The cyst wall is lined partially by ductal epithelium and partly by inflammation and granulation tissue, and it is surrounded by hypercellular spindle cells with smooth muscle features that are possibly derived from the muscularis propria of the duodenum. The process is closely related to so-called groove pancreatitis (i.e., paraduodenal pancreatitis).191–194
Retention Cysts
Retention cysts are unilocular cysts that result from obstruction of a pancreatic duct.131,190,195 They are usually lined by normal-appearing ductal epithelium without prominent cytoplasmic mucin. Superimposed PanIN may be found in some retention cysts.196 Retention cysts with low-grade PanIN closely resemble gastric-type branch duct IPMNs, which can have minimal papilla formation in the most cystic regions of the neoplasm. The distinction between these two entities is largely semantic. Features favoring a diagnosis of IPMN include papilla formation, multiple cysts, specialized (intestinal or oncocytic) epithelium, and lack of non-neoplastic ductal epithelium lining part of the cystic duct.
Other Cysts
Other rare cysts that can occur in the pancreas include parasitic cysts, endometriotic cysts, and congenital foregut (intestinal-type) cysts.
Intraductal Neoplasms
Intraductal tumors have rapidly become a widely studied group of pancreatic neoplasms, and with the increased use of cross-sectional imaging, they are being detected much more frequently.197–212 They often lack a component of invasive carcinoma and are usually readily treatable by surgery.
The tumors have distinctive clinical and radiographic findings. Many cases appear radiographically cystic, and preoperative differentiation of an intraductal neoplasm from true cystic neoplasms, such as serous or mucinous cystic neoplasms, can be difficult.81 Intraductal neoplasms reflect the spectrum of neoplastic progression,197 from the earliest neoplastic changes to invasive carcinoma, but unlike PanIN lesions associated with conventional ductal adenocarcinomas, intraductal neoplasms are clinically detectable, providing an excellent model of preinvasive neoplasia in the pancreas.94
Intraductal Papillary Mucinous Neoplasm
Clinical Features
IPMNs are grossly and radiographically visible tumors characterized by an intraductal proliferation of mucin-producing cells that usually are arranged in a papillary pattern.198–202 The tumors typically are associated with intraluminal mucin accumulation and cystic dilation of the ducts, which may be localized or involve the entire ductal system. Mucin spillage into the duodenum through the ampulla of Vater is an endoscopic finding that is virtually diagnostic of IPMN.203,204
Depending on the location of the primary process and subsequent mechanical changes in the pancreatic ducts, IPMNs may manifest as multilocular cystic masses or abundant papillary nodules. Papilla formation may be a microscopic or macroscopic finding; the latter appear as large, nodular masses in dilated ducts. The various patterns that can occur in IPMNs led to a wide variety of designations for this group of tumors before unification under the term IPMN. Previous terms include mucinous duct ectasia (i.e., ductectatic mucinous cystadenoma),205,206 mucin-producing tumor,207,208 villous adenoma of the pancreatic duct,209 and intraductal papillary tumor.210
IPMNs account for approximately 5% of all pancreatic neoplasms, although they are being reported in increasing numbers and may be more common than previously recognized. Patients with IPMNs are usually in the seventh to eighth decades of life and have nonspecific symptoms. Some patients have a history of pancreatitis. IPMNs in younger, asymptomatic patients are often detected incidentally during abdominal imaging performed for other indications.
Endoscopic findings (e.g., mucin extrusion through the ampulla of Vater) and radiographic findings (e.g., ectasia of the ducts) are diagnostic features. Approximately 80% of IPMNs occur in the head of the pancreas. Based on the extent of involvement of the ductal system, IPMNs are classified as main duct and branch duct types (discussed later).
