CHAPTER 61 Tricuspid and Pulmonary Valvular Disease
The tricuspid and pulmonary valves can fail as a result of disease states, which may lead to stenosis (Figs. 61-1 to 61-3), insufficiency (Figs. 61-4 to 61-6), or a combination of both (Figs. 61-7 to 61-9). Stenosis impedes forward flow, resulting in pressure overload, and insufficiency results in blood flowing backward, leading to volume overload. Tricuspid and pulmonary valvular disease may present in isolation or in conjunction. Clinical presentation, involvement of the cardiac chambers, severity of secondary chamber dysfunction, and impact on systemic and pulmonary vasculature and other viscera depend on which valves are involved, which type of valvular dysfunction predominates, the severity and duration of valvular dysfunction, and the degree of cardiac compensation. Pharmacologic, endovascular, and surgical management for diseased valves relies on accurate and comprehensive noninvasive diagnostic evaluation, using a combination of chest radiography, echocardiography, magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and computed tomography angiography (CTA). Invasive catheterization is no longer used to diagnose primarily tricuspid and pulmonary valvular disease and should be reserved for cases in which flow dynamics require direct assessment. Noninvasive modalities also form the basis for surveillance imaging.
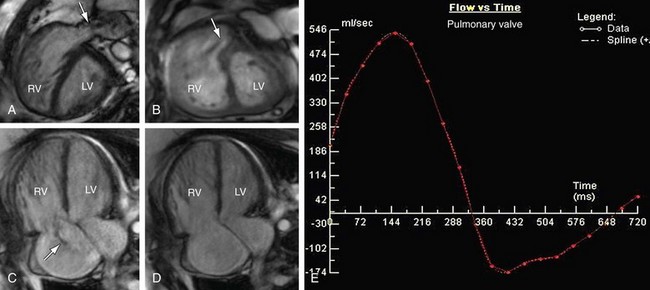
 FIGURE 61-9 Pulmonary stenosis and insufficiency with tricuspid insufficiency—functional analysis. Steady-state free precession short-axis and four-chamber projections from the patient shown in Figure 61-8 are shown in systole (A, C) and diastole (B, D). Turbulent signal (arrows) at the pulmonary valve corresponds to stenosis (A) and insufficiency (B). C, Turbulent signal (arrow) at the tricuspid valve corresponds to insufficiency. E, Quantitative analysis from phase contrast imaging yielded a pulmonary regurgitant fraction of approximately 25%.
FIGURE 61-9 Pulmonary stenosis and insufficiency with tricuspid insufficiency—functional analysis. Steady-state free precession short-axis and four-chamber projections from the patient shown in Figure 61-8 are shown in systole (A, C) and diastole (B, D). Turbulent signal (arrows) at the pulmonary valve corresponds to stenosis (A) and insufficiency (B). C, Turbulent signal (arrow) at the tricuspid valve corresponds to insufficiency. E, Quantitative analysis from phase contrast imaging yielded a pulmonary regurgitant fraction of approximately 25%.
TRICUSPID REGURGITATION
Anatomy and Normal Function of the Tricuspid Valve
The tricuspid valve is the right-sided atrioventricular valve, situated anterior and slightly apical in relation to the left sided mitral atrioventricular valve. It is aligned vertically, with anterior-left lateral angulation, in plane with the right ventricle and an orifice that is the largest of the four cardiac valves. The tricuspid valve is further distinguished from the mitral valve in that the mitral valve anteroseptal border is continuous with the aortic valve apparatus, whereas the tricuspid valve does not have any direct continuity to the pulmonary arterioventricular valve. Structurally, the tricuspid valve is composed of an annulus, three leaflets, and three commissures, which together with the right ventricle’s chordae tendinae, corresponding papillary muscles, and adjacent right atrium and right ventricle myocardium, form the tricuspid valve complex.1 The major function of the complex is to control leaflet aperture and closure. This process is governed by the pressure gradient between the right atrium and right ventricle, which in turn is regulated by preload, afterload, and myocardial contractility. Tricuspid valve opening occurs during ventricular diastole. It is closed during systole, preventing backflow. Physiologic tricuspid regurgitation occurs during systole; however, it may be found in structurally normal hearts of 17% to 65% of adults2–6 and 6.3% to 71% of pediatric patients,6,7 with most regurgitation being trivial to mild.
