Tumors of Major and Minor Ampulla
N. Volkan Adsay
Olca Basturk
Introduction
Tumors of the ampulla of Vater are pathologically and clinically significant (Box 41.1). Because the distal common bile duct and the main pancreatic duct converge at the level of the ampulla, tumors may obstruct two organs, leading to early onset of symptoms. The potential for early detection has improved the prognosis for neoplasms of the ampulla. However, the newer techniques used for local treatment, such as endoscopic polypectomy and transduodenal ampullectomy,1–3 have created challenges in the pathologic analysis of ampullary tumors.
Anatomic and Histologic Considerations
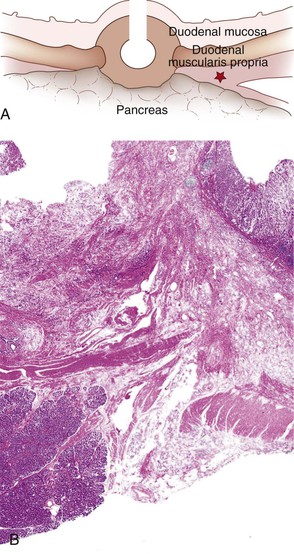
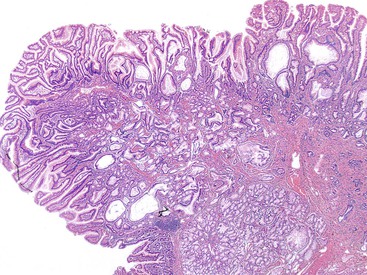
In this chapter, the term ampulla refers to the whole ampullary structure, including all four compartments (Table 41.1). The word papilla refers to the pinnacle (edge) of the ampullary prominence (see Figs. 41.1 and 41.3). For lesions that arise from or are localized preferentially at the duodenal surface of the ampulla, the term ampullary duodenum is used. Although the term periampullary duodenum has been used in publications to describe this region,4 it is not used in this chapter to avoid confusion.
Table 41.1
Terminology
| Term | Synonyms Used in This Chapter | Description |
| Ampulla of Vater (see Fig. 41.1) | Ampulla major | Protuberance in the second portion of the duodenum composed of: (1) duodenal mucosa; (2) papilla of Vater, lined by specialized epithelium; (3) pancreatobiliary-type ductules in sphincter of Oddi musculature, in the wall; (4) distal ends of the common bile and pancreatic ducts |
| Minor ampulla (see Fig. 41.3) | Accessory ampulla | Located 2 cm proximal and anterior to the major ampulla, within the duodenum |
| Papilla of Vater (see Figs. 41.1 and 41.3) | None | Refers specifically to the protuberance (edge of the pinnacle) of the ampulla, which is lined by specialized epithelium, and the wall, which is composed of pancreatobiliary-type ductules embedded in sphincter of Oddi musculature |
| Ampullary duodenum (see Fig. 41.5) | Duodenal surface of ampulla | Duodenum-facing surface of the ampulla, which is mostly lined by intestinal-type epithelium |
| Ampullary duct (see Figs. 41.2 and 41.6) | None | Refers to pancreatobiliary-type ductules and peribiliary mucous glands and tributary ductules, as well as the duct epithelium of the very distal segments of the common bile duct and main pancreatic duct |
The anatomy of the ampulla of Vater can vary considerably between individuals, but in most, the common bile duct and the main pancreatic duct join into a common channel within the wall of the duodenum. Flow of luminal material through the ampulla is regulated by dense fascicles of smooth muscle, called the sphincter of Oddi (Fig. 41.1, A). The mucosa forms folds called plicae. Although the length of the common channel varies,5 it is less than 3 mm long in most persons. In some individuals, the common bile duct and main pancreatic duct are separated by a septum. These anatomic variations are difficult to demonstrate in the routine examination of surgical specimens5a unless dye injection or specimen radiographs are performed.
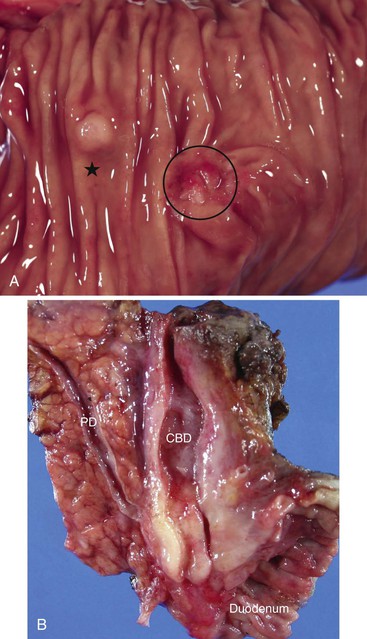
The minor ampulla is also subject to anatomic variations. It is typically located 2 cm proximal and anterior to the major ampulla within the duodenum (Fig. 41.2, A). It can be mistaken for a polyp endoscopically. The dorsal pancreatic duct drains through the minor ampulla. Although this duct normally regresses with fetal maturation, residual duct elements remain in the submucosa of the duodenum in most individuals. The minor ampulla is patent in 40% of the population.6,7 Composition of the minor ampulla varies among individuals.
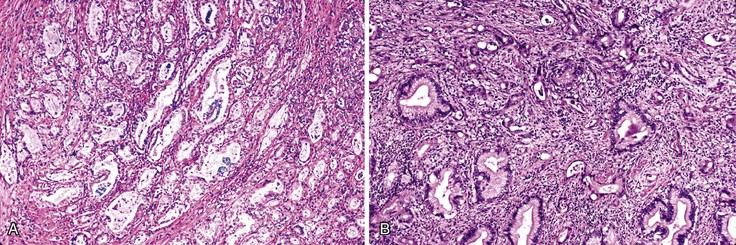
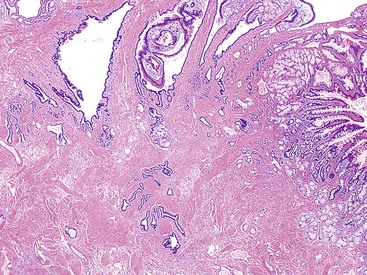
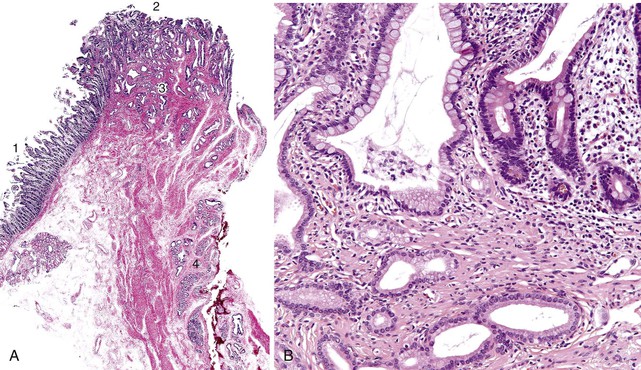
The epithelium of the major and minor ampullae is the small intestinal type on the duodenal surface (Fig. 41.3; see Fig. 41.1). At the edge of the ampullae, where the ducts transition to the duodenal surface (i.e., the papilla), a specialized epithelium with features that resemble gastric-foveolar epithelium with scattered goblet cells is usually seen (see Figs. 41.1, B and 41.3, B). Because of mucinous epithelium, biopsy specimens from this area can be mistaken as representing peptic injury. Pancreatobiliary-type tributary ductules occur in the wall of the ampulla (see Fig. 41.1, B). These structures are usually embedded in dense myoid tissue of the sphincter of Oddi or duodenal musculature (see Fig. 41.1, B).8 The epithelium of the papilla transitions into ductal epithelium. Pancreatic acinar lobules often reside in the wall of the ampulla (see Fig. 41.3, C). They may contain islets of Langerhans. Clusters of neuroendocrine cells may also occur in the minor ampulla but only seldom in major ampulla.9,10
Classification of Ampullary Tumors
Various criteria to determine whether a particular tumor originates in the ampulla or in the nonampullary duodenum are offered in the literature. Inconsistent use of the term periampullary has further complicated the issue. In some publications, it refers to the ampullary region proper, whereas in others, it is used to describe the nonampullary segment of the duodenum.11,12 In the staging section of the protocol for the examination of ampullary tumor specimens published by the College of American Pathologists (CAP),13 only tumors that are intraampullary (i.e., developing within the cavity) are regarded as truly ampullary in origin, whereas in the tumor classification section, other anatomic subregions (e.g., periampullary duodenal, papilla of Vater) are also recognized as part of the ampulla.
