Tricuspid Valvular Disease
 Overview
Overview
The distinction between organic tricuspid regurgitation—where pathology (most commonly rheumatic valve disease, endocarditis, carcinoid valve disease, and trauma) results in structural leaflet damage—and secondary or functional tricuspid regurgitation—where the leaflets appear macroscopically normal—was made in the 1950s. 1 By far the most common reason for tricuspid valve surgery is functional tricuspid regurgitation (TR) in a patient whose primary indication for surgery is mitral valve disease. Functional TR may be due to any combination of right heart dysfunction or dilation, pulmonary hypertension, or left heart dysfunction, and it occurs in the setting of an otherwise normal tricuspid valve. It is commonly seen in patients presenting for mitral, aortic, and coronary bypass surgery, where the indications for concomitant tricuspid repair remain somewhat controversial, and accurate evaluation is complicated by the highly dynamic nature of the dysfunction. Organic tricuspid disease such as endocarditis, carcinoid, and rheumatic valve disease is relatively uncommon in cardiac surgical patients.
 Functional Tricuspid Regurgitation
Functional Tricuspid Regurgitation
Functional TR occurs in the absence of pathology affecting the tricuspid valve leaflets. It results from poor leaflet coaptation due to annular dilation and chordal tethering associated with right ventricular dilation and/or left ventricular (LV) dysfunction (![]() Video 16-1): the most common underlying pathophysiology is congestive heart failure, followed by pulmonary hypertension and aortic or mitral valve dysfunction. It is a common finding in severe ischemic and dilated cardiomyopathy. Significant functional TR is found in up to a third or more of patients presenting for mitral surgery. 2 Although severe TR is a widely accepted indication for surgical intervention, moderate TR is highly dynamic, and its impact on prognosis and functional capacity is not well defined. Indications and optimal strategy for treatment of moderate functional TR at the time of concomitant cardiac surgery remain controversial.
Video 16-1): the most common underlying pathophysiology is congestive heart failure, followed by pulmonary hypertension and aortic or mitral valve dysfunction. It is a common finding in severe ischemic and dilated cardiomyopathy. Significant functional TR is found in up to a third or more of patients presenting for mitral surgery. 2 Although severe TR is a widely accepted indication for surgical intervention, moderate TR is highly dynamic, and its impact on prognosis and functional capacity is not well defined. Indications and optimal strategy for treatment of moderate functional TR at the time of concomitant cardiac surgery remain controversial.
 Functional Anatomy
Functional Anatomy
The tricuspid valve maintains its competence through the integrity of its three valve leaflets, the subvalvular apparatus (which consists of thin chordae arising from the RV papillary muscles attaching to the margins and body of the leaflet), and the saddle-shaped valve annulus. 3 Distortion in the three-dimensional geometry of any of these components results in regurgitation. This can be described using Carpentier’s pathophysiologic triad and classification of regurgitation ( Table 16-1). 4 This terminology is most commonly used in reference to the mitral valve but was applied to the tricuspid valve in his original manuscript on the subject and remains a useful nomenclature. Functional TR is due to RV and annular dilation (which cause type I leaflet dysfunction) and/or papillary muscle displacement and leaflet tethering (which cause type IIIb leaflet dysfunction). Both of these mechanisms, which frequently coexist, result in loss of leaflet coaptation and have been described as occurring in three phases: 5
TABLE 16-1
Carpentier’s Classification of Regurgitation, Listing Lesions Commonly Associated with Each Leaflet Dysfunction
| Valve Dysfunctions | Lesions |
| Type I: Valve dysfunction with normal leaflet motion | Annular dilation and deformation Leaflet perforation |
| Type II: Leaflet prolapse | Chordae rupture Chordae elongation Papillary muscle rupture Papillary muscle elongation |
| Type III: Restricted leaflet motion | |
| IIIa: Diastolic | Leaflet thickening and retraction Commissure fusion Chordae thickening Chordae fusion Calcification |
| IIIb: Systolic | Ventricular aneurysm Ventricular fibrous plaque Ventricular dilation Leaflet entrapment |
From Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. Philadelphia: Saunders; 2010:188.
