12 Tricuspid Atresia
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, left aortic arch, and heart rate of 142 bpm.
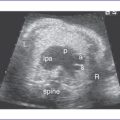
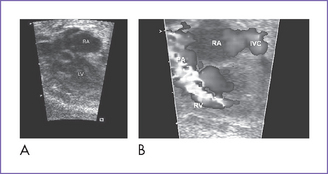
2. The four-chamber view is abnormal (Fig. 12-1), but cardiac axis, position, and size are normal (cardiothoracic ratio = 0.35).
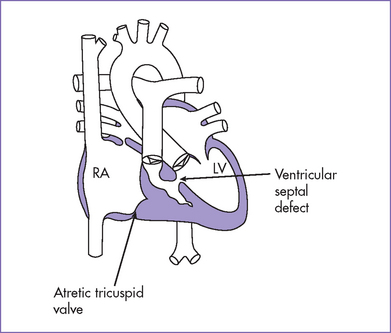
3. The tricuspid valve is atretic (no valve tissue present).
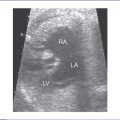
4. The right ventricle (RV) is small (Fig. 12-2) and has mildly reduced function.
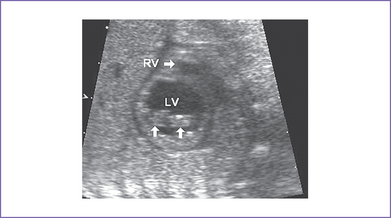
5. The left atrioventricular (AV) valve is patent and opens into a dominant left ventricle (LV) with a narrow jet of mitral regurgitation (4 m/s).
6. There is no evidence of endocardial fibroelastosis.
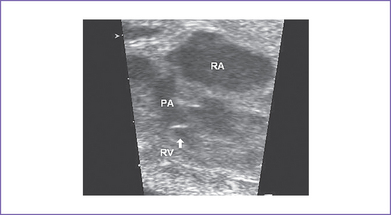
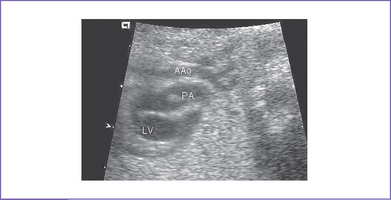
7. The outflow assessment reveals normally related great arteries with clear asymmetry in size (aorta–to–pulmonary artery diameter ratio = 1:0.5).
8. The pulmonary arteries are small and hypoplastic: The right pulmonary artery is 0.26 cm and the left is 0.25 cm. They are anterior and to the left of the aorta.
9. The ascending aorta is large (posterior and to the right) and has normal Doppler blood velocity with antegrade flow.
10. The pulmonary valve is bicuspid and domes mildly in systole.
11. The aortic annulus is of normal size and there is normal Doppler velocity through it.
12. The aortic arch is leftward and the ductal arch is slightly bigger than the aortic arch. The flow is reversed in the ductal arch (aorta to pulmonary) but is normal antegrade through the aortic arch.
13. There is a moderate-sized muscular ventricular septal defect (VSD) with left-to-right shunt to the pulmonary valve (see Fig. 12-1B).
14. The foramen ovale is unrestricted, with mainly right-to-left shunt and normal pulmonary venous flow pattern.
15. The LV Tei index (myocardial performance index) of 0.34 is normal.
D. Fetal management and counseling
1. Amniocentesis could be deferred in view of gestation and the low chance of having chromosomal anomalies with tricuspid atresia.
2. Follow-up included serial antenatal studies at 4-week intervals to assess:
b. Size and direction of flow across the foramen ovale. It could become restricted with an abnormal flow pattern, or the systemic venous Doppler could indicate increasing central venous pressure.
c. Progressive pulmonary outflow obstruction and diminution in VSD size.
d. Branch pulmonary artery growth.
e. Mitral valve anatomy and function.
f. Development of new lesions such as subpulmonary obstruction.