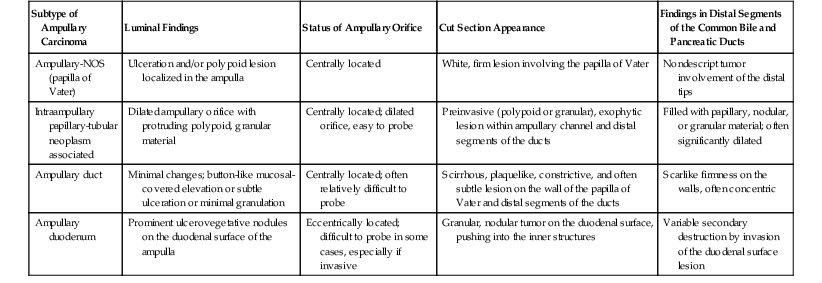
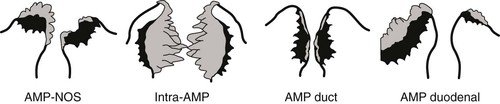
In a more specific definition of the ampulla,4,14 tumors are defined as ampullary if more than 75% of the tumor is localized to or engulfed by the ampulla and the preinvasive component (if any) is localized within one of the four compartments of the ampulla. The four subsets of ampullary tumors (i.e., ampullary-NOS, intraampullary papillary-tubular neoplasm associated, ampullary duct, and ampullary duodenum) are classified according to their primary site of growth4,14 (Table 41.2; see Figs. 41.2 and 41.4 through 41.6):
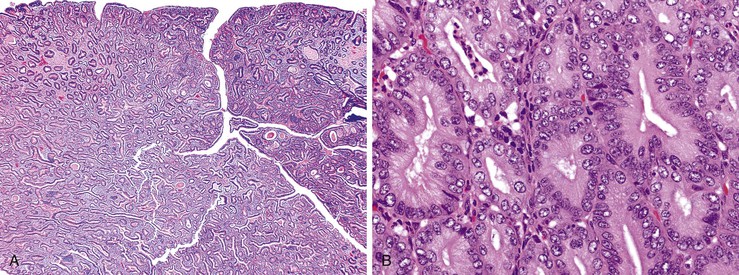
1. Ampullary carcinomas not otherwise specified (NOS) correspond to the edge of the ampullary orifice (i.e., the papilla of Vater) (Fig. 41.7; see Figs. 41.1, B and 41.3, B), which is composed of transitional epithelium with gastric-like features and scattered goblet cells and ductules.
3. Carcinomas of the ampullary duct involve the wall of the papilla of Vater, which is composed of pancreatobiliary-type ductules and muscles of the sphincter of Oddi complex, which merges with the duodenal musculature (see Fig. 41.1).
4. Carcinomas of ampullary duodenum arise from the duodenal surface of the prominence (see Figs. 41.1, A and 41.5).
Table 41.2
Macroscopic Properties of Ampullary Tumors4,14
| Subtype of Ampullary Carcinoma | Luminal Findings | Status of Ampullary Orifice | Cut Section Appearance | Findings in Distal Segments of the Common Bile and Pancreatic Ducts |
| Ampullary-NOS (papilla of Vater) | Ulceration and/or polypoid lesion localized in the ampulla | Centrally located | White, firm lesion involving the papilla of Vater | Nondescript tumor involvement of the distal tips |
| Intraampullary papillary-tubular neoplasm associated | Dilated ampullary orifice with protruding polypoid, granular material | Centrally located; dilated orifice, easy to probe | Preinvasive (polypoid or granular), exophytic lesion within ampullary channel and distal segments of the ducts | Filled with papillary, nodular, or granular material; often significantly dilated |
| Ampullary duct | Minimal changes; button-like mucosal-covered elevation or subtle ulceration or minimal granulation | Centrally located; often relatively difficult to probe | Scirrhous, plaquelike, constrictive, and often subtle lesion on the wall of the papilla of Vater and distal segments of the ducts | Scarlike firmness on the walls, often concentric |
| Ampullary duodenum | Prominent ulcerovegetative nodules on the duodenal surface of the ampulla | Eccentrically located; difficult to probe in some cases, especially if invasive | Granular, nodular tumor on the duodenal surface, pushing into the inner structures | Variable secondary destruction by invasion of the duodenal surface lesion |
NOS, Not otherwise specified.
Preinvasive Neoplasms
Adenomas of the Ampullary Duodenum
Adenomas of the intestinal type, similar to colorectal adenomas, occur in the ampullary duodenum (i.e., duodenal surface of the ampulla).15 In the small intestine, the ampulla is a particularly common site of adenoma development,16 presumably because this is an anatomic site that is prone to chemical or physical injury. Most ampullary adenomas are sporadic, but the ampullary and other parts of the duodenum are the most common sites of extracolonic adenomas in patients with familial adenomatous polyposis (FAP).17–21 Between 80% and 90% of patients with FAP develop multiple adenomas in the duodenum, and 25% have them in the ampullary duodenum. Symptomatic adenomas are usually detected within 10 to 15 years of colectomy in these patients.20 Fortunately, because FAP screening protocols include periodic endoscopic surveillance of the duodenum, adenomas are increasingly detected at an earlier stage of development, while they are asymptomatic.22
Adenomas of ampullary duodenum often extend into the papilla and distal common bile duct and may involve the ductules in the wall of the papilla. More than 75% of the lesion should be located on the duodenal surface of the ampulla to be classified as an adenoma of the ampullary duodenum. Those with complete or near-complete intrapapillary growth are classified as intraampullary papillary-tubular neoplasms.
Clinical Features
Patients with sporadic adenomas of the ampullary duodenum are an average of 60 years. In contrast, FAP patients are typically 20 years younger at the time of diagnosis.16,23–26 Sporadic cases have a female predilection, whereas both sexes are affected equally by FAP.17,18,20,22 Clinical symptoms vary. Larger adenomas may cause bile duct obstruction with jaundice, abdominal pain, and pancreatitis. Smaller adenomas are often asymptomatic. Gastrointestinal hemorrhage is rare and raises concerns regarding the existence of an invasive carcinoma.
Histologically, tubular, villous, and tubulovillous adenomas occur with equal frequency in the sporadic cases, but tubular adenomas are more common in patients with FAP. Villous adenomas tend to be larger than tubular or tubulovillous adenomas. The prevalence of carcinoma in adenomas increases proportionately with the size of the polyp, and villous adenomas are more likely to harbor a carcinoma.
Pathologic Features
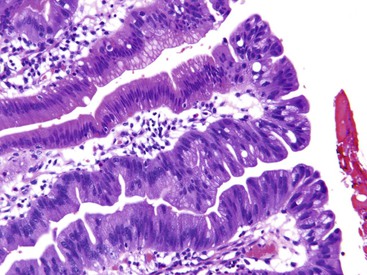
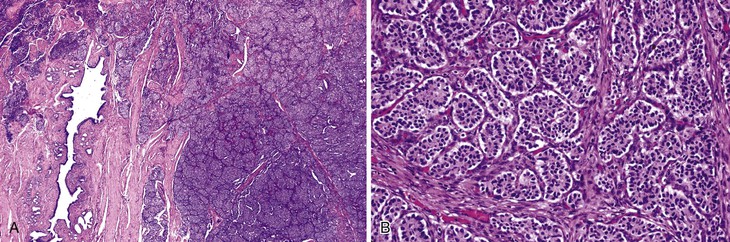
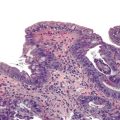
Grossly, most tubular adenomas of ampullary duodenum are bosselated in appearance, whereas most villous adenomas have a feathery or papillary appearance (Fig. 41.8).27,28 Villous adenomas are usually larger than tubular adenomas. Adenomas of the ampullary duodenum are more likely to harbor an invasive carcinoma than similarly sized colorectal adenomas. A firm texture or surface ulceration raises concern about an invasive component. Invasive carcinomas that arise in adenomas are often hidden in the deep creases of the tumor at the base of the polyp and are difficult to detect in surface biopsies.
Microscopically, adenomas of the ampullary duodenum are similar to those that occur in the large intestine. They have complex glands and villi (papillae) lined by mucin-depleted columnar cells with elongated, hyperchromatic, pencil-shaped nuclei and nuclear pseudostratification. The amount of cytoplasmic mucin varies. Goblet cells may be seen, but they typically are not numerous. The appearance of some FAP-related cases may be subtle and may even show surface maturation.
Adenomas are classified as tubular, tubulovillous, or villous, depending on the amount of glandular and papillary architecture. Tubulovillous adenomas contain more than 25% villi,15 and villous adenomas contain more than 75% villi. Tubular, villous, and tubulovillous adenomas occur with equal frequency in sporadic cases, but tubular adenomas are more common in patients with FAP.17–22
By definition, all adenomas are dysplastic. Dysplasia is categorized as low or high grade, depending on the degree of cytologic and architectural atypia and complexity of the epithelium. Low-grade dysplasia consists of pseudostratified, relatively monotonous columnar cells with cigar-shaped, elongated nuclei. The nuclei are lined up in the basal aspect of the cell cytoplasm, and there is no or only mild pleomorphism and loss of nuclear polarity. High-grade dysplasia is characterized by marked nuclear pleomorphism, loss of cell polarity, increased nucleus-to-cytoplasm ratio, and increased mitoses combined with architectural complexity, such as cribriforming, back-to-back glands, and micropapillae formation.
Paneth cells and neuroendocrine cells are particularly common in all ampullary adenomas, and in some cases, they can form more than 50% of the neoplastic cell population, particularly in FAP cases. Immunohistochemical staining typically reveals evidence of intestinal differentiation, such as positivity for cytokeratin 20 (CK20), CDX2, and MUC2.29 CK7 is also commonly positive in these tumors.29,30 Paneth cells label for lysozyme, and the endocrine cell component can be detected with chromogranin or synaptophysin.20,29,31,32
Differential Diagnosis
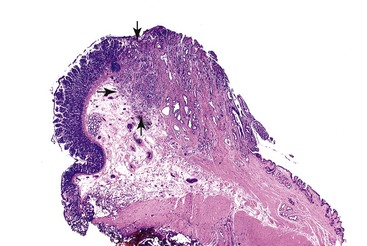
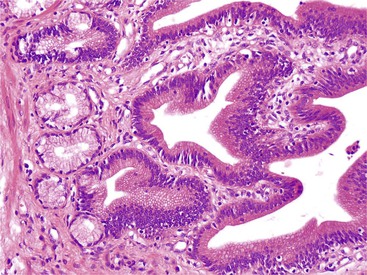
Extension of adenomatous epithelium into the ductules in the wall of the papilla can simulate invasive carcinoma (Fig. 41.9), particularly where continuity with the normal surface epithelium is lost, and high-grade dysplasia is found. Because of the complexity of these ductular structures, it can be difficult or impossible to distinguish in situ disease from true invasion (see Fig. 41.9). Findings such as paradoxical differentiation (i.e., different cytomorphology in the preinvasive and invasive components), cuboidal epithelium, dispersion in a nonlobular and nonorganoid fashion, marked contour irregularities, formation of complex micropapillary tufts or markedly elongated gland units, and severe cytologic atypia favor invasive carcinoma (Table 41.3). Budding and vascular or perineural invasion are helpful findings. However, invasion can be expansile (i.e., nodular or pushing border), not showing the conventional infiltration patterns.