1. An initial phase in which RV and annular dilation are present, with or without TR
2. Significant TR due to progressive annular and RV dilation
3. Papillary muscle displacement as a result of right and/or LV dysfunction causes leaflet tethering and more severe TR.
Annular dilation is an early consequence of RV dilation or dysfunction due to lack of an anatomic fibrous annulus and may increase the annular circumference from around 100 mm to 170 mm. Annular dilation does not occur symmetrically; the posterior and anterior annulus—which are relatively unsupported—dilate more rapidly than the septal annulus ( Fig. 16-1). Structures in close proximity to the tricuspid annulus, and that may be compromised by disease processes or tricuspid valve surgery, include the atrioventricular conduction tissue that lies at the apex of the triangle of Koch (formed by the coronary sinus, septal annulus, and tendon of Todaro), the midportion of the right coronary artery, and the noncoronary cusp of the aortic valve ( Fig. 16-2).
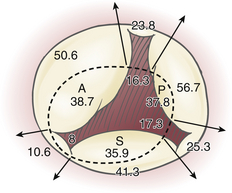
Figure 16-1 Segmental dimensions in millimeters are reported for normal (dotted line) and pathologic conditions. Note greater tendency of anterior (A) and posterior (P) annulus to dilate compared to septal (S) annulus. (Adapted from Deloche A, Guerinon J, Fabiani JN. Etude anatomique des valvulopathies rhumatismales tricuspidiennes: application à l’étude des différentes valvuloplasties. Ann Chir Thorac Cardiovasc. 1973;12:343-349.)
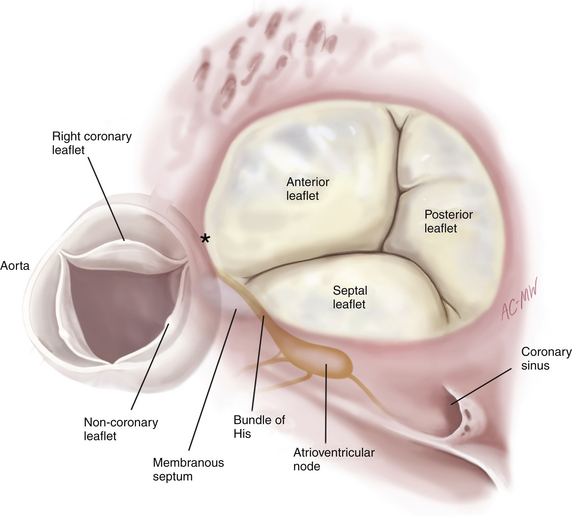
Figure 16-2 Surgeon’s view of tricuspid valve and coronary sinus, showing anatomic structures close to tricuspid annulus. Asterisk denotes commissure between right and noncoronary aortic sinuses. (From Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. Philadelphia: Saunders; 2010:178.)
 Pathophysiology
Pathophysiology
Pulmonary Hypertension
Severe left-sided cardiomyopathy and valvular heart disease are usually characterized by elevated left atrial filling pressures that are directly transmitted to the pulmonary vasculature. Pulmonary hypertension is due to a combination of the resultant increased afterload and compensatory pulmonary vasoconstriction and chronic vascular remodeling, resulting in RV pressure overload, which may directly result in TR. Pulmonary hypertension appears to determine the degree of secondary TR to a limited extent, but the relationship is not a direct one. 6 The finding that functional TR may also develop in the absence of demonstrable pulmonary hypertension suggests additional underlying mechanisms.
