CHAPTER 105 Tropical Diarrhea and Malabsorption
Diarrhea and malabsorption are common in the tropics and most often result from infectious causes. Enteric infections that cause diarrhea are common in tropical countries, a result both of deficient sanitation and the ambient temperature that fosters proliferation of infectious organisms in water and food. Tropical diarrhea affects not only the indigenous population but also visitors to the tropics,1 who are more susceptible than residents to symptomatic enteric infection and who commonly develop diarrhea either during or shortly after their visit.
It has been shown repeatedly that a significant number of asymptomatic and apparently healthy residents in the tropics transiently harbor pathogenic microbes in their gastrointestinal tracts. Up to 15% of the rural population in some countries is infected at any given time by a variety of pathogens, such as Campylobacter, Cryptosporidium, and Giardia species.2–4 By and large, however, a homeostasis is achieved that is perturbed when the person is infected with an overwhelming number of pathogens or when there is impairment of intestinal immunity; this results in diarrheal illness. Residents of the tropics, possibly by virtue of repeated exposure to these or related pathogens, remain asymptomatic, whereas visitors to the tropics quickly develop symptoms upon exposure.
The predominant cause of malabsorption varies with geographic location in the tropics.5–7 In many areas, tropical sprue remains the major cause of malabsorption in adults and a lesser cause of malabsorption in children. Parasitic infections of the small intestine are probably the next most common cause of chronic diarrhea and malabsorption in many areas. In belts of the tropics, celiac disease is now becoming an increasingly diagnosed and appreciated problem and needs to be considered in the differential diagnosis of malabsorption. Intestinal tuberculosis, small intestinal bacterial overgrowth, and pancreatic steatorrhea are other significant causes of malabsorption in the tropics. This chapter deals mainly with tropical sprue and tropical enteropathy, but it discusses very briefly other specific causes of diarrhea and malabsorption in the tropics.
INFECTIOUS DIARRHEA IN THE TROPICS
Acute and chronic infectious diarrhea in the tropics is caused by a variety of bacterial, viral, and parasitic agents (Table 105-1). Although these pathogens affect the indigenous population of the tropics, many of these infected persons remain asymptomatic, probably because of immunity acquired by earlier exposures to the same or related infectious agents. The risk of diarrhea in visitors to the tropics can range up to 55%, depending on their specific travel destination.8
Cholera is the most dramatic form of acute diarrhea, resulting in death from dehydration and electrolyte imbalance, if untreated. The disease occurs in both endemic and epidemic form in the tropics and mainly affects the indigenous population. Only very occasionally does cholera afflict Western travelers.9,10 Cholera is endemic in the Indian subcontinent, particularly in the southern and eastern parts; in the Indonesian islands; in the Philippines; and also in Latin America.
In a study of 17,353 returned travelers from tropical countries, acute diarrhea occurred in 22.2% and chronic diarrhea in 11.3%.9 Parasitic diarrhea (giardiasis and amebiasis) was most common overall, whereas bacterial diarrhea (Campylobacter > Shigella > nontyphoidal Salmonella) was more common in travelers to Southeast Asian countries. Diarrhea in travelers is often geographically determined, being caused by enterotoxin-producing Escherichia coli with travel to South America or to Mexico, by Giardia lamblia and Cryptosporidium in southern Central Asia, and by Campylobacter in Southeast Asia.9,11 Infection with the coccidian parasites occasionally causes diarrhea in travelers to the tropics. Cyclospora cayetanensis first was reported to cause outbreaks and prolonged diarrhea in travelers to Nepal and has subsequently been reported from other regions in the tropics including Southeast Asia, Africa, Turkey, and Latin America.12,13 Blastocystis hominis is another opportunistic protozoan that occasionally causes acute or chronic diarrhea in travelers.14
TROPICAL SPRUE
Tropical sprue remains a significant cause of malabsorption in several tropical countries. The etiology of this disease continues to remain obscure, and it is unlikely that it will ever be conclusively settled given its diminishing presence. Tropical sprue needs to be differentiated from a variety of other conditions that also cause malabsorption in residents of the tropics (Table 105-2).
HIV, human immunodeficiency virus.
DEFINITION
Tropical sprue is a primary (i.e., not caused by other known disease) malabsorption syndrome that occurs in visitors to or residents of the tropics. Baker and Klipstein,15 working in South India and Central America respectively, defined tropical sprue as an intestinal mucosal disease characterized by malabsorption of two or more unrelated nutrient groups (e.g., fat, carbohydrate, vitamins) where other known causes of malabsorption had been excluded. Historically, this definition originated at a time when testing for intestinal absorption was commonplace. The availability of specific diagnostic tests for many of the diseases that cause malabsorption, such as abdominal imaging for chronic pancreatitis or antiendomysial and antitissue transglutaminase antibody for celiac disease, led to a general decline in the use of tests for absorption. In the developed world it is now uncommon to find laboratories that perform tests for absorption, particularly fecal fat estimation.16 The diagnosis of tropical sprue, however, continues to require the demonstration of malabsorption and the exclusion of other specific pathologies including celiac disease, chronic pancreatitis, and parasitic infections.
HISTORY
Modern medical history records the first description of tropical sprue from Barbados in the West Indies by William Hillary in 1759.17 He described this disease (aphthoides chronica) as commencing with severe mouth ulcers and glossitis, followed sequentially by diarrhea, marasmus, and death. The word sprue probably originated from the Dutch term sprouw used to describe a condition characterized by severe aphthous ulceration of the mouth and stomatitis.18 The Dutch used the term Indische Sprouw to describe what appeared to be the same condition occurring in their colonies in Southeast Asia. A similar illness was noted in Europeans who spent time in the Asian colonies including India, Indochina, and China and that went by many names including “chronic diarrhea of the tropics” before being named “sprue” by Manson in 1880.17 Although now primarily described in Westerners who have spent some time in the tropics, descriptions of a malabsorption syndrome in the indigenous population in India date back to the second century bc. The ancient Indian medical textbook the Charaka Samhita19 described an illness (Grahani vyadhi) characterized by glossitis, diarrhea, malabsorption-like stools, and wasting and ascribed to a loss of the digestive fire.