Table 41.3
Morphologic Characteristics of Glands in Invasive and Noninvasive Carcinomas
| Findings | Invasive | Noninvasive |
| Dispersion of glands | +++ | − |
| Lobularity | − | +++ |
| Clustering of evenly shaped glands | − | +++ |
| Contour irregularities | ++ | − |
| Different cytomorphology from the surface | ++ | − |
| Loss of cell polarity | ++ | ± |
+++, Seen; ++, usually seen, ±, may be seen; −, not seen.
Another diagnostic dilemma arises when an underlying invasive carcinoma of the pancreas or bile duct involves the ampullary epithelium by colonization (“cancerization”) of the mucosal basement membrane, which simulates in situ disease (Fig. 41.10), in a process called pseudoadenomatous transformation. Because carcinomas usually have pancreatobiliary (rather than intestinal) differentiation, immunohistochemical staining may reveal positivity for only CK7 (rather than CK7 and CK20) and for MUC1 (rather than MUC2 and CDX2).29 Immunohistochemical stains can help to demonstrate that both components have the same immunophenotype.
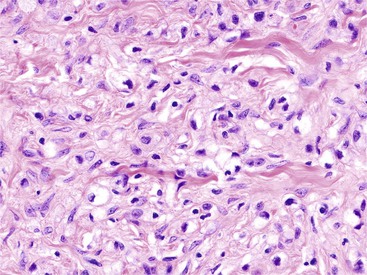
Reactive changes associated with inflammatory processes of the ampulla can mimic an adenoma (Fig. 41.11). In addition to having associated inflammation, reactive atypia usually lacks the degree of nuclear elongation and pseudostratification of adenomas. Paradoxically, reactive nuclei occasionally show more atypia than low-grade adenomas in the form of enlargement and macronuclei, but the high degree of architectural complexity characteristic of high-grade dysplasia is not usually seen in reactive epithelium. Reactive changes often have a higher degree of cytoplasmic eosinophilia, presumably because of retaining normal organelles instead of forming abnormal mucins compared with adenomas.
Adenomatous lesions that occur within the papilla and distal common bile duct (i.e., IAPNs) are similar in appearance to primary pancreatic and biliary intraductal neoplasms. They have several distinctive characteristics that distinguish them from adenomas of the ampullary duodenum.
Intraampullary Papillary-Tubular Neoplasms
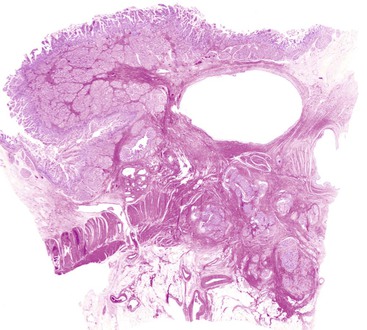
IAPNs are adenomatous (tumoral, intraepithelial, neoplastic) lesions that occur almost exclusively in the ampulla (see Fig. 41.4).14,33 They represent the intraampullary counterpart of intraductal neoplasms of the pancreas and biliary tract (see Chapters 38 and 40). Included in this category of tumors are noninvasive pancreatobiliary neoplasms and intestinal-type adenomas in the ampulla.34 Papillary or polypoid tumors can fill the ampullary channel and distal segment of the common bile duct or main pancreatic duct (see Fig. 41.4). By definition, involvement of the ampullary duodenum and intramucosal extension into the proximal aspect of the common bile duct and main pancreatic duct is minimal (<25%).
Clinical Features
These tumors account for one third of resections performed for a clinical diagnosis of ampullary tumor. The mean age of affected patients is 64 years (range, 27 to 85 years), and the tumors occur predominantly in men (male-to-female ratio of 2.2). Symptoms (e.g., jaundice, pruritus, light stool, dark urine) are usually related to obstruction of the common bile duct, but patients also may have nonspecific symptoms such as abdominal pain and weight loss.
Pathologic Features
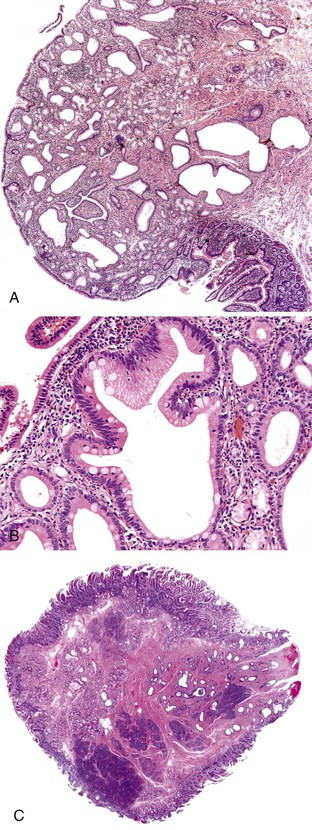
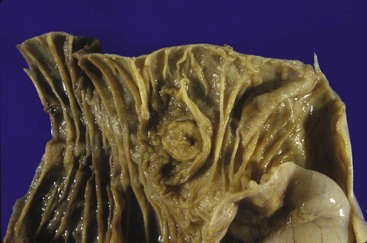
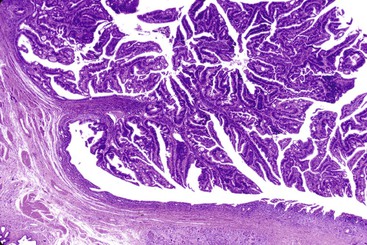
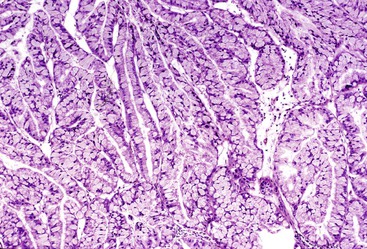
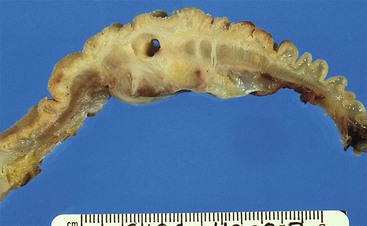
Gross examination of the ampullary duodenum5a typically reveals a hemispheric elevation of intact mucosa, often with a patulous papilla orifice from which nodules of friable granular material protrude into the duodenal lumen (see Fig. 41.4, A). A probe inserted into the common bile duct or main pancreatic duct typically extends into the center of the lesion. Ulceration may be evident, but overt mucinous discharge, characteristic of pancreatic intraductal neoplasms, is seldom encountered. On sectioning, the tumors are characterized by a prominent exophytic growth pattern in the dilated distal bile and pancreatic ducts (see Fig. 41.4B). Obstructive polypoid and light tan, granular nodules, which are often associated with dilation of upstream biliary or pancreatic ducts, may be identified (see Fig. 41.4B). The mean tumor diameter is 2.9 cm.
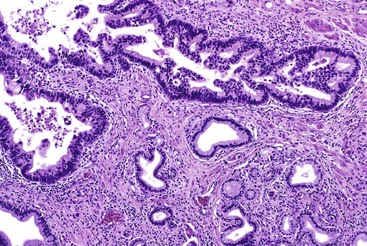
Microscopically, IAPNs show various degrees of papillary or tubular growth (Figs. 41.12 through 41.14). Most have a mixture of these patterns. They exhibit a spectrum of dysplasia, and most cases show a mixture of low- and high-grade dysplasia. The criteria used for low- and high-grade dysplasia is the same as that used for adenomas of ampullary duodenum (discussed earlier). However, some cases exhibit gastric differentiation (see Fig. 41.14) similar to that of intraductal and intracholecystic tumors of the pancreatobiliary tract and foveolar and pyloric adenomas of the stomach. These cases may lack overt cytologic atypia characteristic of intestinal-type adenomas. In most cases, examination reveals foci of high-grade dysplasia. High-grade dysplasia correlates with a papillary configuration. Unlike adenomas of the ampullary duodenum, approximately 50% of IAPNs show mixed (intestinal, gastric, pancreatobiliary) differentiation.
Approximately 75% of IAPNs are associated with invasive carcinoma at the time of diagnosis, but the invasive component is usually less than 1 cm in diameter. Invasion is mostly tubular and often shows a mixture of intestinal (Fig. 41.15) and pancreatobiliary features (Fig. 41.16). The histologic type of invasive carcinoma parallels that of the preinvasive component in many but not all cases.
The hybrid nature of these lesions is reflected in their immunophenotype. More than 50% of cases coexpress CK7 and CK20. Immunostaining for MUC2 and CDX2 is positive in cases with intestinal differentiation. Results for MUC1, MUC5AC, and MUC6 are positive in cases with gastropancreatobiliary differentiation. However, overlaps are common, and a significant proportion of the cases reveal mixed immunophenotype.14
Differential Diagnosis
The most challenging aspect of these lesions is to differentiate invasion from pseudoinvasion. The preinvasive component of these lesions often involves the (tributary) ductules in the wall of the ampulla. The criteria for identifying invasion in adenomas of the ampullary duodenum are applicable in this case (see Table 41.3).