Annular Dilation
Annular dilation results in loss of leaflet coaptation, causing type I regurgitation, and has been shown to be a strong independent predictor of functional TR. 6 It has more recently become apparent that in addition to dilation, several other annular abnormalities characterize functional TR. Three-dimensional (3D) echocardiography has been used to show how valves with functional TR lose their normal saddle-shaped annular geometry, becoming flattened and circular as they dilate, with an associated asymmetric loss of annular contraction. 6
Leaflet Tethering
Leaflet tethering that results in Carpentier type IIIb regurgitation may be the result of chordal or papillary muscle shortening or papillary muscle displacement, most commonly due to ventricular dilation. Functional TR due to septal leaflet tethering may be observed in the context of normal RV function, dimensions, and pulmonary pressure. This really leaves only LV pathology as a potential explanation for functional TR in this setting. The right and left ventricles are interdependent at the septum, where the papillary muscles giving rise to the chordae of the tricuspid valve’s septal leaflet take origin. Where LV pathology results in a dysfunctional or displaced interventricular septum, tethering of the septal tricuspid leaflet may result, even if the RV appears echocardiographically normal.7,8 This may be why LV dysfunction is associated with functional TR. In an analysis of 75 patients with dilated RVs, eccentricity of the RV and tricuspid valve tethering area were most strongly predictive of the severity of functional TR, whereas RV function, dimension, and pulmonary artery pressures were insignificant determinants. 9
 Organic Tricuspid Disease
Organic Tricuspid Disease
Organic tricuspid disease requiring surgery is uncommon ( Table 16-2). Right-sided infective endocarditis is suggestive of intravenous drug use but is also seen in patients with indwelling catheters for dialysis or chemotherapy. Multiple vegetations attached to indwelling catheters and wires and extending over the atrial and ventricular surfaces of the leaflets, which are frequently thickened, are common; leaflet perforations and annular abscesses less so. Trauma can cause TR, most commonly as a result of iatrogenic mechanisms such as endomyocardial biopsies in heart transplant patients, fibrosis around pacing wires, or extraction of long-term intracardiac lines. Blunt trauma is an unusual cause of TR due to papillary muscle rupture (![]() Video 16-1). Rheumatic valve disease, rheumatoid arthritis, systemic lupus erythematosus, and antiphospholipid syndrome are all associated with organic leaflet lesions that may result in significant tricuspid valve dysfunction. Rheumatic tricuspid valve disease is often predominantly functional, but it is occasionally characterized by leaflet involvement with thickened, shortened leaflets and commissural fusion. Carcinoid valve disease usually involves the tricuspid and pulmonary valve, causing very pronounced leaflet thickening and mixed stenotic and regurgitant lesions (regurgitation is usually predominant). In these patients, one of the goals of valve surgery is to facilitate subsequent resection of hepatic metastases. Tricuspid valve stenosis, which is predominantly caused by rheumatic valve disease and occasionally a feature of carcinoid valve disease, is an extremely rare entity in developed countries.
Video 16-1). Rheumatic valve disease, rheumatoid arthritis, systemic lupus erythematosus, and antiphospholipid syndrome are all associated with organic leaflet lesions that may result in significant tricuspid valve dysfunction. Rheumatic tricuspid valve disease is often predominantly functional, but it is occasionally characterized by leaflet involvement with thickened, shortened leaflets and commissural fusion. Carcinoid valve disease usually involves the tricuspid and pulmonary valve, causing very pronounced leaflet thickening and mixed stenotic and regurgitant lesions (regurgitation is usually predominant). In these patients, one of the goals of valve surgery is to facilitate subsequent resection of hepatic metastases. Tricuspid valve stenosis, which is predominantly caused by rheumatic valve disease and occasionally a feature of carcinoid valve disease, is an extremely rare entity in developed countries.