EPIDEMIOLOGY
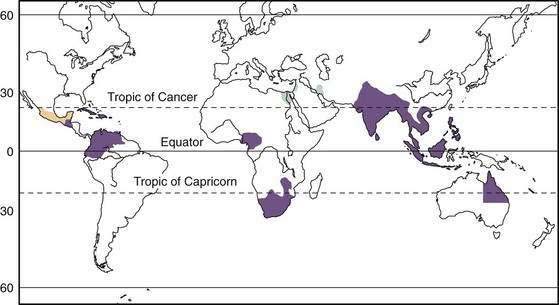
Tropical sprue has been described in South and Southeast Asia, Central America, South America, the Caribbean islands, and parts of Africa (Fig. 105-1). Endemic tropical sprue is most often recognized in visitors who have spent at least a few months in the tropics before returning home,20–22 although tropical sprue or tropical malabsorption also occurs in indigenous residents of the tropics.23–26 It is not clear, however, if the disease that occurs in returning Westerners is the same as the one that occurs in indigenous residents of the tropics. At least in expatriates returning to Europe, it appears that that tropical sprue may be secondary to prolonged infection, or follows an unusual infection, an occurrence that has been called postinfectious malabsorption.27
Tropical sprue also has occurred in epidemic form.5 Epidemics occurred in soldiers and prisoners of war in Asia during the Second World War28,29 and also were reported in South India during the 1960s and 1970s.30 Epidemic tropical sprue affected adults more often than it did children, and exposure during the first wave of an epidemic often conferred protection during the second wave; intrafamilial secondary transmission of disease also was noted. In the early 1960s, epidemic sprue was responsible for the deaths of 30,000 to 40,000 people in South India alone. A seasonal occurrence of tropical sprue was noted in Puerto Rico, where it was common during the first three months of the year.31
Epidemic sprue is no longer reported from South India or other parts of the world. Sporadic tropical sprue also has become relatively rare in some areas of India,7 compared with celiac disease and intestinal tuberculosis, where it once used to represent the major cause of malabsorption and chronic diarrhea. Nonetheless, tropical sprue continues to account for a significant proportion of adult malabsorption and a smaller proportion of childhood malabsorption in South Asia.6,24,25
ETIOLOGY
The etiology of tropical sprue remains unknown. Persistent infection of the small intestine with coliform organisms was postulated to cause the disease in some areas of the world. In Haiti and Puerto Rico, overgrowth of toxin-producing coliforms (Klebsiella spp., Enterobacter cloacae, or E. coli) in the small bowel was associated with feasting and ingesting excessive amounts of long-chain unsaturated fatty acids.32,33 Bacterial overgrowth in the proximal small intestine in patients with tropical sprue also has been noted in other parts of the world, but it has been ascribed to the slow small intestinal transit that characterizes the disease.34 The normal human jejunum contains up to 103 bacteria per milliliter of luminal fluid in Western residents, and bacterial overgrowth is considered to occur when the concentration of bacteria exceeds 105/mL. These criteria, however, do not necessarily hold true for apparently healthy residents of the tropics, and studies in South India have demonstrated that bacterial counts in jejunal luminal fluid ranged up to 105/mL in healthy asymptomatic persons.35 Patients with tropical sprue and asymptomatic persons from the same backgrounds had similar levels and types of bacteria in their proximal small intestine, indicating that bacterial overgrowth probably was not responsible for the malabsorption of tropical sprue. Toxin-producing bacteria were not found in the intestine of patients with tropical sprue in South India.36
Viral particles resembling human enteric coronaviruses have been identified in the stool and jejunal enterocytes of patients with tropical sprue,37 but they also may be present in apparently normal persons, so they have not been definitely implicated in the etiology of sprue. Acute and reversible flattening of the small intestinal mucosa identical to that seen with tropical sprue has been noted in the absence of gluten-sensitive enteropathy and ascribed to a probable viral etiology.38
CLINICAL FEATURES
Although tropical sprue does occur in children, typically the disease affects adults. Presentation is with chronic diarrhea, steatorrhea, glossitis, abdominal distention, prominent bowel sounds or borborygmi, and weight loss (Table 105-3). In expatriates and during epidemics, the illness often begins with fever and watery or, rarely, bloody diarrhea. These symptoms resolve after a week or so to be followed by a lingering diarrhea or steatorrhea associated with weight loss.
Table 105-3 Clinical Features of Tropical Malabsorption and Their Causes
| CLINICAL FEATURE(S) | CAUSE(S) | MECHANISM(S) |
|---|---|---|
| Diarrhea |
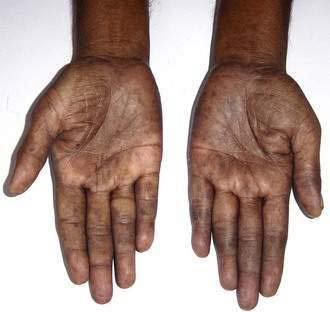
The physical signs that may be found in affected patients (see Table 105-3) (see Chapter 100) include pallor from iron and vitamin B deficiency; angular stomatitis, cheilitis, and glossitis due to vitamin B deficiency; and peripheral edema and skin and hair changes secondary to hypoproteinemia. Hyperpigmentation of the buccal mucosa, palms, and knuckles sometimes is noted (Fig. 105-2) and has been ascribed to vitamin B12 deficiency. Vitamin A deficiency with night blindness, Bitot’s spots, and corneal xerosis and subacute combined degeneration of the spinal cord resulting from vitamin B12 deficiency are much rarer. Fever has been noted in a quarter of patients in South India. Patients are grossly emaciated in the later stages of illness, with muscle weakness, particularly in the proximal muscles.
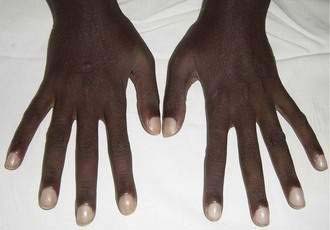
Peripheral neuropathy may be present, but subacute combined degeneration of the spinal cord, once noted in tropical sprue, is no longer found, perhaps because of earlier diagnosis and treatment. The presence of finger clubbing should suggest other illness such as immunoproliferative small intestinal disease, in which this finding is characteristic (Fig. 105-3). Sprue coma, manifest by listlessness and apathy was described in the past and attributed to a deficiency of divalent cations, particularly magnesium. Rarely, a syndrome similar to the Ogilvie syndrome occurs, characterized by abdominal pain and distention with exaggerated bowel sounds secondary to colonic pseudo-obstruction. This surprising finding likely results from disordered intestinal motility in these patients. The clinical features of florid malnutrition probably are less common in expatriates with tropical sprue than in residents with the disease.
