Tumor Immunology
At the conclusion of this chapter, the reader should be able to:
• Compare the characteristics of benign and malignant tumors.
• Describe the epidemiology of cancer in adults and children.
• Explain the characteristics of the three major causative factors in human cancer.
• Compare the stages of carcinogenesis.
• Describe the aspects of cancer-related genes.
• Define and give examples of proto-oncogenes.
• Describe the role of oncogenes.
• Describe the characteristics of the major body defenses against cancer.
• Identify and discuss the characteristics of tumor markers.
• Discuss what’s new in cancer diagnostic testing.
• Compare various modalities for treating cancer.
• Analyze representative case studies.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principle and clinical applications of the prostate-specific antigen procedure.
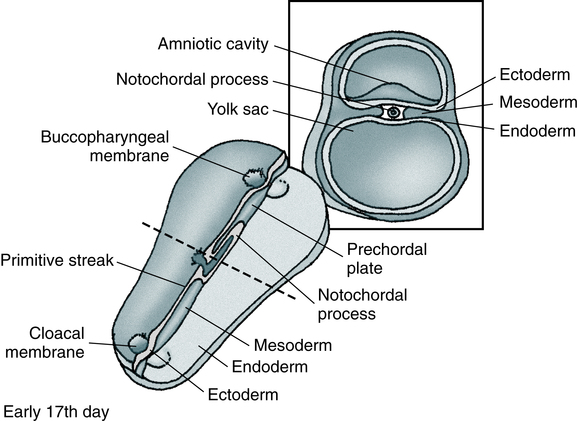
Oncology is that branch of medicine devoted to the study and treatment of tumors. The term tumor is commonly used to describe a proliferation of cells that produces a mass rather than a reaction or inflammatory condition. Tumors are neoplasms and are described as benign or malignant. Most tumors are of epithelial origin (ectoderm, endoderm, or mesoderm); the remaining tumors are of connective tissue origin (Fig. 33-1). The key distinction between benign and malignant tumors is the ability of malignant tumors to invade normal tissue and metastasize to other secondary sites.
Cancer Stem Cells
• Self-renewal when daughter cells retain the same biologic properties as the parent cell
If normal self-renewal is subverted, it becomes abnormal self-renewal. If increased self-renewal occurs, combined with the intrinsic growth potential of stem cells, it may yield a malignant phenotype. It is possible that cancer stem cells can arise by mutation from normal stem cells or mutated progenitor cells (Fig. 33-2).
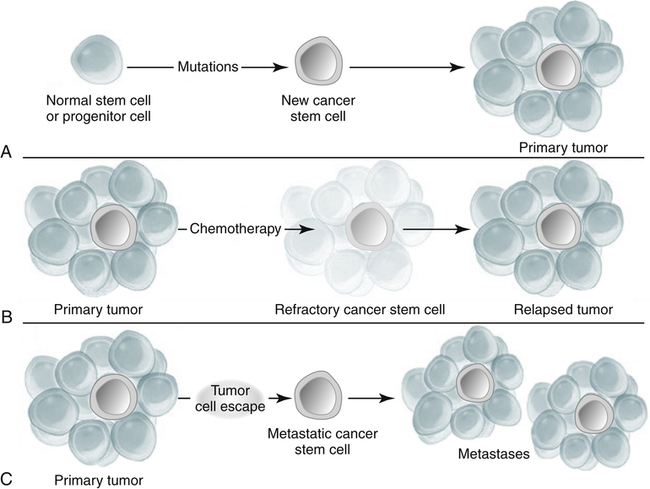
A, Mutation of a normal stem cell or progenitor cell may create a cancer stem cell, which will then generate a primary tumor (Panel A). B, During treatment with chemotherapy, most cells in a primary tumor may be destroyed, but if the cancer stem cells are not eradicated, the tumor may regrow and cause a relapse (Panel B). C, Cancer stem cells arising from a primary tumor may emigrate to distal sites and create metastatic lesions (Panel C). (From Jordan CT, Guzman ML, Noble M: Cancer stem cells, N Engl J Med 355:1253–1260, 2006.)
Types of Tumors
Benign Tumors
Benign tumors are characterized by the following:
Malignant Tumors
Malignant tumors are characterized by the following:
• Increase in the number of cells that accumulate
• Usually, invasion of tissues
• Dissemination by lymphatic spread or by seeding within a body cavity
• Characteristic nuclear cellular features
• Receptors for integrin molecules (e.g., fibronectin), which help malignant cells adhere to extracellular matrix, type IV collagenases, which dissolve basement membranes, and proteases
• Secretion of transforming growth factor α (TGF-α) and transforming growth factor β (TGF-β) to promote angiogenesis and collagen deposition
• Often, recurrence after attempts to eradicate the tumor by surgery, radiation, or chemotherapy
Biologically distinct and relatively rare populations of tumor-initiating cells have been identified in cancers of the hematopoietic system, brain, and breast. Cells of this type have the capacity for self-renewal, the potential to develop into any cell in the overall tumor population, and the proliferative ability to drive continued expansion of the population of malignant cells. The properties of these tumor-initiating cells closely parallel the three features that define normal stem cells. Malignant cells with these functional properties are termed cancer stem cells (Fig. 33-3). Cancer stem cells can be the source of all the malignant cells in a primary tumor.
