21 Trigeminal nerve
Trigeminal Nerve
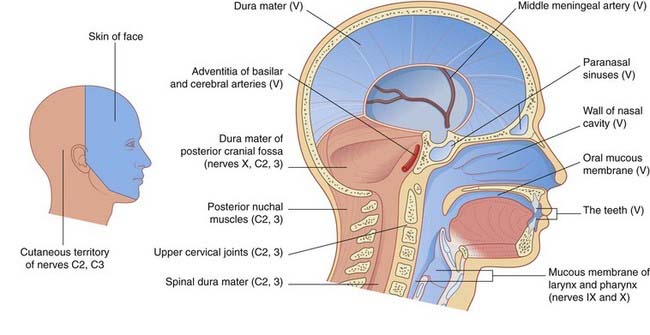
The trigeminal nerve has a very large sensory territory which includes the skin of the face, the oronasal mucous membranes and the teeth, the dura mater, and major intracranial blood vessels. The nerve is also both motor and sensory to the muscles of mastication. The motor root lies medial to the large sensory root at the site of attachment to the pons (Figure 17.16). The trigeminal (Gasserian) ganglion, near the apex of the petrous temporal bone, gives rise to the sensory root and consists of unipolar neurons.
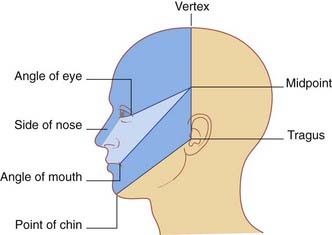
Details of the distribution of the ophthalmic, maxillary, and mandibular divisions are available in gross anatomy textbooks. Accurate appreciation of their respective territories on the face is essential if trigeminal neuralgia is to be distinguished from other sources of facial pain (Clinical Panel 21.1).
Clinical Panel 21.1 Trigeminal neuralgia
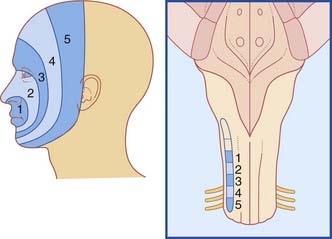
Trigeminal neuralgia is an important condition occurring in middle age or later, characterized by attacks of excruciating pain in the territory of one or more divisions of the trigeminal nerve (usually II and/or III). The patient (who is usually more than 60 years old) is able to map out the affected division(s) accurately. Because it must be distinguished from many other causes of facial pain, the clinician should be able to mark out a trigeminal sensory map (Figure CP 21.1.1). Attacks are triggered by everyday sensory stimuli, e.g. brushing teeth, shaving, and chewing, and the tendency of patients to wince at the onset of attacks accounts for the French term tic doloureux.
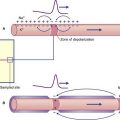
Episodes of paroxysmal facial pain occurring in young adults should raise a suspicion of multiple sclerosis as the cause. Postmortem histology in such cases has revealed demyelination of the sensory root of the trigeminal nerve where it enters the pons. Demyelination of large sensory fibers receiving tactile signals from skin or mucous membranes in trigeminal territory may cause their exposed axons to come into direct contact with unmyelinated axons serving pain receptors. Animal experiments have shown that this type of contact can initiate ephaptic transmission of action potentials between them. It is now widely accepted that the most frequent etiology in later years is vascular compression, usually by a ‘sagging’ posterior cerebral artery in transit around the brainstem. The trigeminal CNS/PNS transition zone (Ch. 6) is several millimeters lateral to the entry zone into the pons, and postmortem histology has provided evidence of the demyelinating effect of chronic pulsatile compression.
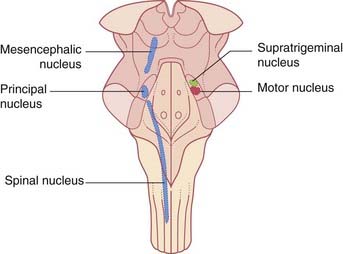
Motor nucleus (Figures 17.16 and 21.1)
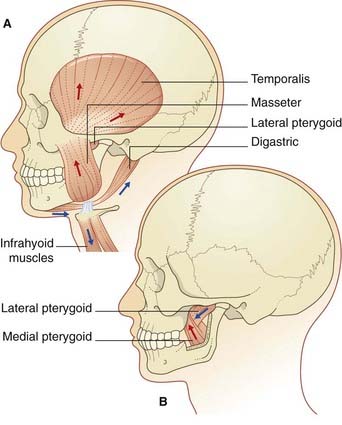
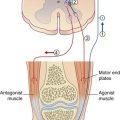
The motor nucleus is the special visceral efferent nucleus supplying the muscles derived from the embryonic mandibular arch. These comprise the masticatory muscles attached to each half of the mandible (Figure 21.2), along with tensor tympani, tensor palati, mylohyoid, and anterior belly of digastric muscle. The nucleus occupies the lateral pontine tegmentum. Embedded in its upper pole is a node of the reticular formation, the supratrigeminal nucleus, which acts as a pattern generator for masticatory rhythm.
Voluntary control is provided by corticonuclear projections from each motor cortex to both motor nuclei, but mainly the contralateral one (Figure 17.3).