TABLE 16-2
Indications for Intervention in Tricuspid Valve Disease
| Indication | Class |
| Severe TR in a patient undergoing left-sided valve surgery | IC |
| Severe primary TR and symptoms despite medical therapy without severe RV dysfunction | IC |
| Severe TS (±TR) with symptoms despite medical therapy ∗ | IC |
| Severe TS (±TR) in a patient undergoing left-sided valve intervention ∗ | IC |
| Moderate organic TR in a patient undergoing left-sided valve surgery | IIaC |
| Moderate secondary TR with dilated annulus (>40 mm) in a patient undergoing left-sided valve surgery | IIaC |
| Severe TR and symptoms after left-sided valve surgery, in the absence of left-sided myocardial, valve, or RV dysfunction and without severe pulmonary hypertension (systolic pulmonary artery pressure >60 mmHg) | IIaC |
| Severe isolated TR with mild or no symptoms and progressive dilation or deterioration of RV function | IIbC |
RV, Right ventricular; TR, tricuspid regurgitation; TS, tricuspid stenosis.
∗Percutaneous technique can be attempted as a first approach if TS is isolated.
From Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230-268.
 Indications for Tricuspid Valve Surgery
Indications for Tricuspid Valve Surgery
Severe symptomatic TR is an American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology class I indication for surgical intervention ( Boxes 16-1 and 16-2).10,11 It is equally accepted that moderate TR in isolation is not an indication for surgery. A large portion of patients fall in a gray area between the two extremes. These are patients who usually present for mitral valve surgery (although occasionally the question arises in patients undergoing aortic valve or coronary bypass surgery) in whom functional TR is an incidental finding. Should these patients undergo tricuspid repair in addition to their primary surgical procedure? The rationale for surgical correction of functional TR in this setting stems from somewhat circumstantial evidence. Over a third of patients undergoing isolated left-sided valve surgery without concomitant tricuspid repair will develop significant TR during long-term follow-up.12,13 Is this associated with poorer clinical outcomes? In nonsurgical series, moderate TR has been associated with increased early and late mortality14,15 and decreased functional outcome; this may be applicable to the population of patients undergoing mitral surgery. Most studies comparing the outcomes of concomitant tricuspid annuloplasty for moderate functional TR at the time of mitral repair to isolated mitral repair have been inadequately powered to demonstrate any clear survival benefit. However, there is limited evidence from two nonrandomized studies suggesting that tricuspid annuloplasty in this setting may be associated with an improvement in both functional status and survival.16,17 This may be due to the beneficial effects of repairing severe (rather than moderate) TR. Although the established consensus is that severe TR should be repaired at the time of concomitant mitral surgery, the indications for repair of lesser degrees of TR are less well defined, and there is wide variation between groups in the prevalence of concomitant tricuspid repair.
 Rationale for Concomitant Tricuspid Repair
Rationale for Concomitant Tricuspid Repair
Moderate Tricuspid Regurgitation
Moderate TR may be identified for the first time on routine intraoperative transesophageal echocardiography (TEE) prior to institution of cardiopulmonary bypass (CPB), and its presence in this setting is highly suggestive of significant valvular dysfunction. Atrioventricular valve regurgitation is usually reduced in severity by at least one grade by the decreased preload, afterload, and RV function that occurs in patients under general anesthesia. 18 Furthermore, quantitative methods may underestimate TR. 19
Traditional consensus was that correction of mitral and aortic valve disease was sufficient to address moderate TR by improving pulmonary hypertension and allowing RV remodeling, but this view has been undermined by available observational data. Tricuspid regurgitation has been reported to either persist or increase in up to two thirds of patients after isolated mitral valve surgery.12,13 Two thirds of patients who undergo isolated mitral surgery and then need reoperative tricuspid surgery for late development of severe TR had only mild tricuspid insufficiency at the time of their initial operation. (Reoperative tricuspid valve surgery is a particularly high-risk procedure, not because of the intrinsic risk of the surgery but because of the associated severe congestive cardiac failure and pulmonary dysfunction.) This appears to be less of a problem in patients undergoing early mitral repair for degenerative valve disease. 20 It is difficult to accurately distinguish between patients in whom moderate TR will or will not progress, so it is increasingly the view that moderate functional TR should probably be repaired at the time of concomitant mitral valve surgery.