With regard to the components of the tricuspid valve complex, the annulus anchors the tricuspid valve to the right trigone of the cardiac fibrous skeleton, providing firm support for the entire complex. It is oval in shape and slightly larger than the mitral valve annulus, demarcating the boundaries of the tricuspid orifice.8 The annulus contracts during systole and reaches maximum size at end-systole.9 The mean major diastolic annulus diameter, area, and fractional shortening during three-dimensional echocardiography are reported to be 3.9 to 4.3 mm, 10 to 18.4 cm2, and 18.5% to 26%, respectively,1,8,10 whereas MRI values are reported to be 4.4 mm, 18.7 cm2, and 27%, respectively.8 Two-dimensional echocardiography underestimates annulus diameter and fractional shortening, with reported four-chamber values of 2.9 to 3.3 mm and 13.5% to 19%, respectively.
The three leaflets, labeled as anterior, posterior, and septal, are asymmetric in size, shape, and function.1 Each is attached to the annulus and separated and supported by the three commissures—anteroposterior, anteroseptal, and posteroseptal. Using three-dimensional echocardiography, Anwar and colleagues1 have reported the valve area to be 4.8 cm2 and each commissure to have an approximate width of 5 mm.
The anterior leaflet is the largest and most mobile, with a semicircular shape and an average width of 3.7cm.11 It is located anteriorly along the right ventricular free wall, extending from the anterolateral to inferolateral margin. The septal leaflet has a semioval shape, paralleling the interventricular septum, from anteroseptal margin to the posterior right ventricle wall. It is typically smaller than anterior leaflet, but is reported to have a width up to 3.6 cm.11 The septal leaflet is the least mobile of the three leaflets. The posterior leaflet extends along the posterior margin of the annulus from the inferolateral margin of the right ventricular free wall to the septum. It is variable in size and morphology, containing one or more (up to four) scallops, with an average width up to 2.8 cm.11
The chordae tendinae and papillary muscles form the subvalvular apparatus. The apparatus generates opposing tension and functions to prevent leaflet prolapse into the right atrium. There are three groups of papillary muscles, labeled as anteroseptal, anteroposterior, and posteroseptal.12 The anteroseptal and anteroposterior group may have up to four muscles, whereas the posteroseptal group may have up to five.12 Each muscle is paired with chordae tendineae which insert onto the leaflets. There are five distinct types of tricuspid valve chordae tendineae, each with variable leaflet distribution and insertion.11
Tricuspid regurgitation most commonly is secondary to other diseases such as left heart failure, pulmonary regurgitation or hypertension, or chamber enlargement, which can result in dilation of the valve annulus.13 Other causes of tricuspid regurgitation include rheumatic heart disease and endocarditis.14
Manifestations of Disease
Clinical Presentation
In the setting of tricuspid regurgitation, decreased cardiac output can cause fatigue and decreased exercise tolerance.15 Right atrial pressure elevation can result in lower extremity edema, hepatic congestion, and abdominal fullness. Chronic severe tricuspid regurgitation can lead to ascites and anasarca.
Patients often have a holosystolic murmur, but this is not common when there is severe regurgitation with equalization of right atrial and ventricular pressures.16 Jugular venous distention occurs in up to 75% of patients and hepatomegaly in 90%.15
Imaging Technique and Findings
Ultrasound
Echocardiography is the mainstay of tricuspid valve imaging, allowing the identification of tricuspid regurgitation and evaluation of the severity and cause. Two-dimensional echocardiography is the traditional method of evaluation and can include color Doppler flow mapping. Three-dimensional echocardiography may hold some advantages over the two-dimensional technique because of the complicated three-dimensional structure of the right ventricle.17 Doppler echocardiography can be used for semiquantitative grading of tricuspid regurgitation or for functional parameters such as change in pressure over time.18,19
Magnetic Resonance Imaging
MRI is able to provide accurate quantitative evaluation of regurgitant volumes. Velocity-encoded cine (VEC) phase contrast MRI of the tricuspid valve can be performed. Because of the motion of the valve during the cardiac cycle, direct measurement of valvular regurgitation is challenging. Another approach for quantification of tricuspid regurgitation is to calculate total right ventricular stroke volume using cine steady-state free precession imaging (see Chapter 15) and the antegrade stroke volume using a VEC MRI sequence prescribed in the pulmonary artery. The regurgitant volume is the difference between total right ventricular stroke volume and forward stroke volume.