Pathologic Features
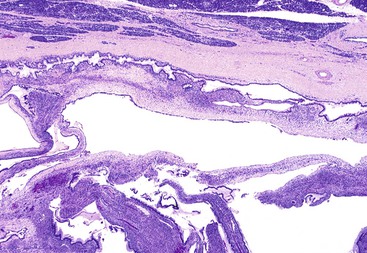
Careful macroscopic examination is imperative to document the intraductal nature of the tumor. The extent of ductal dilation and the amount of gross papilla formation vary between cases and regionally in individual cases (Fig. 40.35). The tumor can primarily involve the major pancreatic duct (i.e., main duct type). However, other cases are limited to involvement of the secondary ducts (i.e., branch duct type), particularly in the uncinate process and in the body and tail of the pancreas.211–213 Branch duct IPMNs may appear as multiple, separate, small cysts grossly and radiographically because of the tortuous nature of the dilated ducts. In these cases, connection to the ductal system may be difficult to demonstrate grossly.
Preoperative designation of IPMNs as main duct or branch duct types is clinically important because the main duct type has a greater likelihood of harboring high-grade dysplasia or invasive carcinoma. Small (<3 cm) branch duct IPMNs may not require surgical resection if they lack other worrisome findings such as mural nodules and interval growth during follow-up.213–217
IPMNs may be localized or multicentric, or rarely, the entire ductal system of the organ may be involved. Thorough sampling of the specimen is necessary for detection of an invasive carcinoma, which is found in approximately 35% of cases.218
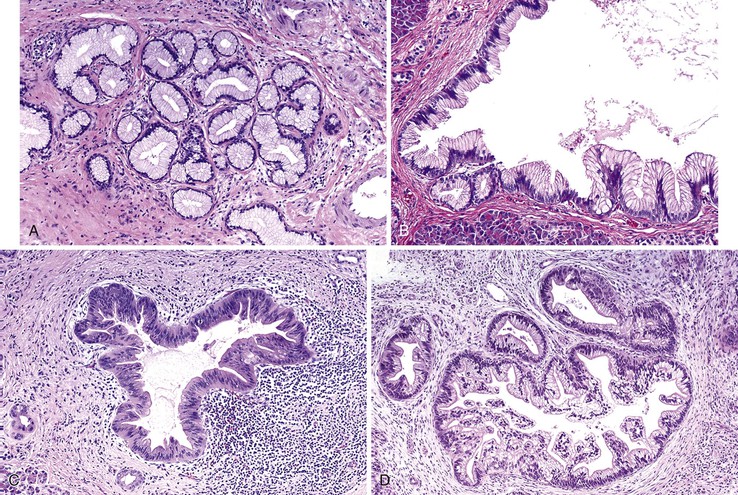
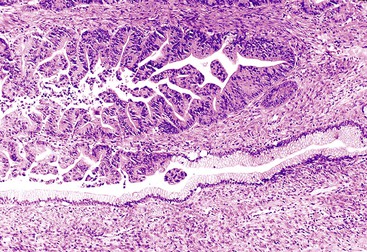
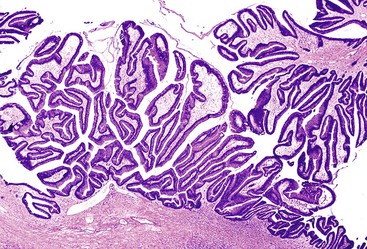
Microscopically, mucinous cells with various degrees of atypia line the cystically dilated ducts of these tumors (Fig. 40.36). Three papillary patterns have been described.94,198,219,220 The intestinal type (35% of cases) is morphologically similar to villous adenomas of the gastrointestinal tract221; the gastric type (50% of cases) is characterized by cells that resemble the foveolar epithelium of the stomach222; and the pancreatobiliary type (15% of cases) has more complex papillae composed of cuboidal cells (Fig. 40.37).223
The papillae in intestinal-type IPMNs are typically long and villiform, although some branching may be seen. The nuclei are elongated and show some degree of pseudostratification and intracellular mucin, depending on the degree of dysplasia (discussed later). Gastric papillae predominantly have a single layer of cells with basally oriented nuclei and abundant mucinous cytoplasm. They often show less exuberant papilla formation. Some cystic ducts may not contain papillae and instead are lined by a flat layer of mucinous epithelium. A tubular pattern with simple, back-to-back glands has been reported as a pyloric gland adenoma of the pancreas (i.e., intraductal tubular adenoma, pyloric gland type), but it is now thought to be a variant of gastric-type IPMN, with which it may be associated (Fig. 40.38).1,224–227 The cells that line the papillae of pancreatobiliary-type IPMNs show the most pronounced degree of cytologic atypia and architectural complexity, with numerous branched papillae, micropapillae, and cribriform areas. The nuclei are typically not pseudostratified but show marked variation in size and shape and have irregular contours and prominent nucleoli. Loss of nuclear polarity is common in these lesions.