Annular Dilation
Because annular dilation is a strong predictor of functional regurgitation, and annular diameter is a less dynamic measure than the regurgitant jet, annular size is commonly used to identify patients who would benefit from tricuspid repair. Obtaining accurate and reproducible measurements of tricuspid annular dimensions, however, is challenging. The asymmetric saddle shape of the tricuspid valve means that even small variations in the angle of the ultrasound beam can result in quite large discrepancies. Tricuspid annular dilation may be defined as an intercommissural distance of over 35 mm in any two-dimensional (2D) echocardiographic view, but the echocardiographic threshold for surgical repair has variously been suggested to be greater than 27 mm in either maximal early systolic or minimal late end-systolic diameters, 21 greater than 40 mm maximum end-systolic diameter in the four-chamber view, 22 greater than 51 mm mean diastolic annulus diameter in the four-chamber view and 54 mm in the short-axis view, 22 or greater than 70 mm in the transgastric view ( Fig. 16-3). 3 Tricuspid dilation may be more accurately analyzed with 3D echocardiography. Intraoperatively, direct assessment can be used to obtain a definitive answer. In the absence of a history of significant TR, reasonable indications for tricuspid annuloplasty include a maximal intercommissural distance greater than 70 mm in the flaccid heart 17 or annulus circumference significantly larger than combined posterior and anterior leaflet surface area.
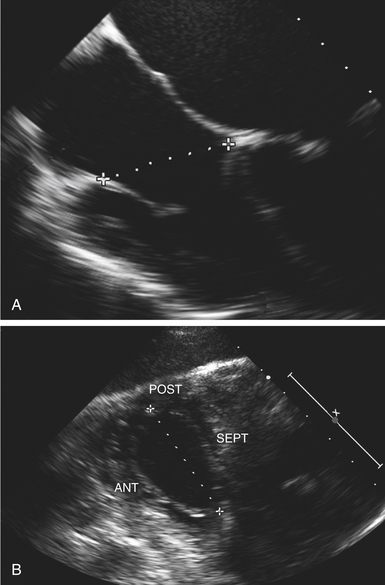
Figure 16-3 Significantly dilated annulus is indicated by a cutoff value of 40 mm in four-chamber view (A) and 70 mm in transgastric view (B). ANT, Anterior; POST, posterior; SEPT, septal. (From Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. Philadelphia: Saunders; 2010:185.)
 Surgical Considerations
Surgical Considerations
The tricuspid valve may be approached via a median sternotomy or right anterolateral thoracotomy, most commonly using an oblique right atriotomy. Bicaval cannulation, which may be direct or indirect (via the right atrial appendage and body of the right atrium, or transjugular or transfemoral in minimally invasive approaches and reoperations), is required together with caval snares and/or vacuum-assisted venous drainage to eliminate airlocks abruptly terminating venous drainage. The additional CPB time required to repair the tricuspid valve is 15 to 20 minutes, which may represent a significant addition to the ischemic time if the repair is performed with the cross-clamp on. There are two alternatives in situations where minimizing ischemic time is a particular priority: (1) performing the tricuspid repair on the beating heart on bypass prior to cross-clamping the aorta (using the opportunity to directly verify placement of the retrograde coronary sinus catheter) or (2) carrying out the tricuspid repair after removal of the cross-clamp during a period of de-airing and reperfusion.
Surgical Treatment of Functional Tricuspid Regurgitation
Tricuspid Annuloplasty
Essentially, there are two surgical approaches to restoring the dilated annulus to its physiologic size and stabilizing an annulus of normal size ( Fig. 16-4). 3 Remodeling annuloplasty permanently fixes the annulus in a systolic position by suturing in a rigid or semirigid ring and reducing the size of the tricuspid orifice (![]() Video 16-2). 4 The alternative to this method is reduction annuloplasty—often using the De Vega suture annuloplasty technique and its derivatives or a completely flexible band to reduce annulus size by using a continuous suture to “purse string” the annulus, relying on continued integrity of the suture and annular contraction and fibrosis to maintain the new annular dimensions. Both methods stabilize the anterior and posterior annulus, which is most at risk of dilation. Depending on the choice of annuloplasty ring, the septal annulus is kept relatively free of sutures, particularly in the anteroseptal commissural area where the conduction tissue is at risk.