MRI also has the advantage of yielding highly accurate and reproducible volumes of the right ventricle, whose complex shape limits the usefulness of formulas used for estimation.20
PULMONARY STENOSIS
Anatomy and Normal Function of the Pulmonary Valve
The right ventricular infundibulum and fibrous semilunar cusps provide the core structural support for the pulmonary valve. Each semilunar cusp extends from the sinotubular junction to merge with the circumferential infundibulum. The valve leaflets are incorporated into the cusps, terminating inferiorly at the ventriculoarterial junction, to define the annulus margin. Superiorly, at the sinotubular junction, the commissures provide zones of attachment for the leaflets, which are directed and open toward the main pulmonary artery.21
Using three-dimensional echocardiography to evaluate adult patients referred for assessment of congenital heart disease, Anwar and associates1 have reported the maximum diameters of the right ventricular outflow tract (infundibulum) and pulmonary valve annulus to be 22 and 19.4 mm, respectively. Two-dimensional echocardiography underestimated both regions, with measurements in the long- and short-axis projections to be 19.7 and 16.4 mm and 16.4 and 13.5 mm, respectively.22
Similar to tricuspid valve function, pulmonary valvular aperture and coaptation is determined by the relative pressure gradient across the valve, between the right ventricle and pulmonary arterial system. This gradient, in turn, is regulated by right ventricular preload, pulmonary afterload, and right ventricular contractility. The valve is open during ventricular systole when pressure in the right ventricle rises above the pressure in the pulmonary system. The valve closes during ventricular diastole, when right ventricular pressure falls below the pulmonary pressure. Physiologic pulmonary regurgitation during diastole, however, may be found in structurally normal hearts of 5% to 92% of adults2–6 and 22% to 75% of pediatric patients,6,7 with most regurgitation being trivial to mild.
More than 95% of the time, pulmonary stenosis is congenital in cause.15 A common association with pulmonary stenosis (PS) is Noonan syndrome. Acquired causes of valvular pulmonary stenosis include rheumatic heart disease and carcinoid syndrome, but isolated pulmonary valve involvement is rare.
Manifestations of Disease
Clinical Presentation
PS usually causes symptoms only when severe. Symptoms include fatigue and dyspnea, lightheadedness on exertion, or syncope.15 Right heart failure can occur with long-standing severe PS. On physical examination, patients have a pulmonary ejection murmur.
Imaging Technique and Findings
Ultrasound
Echocardiography is the primary imaging modality for the evaluation of valvular PS. Characteristic findings include thickened valve leaflets and doming of the valve during systole.23 Continuous-wave Doppler technique can be used to obtain peak velocity. The pressure gradient is derived using the modified Bernoulli equation (see Chapter 17). PS is considered to be mild if the pressure gradient is less than 36 mm Hg, moderate if between 36 and 60 mm Hg, and severe if greater than 60 mm Hg.23
Surgical and Interventional Therapy
Moderate PS with a peak gradient of more than 50 mm Hg and severe PS require intervention.23 Percutaneous balloon valvuloplasty is the usual treatment, but most patients may develop pulmonary regurgitation after valvuloplasty.25 Open or percutaneous prosthetic valve placement are therapeutic options but are less commonly used than balloon valvuloplasty.
PULMONARY REGURGITATION
Pulmonary regurgitation (PR) is most commonly secondary to prior intervention, such as surgery for tetralogy of Fallot or valve repair or valvuloplasty for PS.26,27 Rheumatic heart disease and endocarditis are rare causes of PR.
Manifestations of Disease
Clinical Presentation
PR results in right ventricular enlargement, and chronic severe regurgitation can lead to right ventricular dysfunction, diminished cardiac output, and ultimately heart failure.15 Arrhythmias and sudden death can also occur.28 Patients with PR typically have a diastolic murmur.