HISTOPATHOLOGY
Although tropical sprue typically is a disease of the small intestine, involvement of the stomach and colon has been reported. Many patients with sprue have reduced acid secretion manifest on gastric secretory testing, and examination of biopsy specimens indicated that more than half of patients with tropical sprue had atrophic gastritis.39 These studies, however, were performed before the recognition of Helicobacter pylori and, therefore, their true significance is unknown. Small intestinal biopsy specimens originally were taken using a peroral biopsy capsule, and today it is standard to obtain biopsies from the distal duodenum or proximal jejunum during upper gastrointestinal endoscopy. Biopsies obtained using the capsule were examined with a hand lens or a dissecting microscope and characteristically showed blunting and fusion of the villi. The endoscopic parallel of this finding is scalloping of the duodenal mucosa, seen in patients with villus atrophy of the duodenal mucosa.40 Originally described in celiac disease, it is now appreciated that scalloping is nonspecific and indicates villus atrophy.
Histologically, small intestinal biopsy specimens show varying degrees of villus shortening (atrophy), whereas the crypts are elongated.41–43 In addition to blunting, the villi sometimes show fusion. The normal villus-to-crypt ratio in the jejunal mucosa is 4 : 1 or 5 : 1; in the partial villus atrophy seen with tropical sprue this ratio is reduced to 2 : 1 or 1 : 1. Complete villus atrophy, noted in some patients with celiac disease, is not seen in tropical sprue. The lamina propria of the small intestine is infiltrated by lymphocytes, and there is an increase in intraepithelial lymphocytes. Intraepithelial lymphocytes are a unique T-cell population seen in celiac disease, but they also may be increased in tropical sprue and a number of other diseases.44 Baker and Mathan41 graded the histologic changes in tropical sprue on a scale of 0 to III (Fig. 105-4), which is somewhat different from the Marsh scoring system that is widely used to score celiac disease biopsy specimens.
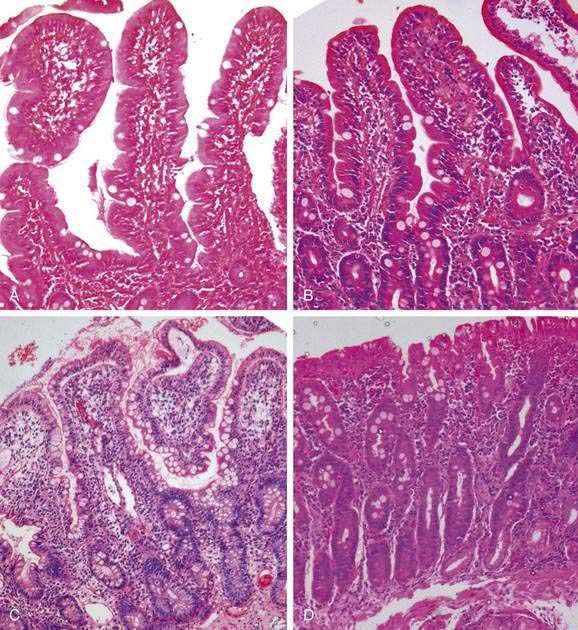
Figure 105-4. Histopathology of tropical sprue. Endoscopic deep duodenal biopsy specimens showing a gradation of mucosal histology according to the grading system of Baker and Mathan41: A, Grade 0: normal. B, Grade I: shortening of the villi, increase in the depth of the glandular layer, and increase in lymphocytic infiltration of the lamina propria and surface epithelial cells. C, Grade II: further increase in the depth of the glandular layer extending up to half the total distance from the crypt base to the villus tip with more cellular infiltration. D, Grade III: the glandular layer occupies more than half the distance from the crypt base to the villus, together with flattening of surface epithelial cells, disorganization of the brush border, and marked cellular infiltration of the lamina propria and surface epithelial cells. The completely flat mucosal surface that occurs in some cases of celiac disease is not seen in tropical sprue (see Fig. 104-2).
Electron microscopy reveals degenerating cells in the crypts of the small intestine,45 a finding similar to that seen in radiation enteritis and in enteritis secondary to chemotherapy. The location of the damaged cells in the base of the crypt suggests that there was damage to the stem cells in the small intestine. In addition to changes noted in the stomach and the small intestine, an infiltration of the colonic mucosa by lymphocytes also is seen in tropical sprue,46 similar to the lymphocytic colitis that may be seen in association with celiac disease.47
PATHOPHYSIOLOGY
The characteristic finding in tropical sprue is villus atrophy in the small intestine accompanied by elongation of the crypts. Ultrastructural studies show the microvilli of the epithelial cells are distorted and grouped. These changes lead to a marked reduction in the total absorptive surface area of the small intestine, which is reflected in reduced xylose absorption.48 Dead epithelial cells at the villus base are extruded more rapidly than normal,49 and this is compensated by increased crypt cell production and enterocyte migration up the villus. In contrast to comparable disorders such as celiac disease, electron microscopy in tropical sprue reveals degenerating and dead cells in the basal region of the crypts, suggesting that there is an intestinal stem cell defect in tropical sprue that prevents adequate restoration of absorptive surface area.
Fat malabsorption in tropical sprue is secondary to epithelial cell damage in the small intestine. It also has been shown that the pancreatic enzyme response to an indirect test such as the Lundh meal is impaired, although pancreatic response to secretin is normal. Such functional pancreatic insufficiency is due to inadequate pancreatic stimulation as a consequence of mucosal disease and is reflected in an abnormal pancreolauryl test in more than 50% of patients with tropical sprue.49 The fat in the stool is largely in the form of free fatty acids, both saturated and unsaturated, depending on the nature of the ingested dietary fat.
Folate and iron deficiency in tropical sprue reflect proximal small intestinal involvement, whereas B12 malabsorption reflects involvement of the terminal ileum. The three-stage Schilling test in tropical sprue evidences mucosal abnormality, because defective vitamin B12 absorption is not corrected either by intrinsic factor or by antibiotic therapy. Bile acid malabsorption also results from terminal ileal involvement and can contribute to diarrhea. Colonic malabsorption of water and electrolytes contributes significantly to diarrhea in tropical sprue and can result from the action of unabsorbed bile acids and free unsaturated fatty acids in the stool, which inhibit colonocyte Na+,K+-ATPase.36,50
Small intestinal transit is prolonged in patients with tropical sprue, and this has been ascribed to the effects of unabsorbed fat on the ileal brake.34 The ileal brake is considered to be mediated via peptide YY, and elevated serum levels of this peptide have been noted in tropical sprue.51
DIAGNOSIS
Malabsorption commonly is established by testing for fecal fat, measuring serum levels of vitamin B12 and folate, and testing for d-xylose absorption (see Chapter 101). Two abnormal tests in the appropriate setting are consistent with tropical sprue in the absence of other causes of malabsorption.