F. Neonatal management
a. Prostaglandin E1 (PGE1) infusion should be started to maintain ductal patency. The hyperoxia test could be deferred given the retrograde flow through the ductus arteriosus in utero consistent with critical pulmonary outflow obstruction.
b. Rarely, if the baby has relatively high systemic saturations but very poor perfusion and evolving evidence of right heart failure and poor cardiac output, an urgent balloon atrial septostomy is required to allow more mixing at the atrial level while surgery is arranged. At times, if the foramen ovale is mildly restrictive before birth, increased pulmonary venous return and higher left atrial pressures after birth can hamper the obligate right-to-left shunt and lead to increasing central venous pressures.
c. Infants with VSD of adequate size and adequate blood flow need to be followed closely for decreasing oxygen saturation resulting from reduction in the size of VSD.
G. Follow-up
1. Quality of life after the Fontan procedure is guarded.
2. Survival after the Fontan procedure (Fontan et al, 1991, results refer mainly to patients with tricuspid atresia).
3. Outcomes after the Fontan procedure have improved significantly over the past decades. Improvements in early survival are in part due to:
a. Better understanding of patient-related risk factors and their application to patient selection.
b. Patient preparation and optimized staging of the Fontan procedure.
c. Procedural modifications (e.g., lateral tunnel or extracardiac Fontan).
4. As patients grow up, they should be followed by an adult congenital heart disease cardiologist for life.
I. Outcome of this case
1. Labor was induced at 38 weeks’ gestation.
2. The baby was born with good Apgar scores and good weight.
3. Moderate cyanosis was noted at birth: Pulse oximeter reading was 77%.
4. Postnatal echocardiography confirmed the diagnosis of tricuspid atresia, hypoplastic right ventricle, and severe critical pulmonary stenosis with a large muscular VSD. There was a large atrial septal defect (ASD) and a patent ductus arteriosus with bidirectional shunting.
6. The baby had a palliative aortopulmonary shunt at I week of age.
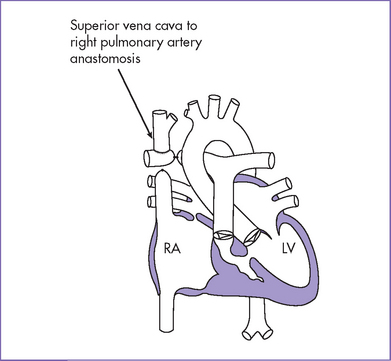
7. At 7 months (6 kg), he had a successful bidirectional Glenn operation.
8. At the age of 2 years, after a diagnostic cardiac catheterization to measure pulmonary vascular resistance, he had a successful extracardiac Fontan operation.
9. Because this neonate was born with tricuspid atresia and pulmonary stenosis, the prognosis is the best of all the types of single ventricle. With a left ventricular pumping chamber, the long-term ventricular function is optimal.
II. YOUR HANDY REFERENCE
A. Tricuspid atresia
a. The incidence of tricuspid atresia has been reported at between 0.3% and 5.3% of infants with congenital heart disease.
b. In most reports, incidence is between 1% and 2.5%.
c. In Allan and Sharland’s (1992) fetal series, incidence is 4%. In this series, out of 84 fetuses with tricuspid atresia, 54 pregnancies were terminated, there were two intrauterine deaths and four infant deaths, and 22 babies survived.
a. The overall prognosis without treatment for children with tricuspid atresia is poor.
b. The surgical options are a single ventricle repair with a bidirectional Glenn at 3 to 6 months (surgical mortality is about 2%-3%), followed by a Fontan operation at 2 years (surgical mortality is 2%-5%).
c. Children with tricuspid atresia usually have the lowest risk in Fontan repair among children undergoing this type of repair.