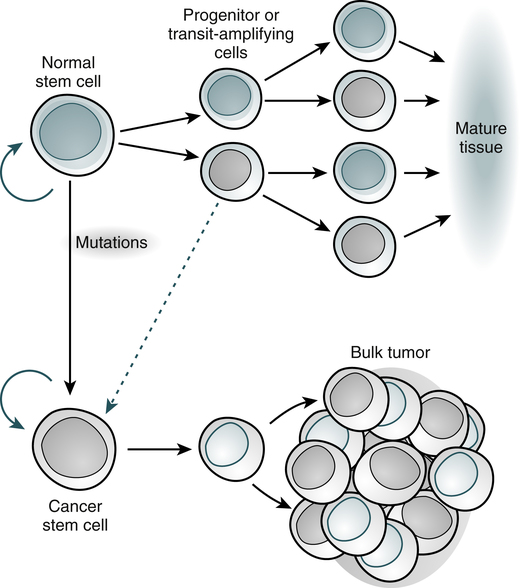
Normal tissues arise from a central stem cell that grows and differentiates to create progenitor and mature cell populations. Key properties of normal stem cells are the ability to self-renew (curved arrow), multilineage potential, and extensive proliferative capacity. Cancer stem cells arise by means of mutation in normal stem cells or progenitor cells and subsequently grow and differentiate to create primary tumors (broken arrow indicates that specific types of progenitors involved in the generation of cancer stem cells are unclear). As with normal stem cells, cancer stem cells can self-renew, give rise to heterogeneous populations of daughter cells, and proliferate extensively. (Adapted from Jordan CT, Guzman ML, Noble M: N Engl J Med 355:1253-1260, 2006.)
Causative Factors in Human Cancer
Environmental Factors
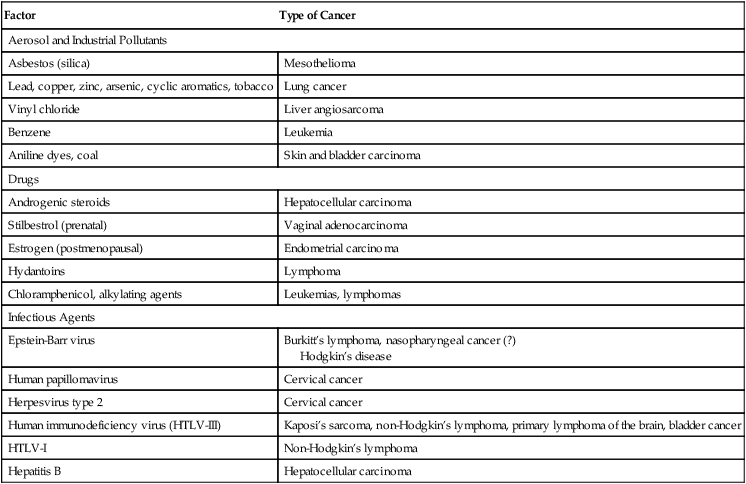
The incidence of cancer has been correlated with certain environmental factors. Table 33-1 lists environmental factors that have been definitively linked with cancer, including aerosol and industrial pollutants, drugs, and infectious agents. Radiation exposure is also known to be associated with specific types of cancer (e.g., acute leukemia, thyroid cancer, sarcomas, breast cancer). Women concerned about organochlorine substances (e.g., polychlorinated biphenyls [PCBs], dioxins, pesticides [DDT, banned in 1972]) can be reassured that available evidence does not suggest an association between exposure to these chemicals and breast cancer.