Video 16-2). 4 The alternative to this method is reduction annuloplasty—often using the De Vega suture annuloplasty technique and its derivatives or a completely flexible band to reduce annulus size by using a continuous suture to “purse string” the annulus, relying on continued integrity of the suture and annular contraction and fibrosis to maintain the new annular dimensions. Both methods stabilize the anterior and posterior annulus, which is most at risk of dilation. Depending on the choice of annuloplasty ring, the septal annulus is kept relatively free of sutures, particularly in the anteroseptal commissural area where the conduction tissue is at risk.
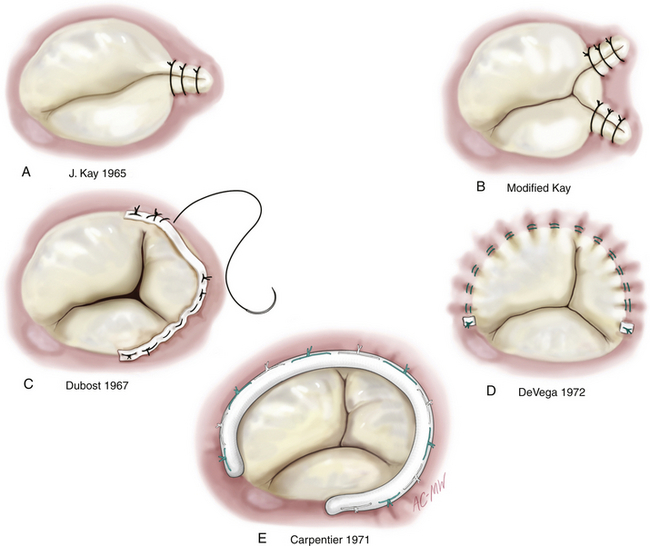
Figure 16-4 Selected techniques of tricuspid annuloplasty. Kay bicuspidalization techniques (A and B) have shown less long-term durability than suture (C and D) and ring (E) annuloplasty techniques and are consequently much less common. (From Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. Philadelphia: Saunders; 2010:195.)
Methods of plicating the posterior annulus or sewing the leaflet edges together have fallen out of favor because long-term results have shown relatively poor freedom from residual and recurrent regurgitation. A remodeling ring may offer the best long-term durability; observational studies suggest that ring repairs are more durable than suture repairs. 23 Data from the surgical literature suggest that over 85% of patients who have a ring annuloplasty will be free from moderate or severe TR 5 to 10 years after surgery.2,23
Tricuspid Valve Replacement
Unlike left-sided valve prostheses, where the primary consideration is balancing the lifetime risk of embolic and hemorrhagic complications associated with mechanical valves against the risk of structural valve degeneration carried by bioprostheses, the main issue in tricuspid valve selection is usually the inability of most patients requiring replacement to comply with anticoagulation. In the case of patients with carcinoid valve disease requiring liver resection (with significant baseline hepatic dysfunction), the difficulties inherent in anticoagulation must be weighed against the levels of tumoral activity, which are inversely related to the longevity of bioprostheses in that position. High serotonin or 5HIAA levels may be associated with severe structural valve degeneration in as little as 2 years. 24 In patients undergoing tricuspid valve replacement, permanent epicardial pacing wires are often placed, in view of the increased risk of postoperative complete heart block and the contraindication to transvalvular pacing wires.
Technical Pitfalls
The tricuspid valve is in close proximity to the noncoronary and right coronary aortic sinuses, atrioventricular conduction tissue, and middle right coronary artery; exposure of the valve requires specific cannulation techniques outlined earlier. There are several potential complications specific to tricuspid surgery. The sinoatrial node is at risk if direct cannulation of the superior vena cava is performed too proximally or caval snares are incorrectly positioned. The atrioventricular node may be injured by suture placement in the region of the apex of the triangle of Koch. Most annuloplasty techniques are designed to avoid this area to reduce the risk of complete heart block and need for a permanent pacemaker, which has been reported to be around 3% after tricuspid surgery. 25 Acute aortic insufficiency is a recognized complication of excessively deep suture placement in the region of the anteroseptal annulus, distorting the adjacent aortic sinuses or even impinging on the aortic valve leaflets.