3. Associated syndromes and extracardiac anomalies.
a. Extracardiac malformations are uncommon.
b. Chromosomal anomalies are uncommon.
a. Pulmonary atresia with intact interventricular septum, where the tricuspid valve is hypoplastic.
b. Double-inlet left ventricle: It could be difficult early in fetal life to see two AV valves, so the appearance is that of a dominant LV and a hypoplastic RV.
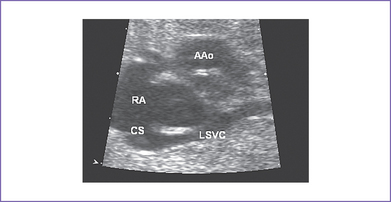
c. With a persistent left SVC, the position of the ASD can be confusing (Fig. 12-4).
5. Clues to fetal sonographic diagnosis.
a. No opening tricuspid valve in the four-chamber view; a muscular ridge separates the RA from the RV.
b. No flow from the RA to RV on color flow mapping.
c. VSD with left-to-right shunt.
d. Transposed great arteries in about 20% to 30%, which may be associated with pulmonary stenosis or subaortic obstruction. In subaortic obstruction, the aortic arch itself is small and there may be coarctation of the aorta. Occasionally, there is neither aortic nor pulmonary outflow obstruction.
e. Pulmonary artery anatomy and flow.
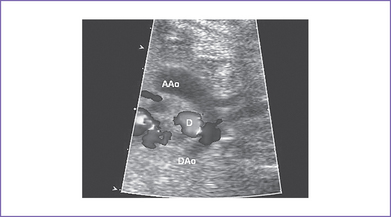
f. The ductus arteriosus can be identified from the aorta supplying the pulmonary arteries (Fig. 12-5). The pattern of flow in it depends on the amount of antegrade flow from the RV (Fig. 12-6).
g. RA to LA flow should be unrestrictive. The venous Doppler blood velocity in the hepatic veins can be monitored to assess this possibility.
6. Cardiovascular profile score.
a. The score may be 10/10 if there are no abnormal signs.
b. The score reflects 2 points for each of five categories: hydrops, venous Doppler, heart size, cardiac function, and arterial Doppler.
c. It is not unusual to see mild venous Doppler changes with:
7. Progression in utero (Figs. 12-7 and 12-8).
a. Possible progressive foramen ovale restriction with evolution of hydrops fetalis.
b. Diminution in VSD size with progressive pulmonary or aortic outflow tract obstruction (depending upon the underlying outflow arrangement and anatomy).
c. Progressive arch hypoplasia where there is subaortic obstruction.
d. Progressive pulmonary artery hypoplasia with more critical pulmonary outflow obstruction.
8. Immediate postnatal management for patients without prenatal diagnosis of tricuspid atresia.
a. Postnatal management depends on:
b. Newborns with some degree of pulmonary outflow obstruction, unless it is associated with reverse flow in the duct in fetal life, typically are assessed as the duct is allowed to close spontaneously.
c. The diagnosis of tricuspid atresia and associated lesions can be made by echocardiography. The size of the interatrial communication is important; if it is inadequate, a balloon atrial septostomy may be needed (rare in tricuspid atresia).
d. With normally connected arteries, a progressive decrease in pulmonary flow is usual over time. It is caused by diminution of the size of the VSD and pulmonary outflow obstruction (usually dynamic).
e. Rarely, there is no significant obstruction to either outflow, and the infant could require a pulmonary artery band to prevent overcirculation and to allow the pulmonary artery pressure and resistance to fall to adequate levels for single ventricle palliation.
f. In infants with tricuspid atresia, TGA, and a restrictive VSD, the Damus–Kaye–Stansel procedure plus an aortopulmonary shunt may be performed in preference to the pulmonary artery banding, which carries a higher mortality than a simple aortopulmonary shunt (5%-15%).
g. A Fontan-type operation is considered the definitive surgery in both types of tricuspid atresia (Fig. 12-9). The Fontan baffle or extracardiac conduit provides a route between the IVC and the pulmonary artery. It may be performed with or without fenestration (depending on the institution and the patient).