Table 33-1
Selected Environmental Factors Associated With Cancer
| Factor | Type of Cancer |
| Aerosol and Industrial Pollutants | |
| Asbestos (silica) | Mesothelioma |
| Lead, copper, zinc, arsenic, cyclic aromatics, tobacco | Lung cancer |
| Vinyl chloride | Liver angiosarcoma |
| Benzene | Leukemia |
| Aniline dyes, coal | Skin and bladder carcinoma |
| Drugs | |
| Androgenic steroids | Hepatocellular carcinoma |
| Stilbestrol (prenatal) | Vaginal adenocarcinoma |
| Estrogen (postmenopausal) | Endometrial carcinoma |
| Hydantoins | Lymphoma |
| Chloramphenicol, alkylating agents | Leukemias, lymphomas |
| Infectious Agents | |
| Epstein-Barr virus | Burkitt’s lymphoma, nasopharyngeal cancer (?) Hodgkin’s disease |
| Human papillomavirus | Cervical cancer |
| Herpesvirus type 2 | Cervical cancer |
| Human immunodeficiency virus (HTLV-III) | Kaposi’s sarcoma, non-Hodgkin’s lymphoma, primary lymphoma of the brain, bladder cancer |
| HTLV-I | Non-Hodgkin’s lymphoma |
| Hepatitis B | Hepatocellular carcinoma |
Host Factors and Disease Associations
The incidence of cancer is 10,000 times greater than expected in patients with an immunodeficiency syndrome. The increased incidence of lymphomas in congenital, acquired, and drug-induced immunosuppression is consistent with the failure of normal immune mechanisms or antigen overstimulation with a loss of normal feedback control. Table 33-2 lists other cancer-related conditions.
Table 33-2
| Disease | Related Cancer |
| Paget’s disease | Osteogenic sarcoma |
| Cryptorchidism | Testicular cancer |
| Neurofibromatosis | Brain tumors, sarcoma |
| Esophageal webbing | Esophageal carcinoma |
| Achlorhydria and pernicious anemia | Gastric carcinoma |
| Cirrhosis | Hepatoma |
| Cholelithiasis | Gallbladder cancer |
| Chronic inflammatory bowel disease | Colon cancer |
| Migratory thrombophlebitis | Adenocarcinoma, especially pancreatic |
| Myasthenia gravis, pure red cell aplasia, T cell disorder | Thymoma |
| Nephrotic syndrome | Membranous carcinomas; lymphomas, especially Hodgkin’s |
Viruses
Viral causes of some cancers are known. Viruses associated with specific cancers are listed in Table 33-1. Nonpermissive cells that prevent an oncogenic RNA or DNA virus from completing its replication cycle often produce changes in the genome that result in the activation of proto-oncogenes or inactivation of suppressor genes.
Cancer-Predisposing Genes
Cancer-predisposing genes may act in the following ways:
• Affect the rate at which exogenous precarcinogens are metabolized to actively carcinogenic forms that can damage the cellular genome directly
• Affect a host’s ability to repair resulting damage to DNA
• Alter the immune ability of the body to recognize and eradicate incipient tumors
• Affect the function of the apparatus responsible for the regulation of normal cell growth and associated proliferation of tissue
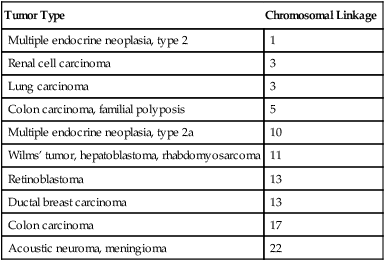
Relatively few cancer-predisposing genes have been described. An absence of functional alleles at specific loci, however, allows the genesis of the malignant process (Table 33-3). For example, individuals with certain mutations in the gene BRCA2 are at a very high risk (up to 85%) for developing breast cancer and other cancers (e.g., ovarian cancer) because a DNA repair path cannot properly repair ongoing wear and tear to the DNA.
Table 33-3
Tumors Associated With Homozygous Loss of Specific Chromosomal Loci
| Tumor Type | Chromosomal Linkage |
| Multiple endocrine neoplasia, type 2 | 1 |
| Renal cell carcinoma | 3 |
| Lung carcinoma | 3 |
| Colon carcinoma, familial polyposis | 5 |
| Multiple endocrine neoplasia, type 2a | 10 |
| Wilms’ tumor, hepatoblastoma, rhabdomyosarcoma | 11 |
| Retinoblastoma | 13 |
| Ductal breast carcinoma | 13 |
| Colon carcinoma | 17 |
| Acoustic neuroma, meningioma | 22 |
Role of Oncogenes
Once an oncogene is activated by mutation, it promotes excessive or inappropriate cell proliferation. Oncogenes have been detected in about 15% to 20% of a variety of human tumors and appear to be responsible for specifying many of the malignant traits of these cells. More than 30 distinct oncogenes, some of which are associated with specific tumor types, have been identified (Table 33-4). Each gene has the ability to evoke many of the phenotypes characteristic of cancer cells.