The intestinal and pancreatobiliary types of IPMNs may have areas of gastric foveolar morphology. However, it is uncommon for an individual IPMN to have intestinal and pancreatobiliary morphology in the same tumor.
Similar to mucinous cystic neoplasms, the dysplasia in IPMNs is graded as low, intermediate, or high grade on the basis of the most severely dysplastic region (Fig. 40.39), emphasizing the need for extensive or complete histologic sampling for proper diagnostic evaluation. Some IPMNs may exhibit a lower degree of dysplasia in the cystic regions than in the nondilated ducts in the surrounding pancreas, and discontinuous foci of invasive carcinoma may be identified. These observations argue for careful gross and histologic examination of the noncystic regions of the pancreas.
IPMNs with low-grade dysplasia are also called intraductal papillary mucinous adenomas, and those with high-grade dysplasia are called intraductal papillary mucinous carcinoma in situ. The morphologic changes in this dysplasia spectrum parallel those seen in PanINs or mucinous cystic neoplasms.
The intestinal, gastric, and pancreatobiliary types of papillae characteristically have different degrees of dysplasia. For example, gastric foveolar IPMNs usually have low- or intermediate-grade dysplasia, intestinal-type IPMNs usually have intermediate- or high-grade dysplasia, and pancreatobiliary-type IPMNs usually have high-grade dysplasia.
Invasive carcinoma, which is identified in one third of cases, is usually a colloid92 or tubular (conventional ductal) type (Fig. 40.40), with each representing approximately 50% of the invasive carcinomas arising in IPMNs. Colloid carcinoma usually is found in intestinal-type IPMNs,92,93 and tubular-type invasive carcinoma may arise in any of the papilla types.228 Invasive carcinoma in IPMNs is usually associated with high-grade dysplasia and is rarely found in IPMNs with gastric papillae.56,198 Invasive carcinoma may be focal or multifocal and may represent most of the tumor mass. The finding of invasive carcinoma should always be mentioned in the diagnosis (i.e., “IPMN with an associated invasive carcinoma”), and the type and extent of invasive carcinoma should be specified.
Special Studies and Molecular Features
Immunohistochemical staining demonstrates that IPMNs express keratins, including CK7, CK8, CK18, and CK19, with the degree of staining for CK20 depending on the type of papillae.202,229 Most IPMNs express CEA, CA 19-9, and MUC5AC.206,230
Expression of MUC1 and MUC2 varies with the type of papillae.56,94,220,231 Expression of MUC1 (mammary-type mucin) is more common in pancreatobiliary-type IPMNs. MUC2 is more commonly expressed in intestinal-type IPMNs. Gastric-type IPMNs do not normally express MUC1 or MUC2. Expression of MUC2 in intestinal-type IPMNs is paralleled by expression of other markers of intestinal differentiation, such as CK20 and CDX2, neither of which is usually detected in pancreatobiliary- and gastric-type IPMNs. MUC6 is preferentially expressed in pancreatobiliary-type IPMNs, suggesting pyloric differentiation.58
These findings have led to the recognition of a separate intestinal pathway of carcinogenesis in the pancreas, which is exemplified by intestinal-type IPMN and the development of colloid-type invasive carcinoma,94 which has intestinal differentiation and a prognosis better than that for other ductal adenocarcinoma variants. Carcinomas that develop in pancreatobiliary-type IPMNs are usually conventional ductal adenocarcinomas.
Many of the molecular alterations in IPMNs are similar to those of ductal adenocarcinoma. A higher number of molecular abnormalities occur in IPMNs with greater degrees of dysplasia. However, mutations in KRAS and TP53 are less common than in ductal adenocarcinoma. Inactivation of CDKN2A occurs in some tumors.232 In contrast to ductal adenocarcinoma, SMAD4 protein expression is typically retained in IPMNs.95 Inactivation of the Peutz-Jeghers gene (STK11) is found in approximately 25% of IPMNs233 but not in ductal adenocarcinomas.