Imaging Technique and Findings
Ultrasound
The diagnosis of PR is usually made by echocardiography, which shows regurgitant color stream, increased velocity measurement. Color Doppler technique can be used to evaluate the severity of PR and to assess right ventricular pressure. The degree of PR can be graded on a 1 to 4 scale.15 Echocardiography can underestimate the degree of PR if the PR is unrestricted and the pulmonary pressure is low.19
Magnetic Resonance Imaging
MRI yields accurate quantitative evaluation of regurgitant volumes using the VEC technique. Because echocardiography has the potential to underestimate the severity of PR, MRI has a key role in the quantification of regurgitation, especially after treatment for tetralogy of Fallot.29
VEC MRI can be used to interrogate flow in the main pulmonary artery and to determine the regurgitant volume and fraction.30
As noted, MRI has the capability of generating highly accurate and reproducible volumes of the right ventricle (see Chapter 15), which is especially important in congenital heart disease, such as tetralogy of Fallot.20 Information on the severity of PR and right ventricular volumes are used to determine the necessity for pulmonary valve repair.30,31
Synopsis of Treatment Options
Medical therapy has not been shown to reduce PR or its effect on the right ventricle.15 Pulmonary valve replacement (Fig. 61-10) is recommended for PR for several indications, most commonly manifestation of symptoms, including arrhythmia, decreased right ventricular function (ejection fraction <40%, as assessed by MRI), and right ventricular enlargement (MRI end-diastolic volume, 160 mL/m2).15
Altrichter PM, Olson LJ, Edwards WD, et al. Surgical pathology of the pulmonary valve: a study of 116 cases spanning 15 years. Mayo Clin Proc. 1989;64:1352-1360.
Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;188:e523-e661.
Bonow RO, Carabello BA, Kanu C, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines: ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84-e231.
Hauck AJ, Freeman DP, Ackermann DM, et al. Surgical pathology of the tricuspid valve: a study of 363 cases spanning 25 years. Mayo Clin Proc. 1988;63:851-863.
Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995-3014.
Lam YY, Kaya MG, Goktekin O, et al. Restrictive right ventricular physiology: its presence and symptomatic contribution in patients with pulmonary valvular stenosis. J Am Coll Cardiol. 2007;50:1491-1497.
Møller JE, Pellikka PA, Bernheim AM, et al. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005;112:3320-3327.
Ramadan FB, Beanlands DS, Burwash IG. Isolated pulmonic valve endocarditis in healthy hearts: a case report and review of the literature. Can J Cardiol. 2000;16:1282-1288.
1 Anwar AM, Geleijnse ML, Soliman OI, et al. Assessment of normal tricuspid valve anatomy in adults by real-time three-dimensional echocardiography. Int J Cardiovasc Imaging. 2007;23:717-724.
2 Berger M, Hecht SR, Van Tosh A, et al. Pulsed and continuous wave Doppler echocardiographic assessment of valvular regurgitation in normal subjects. J Am Coll Cardiol. 1989;13:1540-1545.
3 Choong CY, Abascal VM, Weyman J. Prevalence of valvular regurgitation by Doppler echocardiography in patients with structurally normal hearts by two-dimensional echocardiography. Am Heart J. 1989;117:636-642.
4 Kostucki W, Vandenbossche JL, Friart A, et al. Pulsed Doppler regurgitant flow patterns of normal valves. Am J Cardiol. 1986;58:309-313.
5 Klein AL, Burstow DJ, Tajik AJ, et al. Age-related prevalence of valvular regurgitation in normal subjects: a comprehensive color flow examination of 118 volunteers. J Am Soc Echocardiogr. 1990;3:54-63.
6 Yoshida K, Yoshikawa J, Shakudo M, Akasara T, et al. Color Doppler evaluation of valvular regurgitation in normal subjects. Circulation. 1988;78:840-847.
7 Brand A, Dollberg S, Keren A. The prevalence of valvular regurgitation in children with structurally normal hearts: a color Doppler echocardiographic study. Am Heart J. 1992;123:177-180.
8 Anwar AM, Soliman OI, Nemes A, et al. Value of assessment of tricuspid annulus: real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2007;23:701-705.
9 Tei C, Pilgrim JP, Shah PM, et al. The tricuspid valve annulus: study of size and motion in normal subjects and in patients with tricuspid regurgitation. Circulation. 1982;66:665-671.