Table 33-4
Some Oncogenes Formed by Somatic Mutation of Normal Genetic Loci
| Oncogene | Disorder |
| ab1 | Chronic myelogenous leukemia |
| myc | Burkitt’s lymphoma |
| N-myc | Neuroblastoma |
| EGFR, HER2 | Mammary carcinoma |
| Ras type | Wide variety of tumors |
• Growth factors (e.g., sis oncogene)
• Epidermal growth factor receptors (EGFRs)
• Membrane-associated protein kinases (e.g., src oncogene)
• Membrane-related guanine triphosphate (GTP)–binding proteins (e.g., ras oncogene)
• Cytoplasmic protein kinases (e.g., ras oncogene)
• Transcription regulators located in the nucleus (e.g., c-myc oncogene)
Mechanisms of Activation
• Overexpression of the c-erbB-2 (HER2/neu) oncogene is noted in up to 34% of patients with invasive ductal breast carcinoma and predicts poor survival.
• Activation of the ras proto-oncogene (point mutation) is associated with about 30% of all human cancers. About 25% of patients with acute myelogenous leukemia display this point mutation. Ras is mutated frequently in colon and pancreatic cancers; it appears that ras activation leads to unregulated expression of IL-24 and its receptors.
• Translocation of the abl proto-oncogene from chromosome 9 to chromosome 22 with formation of a large bcr-abl hybrid gene on chromosome 22 (Philadelphia chromosome) results in chronic myelogenous leukemia.
• Inactivation of suppressor genes (point mutations) leads to unrestricted cell division, inactivation of each of the RB1 suppressor genes on chromosome 13 is associated with malignant retinoblastoma in children, and inactivation of the p53 suppressor gene on chromosome 17 accounts for 25% to 50% of all malignancies involving the colon, breast, lung, and central nervous system.
Body Defenses Against Cancer
Although there is no single satisfactory explanation for the success of tumors in escaping the immune rejection process, it is believed that early clones of neoplastic cells are eliminated by the immune response. The growth of malignant tumors is primarily determined by the proliferative capacity of the tumor cells and by the ability of these cells to invade host tissues and metastasize to distant sites. It is believed that malignant tumors can evade or overcome the mechanisms of host defenses (Color Plate 18).
Tumor immunity has the following general features:
1. Tumors express antigens that are recognized as foreign by the immune system of the tumor-bearing host.
2. The normal immune response frequently fails to prevent the growth of tumors.
3. The immune system can be stimulated to kill tumor cells and rid the host of the tumor.
Tumor Markers
Tumor markers are substances present in or produced by tumors that can be used to detect the presence of cancer based on their measurement in blood, body fluids, cells, or tissue (Table 33-5). A tumor marker may be produced by the host in response to a tumor that can be used to differentiate a tumor from normal tissue or to determine the presence of a tumor. Non-neoplastic conditions can also exhibit tumor marker activity (Table 33-6). Some tumor markers are used to screen for cancer, but markers are more often used to monitor recurrence of cancer or determine the degree of tumor burden in the patient. To be of any practical use, the tumor marker must be able to reveal the presence of the tumor while it is still susceptible to destructive treatment by surgical or other means. Tumor markers can be measured quantitatively in tissues and body fluids using biochemical, immunochemical, or molecular tests (Table 33-7).
Table 33-5
| Type of Molecule | Biomarkers in Blood or Body Fluid | Type of Cancer Detected |
| Enzyme | Prostate-specific antigen (PSA) | Prostate |
| Oncofetal proteins | Alpha-fetoprotein (AFP); carcinoembryonic antigen (CEA) | Hepatocellular, germ cell Colorectal |
| Hormones | β-Human chorionic gonadotropin (β-hCG); calcitonin; adrenocorticotropic hormone (ACTH) | Trophoblastic Medullary thyroid Small cell lung |
| Mucins | CA 125, CA 19-9, CA 27.29, CA 15-3 | Ovarian Breast |
| Immunoglobulins | Bence-Jones protein (urine) | Multiple myeloma |
| Genetic alteration | HER2/neu | Breast |
| Other proteins | HE4 | Ovarian |
| Tg | Thyroid | |
| Nuclear matrix protein 22 (NMP-22); bladder tumor–associated antigen (BTA)/complement factor H–related protein (CFHrp) | Bladder |
Adapted from Snyder J: Genomic, proteomic developments in tumor markers, Adv Med Lab Prof 16:42–48, 2004; and Rhea JM, Molinaro RJ: Cancer biomarkers, MLO Med Lab Observer, 43:10–18, 2011.