Sensory nuclei
Pontine nucleus
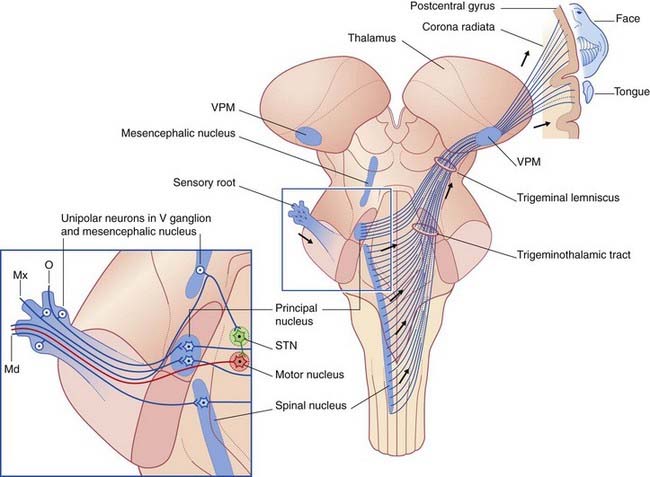
The pontine (principal sensory) nucleus (Figure 17.11) is homologous with the posterior column nuclei (gracile and cuneate). It processes discriminative tactile information from the face and oronasal cavity.
Spinal nucleus
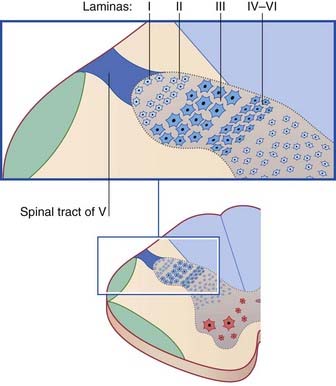
In section, the main spinal nucleus is seen to be an expanded continuation of the outer laminae (I–III) of the posterior horn of the cord (Figure 21.3). The inner three laminae (IV–VI) are relatively compressed. Laminae III and IV are referred to as the magnocellular part of the nucleus. In animals, nociceptive-specific internuncials are found in lamina I. ‘Polymodal’ neurons are in the magnocellular nucleus and correspond to lamina V neurons lower down; they respond to tactile stimuli applied to the trigeminal skin area, also to noxious mechanical stimuli (e.g. pinching the skin with a forceps). Whereas the nociceptive-specific neurons have small receptive fields confined to one territory (a patch of skin or mucous membrane), many of the polymodal neurons show the phenomenon of convergence to a marked degree. In anesthetized animals, a single neuron may be responsive to noxious stimuli applied to a tooth, or to facial skin, or to the temporomandibular joint. This finding provides a plausible basis of explanation for erroneous localization of pain by patients. Examples are given in Clinical Panel 21.2.
Clinical Panel 21.2 Referred pain in diseases of the head and neck
Cervicogenic headache
Experiments on healthy volunteers have demonstrated that noxious stimulation of tissues supplied by the upper cervical nerves may induce pain referred to the head. Tissues tested include the ligaments of the upper cervical joints, the suboccipital muscles, and the sternomastoid and trapezius muscles. The unilateral pain is primarily occipital, as would be expected from the cutaneous distribution of the greater occipital nerve given off by the posterior ramus of nerve C2, but it may radiate to the forehead. Diagnostic features include intensification of the pain by head movement, and temporary abolition by ipsilateral local anesthetic blockade of the greater occipital nerve. A common source of cervicogenic headache in the elderly is spondylosis, a degenerative arthritis in which bony excrescences compress the emerging spinal nerves (Ch. 14). Another source appears to be myofascial disease of the sternomastoid–trapezius continuum close to the base of the skull. Trigger points – tender nodules within the muscles which give rise to occipital pain when compressed – are often detected by physical therapists during palpation of these muscles.
Earache
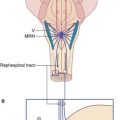
Arrangements for pain modulation appear to be the same as for the spinal cord (Ch. 24). They include the presence of enkephalinergic and GABAergic internuncials in the substantia gelatinosa, and serotonergic projections from the raphe magnus nucleus.
Afferents to the spinal nucleus come from three sources (Figure 21.4):
Topographic representation of the trigeminal territory is onion-like, as in Figure 21.5.
Trigeminothalamic tract and trigeminal lemniscus (Figure 21.6)
The lower part of the trigeminothalamic tract commences in the spinal trigeminal nucleus. Nearly all of these fibers cross the midline before ascending into the pons. This component has features in common with the spinal lemniscus which accompanies it in the brainstem (Figures 17.15–17.21, Figure 17.15, Figure 17.16, Figure 17.17, Figure 17.18, Figure 17.19, Figure 17.20, Figure 17.21), mediating tactile, nociceptive, and thermal sensations. In the pons, it is joined by fibers crossing from the principal sensory nucleus, thus completing the trigeminal lemniscus which terminates in the ventral posterior medial nucleus of thalamus (Ch. 27). From the thalamus, third-order afferents project to the large area of facial representation in the lower half of the somatic sensory cortex.
Mastication
The jaw jerk
The jaw jerk is a tendon reflex elicited by tapping the chin with a downward stroke. The normal response is a twitch of the jaw-closing muscles, because muscle spindle afferents make some direct synaptic contacts upon trigeminal motor neurons. Supranuclear lesions of the motor nucleus (e.g. pseudobulbar palsy, Ch. 18) may be accompanied by an exaggerated (abnormally brisk) jaw jerk.
Foreman PA. Chronic orofacial pain: a clinical challenge. N Z Dent J. 2008;104:44-48.
Gonella MC, Fischbein FC. Disorders of the trigeminal system. Semin Neurol. 2009;29:36-44.
Lambert GA. Pathways for headache. In: Gandevia SC, Burke D, Anthony M, editors. Science and practice in clinical neurology. Cambridge: Cambridge University Press; 1993:284-302.
Pollmann W, Keidel M, Pfaffenrath V. Headache and the cervical spine: a critical review. Cephalalgia. 1997;17:801-816.