10 Anwar AM, Geleijnse ML, Ten Cate FJ, et al. Assessment of tricuspid valve annulus size, shape and function using real-time three-dimensional echocardiography. Interact Cardiovasc Thorac Surg. 2006;5:683-687.
11 Silver MD, Lam JH, Ranganathan N, et al. Morphology of the human tricuspid valve. Circulation. 1971;43:333-348.
12 Joudinaud TM, Flecher EM, Duran CM. Functional terminology for the tricuspid valve. J Heart Valve Dis. 2006;15:382-388.
13 Cohn LH. Tricuspid regurgitation secondary to mitral valve disease: when and how to repair. J Card Surg. 1994;9:237-241.
14 Tang GH, David TE, Singh SK, et al. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation. 2006;114(suppl):I577-I581.
15 Bruce CJ, Connolly HM. Right-sided valve disease deserves a little more respect. Circulation. 2009;119:2726-2734.
16 Salazar E, Levine H. Rheumatic tricuspid regurgitation. Am J Med. 1962;33:111-129.
17 Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463.
18 Imanishi T, Nakatani S, Yamada S, et al. Validation of continuous wave Doppler-determined right ventricular peak positive and negative dP/dt: effect of right atrial pressure on measurement. J Am Coll Cardiol. 1994;23:1638-1643.
19 Zoghbi WA, Enriquez-Sarano M, Foster E, et al. American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777-802.
20 Sechtem U, Pflugfelder PW, Gould RG, et al. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology. 1987;163:697-702.
21 Anderson RH, Freedom RM. Normal and abnormal structure of the ventriculo-arterial junctions. Cardiol Young. 2005;15(suppl 1):3-16.
22 Anwar AM, Soliman OI, ten Cate FJ, et al. True mitral annulus diameter is underestimated by two-dimensional echocardiography as evidenced by real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2007;23:541-547.
23 Bonow R, Carabello B, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84-e231.
24 Hayes C, Gersony W, Driscoll D, et al. Second natural history study of congenital heart defects: results of treatment of patients with pulmonary valvar stenosis. Circulation. 1993;87(suppl):I28-I37.
25 Garty Y, Veldtman G, Lee K, Benson L. Late outcomes after pulmonary valve balloon dilatation in neonates, infants and children. J Invasive Cardiol. 2005;17:18-22.
26 Roos-Hesselink JW, Meijboom FJ, Spitaels SE, et al. Long-term outcome after surgery for pulmonary stenosis (a longitudinal study of 22-33 years). Eur Heart J. 2006;27:482-488.
27 Oechslin E, Harrison D, Harris L, et al. Reoperation in adults with repair of tetralogy of Fallot: indications and outcomes. J Thorac Cardiovasc Surg. 1999;118:245-251.
28 Therrien J, Siu S, Harris L, et al. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001;103:2489-2494.
29 Rebergen SA, Chin JG, Ottenkamp J, et al. Pulmonary regurgitation in the late postoperative followup of tetralogy of Fallot: volumetric quantitation by NMR velocity mapping. Circulation. 1993;88:2257-2266.
30 Varaprasathan GA, Araoz PA, Higgins CB, Reddy GP. Quantification of flow dynamics in congenital heart disease: applications of velocity-encoded cine MR imaging. Radiographics. 2002;22:895-906.