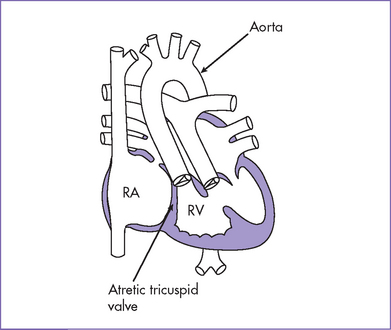
a. Tricuspid atresia is a cyanotic form of congenital heart disease where there is no real or potential direct connection between the RA and RV.
b. In all cases, there is an interatrial communication and almost always a VSD in the outlet septum.
c. There are three types of tricuspid atresia.
d. Other cardiac anomalies are associated with tricuspid atresia.
e. In tricuspid atresia, the systemic venous return has to pass from the RA to the LA via the interatrial communication. Thus, mixing of the systemic venous and pulmonary venous blood occurs in the LA, and this blood then passes into the LV and subsequently to the aorta and pulmonary artery. This systemic and pulmonary admixture results in systemic arterial desaturation.
f. The oxygen saturation depends on the pulmonary blood flow, which is influenced by:
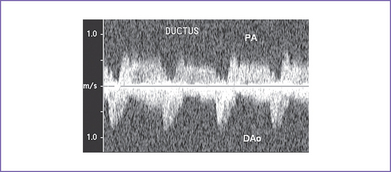
Fig. 12-6 Pulsed Doppler in the ductus arteriosus of the fetus from Figure 12-5 showing left-to-right shunt toward the pulmonary artery (PA) from the descending aorta (DAo).
b. Originally described for patients with tricuspid atresia, the procedure has now been extended to most forms of single-ventricle circulation.
c. There are numerous variations in the surgical approach. The most likely types of Fontan procedure to be encountered today are:
d. The Fontan procedure may be done as a single or a staged procedure. The staged procedure consists of a classic or bidirectional Glenn shunt performed as the first procedure, followed by the completion of the Fontan as a second procedure.
2. Patients who have had a Fontan operation are at risk from the following:
b. Thromboembolism, both systemic and pulmonary.
c. Protein-losing enteropathy.
d. Progressive deterioration of ventricular function with or without AV valve regurgitation, which may be part of the natural history of a patient with a single ventricle.
e. Hepatic dysfunction, usually resulting from hepatic congestion.
f. Right pulmonary vein compression or obstruction caused by compression from the enlarged RA or atrial baffle bulging into the left atrium.
g. Cyanosis: Worsening cyanosis can relate to:
a. Particular attention should be paid to:
b. Investigations are directed toward postoperative sequelae and vary according to the type of operation performed.
4. Indications for reintervention.
a. Residual ASD resulting in a significant right-to-left shunt, symptoms, or cyanosis.
b. Residual shunt secondary to a previous palliative surgical shunt or residual ventricle–to–pulmonary artery connection.
c. Significant systemic AV valve regurgitation.
d. Obstruction in the Fontan circuit.
e. Development of venous collateral channels or pulmonary arteriovenous malformations.
f. Development of sustained atrial flutter/fibrillation. An immediate attempt to restore sinus rhythm is crucial once RA thrombus has been excluded.
h. Pulmonary venous obstruction.
i. High-degree AV block or sick sinus syndrome necessitating pacemaker insertion.
j. Planned closure of a fenestrated Fontan (transcatheter).
a. Patients with systemic AV valve regurgitation might require AV valve repair or replacement.
b. Patients with significant residual shunts might require closure of the residual shunt.
c. Patients with significant obstruction at the Fontan anastomosis may be candidates for balloon angioplasty, stenting, or surgical revision of the Fontan connection.
d. Patients whose anastomosis is a valved conduit (RA–RV connection) might need Fontan revision or conversion to a different form of Fontan.
e. Patients with venous collateral channels might need transcatheter occlusion.