Differentiating IAPNs from their pancreatic and biliary counterparts or adenomas of the ampullary duodenum requires knowledge of the location and distribution of the neoplastic lesion. A nonintestinal phenotype is helpful in distinguishing these lesions from adenomas of the ampullary duodenum, because examination of the latter invariably reveals intestinal differentiation.
Natural History and Prognosis
Noninvasive cases have an excellent prognosis. However, cases with extensive high-grade dysplasia without invasion have recurred. Long-term follow-up is warranted, even in noninvasive cases.
Cases with invasive carcinoma are associated with better survival than conventional (invasive) ampullary carcinomas unaccompanied by an IAPN (3-year survival of 69% versus 44%).14 This survival advantage is likely attributable to early detection of invasion but also reflects differences in tumor biology.4,14,35
Flat Dysplasia
Some ampullary carcinomas arise from flat dysplasia instead of developing from an adenomatous polyp.15 In rare cases, dysplastic epithelial cells grow along the ducts at the periphery of an invasive carcinoma. Flat dysplasia of the ampulla in the absence of invasive carcinoma is rarely seen, presumably because these lesions do not obstruct the ampulla and are usually asymptomatic.
Invasive Adenocarcinomas
Because of the proximity of anatomic structures in the ampulla, it is often difficult to grossly and microscopically distinguish tumors that arise in the distal common bile duct from those that arise in the duodenum, pancreatic head, or true ampulla. Published data regarding ampullary neoplasms often contain references to periampullary tumors. In some studies, the term periampullary is used to define all tumors located in pancreatoduodenectomy specimens, whereas in others, the term is used for pure ampullary tumors. In other publications, the term periampullary refers only to nonampullary segments of the duodenum.11,12,36–38
Classification
The synoptic reporting document of the CAP13 recognizes three categories of ampullary tumors: periampullary-duodenal, papilla of Vater, and intraampullary. However, the tumor-node-metastasis (TMN) staging protocol of the American Joint Committee on Cancer (AJCC),39 which is endorsed by the CAP, considers only intraampullary tumors as ampullary in origin.39 The three categories of tumors recognized by the CAP were further divided into four groups,4 and ampullary carcinomas are classified as follows.
I. Primary ampullary carcinomas (Table 41.4; see Table 41.1)
A. Ampullary carcinomas not otherwise specified (ampullary-NOS) include tumors that are presumed to arise from the papilla of Vater (i.e., the edge of mucosa where the common bile duct and main pancreatic duct merge into the duodenal mucosa) (see Fig. 41.7). Almost 50% of cases that occur in the ampulla fall into this category, presumably because this group of tumors includes those that arise in the papilla of Vater and those that cannot be confidently placed into one of the subsequent categories. With improved gross examination skills and careful evaluation, the percentage of cases in this category is expected to decrease significantly.
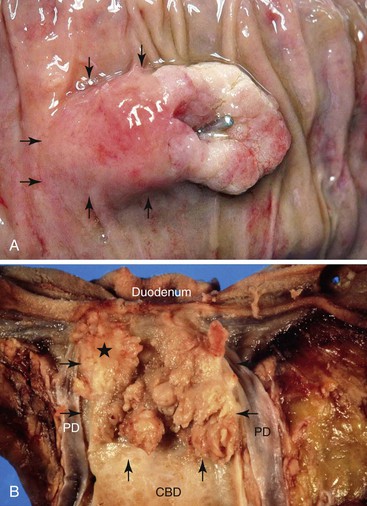
B. Carcinomas associated with IAPNs are characterized by preinvasive nodules located in the ampullary channel (i.e., distal tip of the common bile duct and main pancreatic duct). Gross examination often reveals light tan, friable nodules (see Fig. 41.4). From the duodenal perspective, these tumors show a dilated orifice, from which granular material often protrudes into the lumen of the bowel. Probes placed into the common bile duct and pancreatic duct typically exit into the center of the lesion. Microscopic examination often reveals only a small invasive carcinoma, and the prognosis is relatively good, especially if there is no invasive carcinoma or invasion is limited in amount.
C. Carcinomas of ampullary ducts are scirrhous lesions that circumferentially constrict the distal end of the common bile duct and pancreatic duct, with preservation of the papilla of Vater and ampullary duodenal mucosa. From the duodenal perspective, these tumors typically show a button-like elevation of mucosa or a small, ulcerating lesion (see Fig. 41.2). Microscopically, they often prove to be pancreatobiliary-type carcinomas. Although these tumors are usually less than 2 cm in diameter, they have an aggressive behavior, the worst among the ampullary carcinoma subtypes but significantly better than pancreatic ductal adenocarcinomas.
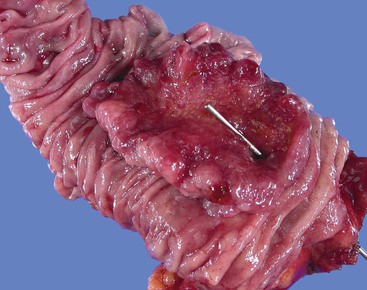
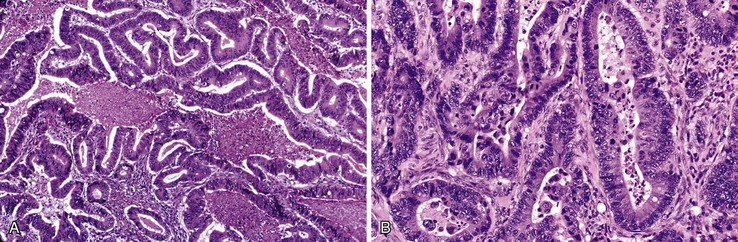
D. Carcinomas of the ampullary duodenum (i.e., periampullary-duodenal tumors) arise from the ampullary duodenum, usually from an adenoma of the ampullary duodenum, forming bulky lesions in which the ampullary orifice is often eccentrically located (see Fig. 41.5). They typically have an intestinal (see Fig. 41.15) or mucinous-intestinal phenotype. Although they are usually very large and produce lymph node metastases, their behavior is often better than expected.
Table 41.4
Clinicopathologic Features of Ampullary Carcinomas14,15
| Feature | AMP-NOS | Intra-AMP | AMP Duct | AMP Duodenum |
| Site-specific Percentage (%) | 55 | 25 | 15 | 5 |
| Baseline Demographics | ||||
| Mean age | 65 | 64 | 69 | 59 |
| Sex (M/F ratio) | 1.5 | 2.2 | 0.9 | 1 |
| Clinical Characteristics | ||||
| Overall tumor size (cm) | 2.5 | 2.9 | 1.9 | 4.7 |
| Invasive tumor size (cm) | 1.8 | 1.5 | 1.7 | 3.4 |
| Invasive histology (intestinal/nonintestinal ratio) | 0.4 | 1.2 | 0.06 | 3 |
| Lymph node metastasis (%) | 42 | 28 | 41 | 50 |
| T stage (T1+T2/T3+T4 ratio) | 1.6 | 5.8 | 0.6 | 2 |
| Survival Rates | ||||
| 1 year (%) | 80 | 88 | 80 | 80 |
| 3 year (%) | 54 | 73 | 41 | 69 |
| 5 year (%) | 39 | 53 | 29 | 55 |
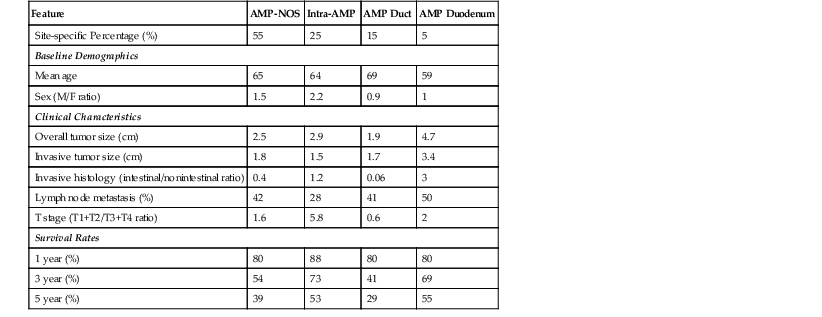
AMP duct, Ampullary duct carcinoma; AMP duodenum, ampullary duodenum carcinoma; AMP-NOS; ampullary carcinoma not otherwise specified; intra-AMP, intraampullary papillary-tubular neoplasm associated; F, female; M, male; T, tumor stage.
II. Secondary ampullary carcinomas
A. Duodenal
Clinical Features
Most malignant neoplasms of the ampulla of Vater are adenocarcinomas. The ampulla is the most common site of all small intestinal adenocarcinomas.23 Overall, approximately 5% of gastrointestinal carcinomas arise from the ampulla.40
Adenocarcinomas develop mainly in adults (mean age, 62 years) and are more common in men than women.40 The estimated lifetime incidence of an ampullary carcinoma is 0.01% to 0.04%.41 Ampullary adenocarcinomas in patients with FAP are diagnosed in younger patients (mean age, 47.5 years). In FAP cases, ampullary adenocarcinomas usually are associated with other neoplasms. Almost 20% of these patients have multiple primary neoplasms.15
Most patients with ampullary adenocarcinoma have symptoms of biliary obstruction. Jaundice accompanied by a palpable, distended gallbladder (i.e., Courvoisier sign) is a classic presentation of ampullary adenocarcinomas, although this constellation of symptoms affects only 15% of patients with these tumors.41–43 Other common symptoms include abdominal pain, weight loss, nausea, and acute or chronic pancreatitis.15 Because even small tumors may obstruct the bile duct, ampullary carcinomas often are less than 2 cm in diameter at the time of diagnosis.44,45
Pathologic Features
The gross findings of ampullary carcinomas vary according to subtype4,5a,14 (see Figs. 41.2 and 41.4 through 41.6). IAPN-associated carcinomas are characterized by granular, polypoid material that fills the ampullary channel (i.e., distal segments of the common bile duct and main pancreatic duct). These tumors protrude into the duodenal lumen through a dilated orifice (see Fig. 41.4).