The quantitative fecal fat estimation of van de Kamer52 is the gold standard for establishing steatorrhea but is not available in most laboratories. Because of difficulties in performing this test, steatorrhea often is assessed qualitatively using Sudan staining of oil (triglyceride) droplets in stool. The acid steatocrit, a test based on the separation of fat from nonfat components by centrifuging acidified stool in a hematocrit tube, was developed as a simple test for the steatorrhea from pancreatic insufficiency in which fecal fat is in the form of triglycerides.53 In the steatorrhea of mucosal disease, however, excreted fat is in the form of free fatty acids and the steatocrit is not reliable.
In contrast to celiac disease, in which involvement of the proximal small intestine predominates and, therefore, serum folate levels are low and vitamin B12 levels are normal, tropical sprue affects the distal small intestine and terminal ileum; as a result, serum folate levels are normal and vitamin B12 levels are low. Vitamin B12 deficiency is common in tropical sprue and is demonstrated best by the three-stage Schilling test. An increase of breath hydrogen after glucose or xylose ingestion has been interpreted as evidence of small bowel bacterial overgrowth, whereas the abnormal lactulose breath hydrogen test suggests prolonged small bowel transit in tropical sprue.34
Fecal examination for occult blood and for parasites is essential. Protozoan parasites that cause diarrhea must be sought using special stains of the feces including modified acid-fast stains. In tropical sprue, characteristic changes observed on small bowel series include thickening of the mucosal folds of the jejunum, loss of the feathery mucosal pattern in the proximal jejunum, and delayed transit through the small intestine (Fig. 105-5). Currently, however, computed tomographic scanning (CT) of the abdomen is more commonly performed than small bowel series. Dilated, featureless, atonic loops of small intestine and dilution of oral contrast, suggesting a hypersecretory state, are found. The main role of CT, however, is to exclude intestinal masses and lymphadenopathy within the mesentery and retroperitoneum, which are characteristic of other inflammatory diseases, such as tuberculosis, lymphoma, parasitic infestations, and eosinophilic enteritis. Capsule endoscopy of the small bowel is sometimes undertaken in patients with small bowel diarrhea after excluding the presence of strictures; however, enteroscopy using push enteroscopes or double-balloon or single-balloon instruments has the advantage of obtaining biopsies and is likely to become the standard for diagnosis in these patients, especially to exclude secondary causes of malabsorption.54,55
Small intestinal mucosal biopsies are taken from the third or fourth part of the duodenum during upper gastrointestinal endoscopy to exclude specific causes of malabsorption and to diagnose tropical sprue. Endoscopy can reveal a scalloped duodenal mucosa similar to that seen in celiac disease, which results from mucosal atrophy and loss of the villus pattern. A similar appearance of the jejunal mucosa also may be found during enteroscopy. Magnification endoscopy, enhanced by application of a 3% acetic acid spray, has been reported to increase the recognition of patchy villus atrophy and to allow targeted mucosal biopsies that can more likely provide a diagnosis in tropical sprue.56 Small intestinal bacterial overgrowth may be demonstrated by quantitative aerobic and anaerobic culture of the small bowel contents aspirated either during endoscopy or by a jejunal tube. Fasting counts exceeding 105/mL of jejunal fluid are considered to indicate bacterial overgrowth.
TREATMENT
Tetracycline 250 mg four times daily (or doxycycline 100 mg once daily) for 6 months is prescribed as specific therapy for tropical sprue.57,58 There is, however, little experience with use of other antibiotics in this condition. A high-calorie, high-protein, fat-restricted diet usually is given to these patients. Restriction of long-chain fatty acids in the diet, and its substitution by medium chain triglycerides, is particularly useful in reducing diarrhea and steatorrhea. Colonic pseudo-obstruction, seen in the rare patient, can require colonic decompression. Relapses of disease after cessation of therapy are known to occur in endemic tropical sprue and require investigation to exclude other causes before resuming therapy.
TROPICAL ENTEROPATHY AND ITS DISTINCTION FROM TROPICAL SPRUE
The issue of whether tropical enteropathy is a milder form (subclinical malabsorption) of tropical sprue remains controversial. Baker and Mathan41 championed a clear distinction between tropical sprue and tropical enteropathy, the former causing symptomatic malabsorption and the latter causing an asymptomatic condition with mild abnormalities of absorption. Menzies and coworkers found that 218 healthy volunteer residents of tropical areas had a higher mean lactulose-to-rhamnose ratio and lower mean five-hour recoveries of 3-O-methyl-d-glucose, d-xylose, and l-rhamnose, indicating higher intestinal permeability, lower mucosal surface area, and lower absorptive capacity than 228 healthy volunteer residents of subtropical and temperate regions.59 Investigation of visiting residents in subtropical and temperate climates suggested that differences in intestinal permeability and absorptive capacity were related to their area of residence rather than area of origin.59
Histologic enteropathy continues to be observed even today in residents of tropical countries, with mucosal biopsy specimens showing blunting of villi and increased cellularity of the lamina propria in persons without overt intestinal disease. It has been postulated that tropical enteropathy might represent an adaptation of the intestine to frequent intestinal infection in childhood. There is some evidence that the mucosal damage of tropical enteropathy is mediated by T cells.60 In a large longitudinal study, Kelly and colleagues performed regular monthly bacteriologic and parasitologic examinations of the stool and annual endoscopic jejunal biopsy with morphometry on 238 asymptomatic adults in Zambia. Five-hour urine xylose excretion was low (16.6 ± 6.9%) in 182 members of a cohort drawn from a township compared with 13 staff members undertaking the study (25.5 ± 4.4%), which in turn was significantly less than that of healthy volunteers in London (33.1 ± 0.7%).2
The mucosal biopsies of those from the Zambian township showed an altered crypt-to-villus ratio of approximately 5 : 3. There were marked changes over time, including reduction in villus height (16%), reduction in xylose absorption (16%), and increase in intestinal permeability (28%) during the peak rainfall months of December and January, in association with an incidence of diarrhea that was higher than in other months.2 Asymptomatic intestinal infections also were noted in approximately half of the participants, and enteropathy was more severe in persons who had been infected with Citrobacter rodentium or hookworm. It has been suggested that the increased intestinal permeability can lead to absorption of endotoxins, systemic inflammation, and growth faltering in Gambian infants.61 However, fecal neopterin excretion, a marker of inflammation, was associated with relative growth failure but did not correlate with intestinal permeability, a marker of tropical enteropathy.62 Attempts to improve tropical enteropathy by oral administration of the probiotic bacterium Lactobacillus GG for 30 days in Malawian children did not reveal any effect of the probiotic on mucosal function.63
Tropical enteropathy is not without clinical consequence. The reduced surface area implies that absorption of energy may be slightly diminished, which might explain a low body mass index in many persons living in the tropics. As an extension of the hygiene hypothesis that relates increased hygiene to an increased prevalence of autoinflammatory diseases, it has been suggested that tropical enteropathy might protect against these inflammatory diseases through poorly understood immune and neuroendocrine mechanisms within the intestinal tract.64 It also has been suggested that tropical enteropathy influences the severity of infectious gastroenteritis and that it has implications for the composition of oral rehydration solution, leading to the use of lower concentrations of sodium and glucose than in the original formulation that was isotonic with plasma.65,66 Ongoing research also addresses the issue of whether tropical enteropathy has an impact on drug absorption, an issue particularly relevant to the management of a number of diseases including infection with the human immunodeficiency virus.