Table 33-6
Non-neoplastic Conditions With Elevated Serum and Plasma Concentrations of Tumor Markers
| Tumor Marker | Concentration in Normal Serum (ng/mL) | Non-neoplastic Conditions |
| CEA | <2.5 | Inflammatory bowel disease, pancreatitis, gastritis, smoker’s chronic bronchitis, alcoholic liver disease, hepatitis |
| AFP | <40 | Pregnancy, regenerating liver tissue after viral hepatitis, chemically induced liver necrosis, partial hepatectomy, cystic fibrosis, ataxia-telangiectasia, premature infants, tyrosinemia |
| β-hCG | Negative | Pregnancy |
| Serum acid phosphatase | Negative | Pregnancy |
| Placental alkaline phosphatase | Negative | Pregnancy |
Table 33-7
| Tumor Markers | Clinical Value |
| CEA | Monitors response to therapy of patients with various types of cancer |
| AFP | Diagnosis of germ cell and hepatic tumors |
| CA 125 | Diagnosis of ovarian cancer |
| β-hCG | Diagnosis of germ cell tumors |
| Prostate acid phosphatase | Diagnosis of prostate cancer |
The search for tumor markers goes back more than 150 years. The earliest identified tumor marker was Bence-Jones protein, a light-chain immunoglobulin, found in patients with multiple myeloma (see Chapter 27). Over the last 15 years, the use of tumor markers in the United States has risen dramatically. Tumor markers play an especially important role in the diagnosis and monitoring of patients with prostate, breast, and bladder cancers.
The list of tumor markers approved by the U.S. Food and Drug Administration (FDA) continues to grow (Table 33-8). Nine of these biomarkers are protein biomarkers identifiable in blood. Other recently approved protein biomarkers can be detected in urine, such as nuclear matrix protein 22, fibrin and fibrinogen degradation products, and bladder tumor antigen for monitoring bladder cancer, and by immunohistochemical methods using tumor tissues, such as estrogen receptor for breast cancer. Additional FDA-approved cancer biomarkers are DNA-based, such as human epidermal growth factor receptor 2 and HER2/neu for breast cancer, and can be assayed by fluorescent in situ hybridization (FISH). Multiple-marker combinations are useful in the management of some cancers (Table 33-9), but the use of more than two markers is questionable.
Table 33-8
Some Common Serum Tumour Markers and Their Clinical Utility∗
| Tumor Type | Cancer Deaths (USA) (%) | Tumour Markers | Specificity | Sensitivity | Tumour Detection | Clinical Utility |
| Lung + bronchus | 28 | Neuron specific enolase | Poor | Poor | Late | Poor |
| Colon + rectum | 9 | Carcinoembryonic antigen (CEA) | Poor | Modest | Late | Modest |
| Breast | 7 | AA 15-3; CEA | Poor | Modest | Late | Modest |
| Pancreas | 6 | CA 19-9: CEA | Poor | Poor | Late | Poor |
| Prostate | 5 | Prostate-specific antigen | Modest | Good | Good | Good |
| Stomach | 2 | CEA; CA 19-9 | Modest | Modest | Late | Poor |
| Ovary | 2.5 | CA 125 | Modest | Modest | Intermediate | Good |
| Liver | 3 | Alpha-feto-protein α-FP | Good | Good | Intermediate | Good |
| Myeloma | 1.9 | Monoclonal protein/FLC | Good | Good | Early | Very good |
| AL amylidosis | 0.3 | Monoclonal protein/FLC | Good | Good | Early | Very good |
| Germ cell | 0.1 | α-FP; human chorionic gonadotrophin (HCG) | Good | Good | Early | Very good |
| Choriocarcinoma | <0.1 | HCG | Good | Good | Early | Very good |
| Neuroendocrine | <0.1 | Chromogranin, A, gastrin | Modest | Good | Early | Very good |
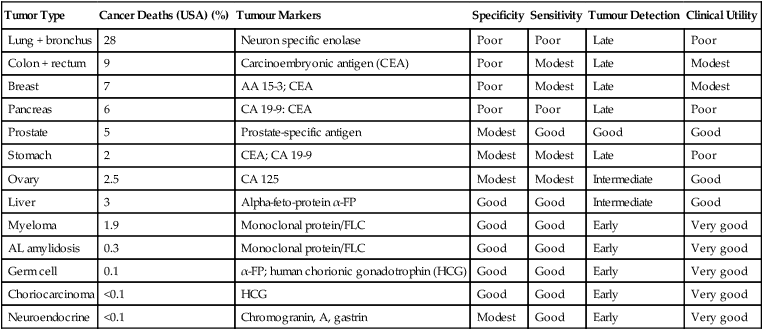
∗All of these are measured using highly sensitive immunoassays apart from monoclonal proteins.
From Bradwell AR: Serum free light chain analysis, Birmingham, UK, 2010, Binding Site, p 2.