Carcinoma of the ampullary duodenum usually manifests as a large, polypoid, and ulcerovegetative mass on the duodenal surface of the ampulla. The orifice of the ampulla is usually located eccentrically in the proximal aspect of the tumor (see Fig. 41.5). In contrast, ampullary duct carcinomas show minimal changes (from the duodenal perspective) such as a button-like mucosal-covered elevation or a small, flat ulcer, and some show subtle and relatively small, plaquelike areas that circumferentially constrict the distal segments of the common bile duct and main pancreatic duct (see Fig. 41.2). Ampullary-NOS carcinomas are often ulcerated, polypoid tumors localized at the papilla of Vater proper. These tumors usually do not show features characteristic of the other subtypes of ampullary tumors.
Ampullary carcinomas are often associated with dilation of the common bile duct and sometimes of the main pancreatic duct. Ductal dilation is usually most striking in cases with a prominent preinvasive component, particularly in IAPNs or tumors of the ampullary duodenum. It is least prominent in ampullary duct tumors. Most ampullary carcinomas are resected by pancreatoduodenectomy. The margins are not usually involved. Extensive ampullary carcinomas are typically unresectable because of distant metastases.
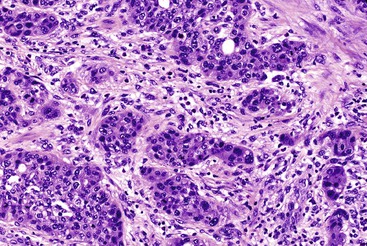
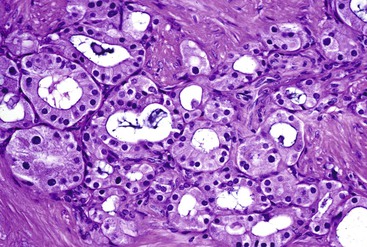
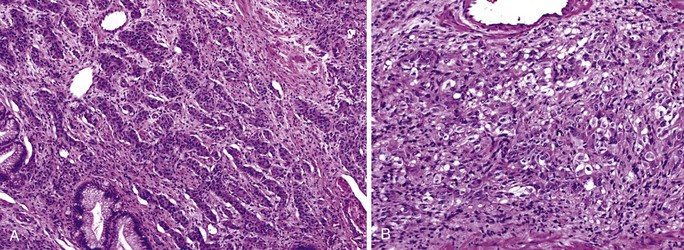
Microscopically, ampullary adenocarcinomas are highly versatile in their morphologic appearance. The microscopic appearance of ampullary adenocarcinomas reflects their origin in intestinal and biliary epithelium, and hybrid phenotypes are identified (see Figs. 41.1, B and 41.3, B). Most invasive adenocarcinomas are tubular. Some are intestinal-type tumors15,40,46,47 that are similar to conventional colonic adenocarcinomas (see Fig. 41.15). Those that resemble pancreatic or bile duct adenocarcinomas are called pancreatobiliary-type adenocarcinomas (see Fig. 41.16). Other histologic types of carcinoma (discussed later) include mucinous (colloid) carcinoma (Fig. 41.17), poorly cohesive carcinoma (with or without signet ring cells) (Fig. 41.18), micropapillary adenocarcinoma (Fig. 41.19),48 clear cell carcinoma, adenosquamous carcinoma, poorly differentiated and undifferentiated carcinomas (Fig. 41.20).15
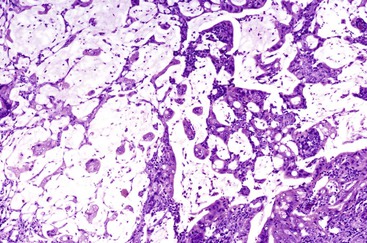
Intestinal-type adenocarcinomas are commonly associated with intestinal-type adenomas. Although they may have any type of gross pattern, most carcinomas of the ampullary duodenum are the intestinal type. Histologically, they are composed of individual glands and cribriform nests of tumor cells with an associated desmoplastic stromal response (see Fig. 41.15). Luminal necrosis is a common feature. The neoplastic glands are often elongated and branched. Many cases exhibit an expansile growth pattern. The cells are columnar and have various amounts of cytoplasmic mucin. Well-differentiated cases may also contain goblet cells. The nuclei are typically elongated, pseudostratified, and have moderate to marked pleomorphism. Rarely, Paneth cell differentiation may be seen,49 and extracellular mucin pools may be identified. An extensive mucinous component (>50% of the tumor volume) justifies a diagnosis of mucinous adenocarcinoma (see Fig. 41.17). Solid nests of cells and single cells are characteristic of poorly differentiated cases (see Fig. 41.20).
Pancreatobiliary-type adenocarcinomas are more commonly associated with an ampullary duct preneoplastic component, usually the flat type,4 consistent with the hypothesis that they arise from the terminal pancreatic or biliary ducts. Some arise from IAPNs, adenomas of the papilla of Vater, or the duodenal adenomas.
Examination of pancreatobiliary-type adenocarcinomas usually reveals a proliferation of individual glands that are relatively small, remarkably well formed, and widely separated and that infiltrate within an extensively desmoplastic stroma (see Fig. 41.16). Some glands are more complex, showing papillary or micropapillary projections. Cribriform glands also may be seen. The cytoplasm of tumor cells contains mucin that in some cases may impart a foamy, clear cell appearance to the tumor. The nuclei are round and moderately atypical. They usually lack the characteristic pseudostratification pattern of intestinal-type adenocarcinomas.
Because the ampulla is a transitional region, it is not surprising that many ampullary carcinomas have a mixed phenotypic appearance. In fact, a significant proportion of cases are difficult to place into one of the categories as intestinal or pancreatobiliary, and there is also significant interobserver variability.14,49a Different regions of a single tumor may show different patterns of growth. Budding is common in all ampullary carcinomas, and areas of budding often exhibit pancreatobiliary-like morphology.50
Ancillary Diagnostic Tests and Molecular Properties
With immunohistochemical staining, ampullary adenocarcinomas express keratins and glycoproteins, some of which vary according to the particular histologic type of tumor. Intestinal-type adenocarcinomas express markers of intestinal differentiation, such as CDX2 and MUC2; CK7 expression varies, and MUC1 staining is usually negative.51 The immunophenotype of pancreatobiliary-type adenocarcinomas resembles that of pancreatic and biliary carcinomas, showing consistent expression of CK7, CK19, and MUC1, whereas staining for MUC2 and CDX2 is usually negative.51 Hybrid (transitional) tumors show a mixed immunophenotype. For example, almost 50% of cases coexpress CK7 and CK20. A variety of glycoproteins, such as carcinoembryonic antigen and B72.3, may be expressed in any histologic subtype. Stains for chromogranin or synaptophysin may reveal scattered neuroendocrine cells in all subtypes.
Other histologic types of carcinoma have an immunophenotype that corresponds to the line of differentiation. For instance, squamous areas within adenosquamous carcinomas express squamous lineage markers such as P63 and K903. Mucinous carcinomas may acquire MUC2 and MUC6 expression. Poorly cohesive carcinomas (with or without signet ring cells) show strong MUC5AC positivity, as some do for MUC6, but they typically lack intestinal differentiation, as indicated by MUC2 and CDX2 positivity. Micropapillary adenocarcinomas show CK7 positivity and reversal of cell polarity with MUC1 (i.e., stroma-facing surface of the cells are positive). Undifferentiated and sarcomatoid carcinomas lose cytokeratin expression but acquire vimentin positivity.
Molecular Features
Ampullary carcinomas reveal molecular alterations similar to those of ductal adenocarcinomas of the pancreas and colorectum.52 KRAS mutations are found in approximately 40% of cases. The pattern of specific mutations is similar to that of colorectal carcinomas.52–56 Increased expression of TP53 is detected in 70% of cases.53,57–60 Sixty-four percent of ampullary carcinomas that arise in FAP contain APC gene mutations, but only 17% of sporadic ampullary carcinomas have mutations in this gene.61 Mutations of the β-catenin gene (CTNNB1) are also uncommon.62 Alterations of the SMAD4 (DPC4) gene are rare in ampullary carcinomas.63 A small proportion of poorly differentiated ampullary carcinomas with morphologic features that resemble medullary carcinomas of the large bowel demonstrate microsatellite instability,63a and in fact, a recent study63b has shown that loss of DNA mismatch repair proteins may be as common as in colorectal cancers, although the earlier literature had conflicting results on this.51,64 In most of these studies, carcinomas arising in different compartments of the ampulla and those with various histologic types, including intestinal and pancreatobiliary, have been analyzed indiscriminately.
Differential Diagnosis
The differential diagnosis of ampullary tumors includes primary tumors of adjacent structures. Gross evaluation is critical to determine the primary site of origin of any tumor of this region, and some guidelines for this have been proposed recently.5a With the principle that most tumors expand uniformly from their site of origin, it is presumed that the center of a neoplasm represents the true primary site. Careful examination of the luminal aspect of the duodenum and well-oriented tissue sections of the ampulla helps to determine the relationship of the tumor to normal anatomic structures.