PROTOZOAN INFECTIONS THAT CAUSE MALABSORPTION
This topic is discussed in detail in Chapter 109.
GIARDIASIS
Infection with the protozoan parasite G. lamblia is most often asymptomatic in persons who live in the tropics.5 Giardiasis can cause diarrhea in immunocompromised persons or in visitors from Western countries to the tropics, and it can cause self-limited illness in children. Diarrhea is associated with the presence of trophozoites in the stool, and the presence of cysts alone should be interpreted with caution. Decreased brush border surface area in the jejunum leads to carbohydrate malabsorption, and associated small intestinal bacterial overgrowth and consequent bile salt deconjugation leads to steatorrhea.
Symptomatic giardiasis responds quickly to treatment with metronidazole, tinidazole, or other imidazoles. Albendazole and nitazoxanide also have been used successfully (Table 105-4).
| DISEASE | SPECIFIC THERAPY | DURATION |
|---|---|---|
| Capillariasis | Thiabendazole 25 mg/kg twice daily or | 20 days |
| Albendazole 400 mg twice daily | 7-10 days | |
| Celiac disease | Gluten free diet | Lifelong |
| Common variable immunodeficiency | Human gamma globulin intravenously every 4 to 6 weeks | Lifelong |
| Bone marrow transplantation | ||
| Cryptosporidiosis | Nitazoxanide 500 mg twice daily or | 3-7 days |
| Paromomycin 500 mg 3 or 4 times daily | ||
| Cyclosporiasis | Trimethoprim-sulfamethoxazole 160 mg/800 mg twice daily or ciprofloxacin 500 mg twice daily | 7 days |
| Followed by trimethoprim-sulfamethoxazole 160 mg/800 mg every other day or three times a week, or ciprofloxacin 500 mg three times a week | 10 weeks | |
| Giardiasis | Metronidazole 400 mg 3 times daily or | 7-14 days |
| Tinidazole 500 mg twice daily | 7-14 days | |
| Albendazole 400 mg twice daily | 7-14 days | |
| Immunoproliferative small intestinal disease stage A | Tetracycline 250 mg 4 times daily | 6 months to 2 years |
| Isosporiasis | Trimethoprim-sulfamethoxazole 160 mg/800 mg twice daily | 10 days |
| followed by 400/80 twice daily | 3 weeks | |
| Microsporidiosis due to Encephalitozoon intestinalis | Albendazole 400 mg twice daily | 2-3 weeks |
| Microsporidiosis due to Enterocytozoon bieuneusi | Nitazoxanide 500 mg twice daily or | 3-7 days |
| Fumagillin 20 mg 3 times daily | 2 weeks | |
| Strongyloidiasis | Thiabendazole 25 mg/kg twice daily or | 3 days |
| Albendazole 400 mg twice daily or | 3-7 days | |
| Ivermectin 200 µg/kg | 1-2 days | |
| Tuberculosis | Isoniazid, rifampicin, pyrazinamide, and ethambutol followed by or | 2 months |
| Two-drug therapy with isoniazid and rifampicin | 4-7 months | |
| Tropical sprue | Tetracycline 250 mg 4 times daily | 6 months |
OTHER PARASITIC INFECTIONS
Other protozoa associated with malabsorption include Cryptosporidium parvum, Isospora belli, Cyclospora cayetanensis, and the microsporidia Enterocytozoon bieneusi and Encephalitozoon intestinalis. Infection with these protozoa is widespread in tropical countries.67 Most infected persons are asymptomatic, but some develop self-limited diarrhea. Endoscopy with intestinal biopsy is helpful in diagnosis.68
HELMINTHIC INFECTIONS THAT CAUSE MALABSORPTION
Helminthic infections also can cause a malabsorption syndrome in the tropics.5 The most common helminthic infections are with Strongyloides stercoralis and Capillaria philippinensis. Infection with Strongyloides stercoralis can cause chronic diarrhea and malabsorption in immunocompetent persons, although immunosuppression, particularly with glucocorticoid use, predisposes to hyperinfection with this parasite. Infection with the human T-lymphotropic virus-1 (HTLV-1) is associated with persistent Strongyloides infection and chronic diarrhea. Diarrhea may be intermittent or persistent, and steatorrhea, anemia, and hypoproteinemia are common. Small bowel series can show changes suggesting mucosal infiltration and ulceration in the duodenum and jejunum. Diagnosis usually is made by examination of feces for the larvae of the parasite, although occasionally the infection is only recognized upon small bowel biopsy. Treatment with thiabendazole, albendazole, or ivermectin is effective.
HIV INFECTION AND AIDS
Since the AIDS epidemic has spread throughout so many tropical countries, it has become necessary to exclude HIV infection in any patient with malabsorption syndrome or chronic diarrhea. Diarrhea and malabsorption lasting for longer than a month is an AIDS-defining condition, is associated with low CD4 counts, and usually is caused by infection of the small intestine with one of the protozoan pathogens mentioned earlier. Studies, however, suggest that chronic diarrhea is neither a very sensitive nor a specific indicator of AIDS in tropical countries, such as India.69 Occurrence of pathogen-negative diarrhea and malabsorption in patients with AIDS has been attributed to possible direct viral (HIV) enteropathy, although this has never been proven. Diarrhea and malabsorption require specific therapy of the opportunistic infection as well as antiretroviral therapy.