Table 33-9
Related Multiple Tumor Markers
| Markers | Comments |
| AFP and β-hCG | Valuable combination in therapy and follow-up in patients with germ cell tumors of the testes. |
| CEA, AFP, and LDH | Combination seems to help differentiate primary liver cancer from liver metastases related to another organ. |
| Ratio of free to total PSA | The ratio may distinguish benign prostatic hypertrophy (BPH) from prostate cancer. |
| CEA and numerous mucin-type markers | May complement each other |
Specific Tumor Markers
Ten protein cancer biomarkers have been FDA-approved for clinical use:
DNA Microarray Technology
New developments in molecular genetics involve DNA microarray technology (see Chapter 14). Cancer can arise not only from mutations in oncogenes and tumor suppressor genes, but also from genes involved in cell cycle control, DNA repair, and apoptosis. Microarrays have the potential to uncover signature gene expression patterns for specific cancers and ultimately assist in the staging of tumors, prognosis, and treatment. Microarrays may help disclose global gene expression pattern differences between healthy and diseased cells as more sensitive and specific diagnostic markers are developed, such as CD44+/CD24− gene expression profile in breast cancer versus normal breast tissue. When differentially expressed genes were used to generate a 186-gene invasiveness gene signature (IGS), the IGS was strongly associated with metastasis-free survival and overall survival for four different types of tumors.
What’s New in Cancer Diagnostic Testing?
Next Generation Sequencing (NGS)
Next Generation Sequencing (NGS) as described in Chapter 14, Molecular Techniques, is another step toward personalized cancer treatment. Three aspects of importance in NGS are:
Continuous Field-Flow Assisted Dielectropheresis (DEP)
1. It permits the isolation of cancer cells from all types of cancer, e.g., lung, prostate, melanoma, breast, pancreatic, and liver.
2. The higher CTC isolation and capture capability provides greater opportunities for downstream analysis of cancer cells for treatment options and monitoring of effectiveness.
3. DEP technology captures the cancer cells in a viable state that allows for additional biological testing.
Modalities For Treating Cancer
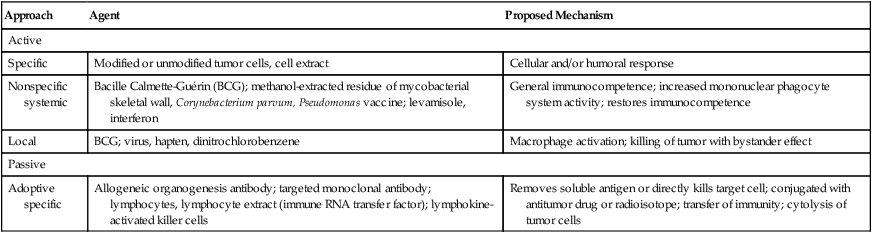
Many different modes of therapy, including angiogenesis inhibitors, which keep tumors from building new blood vessels to supply themselves with food and oxygen, have demonstrated effectiveness in the treatment of cancer (Table 33-10).
Table 33-10
Immunotherapy in Malignant Disease
| Approach | Agent | Proposed Mechanism |
| Active | ||
| Specific | Modified or unmodified tumor cells, cell extract | Cellular and/or humoral response |
| Nonspecific systemic | Bacille Calmette-Guérin (BCG); methanol-extracted residue of mycobacterial skeletal wall, Corynebacterium parvum, Pseudomonas vaccine; levamisole, interferon | General immunocompetence; increased mononuclear phagocyte system activity; restores immunocompetence |
| Local | BCG; virus, hapten, dinitrochlorobenzene | Macrophage activation; killing of tumor with bystander effect |
| Passive | ||
| Adoptive specific | Allogeneic organogenesis antibody; targeted monoclonal antibody; lymphocytes, lymphocyte extract (immune RNA transfer factor); lymphokine-activated killer cells | Removes soluble antigen or directly kills target cell; conjugated with antitumor drug or radioisotope; transfer of immunity; cytolysis of tumor cells |
Chemotherapeutic Agents
Cytokines
Cytokines constitute another group of cancer chemotherapy drugs (see Chapter 5). IFN, IL-2, and colony-stimulating factors (CSFs) have been used to treat certain types of cancer in patients. Currently, IFNs are used to treat patients with hairy cell leukemia, chromic myelogenous leukemia, and multiple myeloma. IL-2 is used in the treatment of renal cell carcinoma and melanoma. CSFs decrease the duration of chemotherapy-induced neutropenia and may permit more dose-intensive therapy.