f. Patients with arteriovenous malformation might need conversion of a classic Glenn shunt to a bidirectional Glenn.
g. Patients with poorly controlled atrial flutter might be candidates for catheter ablation.
h. Conversion of a classic Fontan to a lateral tunnel or external conduit with concomitant atrial maze procedure may be considered for treating serious refractory atrial arrhythmias.
i. If permanent pacing is required, epicardial AV sequential pacing should be employed whenever possible to reduce the risk of thromboembolism.
j. Patients with PLE might be candidates for fenestration in the atrial septum or revision of the Fontan. Alternatively, subcutaneous heparin, octreotide treatment (a synthetic version of the naturally occurring hormone somatostatin), and prednisone therapy have also been tried with variable success. No one therapy seems more successful than the others.
k. Transplantation may be necessary for systemic ventricular failure or intractable PLE.
Note: The role of long-term anticoagulation is contentious. Anticoagulation is recommended in patients with a history of documented atrial flutter/fibrillation, fenestration in the Fontan connection, or spontaneous contrast (smoke) in the right atrium on echocardiography.
III. TAKE-HOME MESSAGE
B. Treatment
1. The counseling and management of tricuspid atresia will depend on the associated lesions. Thus, it is important to define arterial connections and identify pulmonary or systemic obstructive lesions.
2. Children with tricuspid atresia have the lowest risk for Fontan repair among children undergoing this form of treatment (compared to patients with other forms of functionally univentricular hearts, e.g., hypoplastic left heart syndrome).
C. Prognosis
1. Fetal survival in the case of tricuspid atresia and normally related great arteries is possible through the accommodation of the entire systemic return by the widely patent foramen ovale.
2. With both normally related and transposed great arteries, a large VSD allows blood to flow from the well-developed LV to the underdeveloped RV, thus allowing some development of the RV.
3. Postnatal arterial oxygen saturation depends on the respective fraction of the pulmonary and systemic venous return that enters the LV through the patent ductus arteriosus and the VSD (if present).
Allan LD. The outcome of fetal congenital heart disease. Semin Perinatol. 2000;24(5):380-384.
Allan LD, Huggon IC. Counselling following a diagnosis of congenital heart disease. Prenat Diagn. 2004;24(13):1136-1142.
Allan LD, Sharland GK. Prognosis in fetal tetralogy of Fallot. Pediatr Cardiol. 1992;13(1):1-4.
Azakie A, McCrindle BW, Van Arsdell G, et al. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: Impact on outcomes. J Thorac Cardiovasc Surg. 2001;122(6):1219-1228.
Cohen MS, Marino BS, McElhinney DB, et al. Neo-aortic root dilation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. Am Coll Cardiol. 2003;42(3):533-540.
De Vore GR, Siassi B, Platt LD. Fetal echocardiography: The prenatal diagnosis of tricuspid atresia (type Ic) during the second trimester of pregnancy. J Clin Ultrasound. 1987;15(5):317-324.
Ferencz C, Rubin JD, Loffredo CA, Magee CA: Epidemiology of Congenital Heart Disease: The Baltimore–Washington Infant Study, 1981-1989. Perspectives in Pediatric Cardiology, . New York;Futura: 1993;vol 4.
Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240-248.
Jacobs ML, Schneider DJ, Pourmoghadam KK, et al. Total cavopulmonary connection to one lung. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:72-79.
Ro PS, Rychik J, Cohen MS, et al. Diagnostic assessment before Fontan operation in patients with bidirectional cavopulmonary anastomosis: Are noninvasive methods sufficient? J Am Coll Cardiol. 2004;44(1):184-187.
Stamm C, Friehs I, Mayer JEJr, et al. Long-term results of the lateral tunnel Fontan operation. J Thorac Cardiovasc Surg. 2001;121(1):28-41.
Trines J, Hornberger LK. Evolution of heart disease in utero. Pediatr Cardiol. 2004;25(3):287-298.

 B.
B.