INTESTINAL TUBERCULOSIS
Intestinal tuberculosis, comprising ulcerative, hypertrophic, and ulcerohypertrophic varieties, is common in tropical countries. The ulcerative variety of tuberculosis commonly manifests with chronic diarrhea and malabsorption, whereas the hypertrophic variety more commonly causes abdominal pain and intestinal obstruction. In contrast to mucosal diseases such as tropical sprue, abdominal pain is a significant symptom and results from ulceration and partial obstruction of the small bowel. Biochemical evidence of malabsorption can be found in many patients with intestinal tuberculosis, even if the patient does not present with a clinical diagnosis of malabsorption syndrome. The causes of malabsorption in tuberculosis include bacterial overgrowth in a stagnant loop, bile salt deconjugation, and diminished absorptive surface due to ulceration and lymphatic obstruction. The diagnosis is made in most cases by endoscopy with a combination of histology, culture, and polymerase chain reaction tests (see Chapter 107).70
CROHN’S DISEASE
Crohn’s disease is increasing in incidence in tropical countries and now is an important item in the differential diagnosis for any patient in whom tuberculosis is a possible diagnosis. Malabsorption in Crohn’s disease may be due to a number of factors. About a third of patients have small intestinal involvement, and this can reduce the absorptive surface area; extensive small bowel resections ultimately have the same effect. Terminal ileal disease or resections can lead to vitamin B12 deficiency and bile salt malabsorption, and ileocecal valve resections can result in bacterial overgrowth with resultant malabsorption. Approximately 9% of cases of malabsorption were found to be due to Crohn’s disease in an unselected case series from northern India (see Chapter 111).6
CELIAC DISEASE
Celiac disease (gluten sensitive enteropathy), hitherto considered uncommon in the tropics, is increasingly described from northern India and selected areas of sub-Saharan Africa71 and may be unmasked by intestinal infection. The disease often manifests in infancy around the time of weaning, but presentation at later ages, including adulthood, is not uncommon. Celiac disease is differentiated from tropical sprue by the presence of complete villus atrophy in mucosal biopsies. The diagnosis is confirmed by the presence of IgA antiendomysial and antitissue transglutaminase antibodies, although these tests may be negative in persons with selective IgA deficiency. Clinical and histologic responses to gluten withdrawal are important in confirming the diagnosis (see Chapter 104).
PRIMARY IMMUNODEFICIENCY SYNDROMES
Common variable immunodeficiency (CVI) occurs sporadically in residents of the tropics and can manifest as a malabsorption syndrome.72 Recurrent diarrhea, recurrent sinopulmonary infections, and recurrent meningitis are other manifestations of this disease. CVI first may be suspected when a small bowel biopsy shows reduced numbers of plasma cells in the lamina propria or by the finding of nodular lymphoid hyperplasia, which is occasionally associated with the disease. The most common intestinal infection in these patients is giardiasis. Other protozoa, such as Isospora belli, Cryptosporidium parvum, and microsporidia, also can colonize the small bowel and cause malabsorption. Selective IgA deficiency may be associated with a flat mucosa and giardiasis. Bacterial colonization of the upper small bowel occurs in some patients with primary immunodeficiency and causes malabsorption, but it responds quickly to treatment with tetracycline or other antibiotics. Periodic administration of intravenous gamma globulin is the major therapy for patients with CVI.
IMMUNOPROLIFERATIVE SMALL INTESTINAL DISEASE AND SMALL BOWEL LYMPHOMA
Immunoproliferative small intestinal disease (IPSID) and small bowel lymphoma, also termed Mediterranean lymphoma, is not uncommon in the tropics.73 It usually affects socioeconomically disadvantaged persons.
The disease is caused by a clonal proliferation of cells that produce an abnormal alpha heavy chain immunoglobulin, and it can be diagnosed by immunoassay for the alpha heavy chain in the serum. It is suggested that the clonal expansion is driven by an infectious antigen, in a way similar to the link between H. pylori and mucosa-associated lymphoid tissue (MALT) lymphoma of the stomach. Campylobacter jejuni infection has been causally associated with IPSID.74 Mucosal biopsy of the small intestine reveals a dense cellular lymphoplasmacytic infiltrate in the lamina propria, leading to effacement of the crypts.
The disease progresses over several years from a relatively benign infiltration of the entire small intestinal mucosa (stage A) to the development of lymphoplasmacytic and immunoblastic lymphoma (stage C). The disease is staged as other lymphomas by bone marrow examination, by looking for evidence of disease on either side of the diaphragm, and by computed tomography of the abdomen. In patients with stage A disease diagnosed by mucosal biopsy, it is advisable to perform laparoscopy or laparotomy with full-thickness biopsy of the intestine to exclude transmural lymphoma before commencing antibiotic therapy. Areas of bulky tumor are resected before chemotherapy, and full-thickness biopsies of involved areas of intestine and biopsy of enlarged mesenteric nodes are performed. In the premalignant stage A, long-term therapy with antibiotics such as tetracycline can cure the disease. In the more advanced stages of the disease (B and C), chemotherapy or total abdominal irradiation are used (see Chapter 59).
TROPICAL PANCREATITIS AND MALABSORPTION
The disease is likely to be genetically determined, and both disease-inducing and disease-protective mutations have been noted. The most common mutation involves a serine protease (SPINK1) gene and occurs in about 40% of the patients.76 Whether the association of diabetes mellitus in these patients requires a different or an additional mutation is controversial.
Behera B, Mirdha BR, Makharia GK, et al. Parasites in patients with malabsorption syndrome: A clinical study in children and adults. Dig Dis Sci. 2008;53:672-9. (Ref 67.)
Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119-30. (Ref 9.)
Fry LC, Bellutti M, Neumann H, et al. Utility of double-balloon enteroscopy for the evaluation of malabsorption. Dig Dis. 2008;26:134-9. (Ref 55.)
Ghoshal UC, Ghoshal U, Ayyagari A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol. 2003;18:540-7. (Ref 34.)
Hanson JP. Tropical sprue in Far North Queensland. Med J Aust. 2005;182:536-537. (Ref 26.)
Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412-19. (Ref 2.)
Lo A, Guelrud M, Essenfeld H, Bonis P. Classification of villous atrophy with enhanced magnification endoscopy in patients with celiac disease and tropical sprue. Gastrointest Endosc. 2007;66:377-82. (Ref 56.)
Mathan M, Mathan VI, Baker SJ. An electron-microscopic study of jejunal mucosal morphology in control subjects and in patients with tropical sprue in southern India. Gastroenterology. 1975;68:17-32. (Ref 45.)
Morales M, Galván E, Mery CM, et al. Exocrine pancreatic insufficiency in tropical sprue. Digestion. 2001;63:30-4. (Ref 49.)
Owens SR, Greenson JK. The pathology of malabsorption: Current concepts. Histopathology. 2007;50:64-82. (Ref 43.)
Ramakrishna BS, Venkataraman S, Mukhopadhya A. Tropical malabsorption. Postgrad Med J. 2006;82:779-87. (Ref 5.)