When distinguishable from mucosal colonization by an underlying invasive carcinoma (see Fig. 41.10), the location of a residual preinvasive neoplasm suggests its site of origin. Well-developed intestinal differentiation in tumors suggests an ampullary origin, because this feature is virtually nonexistent in primary pancreatic and common bile duct carcinomas. However, there is extensive overlap in the histologic, immunophenotypic, and molecular alterations among carcinomas of the ampulla, pancreas, and bile duct. Therefore, careful dissection and sampling of the pancreatoduodenectomy specimen is crucial for determining the precise origin of the tumor.5a
Natural History and Treatment
Ampullary carcinomas have an overall survival rate far better than pancreatic and distal common bile duct cancers. The 5-year survival rate is approximately 40%.4,65,66 Rates of survival appear to be even better than for nonampullary duodenum cancers.67 This rate may be the result of early detection. Some studies have shown a more favorable outcome for stage-matched pure ampullary carcinomas compared with pancreatic tumors.68 However, this may not be an accurate comparison because the staging parameters used for the two tumors are quite different. Nevertheless, ampullary carcinomas are commonly diagnosed early. Their strategic location at the junction of the common bile duct and main pancreatic duct causes symptoms early in the course of disease.
Ampullary carcinomas often arise from a precursor (adenomatous) lesion, and the mean size of the invasive component is usually significantly smaller than primary pancreatic carcinomas.65 Surgical resectability is an important factor. Positive margins occur in less than 5% of ampullary carcinomas,65 compared with at least 35% of pancreatic tumors. Tumor cell biology may also be a factor in survival rates for ampullary and pancreatic tumors. Some studies have shown that pancreatobiliary-type adenocarcinomas of the ampulla have a worse prognosis than tumors with intestinal differentiation.12,30,69,70 Even pancreatobiliary-type adenocarcinomas that arise in the ampulla have a better prognosis than those that arise from the pancreas or common bile duct, likely because of early detection.4,65
Site-specific subcategories of ampullary tumors have distinct biologic and prognostic properties.4 For instance, IAPN-associated invasive carcinomas often have only a small invasive component (mean, 1.5 cm). Not surprisingly, they have a better prognosis.5a,14 Tumors of the ampullary duodenum are typically associated with a prominent preinvasive component, and although they usually are high stage (i.e., very large size with lymph node metastasis), their prognosis is fairly good. In contrast, ampullary duct tumors often reveal a small (most are <2 cm), invasive carcinoma but still exhibit aggressive behavior. Although ampullary duct tumors are invariably of the pancreatobiliary type, they still have a significantly better prognosis than primary pancreatic or distal bile duct carcinomas.4,66
Other factors associated with prognosis include histologic grade, size of invasive carcinoma, pathologic stage, an adenomatous component,14 and tumor budding.50 Abnormal DNA ploidy, increased tumor proliferation rate, and lymphovascular invasion also have been associated with a poor prognosis.51,71–75
Uncommon Carcinomas
Most uncommon tumors are similar to intestinal- and pancreatobiliary-type carcinomas in terms of their clinical presentation, natural history, and prognosis. The following histologic subtypes are important mainly because of their distinct morphologic patterns of growth.
Mucin production is noted in about 15% of ampullary carcinomas45,76,76a and 10% qualify as mucinous carcinoma, with >50% stromal mucin deposition (see Fig. 41.17). These are mostly mixed-mucinous carcinomas. True colloid carcinomas, in which the carcinoma cells are almost all floating within the mucin, are very uncommon. Mucinous carcinomas of the ampulla are seen more commonly in men and tend to arise from the ampullary duodenum.15 The carcinoma cells often reveal intestinal (rather than pancreatobiliary) phenotype, which is also illustrated by frequent positivity of MUC2 and CDX2. A significant proportion of the cases have mixed ordinary (tubular) adenocarcinoma component. Signet ring cells may be prominent, either confined within the mucin lakes (mucinous signet-ring cell carcinoma), or sometimes infiltrating as cellular/non-mucinous infiltrates into the stroma. Mucinous carcinomas are often fairly large and two-thirds have lymph node metastasis at presentation, but their behavior appears to be less aggressive than what would be expected from advanced tumors. Of note, in a recent study76a mismatch repair protein alteration was found not uncommonly in those with focal mucin production but was very uncommon in those with more profuse mucin formation.
Poorly cohesive cell carcinomas (with or without signet ring cells) are rare.77–79 Unlike mucinous adenocarcinomas, the signet ring cells are individually arranged and infiltrate the stroma in a single-file pattern resembling diffuse-type gastric adenocarcinomas (see Fig. 41.18). Significant extracellular mucin pools are absent. A poorly cohesive cell pattern may be found in other types of ampullary adenocarcinomas. However, this diagnosis is applied when more than 50% of the tumor volume consists of these elements. The differential diagnosis includes metastases from the stomach or breast (i.e., lobular carcinoma). The latter can be distinguished by immunohistochemical staining for hormone receptors. These tumors have gastric differentiation; most are positive for MUC5AC, and more than 50% are positive for MUC6. They usually lack intestinal differentiation; most are negative for CDX2, and staining for MUC2 is positive in only a few cases.79
Adenosquamous carcinomas contain glandular and squamous elements. Adenosquamous carcinomas comprise 1% to 3% of all ampullary primaries.38,80,81 Although any degree of glandular differentiation (in a predominantly squamous neoplasm) is considered sufficient for a diagnosis of adenosquamous carcinoma, by convention, at least 25% of an adenocarcinoma should exhibit squamous differentiation to qualify.15 The glandular areas usually resemble a pancreatobiliary-type adenocarcinoma. The squamous elements grow in sheets and nests, and they are composed of polygonal cells with a generous amount of eosinophilic cytoplasm. Keratinization is usually evident. In cases with a predominantly squamous pattern, stains for mucin may help to identify areas of glandular differentiation. Squamous areas are often positive for P63 or K903.
Clear cell carcinomas resemble renal cell carcinomas, including the characteristic sinusoidal vascular pattern. However, mucin production is also evident.
Micropapillary carcinomas as described in the breast, urinary bladder, and other organs (characterized by small clusters of cells lying within clefts) can occur in the ampulla (see Fig. 41.19).82,83 This can be a focal finding, but it may occasionally be the predominant pattern. In one study, these tumors were found to be aggressive.48
Carcinomas with neuroendocrine differentiation can arise in adenocarcinomas of the ampulla. When the neuroendocrine component is substantial (>50%), the lesion is classified as a high-grade (poorly differentiated) neuroendocrine carcinoma (discussed later). There is emerging evidence in the remainder of the gastrointestinal tract that it may be important to recognize even a small neuroendocrine component, as long as it is convincing. Cis-platinum based therapy may have to be considered for such cases.
Other uncommon carcinomas can occur in the ampulla. Some ampullary adenocarcinomas are too poorly differentiated to permit classification as one of the previously described types. They may be assigned to the group of poorly differentiated, NOS adenocarcinomas (see Fig. 41.20). Rarely, sarcomatoid differentiation may occur in the form of a spindle cell carcinoma (i.e., sarcomatoid carcinoma) or as a biphasic neoplasm showing well-defined glandular elements mixed with distinct sarcomatoid elements (i.e., carcinosarcoma). In both cases, the sarcomatoid region often retains epithelial differentiation (i.e., immunoexpression of keratins). Undifferentiated carcinomas with osteoclast-like giant cells, identical to those that occur in the pancreas, rarely develop in the ampulla.84
Staging of Ampullary Carcinomas
The AJCC TNM staging manual of ampullary cancers defines parameters for the T stage.39 A T1 tumor is confined to the sphincter of Oddi, a T2 tumor invades the duodenum, a T3 tumor invades the pancreas, and a T4 tumor invades peripancreatic soft tissue (Table 41.5). Although this system is useful for intraampullary carcinomas, it is a challenge for ampullary carcinomas that arise from other compartments of this organ.85 For example, a minute carcinoma located in the Papilla of Vater (see Fig. 41.7) is classified as T2 (because of duodenal involvement), but a much larger intraampullary carcinoma confined to the sphincter of Oddi is classified as T1. This may explain why survival rates for T1 tumors are worse than those for T2 tumors according to the AJCC document.39 It has been proposed to stage the cases with duodenal submucosal involvement as T1b.85a
Table 41.5
American Joint Committee on Cancer Tumor Staging for Ampulla of Vater Cancers
| Tumor Stage | Definition |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of a primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to the ampulla of Vater or sphincter of Oddi |
| T2 | Tumor invades the duodenal wall |
| T3 | Tumor invades the pancreas |
| T4 | Tumor invades the peripancreatic soft tissues or other adjacent organs or structures other than pancreas |
Data from Edge SE, Byrd DR, Compton CC, et al, eds. Ampulla of Vater. In: AJCC Cancer Staging Manual and Handbook. 7th ed. New York: Springer; 2010:235-240.
The AJCC TNM staging scheme presumes that the ampulla is (concentrically) surrounded by the duodenum and pancreas. However, in the posterior aspect, there is no pancreatic tissue, and ampullary carcinomas may invade directly into the periduodenal or peripancreatic soft tissues and duodenal serosa5a (Fig. 41.21). A significant proportion of ampullary carcinomas involves this anatomic region. Some authorities classify these tumors as duodenal and report periduodenal soft tissue involvement as T3 and serosal involvement as T4.85
Neuroendocrine Neoplasms and Related Tumors
Neuroendocrine cells are uncommon within the pancreatobiliary portion of the ampulla.86 The overlying duodenal epithelium contains the same amount of neuroendocrine cells as the surrounding nonampullary duodenal mucosa. Nevertheless, a variety of neuroendocrine neoplasms occur in the ampulla.