Effects of Drug-Induced Immunosuppression
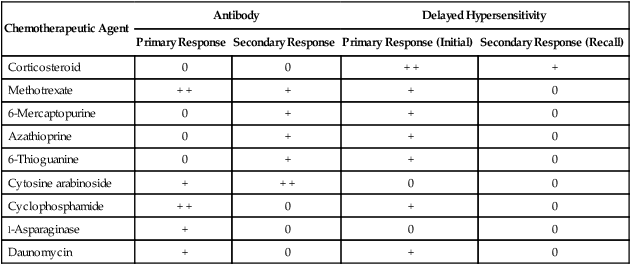
Drugs used to treat malignancies such as solid tumors or leukemia can have profoundly suppressive effects on the inflammatory response, delayed hypersensitivity, and specific antibody production (Table 33-11). Examples of the immune depression induced by drugs include depletion of T cells by corticosteroids, caused by the blocking of egress from the bone marrow into the circulation, and dysfunction of the antibody response, caused by folate antagonists and purine analogues. Thus, infection secondary to immune suppression is a major cause of death in cancer patients beginning therapy and those who are in clinical remission.
Table 33-11
Effects of Chemotherapy on the Immune Response
| Chemotherapeutic Agent | Antibody | Delayed Hypersensitivity | ||
| Primary Response | Secondary Response | Primary Response (Initial) | Secondary Response (Recall) | |
| Corticosteroid | 0 | 0 | + + | + |
| Methotrexate | + + | + | + | 0 |
| 6-Mercaptopurine | 0 | + | + | 0 |
| Azathioprine | 0 | + | + | 0 |
| 6-Thioguanine | 0 | + | + | 0 |
| Cytosine arabinoside | + | + + | 0 | 0 |
| Cyclophosphamide | + + | 0 | + | 0 |
| l-Asparaginase | + | 0 | 0 | 0 |
| Daunomycin | + | 0 | + | 0 |
Recent Advances
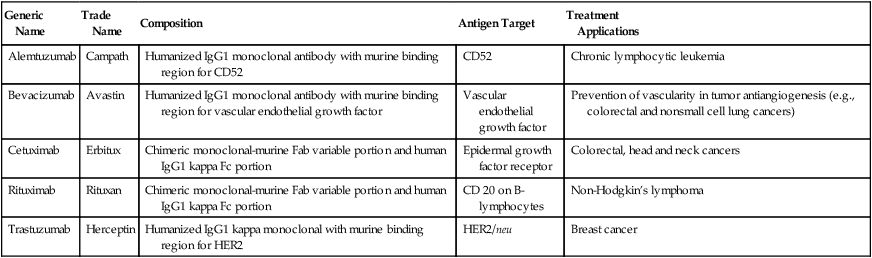
Monoclonal antibody (MAb) technology began with the winning contribution of Köhler, Milstein, and Jerne, who won the Nobel Prize in Physiology or Medicine in 1984. This led to great expectation that MAbs would provide effective targeted therapy for cancer. After early enthusiasm for MAbs, clinical trials were disappointing in the 1980s and early 1990s with one exception, antiidiotype antibodies in follicular lymphoma. When success was finally observed in hematologic malignancies, the importance of the antigen target specificity and developing humanized MAbs was recognized. The major success of mAB therapy has been seen with anti-CD20 MAbs. Anti-CD20 rituximab (Table 33-12) was the first MAb to be approved by FDA for use in relapsed indolent lymphoma. Today, rituximab is widely accepted to be the single most important factor leading to improved prognosis in a range of B cell lymphomas and, more recently, in B cell chronic lymphocytic leukemia (CLL). However, some patients develop resistance to rituximab, which provides a challenge for research.
Table 33-12
Current FDA-Approved Antibodies for Cancer Treatment
| Generic Name | Trade Name | Composition | Antigen Target | Treatment Applications |
| Alemtuzumab | Campath | Humanized IgG1 monoclonal antibody with murine binding region for CD52 | CD52 | Chronic lymphocytic leukemia |
| Bevacizumab | Avastin | Humanized IgG1 monoclonal antibody with murine binding region for vascular endothelial growth factor | Vascular endothelial growth factor | Prevention of vascularity in tumor antiangiogenesis (e.g., colorectal and nonsmall cell lung cancers) |
| Cetuximab | Erbitux | Chimeric monoclonal-murine Fab variable portion and human IgG1 kappa Fc portion | Epidermal growth factor receptor | Colorectal, head and neck cancers |
| Rituximab | Rituxan | Chimeric monoclonal-murine Fab variable portion and human IgG1 kappa Fc portion | CD 20 on B-lymphocytes | Non-Hodgkin’s lymphoma |
| Trastuzumab | Herceptin | Humanized IgG1 kappa monoclonal with murine binding region for HER2 | HER2/neu | Breast cancer |
• Vaccination with killed tumor cells or with tumor antigens or peptides. New research studies have suggested that anti-CD20 MAb may induce an adaptive antitumor immune response or vaccination effect, which may underlie the durable remissions experienced by some patients after anti-CD20 MAb treatment.