GNAS is mutated in 60% of IPMNs, and RNF43, a gene coding for a protein with intrinsic E3 ubiquitin ligase activity, is mutated in 75%.155,157 APC is mutated in approximately 25% of IPMNs. None of these three genes is altered in conventional ductal adenocarcinomas, although RNF43 mutations do occur in mucinous cystic neoplasms.155
Differential Diagnosis
The differential diagnosis of IPMNs and mucinous cystic neoplasms is discussed later (see Differential Diagnosis of Cystic and Intraductal Lesions). IPMNs should be distinguished from PanIN, small incidental ectatic ducts,234 and retention cysts (i.e., secondary cystic dilation of obstructed ducts), all of which are intraductal processes that may contain mucinous lining cells. Small IPMNs are essentially indistinguishable from large foci of PanIN. The criteria offered to separate these two related types of preinvasive neoplasms are admittedly arbitrary and allow some degree of overlap.131 Nevertheless, PanINs are usually incidental, microscopic (i.e., not radiographically detectable) lesions that are less than 0.5 cm in the maximal diameter, whereas IPMNs are usually radiographically or macroscopically detectable masses or cysts that are more than 1.0 cm in diameter.
It is relatively common for mucinous lesions to be found in small ducts near or distant from ducts involved by IPMNs. In some cases, the morphology of the epithelium in the small ducts is distinct from that of the IPMN, which supports the argument that additional foci or PanIN can be found in surrounding pancreas. However, it is often difficult to determine whether the small duct lesion represents extension of the IPMN. Comparative mutational studies suggest that two adjacent ducts are more likely to have the same KRAS and GNAS mutations (reflecting involvement by a single neoplasm) compared with topographically separate locules, which often contain genetically distinct lesions.157
Another diagnostic challenge is recognition of focal invasive colloid carcinoma and its distinction from benign leakage of mucin into the surrounding stroma that is related to rupture of the involved pancreatic ducts. When mucin leaks into the stroma—a rare event in IPMNs—the amount is limited. The mucin in these cases is typically located adjacent to an involved duct, and it is devoid of free-floating neoplastic epithelial cells. Any epithelium usually is found in continuity with the epithelium of the intraductal component. Mucin leakage is more likely to produce an inflammatory reaction than mucin associated with an invasive colloid carcinoma. When acellular mucin pools lacking an inflammatory response are found in the stroma in IPMNs, a careful search for diagnostic evidence of invasive colloid carcinoma should be undertaken at multiple histologic levels.
Natural History and Treatment
The overall 5-year survival rate of patients with IPMN is relatively good.215,228 More than 75% of patients are free of disease after 5 years. Even patients with an associated invasive carcinoma have a good prognosis, particularly if the carcinoma is the colloid type or the amount of invasive carcinoma is small. Invasive carcinomas less than 0.5 cm in the greatest dimension have been designated minimally invasive and have an excellent prognosis.235 Cases with a significant component of invasive tubular-type adenocarcinoma pursue an aggressive course, similar to that of conventional ductal carcinoma that arises without a preexisting IPMN.236
IPMNs are a precursor to and a marker of invasive carcinoma.198 Invasive carcinoma may occur in or distant from the intraductal component.237 The multicentric nature of IPMNs raises the issue of whether total pancreatectomy is the best form of management for these tumors. However, most patients treated with conservative resection of grossly visible disease (usually a pancreatoduodenectomy) do not experience local recurrences, even if the intraductal component extends to the pancreatic ductal margins.215,228 The highest grade of dysplasia in the IPMN was a better predictor of recurrence than the status of the margin in one study.215 Conversely, some patients suffer recurrence despite complete resection (with negative margins), which raises the possibility of tumor multicentricity, as does the observation that invasive carcinoma distant from the cystic lesion may develop in patients with IPMNs under surveillance may.238
Additional long-term follow-up is needed to determine the optimal treatment for patients with a positive ductal margin. Different institutions are using different approaches, but most are tailored to each patient. However, patients with invasive carcinoma should be treated similarly to patients with conventional ductal adenocarcinoma.