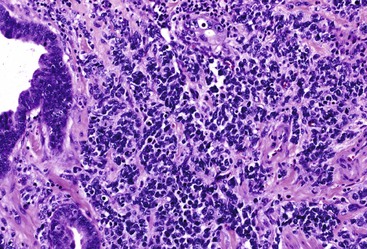
Ampullary neuroendocrine neoplasms are divided into well-differentiated and poorly differentiated categories, which do not appear to be closely related. Well-differentiated neuroendocrine neoplasms are essentially carcinoid tumors (Fig. 41.22), although some have distinct clinical and pathologic features. In addition to carcinoid-type tumors, not otherwise specified, clinically functional gastrinomas may arise within the periampullary region, as well as histologically distinctive glandular duodenal carcinoids, which contain somatostatin. These tumors have been called ampullary somatostatinomas (Fig. 41.23). A distinct but related entity is gangliocytic paraganglioma, a neoplasm that combines features of a carcinoid tumor with those of a nerve sheath tumor (Fig. 41.24). Poorly differentiated neuroendocrine carcinomas include small cell (Fig. 41.25) and large cell variants.
Well-Differentiated Neuroendocrine Tumors
Clinical Features
Well-differentiated neuroendocrine tumors (WDNETs), previously referred to as carcinoids, comprise approximately 3% of all tumors in the ampulla. They arise in adults in their 40s and 50s and have a male predominance.87–90 Most are solitary and sporadic, but this region is a common site of WDNETs in multiple endocrine neoplasia (MEN) I, including gastrinomas associated with Zollinger-Ellison syndrome.91–93 Ampullary somatostatinomas develop in patients with neurofibromatosis.94–99
Most ampullary and duodenal WDNETs are clinically nonfunctional, with the exception of gastrinomas. Somatostatin-producing pseudoglandular tumors are not clinically functional, in contrast to some that occur within the pancreas.89,98,100,101 Carcinoid syndrome is rare.89
The clinical presentation is similar to that of other types of ampullary tumors.89,90,102 Duodenal gastrinomas may be difficult to identify clinically because of their small size. In patients with MEN I, it can be difficult to determine which of the multiple, small neuroendocrine neoplasms in the pancreas and duodenum are functional.103 Because most sporadic gastrinomas arise in the head of the pancreas or duodenum (or, rarely, within the peripancreatic lymph nodes), pancreatoduodenectomy has been advocated for patients with Zollinger-Ellison syndrome to remove the gastrinoma triangle.91 Because most pancreatic gastrinomas are sizable, the ampulla and duodenum in the gastrinoma triangle are the primary locations for most grossly inapparent tumors.103
Pathologic Features
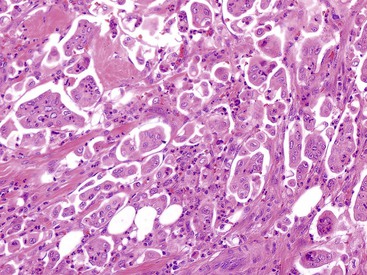
WDNETs are usually small (3 cm in diameter or less), well-demarcated, submucosal nodules composed of uniform, tan tissue.88 Those arising in the ampulla may surround the distal pancreatic and bile ducts. Histologically, most nonfunctional cases (e.g., gastrinomas) are indistinguishable. They resemble their counterparts from other parts of the gastrointestinal (GI) tract, such as the stomach or pancreas (see Fig. 41.22).88 Typically, nests and ribbons of uniform cells are surrounded by some degree of hyalinized stroma. Despite being well circumscribed, these tumors usually infiltrate the surrounding tissues, entrap periampullary ductules within the tumor, and produce lymph node metastases, particularly by tumors that are larger than 2 cm in the greatest dimension. Individual cells have a modest amount of pale to eosinophilic cytoplasm. The nuclei are uniform in size and shape and contain stippled chromatin. The proliferative rate is usually low. Most cases have fewer than 10 mitoses per 10 high-power fields, and many cases have no more than 1 to 2 mitoses per 10 high-power fields. Necrosis may be focal and punctate, but it is usually absent.
Ampullary somatostatinomas (i.e., glandular psammomatous neuroendocrine tumor of the duodenum) have distinctive features (see Fig. 41.23).88,95–99,104,105 Although examination of any type of WDNET can reveal focal lumen formation, ampullary somatostatinomas are extensively gland forming. They usually show individual tubular glands that infiltrate the stroma (see Fig. 41.23). Some cribriform glands may also occur. The tumor cells have relatively abundant cytoplasm, which is eosinophilic and finely granular. Psammoma bodies are often found within glandular lumina, and luminal mucin may be seen. In some cases, the nuclei are basally oriented and uniform in appearance. The mitotic rate is low. Ampullary somatostatinomas have more frequent metastases than other types of duodenal WDNETs in this region.88 In approximately one fourth of patients, ampullary somatostatinomas are associated with neurofibromatosis. In some cases, neurofibromatosis is evident in the resection specimen because of abnormal nerves that have a spectrum of neurofibroma-like changes or because an incidental gastrointestinal stromal tumor is found.94,95,97,99,100,104 A distinctive type of sclerotic change within uninvolved blood vessels has been identified in ampullary somatostatinomas.
On immunohistochemical staining, all of these tumors express neuroendocrine markers such as chromogranin and synaptophysin. Staining for keratin is also positive. A variety of peptides and bioamines can be detected, even in cases that are clinically nonfunctional.88,95,98,106–109 Gastrinomas usually label for gastrin, and somatostatinomas typically express somatostatin. The Ki67 labeling index for all types of duodenal and ampullary WDNETs is usually less than 10%.
Differential Diagnosis
The differential diagnosis depends on the specific histology of the tumor. Cases that show a nested or trabecular architecture may be confused with a poorly differentiated neuroendocrine carcinoma, but the latter neoplasms have a high mitotic rate (more than 10 mitoses per 10 high-power fields), extensive necrosis, and nuclear pleomorphism. The Ki67 labeling index is typically greater than 50%.110 Gland-forming examples may resemble adenocarcinomas, but the lack of a high degree of nuclear atypia, mitotic activity, and intracellular mucin helps to separate adenocarcinomas from carcinoid tumors. Gland-forming somatostatinomas can be mistaken for normal Brunner glands in endoscopic biopsies.
Poorly Differentiated Neuroendocrine Carcinoma
Poorly differentiated (high-grade) neuroendocrine carcinomas are categorized as small cell or large cell types. They are uncommon carcinomas of the ampulla, but based on the numbers of reported cases, they are more common in this anatomic location than in the pancreas.71,123–131
The clinical features are similar to other types of ampullary carcinomas.131 The mean age at diagnosis is 69 years, and most patients are men. Many cases of poorly differentiated neuroendocrine carcinomas are associated with ampullary adenomas, and an adenocarcinoma component may be found.
The histologic features resemble those that arise in the lung. Small cell carcinomas contain sheets and nests of small cells with minimal cytoplasm, fusiform nuclei, finely granular chromatin, and inconspicuous nucleoli (see Fig. 41.25). Focal squamous differentiation may occur.132 Large cell neuroendocrine carcinomas possess larger cells with moderate amounts of cytoplasm and round to oval nuclei, often with prominent nucleoli.
The high-grade nature of poorly differentiated neuroendocrine carcinomas is reflected in their high proliferation rate, which is usually more than 10 mitoses per 10 high-power fields (by definition) and often in the range of 40 to 60 in large cell neuroendocrine carcinoma to more than 80 in small cell carcinoma. The Ki67 labeling index for these tumors is typically more than 50%.110 Necrosis is usually extensive. Both types of tumors immunostain for chromogranin and synaptophysin, although this is typically more focal than in well-differentiated neuroendocrine neoplasms. A diagnosis of small cell carcinoma does not require expression of neuroendocrine markers when classic cytologic features are evident.
These tumors are highly aggressive. Rapid dissemination and death from disease usually occurs within 2 years of diagnosis.127,129
Gangliocytic Paraganglioma
Gangliocytic paraganglioma is a rare tumor that occurs almost exclusively in the ampullary region (Fig. 41.24). Patient age at diagnosis varies widely (mean, 53 years), and men are affected more often than women.88,111–113 Most gangliocytic paragangliomas are benign, and cases with metastases are rare.
Gangliocytic paragangliomas are usually 1 to 3 cm in diameter, nodular, and occur in the submucosa within a few centimeters of the ampulla. Some are pedunculated.15 Microscopically, gangliocytic paragangliomas contain three distinct cellular components, although in proportions that vary widely between cases and even within an individual tumor (see Fig. 41.24). Carcinoid-like elements form nests and ribbons of cells. They resemble cells of a typical carcinoid tumor or paraganglioma. Scattered, large, ganglion-like cells contain large nuclei with prominent nucleoli and granular cytoplasm. Spindle cells resemble peripheral nerve elements, and they may contain Schwann cells. The various cellular components are usually intimately intermixed, although some cases may show a rather sharp segregation between the different cellular elements.