Ranjan P, Ghoshal UC, Aggarwal R, et al. Etiological spectrum of sporadic malabsorption syndrome in northern Indian adults at a tertiary hospital. Indian J Gastroenterol. 2004;23:94-8. (Ref 6.)
Rickles FR, Klipstein FA, Tomasini J, et al. Long-term follow-up of antibiotic-treated tropical sprue. Ann Intern Med. 1972;76:203-10. (Ref 57.)
Thomas PD, Forbes A, Green J, et al. Guidelines for the investigation of chronic diarrhoea, 2nd edition. Gut. 2003;52(Suppl 5):v1-15. (Ref 16.)
Wahnschaffe U, Ignatius R, Loddenkemper C, et al. Diagnostic value of endoscopy for the diagnosis of giardiasis and other intestinal diseases in patients with persistent diarrhea from tropical or subtropical areas. Scand J Gastroenterol. 2007;42:391-6. (Ref 68.)
Westergaard H. Tropical sprue. Curr Treat Options Gastroenterol. 2004;7:7-11. (Ref 58.)
1. Guerrant RL, Oria R, Bushen OY, et al. Global impact of diarrheal diseases that are sampled by travelers: The rest of the hippopotamus. Clin Infect Dis. 2005;41(Suppl 8):S524-30.
2. Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412-19.
3. Mathan VI, Rajan DP, Klipstein FA, Engert RF. Enterotoxigenic Campylobacter jejuni among children in South India. Lancet. 1984;2:981.
4. Kang G, Sheela S, Ramakrishna BS. Asymptomatic Cryptosporidium carriage in rural South Indians. Ind J Med Microbiol. 1998;16:112-14.
5. Ramakrishna BS, Venkataraman S, Mukhopadhya A. Tropical malabsorption. Postgrad Med J. 2006;82:779-87.
6. Ranjan P, Ghoshal UC, Aggarwal R, et al. Etiological spectrum of sporadic malabsorption syndrome in northern Indian adults at a tertiary hospital. Indian J Gastroenterol. 2004;23:94-8.
7. Thakur B, Mishra P, Desai N, et al. Profile of chronic small-bowel diarrhea in adults in Western India: A hospital-based study. Trop Gastroenterol. 2006;27:84-6.
8. Dupont HL. Travel medicine and principles of safe travel. Trans Am Clin Climatol Assoc. 2008;119:1-27.
9. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119-30.
10. Tarantola A, Vaucel J, Laviolle C, et al. A cluster of Vibrio cholerae O1 infections in French travelers to Rajasthan (India), May 2006. J Travel Med. 2008;15:273-7.
11. Weitzel T, Wichmann O, Mühlberger N, et al. Epidemiological and clinical features of travel-associated cryptosporidiosis. Clin Microbiol Infect. 2006;12:921-4.
12. Hoge CW, Shlim DR, Rajah R, et al. Epidemiology of diarrhoeal illness associated with coccidian-like organism among travellers and foreign residents in Nepal. Lancet. 1993;341:1175-9.
13. Karanja RM, Gatei W, Wamae N. Cyclosporiasis: An emerging public health concern around the world and in Africa. Afr Health Sci. 2007;7:62-7.
14. Sohail MR, Fischer PR. Blastocystis hominis and travelers. Travel Med Infect Dis. 2005;3:33-8.
15. Baker SJ, Klipstein FA. Regarding the definition of tropical sprue. Gastroenterology. 1970;58:717-1.
16. Thomas PD, Forbes A, Green J, et al. Guidelines for the investigation of chronic diarrhoea, 2nd edition. Gut. 2003;52(Suppl 5):v1-15.
17. Booth CC. The first description of tropical sprue (William Hillary). Gut. 1964;5:45-50.
18. Bartholomew C. William Hillary and sprue in the Caribbean: 230 years later. Gut. 1989;30:17-21.
19. Sharma RK, Dash VB, editors, Caraka samhita, vol 4, 2002, translators and editors. Varanasi: Chowkhamba Sanskrit Series
20. Pittman FE, Pittman JC. Tropical sprue in American servicemen following return from Vietnam. Am J Dig Dis. 1976;21:393-8.
21. Klipstein FA. Tropical sprue in travelers and expatriates living abroad. Gastroenterology. 1981;80:590-600.
22. Peetermans WE, Vonck A. Tropical sprue after travel to Tanzania. J Travel Med. 2000;7:33-4.
23. Baker SJ, Mathan VI. Syndrome of tropical sprue in South India. Am J Clin Nutr. 1968;21:984-93.
24. Mittal SK, Rajeshwari K, Kalra KK, et al. Tropical sprue in north Indian children. Trop Gastroenterol. 2001;22:146-8.
25. Khokhar N, Gill ML. Tropical sprue revisited. J Pak Med Assoc. 2004;54:133-4.
26. Hanson JP. Tropical sprue in Far North Queensland. Med J Aust. 2005;182:536-7.
27. Cook GC. “Tropical sprue”: Some early investigators favoured an infectious cause, but was a coccidian protozoan involved? Gut. 1997;40:428-9.
28. Lim ML. A perspective on tropical sprue. Curr Gastroenterol Rep. 2001;3:322-7.
29. Girdwood RH. Fifty years after: Some experiences of the medical care of prisoners of war. Scott Med J. 1993;38:120-4.
30. Mathan VI, Baker SJ. Epidemic tropical sprue and other epidemics of diarrhea in South Indian villages. Am J Clin Nutr. 1968;21:1077-87.
31. Klipstein FA, Corcino JJ. Seasonal occurrence of overt and subclinical tropical malabsorption in Puerto Rico. Am J Trop Med Hyg. 1974;23:1189-96.
32. Klipstein FA, Short HB, Engert RF, et al. Contamination of the small intestine by enterotoxigenic coliform bacteria among the rural population of Haiti. Gastroenterology. 1976;70:1035-41.
33. Klipstein FA, Engert RF, Short HB. Enterotoxigenicity of colonising coliform bacteria in tropical sprue and blind-loop syndrome. Lancet. 1978;2:342-4.
34. Ghoshal UC, Ghoshal U, Ayyagari A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol. 2003;18:540-7.
35. Bhat P, Shantakumari S, Rajan D, et al. Bacterial flora of the gastrointestinal tract in southern Indian control subjects and patients with tropical sprue. Gastroenterology. 1972;62:11-21.
36. Ramakrishna BS, Mathan VI. Role of bacterial toxins, bile acids and free fatty acids in colonic water malabsorption in tropical sprue. Dig Dis Sci. 1987;32:500-5.