Outcomes of Tricuspid Surgery
Isolated Tricuspid Surgery
Tricuspid valve surgery has been associated with operative mortality of up to 25% in historic series. 26 Currently, isolated tricuspid valve surgery has an associated mortality of 10% to 15% in national registries27,28—much higher than that for isolated surgery of other valves, reflecting the significant burden of comorbidity in these patients. The apparent decrease in mortality compared to historical series has probably been achieved through incremental changes in patient selection and preoperative optimization; refining strategies for mediastinal reentry; more aggressive use of inhaled pulmonary vasodilators (e.g., nitric oxide, epoprostenol, and oral pulmonary inodilators like sildenafil); management of RV dysfunction using inotropes and mechanical support where necessary; and prevention and effective treatment of end-organ dysfunction, coagulopathy, and sepsis in the postoperative period. Prolonged dependence on mechanical ventilation and inotropic support is common. Long-term data on event-free survival are limited. In one recent series, the 10-year survival of patients undergoing isolated tricuspid repair was 69%, compared to 50% for those undergoing tricuspid valve replacement. Ten-year freedom from reoperation for patients with bioprostheses has been reported to be 95% and for those with mechanical valves, 80%.29,30 The combined challenges of hepatic dysfunction, right heart failure, and carcinoid crises mean that valve replacement for carcinoid disease is associated with a mortality of 10% to 20%.
Concomitant Tricuspid Repair
In contrast to outcomes for isolated tricuspid surgery, the incremental mortality risk posed by concomitant tricuspid annuloplasty at the time of mitral valve surgery in contemporary practice appears to be almost negligible. 17 Tricuspid valve repair takes an additional 15 to 20 minutes but can be performed with the cross-clamp off and the heart perfused and beating, so although the procedure does add significantly to the CPB time, there is no particular need to prolong the ischemic clamp time if this is a concern. The incidence of heart block requiring pacemaker insertion is potentially greater with concomitant tricuspid surgery, but this has not been shown to be the case in most comparative series 17 and is highly dependent on choice of annuloplasty technique. Similarly, the theoretical incremental risk of postoperative bleeding incurred by the addition of a right atriotomy suture line has not been confirmed by studies available to date. 17 Midterm freedom from significant TR appears significantly greater in patients with tricuspid annular dilation or moderate functional TR undergoing concomitant tricuspid annuloplasty, compared to those undergoing isolated mitral surgery, with some evidence of associated improvement in functional status.17
References
1. Anyanwu, A. C., Chikwe, J., Adams, D. H. Tricuspid valve repair for treatment and prevention of secondary tricuspid regurgitation in patients undergoing mitral valve surgery. Curr Cardiol Rep. 2008; 10:110–117.
2. Chan, V., Burwash, I. G., Lam, B. K., et al. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg. 2009; 88:1209–1215.
3. Carpentier, A., Adams, D. H., Filsoufi, F. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. Philadelphia: Saunders; 2010.
4. Carpentier, A. Cardiac valve surgery–the “French correction. ”. J Thorac Cardiovasc Surg. 1983; 86:323–337.
5. Dreyfus, G. D., Chan, K. M. Functional tricuspid regurgitation: a more complex entity than it appears. Heart. 2009; 95:868–869.
6. Park, Y. H., Song, J. M., Lee, E. Y., Kim, Y. J., Kang, D. H., Song, J. K. Geometric and hemodynamic determinants of functional tricuspid regurgitation: a real-time three-dimensional echocardiography study. Int J Cardiol. 2008; 124:160–165.
7. Fukuda, S., Saracino, G., Matsumura, Y., et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation. 2006; 114:I492–I498.