• Enhancement of cell-mediated immunity to tumors by expressing costimulators and cytokines and treating with cytokines that stimulate the proliferation and differentiation of T lymphocytes and NK cells.
• Nonspecific stimulation of the immune system by the local administration of inflammatory substances or by systemic treatment with agents that function as polyclonal activators of lymphocytes.
• For the first time in the history of cancer treatment, gene therapy has apparently succeeded in shrinking and even eradicating large metastatic tumors. Inserting genes into a patient’s cells enables the body to fight a disease on its own, without medication.
Passive immunotherapy consists of the following:
• Adoptive cellular therapy by transferring cultured immune cells with antitumor reactivity into a tumor-bearing host.
• Administration of tumor-specific MAbs for specific tumor immunotherapy.
What’s New in Drug Therapy?
The list of drugs used for cancer therapy continues to grow. The new therapeutic agents target various modes of action and applications (Table 33-13).
Table 33-13
Targeted Therapeutic Agents in Cancer
| Classification | Gene | Genetic Alteration |
Drug | Application |
| Nonreceptor tyrosine kinase | ABL | Translocation (BCR-ABL) |
Imatinib | Chronic myelogenous leukemia |
| Receptor, tyrosine kinase | ECFR | Mutation, amplification | Gefitinib, erlotinib | Lung cancer, glioblastoma |
| Serine-threonine-lipid kinase | PI3K | PIK3CA mutations | BEZ235 | Colorectal, breast, gastric cancer, glioblastoma |
| DNA damage or repair | BRCA1 and BRCA2 |
Mutation (synthetic lethal effect) |
Olaparib, MK-4827 (PARP inhibitor) | Breast, ovarian cancer |
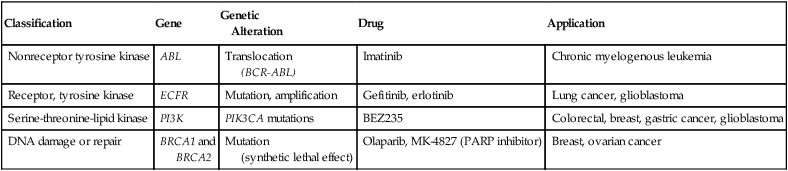
Adapted from McDermott U, Downing JR, Stratton MR: Genomics and the continuum of cancer care, N Engl J Med 364:340–350, 2011.
Chapter Highlights
• Tumors are neoplasms described as benign or malignant. A benign neoplasm is a nonspreading tumor; a malignant neoplasm is a growth that infiltrates tissues, metastasizes, and often recurs after attempts to remove it surgically.
• A malignant neoplasm can be referred to as carcinoma or cancer.
• The incidence of cancer has been correlated with certain environmental factors (e.g., occupational exposure to known carcinogenic agents) and host susceptibility.
• Cancer often begins when a carcinogenic agent damages the DNA of a critical gene in a cell. The mutant cell multiplies and the succeeding generations of cells aggregate to form a malignant tumor.
• Proto-oncogenes act as central regulators of the growth in normal cells and are antecedents of oncogenes.
• The genetic targets of carcinogens, oncogenes, have been associated with various tumor types, largely from preexisting genes present in the normal human genome. Oncogenes are considered altered versions of normal genes that promote excessive or inappropriate cell proliferation.
• Various RNA and DNA viruses have been associated with human malignancies (e.g., Epstein-Barr virus, certain papillomaviruses).
• Viruses carry viral oncogenes into target cells, where they become firmly established. Clonal descendants then carry the viral genes, which maintain the malignant phenotype of the cell clones.
• A very different class of cancer genes was discovered rather recently. Tumor-suppressing genes (antioncogenes) in normal cells appear to regulate the proliferation of cell growth. When this type of gene is inactivated, a block to proliferation is removed and cells begin a program of deregulated growth, or the genetically depleted cell itself may proliferate uncontrollably.
• No single satisfactory explanation exists for the success of tumors in escaping the immune rejection process. It is believed that early clones of neoplastic cells are eliminated by the immune response.
• Cells, rather than immunoglobulins, are believed to dominate tumor immunity.
• Four types of identified tumor antigens are tumor-specific antigens on chemically induced tumors, tumor-associated antigens on virally induced tumors, carcinofetal antigens, and spontaneous tumor antigens.
• A tumor marker is a characteristic of a neoplastic cell that can be detected in plasma or serum. Markers may be useful in the diagnosis and selection of different treatment approaches, monitoring therapies, and determining prognosis.
• Tumor markers include CEA, AFP, β-hCG, neuron-specific enolase, prostatic acid phosphatase, and placental alkaline phosphatase.
• Various modalities are used to treat cancer. In addition to the classic therapies, newer therapies (e.g., monoclonal antibodies) are being used.