37. Baker SJ, Mathan M, Mathan VI, et al. Chronic enterocyte infection with coronavirus. One possible cause of the syndrome of tropical sprue? Dig Dis Sci. 1982;27:1039-43.
38. Goldstein NS. Non–gluten sensitivity–related small bowel villous flattening with increased intraepithelial lymphocytes: Not all that flattens is celiac sprue. Am J Clin Pathol. 2004;121:546-50.
39. Vaish SK, Sampathkumar J, Jacob R, Baker SJ. The stomach in tropical sprue. Gut. 1965;6:458-65.
40. Tawil SC, Brandt LJ, Bernstein LH. Scalloping of the valvulae conniventes and mosaic mucosa in tropical sprue. Gastrointest Endosc. 1991;37:365-6.
41. Baker SJ, Mathan VI. Tropical enteropathy and tropical sprue. Am J Clin Nutr. 1972;25:1047-55.
42. Brunser O, Eidelman S, Klipstein FA. Intestinal morphology of rural Haitians. A comparison between overt tropical sprue and asymptomatic subjects. Gastroenterology. 1970;58:655-68.
43. Owens SR, Greenson JK. The pathology of malabsorption: Current concepts. Histopathology. 2007;50:64-82.
44. Kakar S, Nehra V, Murray JA, et al. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol. 2003;98:2027-33.
45. Mathan M, Mathan VI, Baker SJ. An electron-microscopic study of jejunal mucosal morphology in control subjects and in patients with tropical sprue in southern India. Gastroenterology. 1975;68:17-32.
46. Puri AS, Khan EM, Kumar M, et al. Association of lymphocytic (microscopic) colitis with tropical sprue. J Gastroenterol Hepatol. 1994;9:105-7.
47. Nyhlin N, Bohr J, Eriksson S, Tysk C. Microscopic colitis: A common and an easily overlooked cause of chronic diarrhoea. Eur J Intern Med. 2008;19:181-6.
48. Rolston DD, Mathan VI. Xylose transport in the human jejunum. Dig Dis Sci. 1989;34:553-8.
49. Morales M, Galván E, Mery CM, et al. Exocrine pancreatic insufficiency in tropical sprue. Digestion. 2001;63:30-4.
50. Ramakrishna BS, Mathan VI. Absorption of water and sodium and activity of adenosine triphosphatases in the rectal mucosa in tropical sprue. Gut. 1988;29:665-8.
51. Adrian TE, Savage AP, Bacarese-Hamilton AJ, et al. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90:379-84.
52. Van de Kamer JH, Huinink HB, Weyers HA. Rapid method for the determination of fat in feces. J Biol Chem. 1949;147:347-55.
53. Dumasy V, Delhaye M, Cotton F, Deviere J. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol. 2004;99:1350-4.
54. Sharma BC, Bhasin DK, Makharia G, et al. Diagnostic value of push-type enteroscopy: A report from India. Am J Gastroenterol. 2000;95:137-40.
55. Fry LC, Bellutti M, Neumann H, et al. Utility of double-balloon enteroscopy for the evaluation of malabsorption. Dig Dis. 2008;26:134-9.
56. Lo A, Guelrud M, Essenfeld H, Bonis P. Classification of villous atrophy with enhanced magnification endoscopy in patients with celiac disease and tropical sprue. Gastrointest Endosc. 2007;66:377-82.
57. Rickles FR, Klipstein FA, Tomasini J, et al. Long-term follow-up of antibiotic-treated tropical sprue. Ann Intern Med. 1972;76:203-10.
58. Westergaard H. Tropical sprue. Curr Treat Options Gastroenterol. 2004;7:7-11.
59. Menzies IS, Zuckerman MJ, Nukajam WS, et al. Geography of intestinal permeability and absorption. Gut. 1999;44:483-9.
60. Veitch AM, Kelly P, Zulu IS, et al. Tropical enteropathy: A T-cell-mediated crypt hyperplastic enteropathy. Eur J Gastroenterol Hepatol. 2001;13:1175-81.
61. Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332-8.
62. Campbell DI, McPhail G, Lunn PG, et al. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: Association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39:153-7.
63. Galpin L, Manary MJ, Fleming K, et al. Effect of Lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. Am J Clin Nutr. 2005;82:1040-5.
64. Bickler SW. Tropical enteropathy protects against Western diseases in environments of poor sanitation. Med Hypotheses. 2006;67:146-50.
65. Rosenberg IH. Tropical enteritis: Nutritional consequences and connections with the riddle of cholera. J Nutr. 2003;133:333S-335S.
66. Kukuruzovic RH, Brewster DR. Small bowel intestinal permeability in Australian aboriginal children. J Pediatr Gastroenterol Nutr. 2002;35:206-12.
67. Behera B, Mirdha BR, Makharia GK, et al. Parasites in patients with malabsorption syndrome: A clinical study in children and adults. Dig Dis Sci. 2008;53:672-9.
68. Wahnschaffe U, Ignatius R, Loddenkemper C, et al. Diagnostic value of endoscopy for the diagnosis of giardiasis and other intestinal diseases in patients with persistent diarrhea from tropical or subtropical areas. Scand J Gastroenterol. 2007;42:391-6.
69. Kaushik P, Pandey RM, Pande JN. Evaluation of the WHO clinical case definition of AIDS in the Indian context. Suggesting a prediction rule. Medical Princ Pract. 2000;9:42-51.
70. Balamurugan R, Venkataraman S, John KR, Ramakrishna BS. PCR amplification of the IS6110 insertion element of Mycobacterium tuberculosis in fecal samples from patients with intestinal tuberculosis. J Clin Microbiol. 2006;44:1884-6.
71. Yachha SK, Poddar U. Celiac disease in India. Indian J Gastroenterol. 2007;26:230-7.
72. Verma S, Sharma PK, Sivanandan S, et al. Spectrum of primary immune deficiency at a tertiary care hospital. Indian J Pediatr. 2008;75:143-8.
73. Nair S, Mathan M, Ramakrishna BS, Mathan VI. Immunoproliferative small intestinal disease in south India: A clinical and immunomorphological study. J Gastroenterol Hepatol. 1998;13:1207-1211.
74. Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med. 2004;350:239-48.
75. Balakrishnan V, Unnikrishnan AG, Thomas V, et al. Chronic pancreatitis. A prospective nationwide study of 1,086 subjects from India. JOP. 2008;9:593-600.
76. Schneider A, Suman A, Rossi L, et al. SPINK1/PSTI mutations are associated with tropical pancreatitis and type II diabetes mellitus in Bangladesh. Gastroenterology. 2002;123:1026-30.