8. Fukuda, S., Gillinov, A. M., McCarthy, P. M., et al. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2006; 114:I582–I587.
9. Kim, H. K., Kim, Y. J., Park, J. S., et al. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006; 98:236–242.
10. Bonow, R. O., Carabello, B. A., Kanu, C., et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease), developed in collaboration with the Society of Cardiovascular Anesthesiologists, endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006; 114:e84–231.
11. Vahanian, A., Baumgartner, H., Bax, J., et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007; 28:230–268.
12. Porter, A., Shapira, Y., Wurzel, M., et al. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis. 1999; 8:57–62.
13. Matsunaga, A., Duran, C. M. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation. 2005; 112:I453–I457.
14. Sagie, A., Schwammenthal, E., Newell, J. B., et al. Significant tricuspid regurgitation is a marker for adverse outcome in patients undergoing percutaneous balloon mitral valvuloplasty. J Am Coll Cardiol. 1994; 24:696–702.
15. Nath, J., Foster, E., Heidenreich, P. A. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004; 43:405–409.
16. Calafiore, A. M., Gallina, S., Iaco, A. L., et al. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. Ann Thorac Surg. 2009; 87:698–703.
17. Dreyfus, G. D., Corbi, P. J., Chan, K. M., Bahrami, T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005; 79:127–132.
18. Grewal, K. S., Malkowski, M. J., Piracha, A. R., et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol. 2000; 85:199–203.
19. Grossmann, G., Stein, M., Kochs, M., et al. Comparison of the proximal flow convergence method and the jet area method for the assessment of the severity of tricuspid regurgitation. Eur Heart J. 1998; 19:652–659.
20. Yilmaz, O., Suri, R. M., Dearani, J. A., et al. Functional tricuspid regurgitation at the time of mitral valve repair for degenerative leaflet prolapse: the case for a selective approach. J Thorac Cardiovasc Surg. 2011; 142:608–613.
21. Ubago, J. L., Figueroa, A., Ochoteco, A., Colman, T., Duran, R. M., Duran, C. G. Analysis of the amount of tricuspid valve anular dilatation required to produce functional tricuspid regurgitation. Am J Cardiol. 1983; 52:155–158.
22. Come, P. C., Riley, M. F. Tricuspid anular dilatation and failure of tricuspid leaflet coaptation in tricuspid regurgitation. Am J Cardiol. 1985; 55:599–601.
23. McCarthy, P. M., Bhudia, S. K., Rajeswaran, J., et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004; 127:674–685.
24. Castillo, J. G., Filsoufi, F., Rahmanian, P. B., Zacks, J. S., Warner, R. R., Adams, D. H. Early bioprosthetic valve deterioration after carcinoid plaque deposition. Ann Thorac Surg. 2009; 87:321.
25. Ghoreishi, M., Brown, J. M., Stauffer, C. E., et al. Undersized tricuspid annuloplasty rings optimally treat functional tricuspid regurgitation. Ann Thorac Surg. 2011; 92:89–95. [discussion 6].
26. King, R. M., Schaff, H. V., Danielson, G. K., et al. Surgery for tricuspid regurgitation late after mitral valve replacement. Circulation. 1984; 70:I193–I197.
27. Rankin, J. S., Hammill, B. G., Ferguson, T. B., Jr., et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg. 2006; 131:547–557.
28. Bridgewater, B., Keogh, B., The Society for Cardiothoracic Surgery in Great Britain and Ireland Sixth National Adult Cardiac Surgical Database Report 2008: Dendrite Clinical Systems, 2009.
29. Moraca, R. J., Moon, M. R., Lawton, J. S., et al. Outcomes of tricuspid valve repair and replacement: a propensity analysis. Ann Thorac Surg. 2009; 87:83–88. [discussion 8-9].
30. Filsoufi, F., Anyanwu, A. C., Salzberg, S. P., Frankel, T., Cohn, L. H., Adams, D. H. Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg. 2005; 80:845–850.