Each component has a different immunohistochemical staining pattern.114–119 Epithelioid cells are usually keratin positive, although often not as diffusely as typical carcinoid tumors. Staining for chromogranin, synaptophysin, pancreatic polypeptide, and somatostatin is usually positive. Other peptides, such as vasoactive intestinal polypeptide and glucagon, are less frequently positive. Ganglion-like cells express only synaptophysin; staining for keratin and chromogranin is negative. The spindle cell component stains positively for S100 protein and neurofilaments. These findings suggest that gangliocytic paragangliomas contain elements of endodermal (i.e., carcinoid-like cells) and neuroectodermal (i.e., Schwann and ganglion-like cells) origin, which is an unusual combination of findings that has evoked some intriguing hypotheses regarding the possible origin.112–114,119,120
Consistent expression of pancreatic polypeptide, which is normally produced by islet cells derived from the embryonic ventral lobe of the gland, and the location of gangliocytic paragangliomas along the course of developmental migration of the ventral pancreas suggest a relationship to pancreatic development. However, it is unknown why these tumors occur only in the duodenum and not in the pancreas. Other investigators have suggested a relationship to an embryonic structure, the sympathetico-insular complex, which contains islet cells and branches of sympathetic nerves, including ganglion cells.119,121
Some have proposed that only the epithelial component of gangliocytic paragangliomas is truly neoplastic. The neuroectodermal components may be reactive and derived from the myenteric plexus. However, the observation of Schwann cell elements within metastases argues against this theory. Even when metastatic, these tumors seem to be benevolent.114,117,122
Mesenchymal Neoplasms
Few types of mesenchymal neoplasms arise specifically in the ampulla. Tumors of the duodenal wall, such as GI stromal tumors or submucosal lipomas, may involve the ampulla secondarily and manifest clinically with obstructive jaundice.15 Neurofibroma, ganglioneuroma, rhabdomyosarcoma, and Kaposi sarcoma have been reported in the ampullary region, and their pathologic features are similar to those that arise in other anatomic locations.133–139 Sarcomas should be distinguished from sarcomatoid carcinomas, which are more common and show immunohistochemical evidence of epithelial differentiation (i.e., keratin expression) and a separate, morphologically recognizable carcinoma component.
Tumor-like Lesions
A variety of tumor-like lesions can involve the ampulla and simulate a neoplasm clinically, radiographically, and sometimes pathologically.
Paraduodenal Pancreatitis
Paraduodenal pancreatitis140–143 is a distinctive variant of pancreatitis that occurs in the duodenal wall adjacent to pancreatic tissue (Fig. 41.26). It often centers on the accessory duct and minor ampulla. Paraduodenal pancreatitis is the most common cause of a benign condition that is mistaken for cancer. A significant proportion of paraduodenal pancreatitis cases are initially diagnosed as periampullary cancer or pancreatic cancer, a phenomenon previously referred to as groove pancreatitis or cystic dystrophy in heterotopic pancreas.
Paraduodenal pancreatitis is characterized by an exuberant myofibroblastic proliferation arranged in fascicles. The process narrows the duodenal lumen and scars the duodenal wall. The duodenal mucosa often acquires a nodular or cobblestone appearance. The duodenal mucosa is often thickened and contains Brunner gland hyperplasia in addition to myoid hyperplasia. On sectioning, the duodenal wall musculature, especially in the vicinity of the minor ampulla, has a trabeculated appearance that is often accompanied by a cystic change. In some cases, cyst formation may be prominent, with lesions as large as several centimeters in diameter (i.e., paraduodenal wall cysts) (Fig. 41.27).
Microscopic examination often reveals round (well-circumscribed), small lobules of pancreatic tissue (i.e., myoadenomatosis pattern) or various sizes of ducts (i.e., cystic dystrophy in heterotopic pancreas) scattered among myoid stromal cells. These ducts may contain inspissated acinar enzymes. Some cysts are devoid of epithelium. Instead, they are lined by a more cellular fibroblastic tissue. Occasionally, the lining fibroblasts may appear epithelioid and raise concern about the possibility of a sarcomatoid carcinoma. The cystic contents may extravasate and cause a foreign-body giant cell reaction and stromal eosinophilia.143
Overall, clinically, the lesion displays several stigmata associated with cancer, including a highly infiltrative appearance, dilation of the common bile duct, and narrowing of portal and mesenteric blood vessels. A tubulocystic change in the vicinity of the accessory duct and duodenal wall (in the minor ampullary region) are highly specific features seen on magnetic resonance imaging.
The reasons this process develops mainly around the minor (accessory) ampulla or accessory duct are unknown. In some cases, pancreas divisum (i.e., persistence of embryologic-type, dorsal-ventral separation of drainage systems) is suspected. One possibility is occlusion of a functionally overactive accessory duct (by unknown mechanisms), perhaps triggered by alcohol abuse. However, the macroscopic and microscopic morphologic findings are quite distinctive.
This process appears to have some consistent clinical associations. Most patients are young men in their 40s. Patients often have a history of alcohol abuse, and some have a history of hypertension.
Adenomyomatous Hyperplasia
Adenomyomatous hyperplasia of the ampulla (i.e., adenomyoma) may mimic carcinoma. In some studies, 60% of autopsy cases had this lesion.144 Some pathologists define this entity as having at least a 5-mm thickness of the ampullary musculature. Because the normal ampulla contains tubules within dense muscular tissue (see Fig. 41.1, B), it is difficult to determine specific criteria for adenomyomatous hyperplasia.
In some patients with obstructive jaundice and a suspected tumor in the ampulla, the only pathologic finding proves to be a thickened or exaggerated ampulla (Fig. 41.28). The diagnosis relies heavily on clinical findings, particularly raised intraampullary pressure, combined with exclusion of other pathologic conditions.
Other Tumor-Like Conditions
Papillary hyperplasia (Fig. 41.29) is a poorly characterized type of intraampullary lesion that consists of an exaggeration of the normal papillae.15 The papillae are lined by biliary-type epithelium and sometimes contain an expanded fibrovascular core. Cases with inflammation may also have reactive epithelial changes. However, the degree of nuclear pseudostratification characteristic of intestinal-type adenomas and the marked degree of atypia of true papillary neoplasms are absent in this condition. The diagnosis rests on the recognition that the extent of papilla formation exceeds that normally found in the ampulla.
Pancreatic heterotopia can occur throughout the upper GI tract, including the ampulla of Vater,145,146 although symptomatic cases at this anatomic site are rare. Pancreatic acini, ducts, and islets are found adjacent to ducts within the muscularis of the sphincter of Oddi. Pancreatic tissue is commonly associated with the minor ampulla and represents residua from involution of the terminal ventral embryonic pancreatic duct. In some cases, there is no acinar or neuroendocrine tissue, only scattered ducts, which are usually embedded within hyperplastic smooth muscle bundles. Brunner gland hyperplasia, which consists of nodules of histologically normal Brunner glands mixed with smooth muscle fibers, also can affect the ampulla.147–149
Gross Evaluation and the Surgical Pathology Report
Classification of ampullary tumors and their differentiation from neoplasms of nearby sites rely heavily on gross examination.5a Opening the duodenum along the antiampullary side and placing a probe into and through the common bile duct to locate the ampullary orifice in the lesion provides the key findings. For example, ampullary duodenal tumors most often form large, variegated, ulcerovegatative lesions on the duodenal surface of the ampulla with an eccentric location of the orifice. IAPNs typically have a centrally located orifice with evenly distributed granular or polypoid protrusions into the duodenal lumen. The ampullary duct cases show minimal changes and are often covered by mucosa, and the common bile duct is usually difficult to probe.
Different methods may be used to section the ampulla as long as all the important parameters are documented. Transverse (amputative) sectioning of the ampulla may allow documentation of intraampullary tumor spread beyond the sphincter of Oddi. However, this approach typically fails to capture the extent of a carcinoma that arises from the edge of the papilla of Vater. Evaluation of the groove region (i.e., posterior aspect of the ampulla that is not covered by pancreatic tissue) usually requires specific attention to this area. It is advisable to ink the pancreatoduodenal soft tissue, open the ducts, and obtain a perpendicular section, including the tumor with the duodenal serosa.
Standard information should be included in the surgical pathology report for ampullary carcinomas. Site-specific classification should be provided as ampullary-NOS, ampullary duodenum, ampullary duct, or IAPN-associated tumors (see Fig. 41.6). Histologic typing of carcinoma as intestinal (see Fig. 41.15), pancreatobiliary (see Fig. 41.16), or others should be provided separately, if feasible. Cases with unclassifiable patterns should be documented as such. For mixed-pattern cases, the types identified in the diagnostic section and their proportions should be provided in a comment. For cases with a preinvasive (adenomatous) component, the available characteristics should be provided separately, including the location (i.e., ampullary duodenal adenoma or IAPN), size, and cell lineage.
The size of an invasive carcinoma should be documented separately by direct measurement or estimation of the percentage of the overall tumor.4,150 In staging ampullary carcinomas, most investigators still report duodenal involvement (including duodenal mucosal involvement) as T2, although this leads to serious overstaging of small tumors arising in the papilla of Vater (because they also involve the duodenal mucosa), and all ampullary duodenal cases become T2 by default.85 Staging protocols are under development for these cases.151 For cases with invasion into the periduodenal soft tissue at the groove area, most authorities advocate designating them as T3, not T4 (i.e., not as peripancreatic soft tissue). Duodenal serosal involvement should be regarded as T4, as is the case for any other GI organ.85
Reporting of ampullary carcinomas should include all the conventional parameters for cancer reporting, such as vascular and perineural invasion, margin status, and lymph node status. A pancreatoduodenectomy specimen typically contains more than 12 lymph nodes.5a,152 Orange peeling of the peripancreatic soft tissues before sectioning the pancreatic head can help to ensure maximal node recovery, because the lymph nodes are typically embedded in the surface indentations of the pancreas and can be difficult to harvest after the specimen has been dissected.152
Acknowledgment
We are indebted to Dr. Serdar Balci, Dr. Burcu Saka, Dr. Pelin Bagci, Ms. Rhonda Everett, and Ms. Leslie Ducato for their extensive contributions in the preparation of this chapter.