31 Therrien J, Siu SC, McLaughlin PR, et al. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670-1675.

 FIGURE 61-1
FIGURE 61-1
 FIGURE 61-2
FIGURE 61-2
 FIGURE 61-3
FIGURE 61-3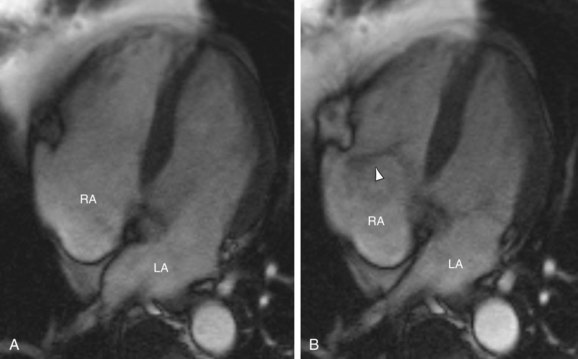
 FIGURE 61-4
FIGURE 61-4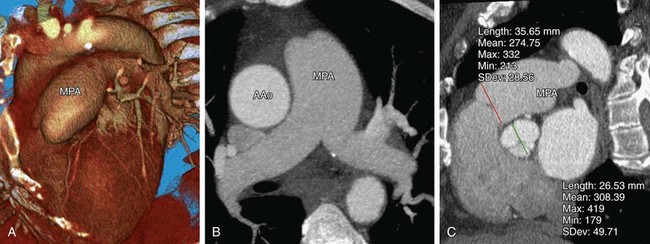
 FIGURE 61-5
FIGURE 61-5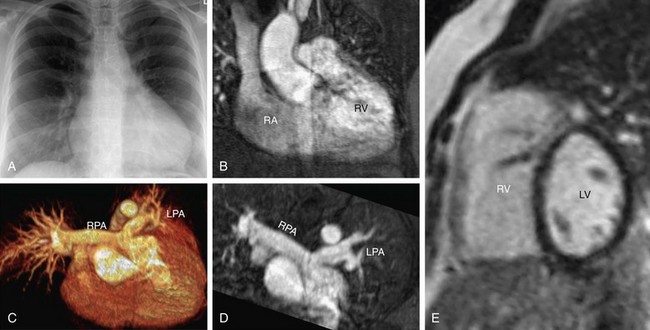
 FIGURE 61-6
FIGURE 61-6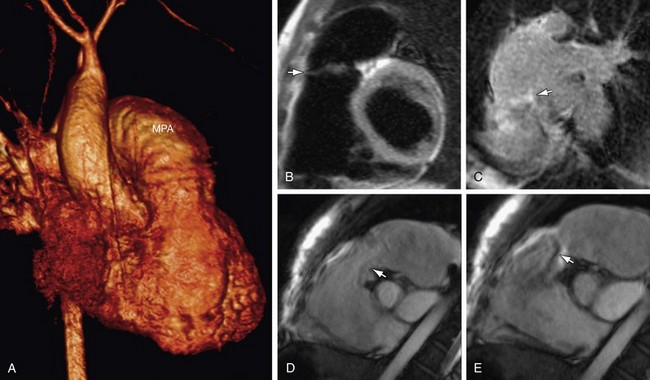
 FIGURE 61-7
FIGURE 61-7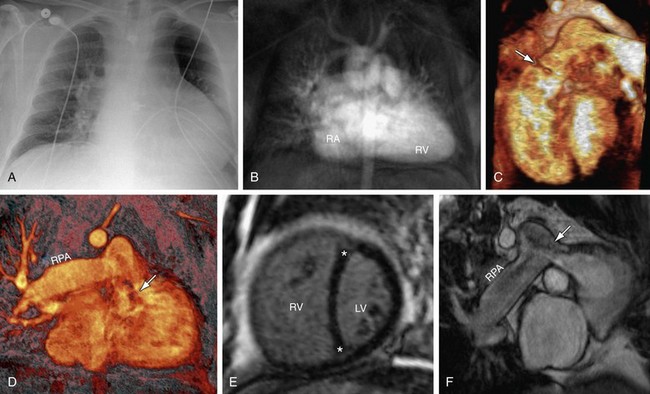
 FIGURE 61-8
FIGURE 61-8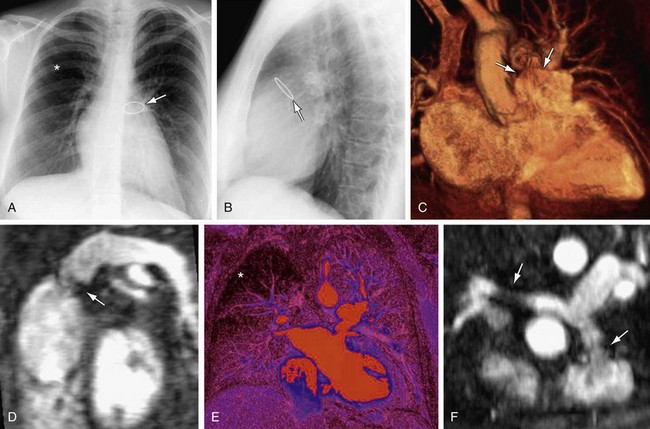
 FIGURE 61-10
FIGURE 61-10